Abstract
The extracellular matrix of articular cartilage is structurally specialized for efficient absorption of mechanical impact. In particular, giant aggregates of the large chondroitin sulfate proteoglycan, aggrecan, with the glycosaminoglycan, hyaluronan, allow cartilage to resist compressive load. Proteolysis of aggrecan by members of the proteinase family ADAMTS (A disintegrin-like and metalloproteinase domain with thrombospondin type 1 motif), was identified as an early step in the inexorable destruction of cartilage in osteoarthritis (OA). Of the investigated proteinases, ADAMTS5 has emerged as a principal mediator of aggrecan loss in OA, convincingly so in mouse models, and with high probability in humans. ADAMTS5 has a bipartite organization, comprising a proteinase domain and an ancillary domain containing exosites for interaction with aggrecan and other substrates. In a recent issue of this journal, Santamaria et al. characterized anti-ADAMTS5 monoclonal antibodies isolated from a phage display library. By blocking the catalytic site of the ADAMTS5 immunogen with a synthetic inhibitor, the authors of the paper biased selection of antibodies to the ancillary domain. This work, together with other antibodies targeting ADAMTS5, offers diverse, high-affinity and, as far as can be determined, selective aggrecanase inhibitors. Mapping of their epitopes provided novel insights into ADAMTS5 interactions with aggrecan. These monoclonal antibodies deserve continued investigation for potential arthritis therapy, although their successful use will require a comprehensive understanding of the physiological roles of ADAMTS5, and its regulation, intrinsic properties and intermolecular interactions.
Keywords: ADAMTS proteinase, aggrecan, arthritis, articular cartilage, metalloproteinase, osteoarthritis
Osteoarthritis (OA) is a common ‘degenerative’ disorder of synovial joints resulting from loss of articular cartilage, reactive changes in subchondral bone and varying degrees of synovial inflammation. Its manifestations are joint pain, joint stiffness and, periodically, joint swelling, which reduce mobility and adversely affect the quality of life. Although it can affect any synovial joint, OA of the hip, knee, spine and hands commands the most clinical attention. OA is a progressive, irreversible condition that is common in both sexes after the fifth decade of life. Its incidence and severity within the population increase steadily thereafter, portending epidemic proportions as life expectancy improves globally. Current pharmacological treatment addresses the symptoms of OA without modifying the disease itself. Definitive resolution of OA is provided only by joint arthroplasties, which are major, expensive, surgical procedures requiring advanced medical facilities, unavailable in many parts of the world.
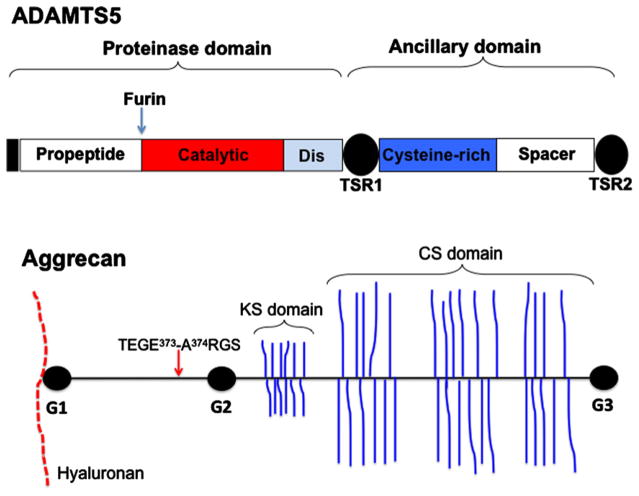
These imperatives, especially the prospect of a large ageing population, have driven investigation of OA mechanisms as a gateway to the development of disease-modifying therapy. The current view of OA pathobiology is that it is a disease of the entire joint, but arises from progressive loss of articular cartilage, with secondary effects on underlying bone and articular soft tissues. Articular cartilage comprises chondrocytes, present at a relatively low cell density, and an abundant extracellular matrix, the components and networks of which have evolved specifically to absorb mechanical impact. Aggrecan, a large sulfated proteoglycan that forms giant supramolecular aggregates with the glycosaminoglycan (GAG) hyaluronan (HA) [1,2], is a major cartilage constituent. Aggrecan core protein comprises three globular domains – G1, G2 and G3 – and the region between the G2 and G3 domains is modified by attachment of the GAGs keratan sulfate (KS) and chondroitin sulfate (CS) [3] (Figure 1). Extensive sulfation of KS/CS and aggregation with HA generates a substantial fixed negative charge that renders the aggregates highly hydrated, and the resulting swelling pressure confers the desired viscoelastic properties to cartilage. HA is bound by the G1 domain in a ternary complex which includes a cartilage link protein, whereas the G3 domain interacts with several matrix molecules such as fibulin-1 and -2, fibrillin-1, and tenascin-C and -R [3]. Thus, aggrecan contributes unique intrinsic properties and is an indispensable component of a crucial network in cartilage matrix.
