Abstract
The detonation of an improvised nuclear device would produce prompt radiation consisting of both photons (gamma rays) and neutrons. While much effort in recent years has gone into the development of radiation biodosimetry methods suitable for mass triage, the possible effect of neutrons on the endpoints studied has remained largely uninvestigated. We have used a novel neutron irradiator with an energy spectrum based on that 1–1.5 km from the epicenter of the Hiroshima blast to begin examining the effect of neutrons on global gene expression, and the impact this may have on the development of gene expression signatures for radiation biodosimetry. We have exposed peripheral blood from healthy human donors to 0.1, 0.3, 0.5 or 1 Gy of neutrons ex vivo using our neutron irradiator, and compared the transcriptomic response 24 h later to that resulting from sham exposure or exposure to 0.1, 0.3, 0.5, 1, 2 or 4 Gy of photons (X rays). We identified 125 genes that responded significantly to both radiation qualities as a function of dose, with the magnitude of response to neutrons generally being greater than that seen after X-ray exposure. Gene ontology analysis suggested broad involvement of the p53 signaling pathway and general DNA damage response functions across all doses of both radiation qualities. Regulation of immune response and chromatin-related functions were implicated only following the highest doses of neutrons, suggesting a physiological impact of greater DNA damage. We also identified several genes that seem to respond primarily as a function of dose, with less effect of radiation quality. We confirmed this pattern of response by quantitative real-time RT-PCR for BAX, TNFRSF10B, ITLN2 and AEN and suggest that gene expression may provide a means to differentiate between total dose and a neutron component.
INTRODUCTION
In the event of detonation of an improvised nuclear device (IND), very large numbers of people would receive radiation exposures and would need to be assessed for radiological injury. Many approaches are being developed to provide biological dosimetry suitable for radiological triage in such an event, including automation of cytogenetic assays, and newer assays based on gene expression or metabolomics. The majority of such assays have so far only been developed for photon biodosimetry, generally focusing on gamma-ray and X-ray exposures. The radiation from an IND detonation is expected to also have a neutron component, however, with an energy spectrum similar to that produced by the detonation of the gun-type 15 kT device exploded at Hiroshima (1). Current modeling predicts that the neutron contribution of a ground burst IND in an urban environment is likely to be much higher than that from the air burst at Hiroshima, with neutrons expected to contribute around 6–27% of the dose to bone marrow in the 0.75–4 Gy range (2). This emphasizes the potential for significant neutron contribution to radiological injury. As neutrons have a high relative biological effectiveness compared to photons (3), it will be important to be able to detect and account for the neutron contribution to total dose to optimize radiological triage and appropriate injury treatment.
In order to study the impact of IND-spectrum neutrons on biodosimetry assays, we have recently developed a broad-spectrum neutron irradiator at the Columbia University Radiological Research Accelerator Facility (RARAF) (4, 5). This irradiator is capable of exposing cells, blood and small animals to neutrons with an energy spectrum mirroring that produced at 1–1.5 km from the epicenter in Hiroshima (4, 5). This is a distance where many exposed survivors would be expected in the event of an IND detonation.
We are also developing new approaches to radiation biodosimetry, and we and others have previously shown that differential gene expression is a promising approach (6–12). The signaling pathways leading from DNA damage to altered gene expression are complex, and can result in different gene expression profiles in response to different characteristics of a radiation exposure. For instance, gene expression profiles also show the potential to discriminate between acute exposures and more protracted low-dose-rate exposures (13–16), such as could occur from exposure to fallout after an IND detonation or exposure to radionuclides dispersed by a dirty bomb.
In the present study, we started by investigating the impact of neutrons on gene expression for application to biodosimetry. We have exposed peripheral blood from volunteer human donors ex vivo to either photons (X rays) or IND-spectrum neutrons using the RARAF source, and measured global gene expression 24 h later using whole-genome microarrays. In our initial biodosimetry studies, we found our IND-spectrum neutrons to have a relative biological effectiveness (RBE) of approximately 4 for induction of cytogenetic damage using the in vitro cytokinesis-block micronucleus assay (4). Basing our initial gene expression study on this finding, we began by comparing global gene expression 24 h after neutron doses of 0.25, 0.5 or 1 Gy or X-ray doses of 1, 2 or 4 Gy. In subsequent experiments, we added a 0.1 Gy neutron dose and matched X-ray doses from 0.1 to 1 Gy.
While we have found broad similarities in the response to these two radiation qualities at the level of genes modulated and biological processes implicated, there was a generally heightened response to neutrons compared with the same dose of X rays, consistent with the general observation of RBEs >1. However, we did identify some radiation responsive genes without an apparent RBE effect, suggesting that a gene expression approach combining genes with these different neutron-response patterns may provide a means of distinguishing high-LET exposure from total radiation dose.
METHODS
Blood Culture and Irradiation
All experiments involving human subjects were approved by the Columbia University Medical Center Institutional Review Board IRB no. 4 under protocol no. IRB-AAAF2671, and were conducted according to the principles expressed in the Declaration of Helsinki. Blood was collected from healthy volunteer donors (a total of 6 males and 6 females) into tubes containing sodium citrate. Neutron exposures used an accelerator-based irradiator that produces a neutron energy spectrum modeled on the Hiroshima spectrum 1–1.5 km from the epicenter and similar to that expected from an improvised nuclear device (4). Four irradiation runs were performed, each with blood from three different donors. The first two runs used neutron doses of 0.25, 0.5 or 1 Gy and X-ray doses of 1, 2, or 4 Gy plus a sham-exposed control. The last two runs used doses of 0.1, 0.3, 0.5 or 1 Gy of either neutrons X-rays, plus a sham-exposed control.
For neutron irradiations, 6–18 blood samples were placed in adjacent positions on an eighteen position Ferris wheel rotating around the particle beam at an angle of 60° and a distance of 17.5 cm from the beam’s impingement on a beryllium target. Blood tubes were placed in holders designed to provide isotropic exposures as the wheel rotates around the target at about half a revolution per minute. The tubes were turned end-to-end half-way through the exposure, to ensure an equal dose to the whole sample. When there were empty positions on the wheel, two tubes containing water blanks were placed at either end of the string of blood to ensure a uniform scatter dose. A total beam current of 18 μA was used, resulting in a neutron dose rate of 1.55 Gy/ h with an additional 0.33 Gy/h (21%) from to gamma rays. The dose rate was adjusted to deliver the 0.5 Gy dose in 10 rotations (20 min). Further details of the neutron spectrum (5) and dosimetry (4) have been published elsewhere.
X-ray irradiations were performed using a Westinghouse Coronado orthovoltage X-ray machine running at 250 kVp and 15 mA with 0.5 mm copper + 1 mm aluminum filtration. X rays were delivered at a dose rate of 1.23 Gy/min, as determined using a Victoreen model 570 condenser R meter with a 250r chamber.
After irradiation, the blood samples were mixed with an equal volume of RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum plus 100 U/ml penicillin and 100 μg/ ml streptomycin, and incubated in a humidified 37°C incubator with 5% CO2 for 24 h before RNA isolation.
Microarray Analysis
RNA was isolated using the PerfectPure RNA Kit (5 Prime Inc., Gaithersburg, MD), and globin transcript reduction was performed using GLOBINclear-Human Kit (Life Technologies, Grand Island, NY). RNA was quantified using the NanoDrop ND1000 (Thermo-Fisher, Waltham, MA) and RNA integrity was monitored with the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). For hybridization, 100 ng RNA from each sample was converted to cyanine-3 labeled cRNA using the One-Color Low Input Quick Amp Labeling Kit (Agilent Technologies), fragmented, hybridized to Agilent Whole Human Genome Microarrays v 2, 4×44K (G4112F) and washed as previously described elsewhere (15). The microarrays were scanned using the Agilent DNA Microarray Scanner (G2505B) and Agilent Feature Extraction Software (v 10.7) was used with default parameters for data extraction. The microarray data are available from the Gene Expression Omnibus (17) using accession number GSE90909.
Processed signal intensities were imported into BRB-ArayTools (v 4.3.2) (18), log2-transformed, median normalized and then filtered for significant expression and variation as described elsewhere (15). BRB-ArrayTools class comparison analysis was used to identify differentially expressed genes with P < 0.001 using an F test (for comparisons of multiple groups) or random-variance t test (for comparisons of one dose vs. control). Benjamini and Hochberg false discovery rates were also calculated for each gene to allow controlling for false positives. Prism (GraphPad Software, San Diego, CA) was used for curve fitting and linearity testing for RBE comparisons.
Gene Ontology and Network Analyses
For each dose, the list of differentially expressed genes was analyzed using the functional annotation tool of the Database for Annotation Visualization and Integrated Discovery (DAVID; v 6.7). Gene ontology terms and biological functions with a Benjamini-corrected P value <0.05 were considered significantly over-represented within a gene list.
The lists of differentially expressed genes from each dose and their fold-change values were also imported into Ingenuity™ Pathways Analysis (IPA) (http://www.ingenuity.com). The Ingenuity Upstream Regulator Analysis tool was used to predict potential upstream regulators that may be either activated or inhibited after irradiation based on the gene expression profile observed for each X-ray and neutron dose. Regulators with a z-score ≥2 were considered to have a significant prediction of activation, and those with a z-score ≤2 to have a significant prediction of inhibition in response to radiation.
Quantitative Real-Time RT-PCR
cDNA was prepared using the High-Capacity cDNA Archive Kit (Life Technologies), and real-time quantitative RT-PCR (qRT-PCR) was performed for selected genes using Taqman chemistry and the ABI 7900 Real Time PCR System as described previously (15). Assays were purchased from Thermo Fisher for the following genes: BAX (Hs00180269_m1), TNFRSF10B (Hs00366278_m1), ITLN2 (Hs00365614_m1), AEN (Hs00901422_m1), VWCE (Hs00328069_m1), FDXR (Hs00244586_m1), PHLDA3 (Hs00385313_m1), EDA2R (Hs00939736_m1) and ACTB (Hs99999903_m1). The ΔΔCT method was used to calculate expression relative to controls as described previously (19), using normalization to ACTB expression.