Figure 1. ADAMTS5 and aggrecan, the protagonists of this commentary.
The schematic shows the domain structure of ADAMTS5 and aggrecan. The furin cleavage site in ADAMTS5 and the interglobular domain cleavage site for ADAMTS5 in aggrecan are shown. Dis, disintegrin-like module.
The swelling pressure exerted by HA–aggrecan is constrained by a network of collagen II-rich fibrils, associated with small leucine-rich proteoglycans [4]. Through the compressive resilience provided by HA–aggrecan aggregates, and the resistance to shear and tension provided by the fibrils, cartilage efficiently absorbs multiaxial loads. Loss of aggrecan, an early hallmark of OA, results not from physical ‘wear and tear’, but from an active, protease-mediated catabolic process, the instigating factors of which include joint trauma, joint malalignment or genetic variations that weaken cartilage extracellular matrix. Aggrecan depletion is believed to expose surface molecules on collagen fibrils and, subsequently, the collagen II fibrils themselves to proteolytic degradation [5,6], by which point the disease process is well nigh irreversible. Evidence also exists for feedback loops in which the products of cartilage catabolism potentiate joint damage [7–9]. For these reasons, aggrecan catabolism became a major focus of many academic and pharmaceutical laboratories.
The paper by Santamaria et al. [10] in the Biochemical Journal, as well as other recent successes in developing selective, high-affinity, inhibitory antibodies [11,12], is the culmination of almost three decades of intense research on mechanisms of aggrecan proteolysis. The sequence of discoveries tells a remarkable scientific story. The concept of ‘aggrecanase’ as a catabolic entity distinct from known matrix-degrading proteinases, then chiefly the matrix metalloproteinases (MMPs), first arose in the early 1990s. Sandy et al. [13,14] noted that the bulk of aggrecan found in OA synovial fluid had the N-terminus A374RGS (mature human/bovine aggrecan sequence enumeration), and attributed cleavage at the Glu373–Ala374 peptide bond in the ‘interglobular domain’ (IGD), i.e. between G1 and G2, to a putative novel proteinase (Figure 1). In contrast, cartilage matrix contained the remnant N-terminal, hyaluronan-binding region of aggrecan (Figure 1). The development of antibodies specifically detecting the new IGD N- and C-termini (neoepitope antibodies) [15], and other neoepitopes within the CS-bearing domain [16], was a major advance, enabling the discovery of aggrecanase. Anti-A374RGS neoepitope antibodies identified aggrecanase-derived cleavage products in OA synovial fluid and showed intense staining in OA cartilage. Staining was also evident in histologically normal cartilage, suggesting that OA could be incipient with a long latency, although the staining could also have arisen from physiological matrix turnover in the chondrocyte pericellular matrix [17]. The Glu373–Ala374 cleavage was detectable in aggrecan fragments released from retinoic acid or interleukin-1-treated cartilage explants and chondrocytes, providing a system for in vitro manipulation, characterization and finally isolation of the putative aggrecanase(s) [18,19]. The identity of aggrecanase remained a mystery for a while, because cognate matrix-degrading enzymes such as MMPs were unable to reproduce the activity efficiently, until Tortorella et al. [20] identified a disintegrin-like and metalloproteinase domain with thrombospondin type 1 motif 4 (ADAMTS4) as aggrecanase-1. Subsequently, ADAMTS5 (redundantly numbered ADAMTS11), which was concurrently cloned in a search for novel metalloproteinases, was identified as aggrecanase-2 [21,22]. Joint protection in mice that had an aggrecan knock-in mutation to prevent cleavage of Glu373–Ala374 provided compelling justification for targeting ADAMTS-mediated aggrecanolysis [5]. The resistance of Adamts5- but not Adamts4-mutant mice to mechanical instability or inflammation-induced cartilage aggrecan loss was a pivotal discovery that pointed to ADAMTS5 as the major aggrecanase in mice [23,24].