RESULTS
Microarray Analysis
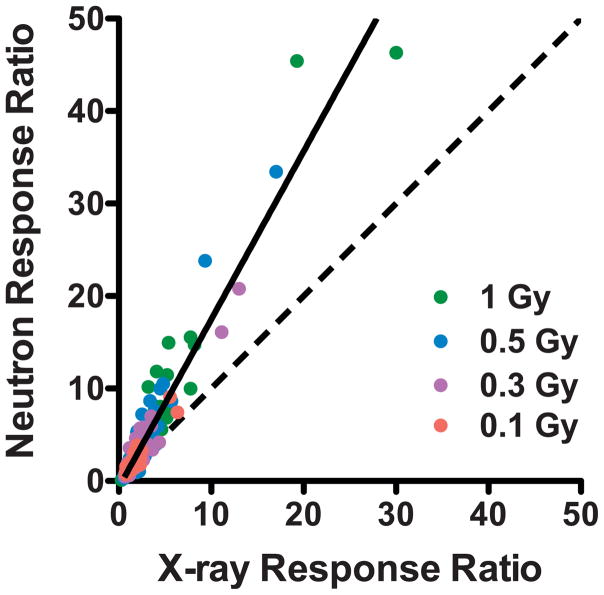
Global gene expression levels were measured in ex vivo irradiated donor blood samples 24 h after exposure to photons (X rays), or to IND-spectrum neutrons using Agilent whole-genome microarrays and a one-color work-flow. Class comparison in BRB-ArrayTools was first used to identify genes significantly differentially expressed (P < 0.001 and FDR<5%) as a function of dose of neutrons (0.1–1 Gy) or X rays (0.1–4 Gy), yielding 201 neutron responsive genes and 325 X-ray responsive genes, with 125 genes responding to both (Supplementary Table S1; http://dx.doi.org/10.1667/RR0004CC.1.S1). Comparison of induced expression levels relative to controls for these 125 genes showed a trend of greater response to neutrons than to the same dose of X rays (Fig. 1). Class comparison was also used to identify significantly differentially expressed genes for each dose of neutrons or X rays individually (Supplementary Table S1). These results are summarized in Table 1.
FIG. 1.
Comparison of the fold-change in gene expression relative to controls after irradiation of blood to equal doses of X rays and neutrons. Points are plotted individually for each dose (pink, 0.1 Gy; purple, 0.3 Gy; blue, 0.5 Gy; green, 1 Gy) for each of the 125 genes that were significantly differentially expressed after both photon and neutron exposure. The dashed line indicates an identical response to both qualities of radiation (RBE = 1) and the solid line is a linear fit to all data points (slope = 1.8).
TABLE 1.
Number of Features Differentially Expressed (P < 0.001) at Each Radiation Dose Relative to Controls
| Dose (Gy) | X ray
|
Neutron
|
||
|---|---|---|---|---|
| Up | Down | Up | Down | |
| 0.1 | 14 | 0 | 44 | 3 |
| 0.3a | 33 | 4 | 87 | 37 |
| 0.5 | 49 | 1 | 228 | 187 |
| 1 | 152 | 87 | 317 | 301 |
| 2 | 149 | 96 | NAb | NA |
| 4 | 149 | 58 | NA | NA |
Data from 0.25–0.3 Gy neutron exposures were analyzed together.
Not applicable, dose not used.
Gene Ontology Analysis
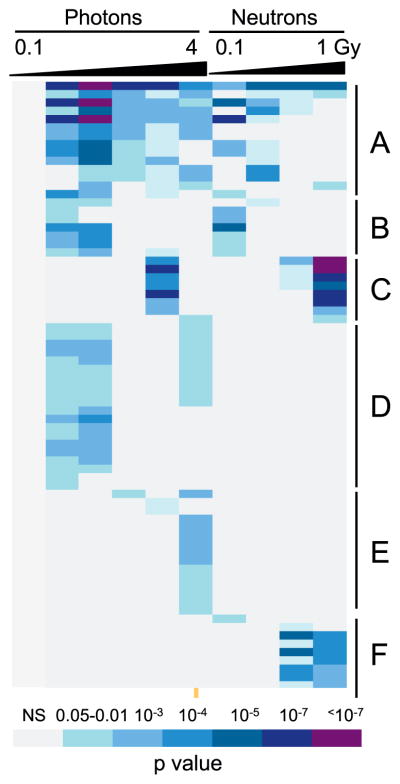
The Functional Annotation Tool of DAVID (20, 21) was used to find gene ontology (GO) terms significantly over-represented among the differentially expressed genes at each dose of X rays or neutrons (Fig. 2 and Supplementary Table S2; http://dx.doi.org/10.1667/RR0004CC.1.S2). In all, 112 GO terms were identified as significant (Benjamini-corrected P value <0.05), with those related to TP53 signaling and general DNA damage response showing the broadest representation across all tested doses of both radiation qualities, with the exception of 0.1 Gy X rays, for which no significant GO terms were found. Forty-six of the GO terms were only over-represented among genes significantly differentially expressed after the highest dose of neutrons tested (1 Gy) and these represented mainly immune response and chromatin-related functions.