Similar to other ADAMTS proteinases, ADAMTS5 (see Figure 1) has a proteinase domain and a large non-catalytic (or ancillary) domain [22,25]. The proteinase domain comprises the propeptide, which needs to be excised by proprotein convertases to reveal proteolytic activity [26,27], the catalytic module and the disintegrin-like module (see Figure 1). The crystal structure of ADAMTS5 has shown that the catalytic and disintegrin-like modules both participate in substrate engagement [28,29]. ADAMTS5 comprising only the proteinase domain cleaved native aggrecan inefficiently, implying the requirement for additional contacts between the ancillary domain (termed ‘exosites’) and aggrecan [30,31]. This finding was consistent with the role of the ancillary domains of other ADAMTS proteinases in cleavage of native substrates, e.g. cleavage of von Willebrand factor by ADAMTS13 [32] and procollagen I processing by ADAMTS2 [33]. Santamaria et al. [10] postulated that exosites could be specific for different ADAMTS5 substrates; thus, antibodies or other molecules targeting ADAMTS5 exosites required for aggrecan cleavage may spare catalytic activity towards other substrates.
Santamaria et al. [10] used recombinant ADAMTS5 which included the spacer module, and they blocked the catalytic cleft with the peptidomimetic, broad-spectrum, zinc-chelating, active-site metalloproteinase inhibitor GM6001 to enhance isolation of ancillary domain antibodies from a phage antibody library. They used surface plasmon resonance, domain-specific deletion constructs of ADAMTS5, spatial occlusion of the catalytic domain by the endogenous inhibitor tissue inhibitor of metalloproteinases 3 (TIMP3) [34] and molecular modelling to define the epitopes for these antibodies [10]. The four selected antibodies showed inhibitory activity against ADAMTS5, but not against ADAMTS4 or a panel of selected metalloproteinases, and bound specifically to ADAMTS5 but not to ADAMTS4 in surface plasmon resonance assays. The antibodies 2D3 and 2D11 bound to distinct surfaces in the catalytic/disintegrin-like modules, the epitope for 2D5 was at the interface between the catalytic/disintegrin-like modules and TS type 1 module 1 (TSR1), and 2B9 bound to the ADAMTS5 spacer. Following their previous work [31], the paper by Santamaria et al. [10] shows that the spacer is crucial for cleavage of aggrecan, via interaction with the aggrecan core protein but not with the GAG chains, which bind to the ADAMTS5 cysteine-rich domain (see Figure 1). Interestingly, 2B9 did not inhibit aggrecan release from a chondrocyte monolayer because in this system the prevalent form of ADAMTS5 lacked the spacer, and hence the antibody’s epitope [10]. This leads to legitimate concerns about inhibitory antibodies to the ancillary domain, and the need for a detailed knowledge of truncated ADAMTS5 isoforms in OA.
Although Santamaria et al. [10] did not test their antibodies in animal models, two recent publications demonstrated the efficacy of aggrecanase inhibition in vivo using monoclonal antibodies. Chiusaroli et al. [11] identified CRB0017, a recombinant ADAMTS5 monoclonal antibody of high affinity and selectivity against the spacer. Intra-articular injection of CRB0017 in STR/ort male mice, which spontaneously develop OA, resulted in significant chondroprotection [11]. Larkin et al. [12] developed selective high-affinity antibodies against ADAMTS4 and ADAMTS5. They demonstrated that the ADAMTS5 antibody GSK2394002 was chondroprotective in both mice and cynomolgus monkeys, and could reduce pain-associated allodynia in mice [12]. GSK2394002 recognizes an epitope spanning the catalytic and disintegrin-like modules and appears to work by an ‘allosteric lock effect’ on ADAMTS5’s active site [12]. Molecular imaging demonstrated its successful targeting to cartilage after intraperitoneal injection. Although ADAMTS5 is unequivocally implicated as the chief aggrecanase in mice, the identity of the major human aggrecanase is somewhat controversial [35,36]. Larkin et al. [12] suggest that ADAMTS5 is the primary target for human OA as well, because anti-ADAMTS5 antibodies effectively suppressed release of the ARGS epitope from human knee cartilage explants.
By any measure, the story of aggrecanase leading up to its selective targeting is a notable success of modern biomedical research, and a fine example of academic–industrial collaboration and synergy. How does one build on this success while being mindful of the wisdom accrued from previous failure? The aggrecanase armamentarium available for prospective OA therapy also includes several small-molecule, active-site inhibitors (reviewed in Dancevic and McCulloch [37]). Small-molecule, active-site inhibitors are cheaper than antibody drugs and orally bioavailable, but often lack the exquisite specificity of well-characterized monoclonal antibodies, a lesson learned from failed attempts to treat cancer with MMP inhibitors [38]. In addition to the lack of fine specificity of active-site inhibitors, this failure revealed how little was known about the complex biology of proteinases in cancer and normal tissue turnover [38]. The human genome carries little dead weight, and ADAMTS5, which has evolved over millennia, is required for cardiovascular and limb development, widely expressed in adult mice, and potentially involved in wound healing via processing of versican and/or other substrates [39–44]. All inhibitors have potential side effects. Santamaria et al. [10] suggest that exosite-specific antibodies could be one way of selectively inhibiting proteolysis of aggrecan, which is located primarily in cartilage, fibrocartilage of tendons and menisci, and the brain, which is protected by the blood–brain barrier. What is needed now is an in-depth understanding of the physiological roles of ADAMTS5, including comprehensive understanding of its substrate repertoire, which currently includes aggrecan, versican and brevican, located in the brain. The blockage of the turnover of versican, which is present in the cardiovascular system and elsewhere, is probably inadvisable and may lead to collateral damage. Consideration could be given to dosing or delivery methods that maximize the effect on cartilage, but spare other tissues. Some possibilities include intra-articular delivery, intermittent infrequent dosing and combinations of low-dose anti-ADAMTS5 with drugs targeting other OA pathways. The catabolic effect of cartilage breakdown products [7–9] makes a case for early treatment to interrupt a feed-forward cycle of joint destruction, an approach that could be facilitated by advances in OA biomarkers.
Acknowledgments
FUNDING
Work in S.S Apte’s laboratory is supported by the National Institutes of Health award [HL107147] from the NHLBI-supported Program of Excellence in Glycosciences.
Abbreviations
- ADAMTS
adamalysin-like metalloproteinases with TS motif
- CS
chondroitin sulfate
- GAG
glycosaminoglycan
- HA
hyaluronan
- IGD
interglobular domain
- KS
keratan sulfate
- MMP
matrix metalloproteinase
- OA
osteoarthritis
- TS
thrombospondin
References
- 1.Hascall VC, Calabro A, Midura RJ, Yanagishita M. Isolation and characterization of proteoglycans. Methods Enzymol. 1994;230:390–417. doi: 10.1016/0076-6879(94)30026-7. [DOI] [PubMed] [Google Scholar]
- 2.Hascall VC, Heinegard D. Aggregation of cartilage proteoglycans. I The role of hyaluronic acid. J Biol Chem. 1974;249:4232–4241. [PubMed] [Google Scholar]
- 3.Aspberg A. The different roles of aggrecan interaction domains. J Histochem Cytochem. 2012;60:987–996. doi: 10.1369/0022155412464376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinegard D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7:50–56. doi: 10.1038/nrrheum.2010.198. [DOI] [PubMed] [Google Scholar]
- 5.Little CB, Meeker CT, Golub SB, Lawlor KE, Farmer PJ, Smith SM, Fosang AJ. Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair. J Clin Invest. 2007;117:1627–1636. doi: 10.1172/JCI30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pratta MA, Yao W, Decicco C, Tortorella MD, Liu RQ, Copeland RA, Magolda R, Newton RC, Trzaskos JM, Arner EC. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278:45539–45545. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- 7.Homandberg GA, Meyers R, Xie DL. Fibronectin fragments cause chondrolysis of bovine articular cartilage slices in culture. J Biol Chem. 1992;267:3597–3604. [PubMed] [Google Scholar]