FIG. 2.
Gene ontology analysis of differentially expressed genes by dose. GO terms broadly represented across doses after both photon and neutron irradiations (panel A) were dominated by TP53 signaling and apoptosis functions. Similar functions were also seen among GO terms significant only at lower doses of both radiation qualities (panel B). Terms significant only at higher doses of both radiations (panel C) included NK-cell mediated immunity and glycoproteins. Categories that were only seen as significant after X-ray irradiation (panel D) included additional cell death and apoptosis and DNA repair functions, while those significant only after higher X-ray doses (panel E) included TNF and cell membrane functions, as well as additional apoptotic functions. GO terms that appeared as significant only after neutron exposure (panel F) were related to histones and the nucleosome. An additional 46 GO terms were enriched only among genes differentially expressed after 1 Gy neutrons (see Supplementary Table S2; http://dx.doi.org/10.1667/RR0004CC.1.S2), representing mainly immune-related and additional chromatin functions. The colors represent Benjamini-corrected P values as indicated in the color key; NS= not significant (corrected P > 0.05). The columns represent, from left to right, doses of 0.1, 0.3, 0.5, 1, 2 and 4 Gy of X rays, and 0.1, 0.3, 0.5 and 1 Gy of neutrons. A fully-annotated version is available in Supplementary Table 2.
Upstream Network Analysis
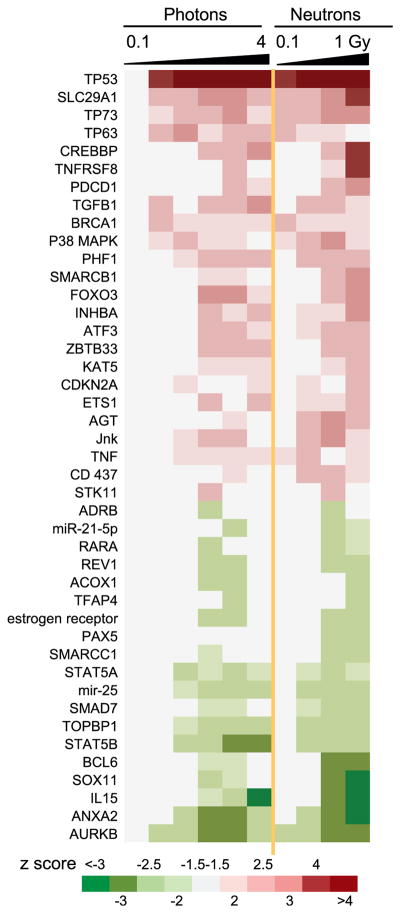
Upstream Regulator Analysis was performed in Ingenuity Pathways Analysis (IPA) to identify potential regulators of the gene expression patterns seen after different doses of X rays or neutrons (Fig. 3). No upstream regulators were predicted to be significantly activated (z ≥ 2) or suppressed (z ≤ –2) after 0.1 Gy X rays, but all other doses yielded predictions of activity changes in multiple upstream regulators. TP53 showed the strongest prediction of activation across all doses, with the exception of the 0.1 Gy X-ray dose. SLC29A1, TP73 and TP63, were also broadly predicted to be activated, while AURKB scored the broadest prediction of suppression across the study.
FIG. 3.
Potential upstream regulators of differentially expressed genes by dose predicted using Ingenuity Upstream Regulator Analysis in IPA. Potential regulators significant (|z|>2) at two or more doses are shown. The columns represent, from left to right, doses of 0.1, 0.3, 0.5, 1, 2 and 4 Gy of X rays, and 0.1, 0.3, 0.5 and 1 Gy neutrons. The colors represent positive (red, indicating activation of regulator) and negative (green, indicating suppression of regulator activity) z scores as indicated in the color key. Note that z scores with absolute value between 1.5 and 2 have also been colored to illustrate potential activity trends.