- 8.Johnson A, Smith R, Saxne T, Hickery M, Heinegard D. Fibronectin fragments cause release and degradation of collagen-binding molecules from equine explant cultures. Osteoarthritis Cartilage. 2004;12:149–159. doi: 10.1016/j.joca.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda T, Tchetina E, Ohsawa K, Roughley PJ, Wu W, Mousa A, Ionescu M, Pidoux I, Poole AR. Peptides of type II collagen can induce the cleavage of type II collagen and aggrecan in articular cartilage. Matrix Biol. 2006;25:419–429. doi: 10.1016/j.matbio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Santamaria S, Yamamoto K, Botkjaer K, Tape C, Dyson MR, McCafferty J, Murphy G, Nagase H. Anti-body-based exosite inhibitors of ADAMTS-5 (Aggrecanase-2) Biochem J. 2015;471:391–401. doi: 10.1042/BJ20150758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiusaroli R, Visentini M, Galimberti C, Casseler C, Mennuni L, Covaceuszach S, Lanza M, Ugolini G, Caselli G, Rovati LC, et al. Targeting of ADAMTS5’s ancillary domain with the recombinant mAb CRB0017 ameliorates disease progression in a spontaneous murine model of osteoarthritis. Osteoarthritis Cartilage. 2013;21:1807–1810. doi: 10.1016/j.joca.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Larkin J, Lohr TA, Elefante L, Shearin J, Matico R, Su JL, Xue Y, Liu F, Genell C, Miller RE, et al. Translational development of an ADAMTS-5 antibody for osteoarthritis disease modification. Osteoarthritis Cartilage. 2015;23:1254–1266. doi: 10.1016/j.joca.2015.02.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandy JD, Boynton RE, Flannery CR. Analysis of the catabolism of aggrecan in cartilage explants by quantitation of peptides from the three globular domains. J Biol Chem. 1991;266:8198–8205. [PubMed] [Google Scholar]
- 14.Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu373–Ala374 bond of the interglobular domain. J Clin Invest. 1992;89:1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes CE, Caterson B, Fosang AJ, Roughley PJ, Mort JS. Monoclonal antibodies that specifically recognize neoepitope sequences generated by ‘aggrecanase’ and matrix metalloproteinase cleavage of aggrecan: application to catabolism in situ and in vitro. Biochem J. 1995;305(Pt 3):799–804. doi: 10.1042/bj3050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tortorella MD, Pratta M, Liu RQ, Austin J, Ross OH, Abbaszade I, Burn T, Arner E. Sites of aggrecan cleavage by recombinant human aggrecanase-1 (ADAMTS- 4) J Biol Chem. 2000;275:18566–18573. doi: 10.1074/jbc.M909383199. [DOI] [PubMed] [Google Scholar]
- 17.Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, Singer II, Donatelli SA, Weidner JR, Williams HR, et al. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest. 1997;100:93–106. doi: 10.1172/JCI119526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arner EC, Pratta MA, Trzaskos JM, Decicco CP, Tortorella MD. Generation and characterization of aggrecanase. A soluble, cartilage-derived aggrecan-degrading activity. J Biol Chem. 1999;274:6594–6601. doi: 10.1074/jbc.274.10.6594. [DOI] [PubMed] [Google Scholar]
- 19.Lark MW, Gordy JT, Weidner JR, Ayala J, Kimura JH, Williams HR, Mumford RA, Flannery CR, Carlson SS, Iwata M, et al. Cell-mediated catabolism of aggrecan. Evidence that cleavage at the ‘aggrecanase’ site (Glu373–Ala374) is a primary event in proteolysis of the interglobular domain. J Biol Chem. 1995;270:2550–2556. doi: 10.1074/jbc.270.6.2550. [DOI] [PubMed] [Google Scholar]
- 20.Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, Rosenfeld SA, Copeland RA, Decicco CP, Wynn R, et al. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins [see comments] Science. 1999;284:1664–1666. doi: 10.1126/science.284.5420.1664. [DOI] [PubMed] [Google Scholar]
- 21.Abbaszade I, Liu RQ, Yang F, Rosenfeld SA, Ross OH, Link JR, Ellis DM, Tortorella MD, Pratta MA, Hollis JM, et al. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem. 1999;274:23443–23450. doi: 10.1074/jbc.274.33.23443. [DOI] [PubMed] [Google Scholar]
- 22.Hurskainen TL, Hirohata S, Seldin MF, Apte SS. ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. J Biol Chem. 1999;274:25555–25563. doi: 10.1074/jbc.274.36.25555. [DOI] [PubMed] [Google Scholar]
- 23.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 24.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 25.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longpre JM, McCulloch DR, Koo BH, Alexander JP, Apte SS, Leduc R. Characterization of proADAMTS5 processing by proprotein convertases. Int J Biochem Cell Biol. 2009;41:1116–1126. doi: 10.1016/j.biocel.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Malfait AM, Arner EC, Song RH, Alston JT, Markosyan S, Staten N, Yang Z, Griggs DW, Tortorella MD. Proprotein convertase activation of aggrecanases in cartilage in situ. Arch Biochem Biophys. 2008;478:43–51. doi: 10.1016/j.abb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Mosyak L, Georgiadis K, Shane T, Svenson K, Hebert T, McDonagh T, Mackie S, Olland S, Lin L, Zhong X, et al. Crystal structures of the two major aggrecan degrading enzymes, ADAMTS4 and ADAMTS5. Protein Sci. 2008;17:16–21. doi: 10.1110/ps.073287008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shieh HS, Mathis KJ, Williams JM, Hills RL, Wiese JF, Benson TE, Kiefer JR, Marino MH, Carroll JN, Leone JW, et al. High resolution crystal structure of the catalytic domain of ADAMTS-5 (aggrecanase-2) J Biol Chem. 2008;283:1501–1507. doi: 10.1074/jbc.M705879200. [DOI] [PubMed] [Google Scholar]
- 30.Fushimi K, Troeberg L, Nakamura H, Lim NH, Nagase H. Functional differences of the catalytic and non-catalytic domains in human ADAMTS-4 and ADAMTS-5 in aggrecanolytic activity. J Biol Chem. 2008;283:6706–6716. doi: 10.1074/jbc.M708647200. [DOI] [PubMed] [Google Scholar]
- 31.Gendron C, Kashiwagi M, Lim NH, Enghild JJ, Thogersen IB, Hughes C, Caterson B, Nagase H. Proteolytic activities of human ADAMTS-5: comparative studies with ADAMTS-4. J Biol Chem. 2007;282:18294–18306. doi: 10.1074/jbc.M701523200. [DOI] [PubMed] [Google Scholar]
- 32.Zheng X, Nishio K, Majerus EM, Sadler JE. Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13. J Biol Chem. 2003;278:30136–30141. doi: 10.1074/jbc.M305331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colige A, Ruggiero F, Vandenberghe I, Dubail J, Kesteloot F, Van Beeumen J, Beschin A, Brys L, Lapiere CM, Nusgens B. Domains and maturation processes that regulate the activity of ADAMTS-2, a metalloproteinase cleaving the aminopropeptide of fibrillar procollagens type I, II, III and V. J Biol Chem. 2005;280:34397–34408. doi: 10.1074/jbc.M506458200. [DOI] [PubMed] [Google Scholar]
- 34.Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5) J Biol Chem. 2001;276:12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- 35.Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 36.Tortorella MD, Malfait AM. Will the real aggrecanase(s) step up: evaluating the criteria that define aggrecanase activity in osteoarthritis. Curr Pharm Biotechnol. 2008;9:16–23. doi: 10.2174/138920108783497622. [DOI] [PubMed] [Google Scholar]
- 37.Dancevic CM, McCulloch DR. Current and emerging therapeutic strategies for preventing inflammation and aggrecanase-mediated cartilage destruction in arthritis. Arthritis Res Ther. 2014;16:429. doi: 10.1186/s13075-014-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shay G, Lynch CC, Fingleton B. Moving targets: Emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015:200–206. doi: 10.1016/j.matbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupuis LE, McCulloch DR, McGarity JD, Bahan A, Wessels A, Weber D, Diminich AM, Nelson CM, Apte SS, Kern CB. Altered versican cleavage in ADAMTS5 deficient mice; a novel etiology of myxomatous valve disease. Dev Biol. 2011;357:152–164. doi: 10.1016/j.ydbio.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hattori N, Carrino DA, Lauer ME, Vasanji A, Wylie JD, Nelson CM, Apte SS. Pericellular versican regulates the fibroblast-myofibroblast transition: a role for ADAMTS5 protease-mediated proteolysis. J Biol Chem. 2011;286:34298–34310. doi: 10.1074/jbc.M111.254938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCulloch DR, Goff CL, Bhatt S, Dixon LJ, Sandy JD, Apte SS. Adamts5, the gene encoding a proteoglycan-degrading metalloprotease, is expressed by specific cell lineages during mouse embryonic development and in adult tissues. Gene Expr Patterns. 2009;9:314–323. doi: 10.1016/j.gep.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCulloch DR, Nelson CM, Dixon LJ, Silver DL, Wylie JD, Lindner V, Sasaki T, Cooley MA, Argraves WS, Apte SS. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev Cell. 2009;17:687–698. doi: 10.1016/j.devcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nandadasa S, Foulcer S, Apte SS. The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis. Matrix Biol. 2014;35:34–41. doi: 10.1016/j.matbio.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velasco J, Li J, Dipietro L, Stepp MA, Sandy JD, Plaas A. ADAMTS5 ablation blocks murine dermal repair through CD44-mediated aggrecan accumulation and modulation of TGFbeta1 signaling. J Biol Chem. 2011;286:26016–26027. doi: 10.1074/jbc.M110.208694. [DOI] [PMC free article] [PubMed] [Google Scholar]