Relative Biological Effectiveness of IND-Spectrum Neutrons
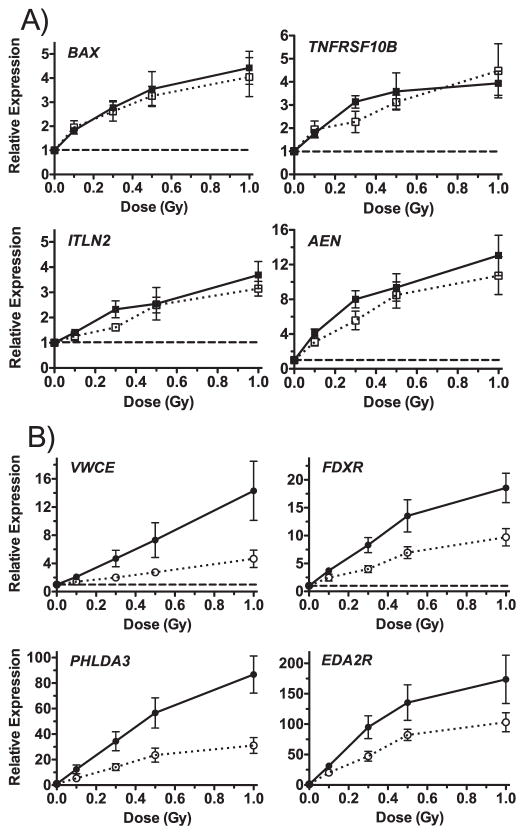
We used the microarray data from the 125 features that responded significantly to dose after both X rays and neutrons (Fig. 1), to examine the neutron RBE for gene expression. In this context, we define RBE as the ratio of X-ray dose compared to neutron dose required to produce the equivalent fold-change in gene expression (22). None of the dose-response curves departed significantly from linearity (P > 0.05), so we used a linear equation to fit the neutron dose response for each gene feature as measured by microarray. The calculated RBE varied both by gene and by dose, with most genes showing RBE > 1 for neutrons across the dose range. Some genes, however, appeared to have a RBE close to 1 at all doses. To verify these two general patterns, we used real-time quantitative RT-PCR (c) to measure the expression of several genes in the matched dose samples (0.1, 0.3, 0.5 and 1 Gy). BAX, TNFRSF10B, ITLN2 and AEN were selected to represent genes with RBE ≈ 1 and VWCE, FDXR, PHLDA3 and EDA2R were selected to represent those with RBE > 1. The dose-response patterns (Fig. 4) agreed well with those based on microarray measurements, and suggested that while most genes appear to respond more robustly to IND-spectrum neutrons than to X rays, some respond only as a function of dose, at least at doses of 1 Gy or less. Calculating RBEs for these genes using the qRT-PCR data produced values around 1 as predicted for BAX, TNFRSF10B, ITLN2 and AEN and higher values for VWCE, FDXR and PHLDA3, with intermediate values for EDA2R (Table 2).
FIG. 4.
Real-time quantitative RT-PCR of genes showing different RBE relationships by microarray analysis. Panel A: BAX, TNFRSF10B, ITLN2 and AEN had an RBE close to 1 across the dose range tested for neutron exposure (solid symbols and lines) compared to X-ray exposure (open symbols and dotted lines). Panel B: VWCE, FDXR, PHLDA3 and EDA2R showed a greater effect after neutron exposure (solid symbols and lines) in comparison to X-ray exposure (open symbols and dotted lines). Points are the mean of measurements in 5 different donors (4 for ITLN2) and error bars are SEM. The dashed lines indicate the level in sham controls.
TABLE 2.
IND-Spectrum Neutron RBE Values Calculated from qRT-PCR Data
| Gene | 0.1 Gy | 0.3 Gy | 0.5 Gy | 1 Gy |
|---|---|---|---|---|
| BAX | 0.4 | 0.7 | 0.9 | 1.3 |
| TNFRSF10B | 0.4 | 0.9 | 0.9 | 1.1 |
| ITLN2 | 1.1 | 1.4 | 1.0 | 1.3 |
| AEN | 0.7 | 0.9 | 0.9 | 1.4 |
| VWCE | 3.2 | 3.9 | 3.7 | 3.6 |
| FDXR | 1.3 | 1.9 | 1.6 | 2.2 |
| PHLDA3 | 2.1 | 2.1 | 2.0 | 3.1 |
| EDA2R | 1.0 | 1.3 | 1.2 | 2.0 |
DISCUSSION
In this study, we have examined the novel transcriptomic response in human peripheral blood to neutrons with an energy spectrum that would be expected at a survivable distance from an IND. Unsurprisingly, many of the same genes respond to both X rays and neutrons, with a generally greater fold-change observed in response to neutrons compared with the same dose of X rays. Overall, we observed significant changes in expression of more genes after neutron exposure compared with X-ray exposure. However, this does not necessarily represent unique gene expression responses to neutron irradiation. Some of these genes may respond at times that were not captured in this experiment, or may require higher doses of X rays. The more profound biological damage inflicted on cells by neutrons could contribute to such differences in timing and dose-response relationships.
Gene ontology analysis of the significantly differentially expressed genes at each dose and radiation quality revealed changing patterns of biological response at increasing doses. Gene ontology analysis can provide insight into the biological ramifications of the transcriptomic responses to different exposures, by revealing the biological processes and pathways that are disproportionately represented among the responding genes. As in many radiation-response studies, we found that functions related to p53 pathway signaling and apoptosis were broadly over-represented across the doses tested for both X rays and neutrons. Many of these functions appeared to be significant either only at lower doses (Fig. 2B) or only after X-ray exposure (Fig. 2D and E). This does not mean that genes in these pathways and biological functions were not differentially expressed after the higher doses, however, but is likely to be a reflection of the way significance is calculated. As larger numbers of genes are differentially expressed at higher doses, particularly for neutrons (Table 1), the number of responding genes that participate in specific sub-processes no longer represents a significantly disproportionate percentage of the total number of responding genes.
Additional biological processes were implicated in the transcriptional response to the higher doses of neutrons, suggesting a damage threshold for their response that was exceeded by a 1 Gy dose of neutrons but not by a 4 Gy dose of X rays and reflecting the larger number of genes involved in the neutron response. These processes were almost exclusively involved in either chromatin function or immune response.
Interestingly, immune and inflammatory response genes represent a major component of the human radiation-response in vivo, but are infrequently seen to be responsive in radiation studies using ex vivo models (7, 9, 19). The significant involvement of immune functions in the transcriptional response to 1 Gy neutron ex vivo irradiation may suggest a higher damage threshold for the response of these genes in blood exposed in culture, rather than an absolute dependence on signaling from damaged non-blood tissues to elicit a response. In this case, the response of genes in these pathways may show a heightened sensitivity in vivo to lower doses of neutrons, implying the possibility that neutrons may have a disproportionately large impact on immune function and inflammatory response in comparison to photon exposure.
Altered expression of histone genes and other genes involved with chromatin structure and function has also been reported in response to radiation exposure, including experiments using high-LET radiation (23). Similar to the pattern seen for immune responses, “chromatin packaging and remodeling” was found to be a significantly over-represented biological process among genes responding in patients who were undergoing 3.75 Gy total-body irradiation, but not in ex vivo irradiated human blood (9).
In contrast to the results of our gene ontology analysis, the pattern of IPA-predicted upstream regulatory factors was very similar for X rays and neutrons (Fig. 3). Consistent with the finding that activation of p53 pathway signaling dominated the gene ontology results, TP53 was also the upstream regulatory factor most strongly predicted to be activated by both X rays and neutrons. The predominance of p53 activation is a long-standing theme in the study of gene expression responses to ionizing radiation, in cell lines (24, 25), in animals (13, 26) and in human blood exposed ex vivo (8, 27, 28) or in vivo (9, 29). Other regulatory factors predicted by our IPA analysis to be upstream of the observed gene expression changes are also known mediators of radiation response. This includes regulatory factors predicted to be activated, such as TGFB1 (30), BRCA1 (31), ATF3 (32), p38 MAPK, JNK and TNF (33), as well as factors predicted to be suppressed, such as STAT5B (34) and AURKB (35).
Initially in this study, we compared global gene expression at 24 h after sham exposure, 0.25, 0.5 or 1 Gy neutron doses vs. 24 h after 1, 2 or 4 Gy X-ray does. This experiment structure was based on our earlier finding of an RBE of around 4 for the induction of cytogenetic damage using the same neutron source (4). We subsequently extended the dose range of our experiment, to include a 0.1 Gy neutron dose and the lower X-ray doses. As neutrons would only make up a small percentage of the total dose received, for biodosimetry purposes the relative response of gene expression to low-dose neutron exposure would be more important to understand than the response to doses greater than 1 Gy. Although we found that neutrons did produce generally larger changes from control gene expression levels than those seen after the same X-ray dose, the extent of the enhanced response to neutrons varied both by dose and by gene. When we plotted the neutron response ratio vs. the X-ray response ratio at the same dose for all significantly dose-responsive genes (Fig. 1), a line fitted to all points had a slope of 1.8, suggesting an overall RBE for gene expression closer to 2 than to 4.
Of all the genes for which we validated expression using qRT-PCR, PHLDA3 and VWCE showed the most consistently elevated RBE at all doses examined up to 1 Gy (Table 2), a little over 2 for PHLDA3 and a little over 3 for VWCE. Within the <1 Gy dose range, we also confirmed the apparent lack of RBE for several other genes: BAX, TNFRSF10B, ITLN2 and AEN. This finding was consistent with the predicted RBEs calculated from the microarray data within this low-dose range. It should be noted that using the microarray data for X-ray irradiation up to 4 Gy, even these genes showed RBE values greater than 1 at the higher doses (see the “Common” tab in Supplementary Table S1; http://dx.doi.org/10.1667/RR0005CC.1.S1).
Among the RBE values calculated from the microarray data we generally found higher RBEs at the higher doses (Supplementary Table S1; http://dx.doi.org/10.1667/RR0005CC.1.S1). This is in contrast to the pattern generally seen for cytogenetic endpoints, where the RBE is generally higher at lower doses (4). The pattern of RBE with dose reflects the difference in the shapes of the dose-response curves for gene expression and chromosome aberrations. Dose-response curves for endpoints requiring multiple chromosome breaks, such as translocations or micronucleus formation, tend to be linear with dose for high-LET radiations and linear quadratic for X rays (22). In contrast, gene expression changes are regulated by signal transduction pathways, rather than resulting from direct DNA damage. For most genes, this produces generally linear-dose-response curves, with a tendency to flatten at higher doses where signaling saturates. We observed this pattern in many genes in this study for both X-ray and neutron exposures (Fig. 4). In this study, we focused mainly on the lower part of the neutron dose-response curve, as neutron doses in excess of 1 Gy are likely to be less relevant to radiological triage following an IND.
In this study, we examined the global gene expression response of human peripheral blood to neutrons with an energy spectrum relevant to exposures from an IND. The dose-dependent patterns of gene expression in response to different radiation quality may represent a valuable means to estimate both total dose and radiological injury, while also distinguishing the neutron component of an exposure after an IND event. Genes with a neutron RBE close to 1 (such as BAX and AEN) could be useful for establishing overall radiation dose irrespective of radiation quality. The inclusion in biodosimetric signatures of genes with a high-neutron RBE (such as VWCE and PHLDA3) could be used to distinguish a neutron component, which may indicate greater radiological injury. Discordance between separate dose estimates made using these two groups of genes would provide an indicator that a neutron component was involved in the exposure. Further studies are warranted to establish if gene expression can be used to estimate the percentage of the neutron component in mixed neutron-photon exposures.
Supplementary Material
Acknowledgments
Analyses were performed using BRB-ArrayTools developed by Dr. Richard Simon and the BRB-ArrayTools Development Team. Irradiations were performed at the RARAF, an NIH supported Research Center through NIBIB grant no. 5P41EB-002033. This work was supported by the Center for High-Throughput Minimally-Invasive Radiation Biodosimetry, National Institute of Allergy and Infectious Diseases grant no. U19AI067773.
References
- 1.Egbert SD, Kerr GD, Cullings HM. DS02 fluence spectra for neutrons and gamma rays at Hiroshima and Nagasaki with fluence-to-kerma coefficients and transmission factors for sample measurements. Radiat Environ Biophys. 2007;46:311–25. doi: 10.1007/s00411-007-0120-5. [DOI] [PubMed] [Google Scholar]
- 2.Kramer K, Li A, Madrigal J, Sanchez B, Millage K. Report DTRA-TR-13-045 (R1): Monte Carlo modeling of the initial radiation emitted by a nuclear device in the national capitol region. Fort Belvoir, VA: Defense Threat Reduction Agency; 2016. [Google Scholar]
- 3.Pandita TK, Geard CR. Chromosome aberrations in human fibroblasts induced by monoenergetic neutrons. I. Relative biological effectiveness. Radiat Res. 1996;145:730–9. [PubMed] [Google Scholar]
- 4.Xu Y, Randers-Pehrson G, Turner HC, Marino SA, Geard CR, Brenner DJ, et al. Accelerator-based biological irradiation facility simulating neutron exposure from an improvised nuclear device. Radiat Res. 2015;184:404–10. doi: 10.1667/RR14036.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y, Randers-Pehrson G, Marino SA, Garty G, Harken A, Brenner DJ. Broad energy range neutron spectroscopy using a liquid scintillator and a proportional counter: Application to a neutron spectrum similar to that from an improvised nuclear device. Nucl Instrum Methods Phys Res A. 2015;794:234–9. doi: 10.1016/j.nima.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amundson SA, Bittner M, Meltzer P, Trent J, Fornace AJ., Jr Induction of gene expression as a monitor of exposure to ionizing radiation. Radiat Res. 2001;156:657–61. doi: 10.1667/0033-7587(2001)156[0657:iogeaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Dressman HK, Muramoto GG, Chao NJ, Meadows S, Marshall D, Ginsburg GS, et al. Gene expression signatures that predict radiation exposure in mice and humans. PLoS Med. 2007;4:e106. doi: 10.1371/journal.pmed.0040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul S, Amundson SA. Development of gene expression signatures for practical radiation biodosimetry. Int J Radiat Oncol Biol Phys. 2008;71:1236–44. doi: 10.1016/j.ijrobp.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul S, Barker CA, Turner HC, Mclane A, Wolden SL, Amundson SA. Prediction of in vivo radiation dose status in radiotherapy patients using ex vivo and in vivo gene expression signatures. Radiat Res. 2011;175:257–65. doi: 10.1667/RR2420.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badie C, Kabacik S, Balagurunathan Y, Bernard N, Brengues M, Faggioni G, et al. Laboratory intercomparison of gene expression assays. Radiat Res. 2013;180:138–48. doi: 10.1667/RR3236.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tucker JD, Joiner MC, Thomas RA, Grever WE, Bakhmutsky MV, Chinkhota CN, et al. Accurate gene expression-based biodosimetry using a minimal set of human gene transcripts. Int J Radiat Oncol Biol Phys. 2014;88:933–9. doi: 10.1016/j.ijrobp.2013.11.248. [DOI] [PubMed] [Google Scholar]
- 12.Lucas J, Dressman HK, Suchindran S, Nakamura M, Chao NJ, Himburg H, et al. A translatable predictor of human radiation exposure. PLoS One. 2014;9:e107897. doi: 10.1371/journal.pone.0107897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul S, Ghandhi SA, Weber W, Doyle-Eisele M, Melo D, Guilmette R, et al. Gene expression response of mice after a single dose of 137CS as an internal emitter. Radiat Res. 2014;182:380–9. doi: 10.1667/RR13466.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghandhi SA, Weber W, Melo D, Doyle-Eisele M, Chowdhury M, Guilmette R, et al. Effect of 90Sr internal emitter on gene expression in mouse blood. BMC Genomics. 2015;16:586. doi: 10.1186/s12864-015-1774-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghandhi SA, Smilenov LB, Elliston CD, Chowdhury M, Amundson SA. Radiation dose-rate effects on gene expression for human biodosimetry. BMC Med Genomics. 2015;8:22. doi: 10.1186/s12920-015-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul S, Smilenov LB, Elliston CD, Amundson SA. Radiation dose-rate effects on gene expression in a mouse biodosimetry model. Radiat Res. 2015;184:24–32. doi: 10.1667/RR14044.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–10. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon R, Lam A, Li M-C, Ngan M, Menenzes S, Zhao Y. Analysis of gene expression data using BRB-Array Tools. Cancer Informatics. 2007;2:11–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Amundson SA, Grace MB, Mcleland CB, Epperly MW, Yeager A, Zhan Q, et al. Human in vivo radiation-induced biomarkers: gene expression changes in radiotherapy patients. Cancer Res. 2004;64:6368–71. doi: 10.1158/0008-5472.CAN-04-1883. [DOI] [PubMed] [Google Scholar]
- 20.Dennis G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 21.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 22.Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 7. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 23.Meador JA, Ghandhi SA, Amundson SA. p53-independent downregulation of histone gene expression in human cell lines by high- and low-let radiation. Radiat Res. 2011;175:689–99. doi: 10.1667/rr2539.1. [DOI] [PubMed] [Google Scholar]
- 24.Amundson SA, Bittner M, Chen Y, Trent J, Meltzer P, Fornace AJ., Jr Fluorescent cDNA microarray hybridization reveals complexity and heterogeneity of cellular genotoxic stress responses. Oncogene. 1999;18:3666–72. doi: 10.1038/sj.onc.1202676. [DOI] [PubMed] [Google Scholar]
- 25.Amundson SA, Do KT, Vinikoor LC, Lee RA, Koch-Paiz CA, Ahn J, et al. Integrating global gene expression and radiation survival parameters across the 60 cell lines of the National Cancer Institute Anticancer Drug Screen. Cancer Res. 2008;68:415–24. doi: 10.1158/0008-5472.CAN-07-2120. [DOI] [PubMed] [Google Scholar]
- 26.Lane DP. Exploiting the p53 pathway for the diagnosis and therapy of human cancer. Cold Spring Harb Symp Quant Biol. 2005;70:489–97. doi: 10.1101/sqb.2005.70.049. [DOI] [PubMed] [Google Scholar]
- 27.Kabacik S, Mackay A, Tamber N, Manning G, Finnon P, Paillier F, et al. Gene expression following ionising radiation: identification of biomarkers for dose estimation and prediction of individual response. Int J Radiat Biol. 2011;87:115–29. doi: 10.3109/09553002.2010.519424. [DOI] [PubMed] [Google Scholar]
- 28.Knops K, Boldt S, Wolkenhauer O, Kriehuber R. Gene expression in low- and high-dose-irradiated human peripheral blood lymphocytes: possible applications for biodosimetry. Radiat Res. 2012;178:304–12. doi: 10.1667/rr2913.1. [DOI] [PubMed] [Google Scholar]
- 29.Filiano AN, Fathallah-Shaykh HM, Fiveash J, Gage J, Cantor A, Kharbanda S, et al. Gene expression analysis in radiotherapy patients and C57BL/6 mice as a measure of exposure to ionizing radiation. Radiat Res. 2011;176:49–61. doi: 10.1667/RR2419.1. [DOI] [PubMed] [Google Scholar]
- 30.Andarawewa KL, Paupert J, Pal A, Barcellos-Hoff MH. New rationales for using TGFbeta inhibitors in radiotherapy. Int J Radiat Biol. 2007;83:803–11. doi: 10.1080/09553000701711063. [DOI] [PubMed] [Google Scholar]
- 31.Gilmore PM, Quinn JE, Mullan PB, Andrews HN, Mccabe N, Carty M, et al. Role played by BRCA1 in regulating the cellular response to stress. Biochem Soc Trans. 2003;31:257–62. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 32.Fan F, Jin S, Amundson SA, Tong T, Fan W, Zhao H, et al. ATF3 induction following DNA damage is regulated by distinct signaling pathways and over-expression of ATF3 protein suppresses cells growth. Oncogene. 2002;21:7488–96. doi: 10.1038/sj.onc.1205896. [DOI] [PubMed] [Google Scholar]
- 33.Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Herok R, Konopacka M, Polanska J, Swierniak A, Rogolinski J, Jaksik R, et al. Bystander effects induced by medium from irradiated cells: similar transcriptome responses in irradiated and bystander K562 cells. Int J Radiat Oncol Biol Phys. 2010;77:244–52. doi: 10.1016/j.ijrobp.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 35.Tao Y, Leteur C, Calderaro J, Girdler F, Zhang P, Frascogna V, et al. The aurora B kinase inhibitor AZD1152 sensitizes cancer cells to fractionated irradiation and induces mitotic catastrophe. Cell Cycle. 2009;8:3172–81. doi: 10.4161/cc.8.19.9729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.