Abstract
We conducted quantitative cellular respiration analysis on samples taken from human breast cancer (HBC) and human colorectal cancer (HCC) patients. Respiratory capacity is not lost as a result of tumor formation and even though, functionally, complex I in HCC was found to be suppressed, it was not evident on the protein level. Additionally, metabolic control analysis was used to quantify the role of components of mitochondrial interactosome. The main rate-controlling steps in HBC are complex IV and adenine nucleotide transporter, but in HCC, complexes I and III. Our kinetic measurements confirmed previous studies that respiratory chain complexes I and III in HBC and HCC can be assembled into supercomplexes with a possible partial addition from the complex IV pool. Therefore, the kinetic method can be a useful addition in studying supercomplexes in cell lines or human samples. In addition, when results from culture cells were compared to those from clinical samples, clear differences were present, but we also detected two different types of mitochondria within clinical HBC samples, possibly linked to two-compartment metabolism. Taken together, our data show that mitochondrial respiration and regulation of mitochondrial membrane permeability have substantial differences between these two cancer types when compared to each other to their adjacent healthy tissue or to respective cell cultures.
1. Introduction
The field of cellular bioenergetics is gaining increased attention and studies performed during the last years have shown that targeting cancer cell energy metabolism may be a new and promising area for selective tumor treatment [1]. The literature describing changes in energy metabolism and mitochondrial function during carcinogenesis is, unfortunately, full of contradictions. Majority of previous studies about the bioenergetics of malignant tumors were performed in vitro on different cell models with the conclusion that cancer cells have increased glucose uptake and, due to mitochondrial damage, it is not metabolized via oxidative phosphorylation (OXPHOS) [2–4]. It is clear that for many tumors, glycolysis is the main energy provider, but in others, OXPHOS is still crucial for survival and progression and produces necessary ATP [1, 5, 6]. Recently, a new concept for tumor metabolism was proposed—metabolic coupling between mitochondria in cancer cells and catabolism in stromal cells—which promotes tumor growth and development of metastases. In other words, tumor cells induce reprogramming in surrounding nontumor cells so that the latter acquire the Warburg phenotype [7] and start producing and exporting the necessary fuels for the anabolic cancer cells (“reverse Warburg”). The cancer cells will then metabolize these fuels via their tricarboxylic acid cycle and OXPHOS [8–10]. Complex interplay between developing cancer cells and host physiology, possibly mediated by “waves” of gene expression in the tumor [11, 12], can only develop in vivo and therefore in vitro studies cannot give conclusive information about the functional activity and capacity of OXPHOS in human samples. In vitro models ignore many factors arising from the tumor microenvironment (TME), which can and will exert significant effects in vivo. TME consists of nonmalignant cells, soluble growth factors, signaling molecules, and extracellular matrix that support tumor progression [13], but high heterogeneity within cancers cell population on top of it contributes to even further complexity in clinical samples [14]. At the same time, the metabolic profiles of tumor cells that are grown in culture have significant variations primarily due to the culture conditions, such as concentrations of glucose, glutamine, and/or fetal serum. Cells grown in glucose-free medium display relatively high rates of oxygen consumption, but cultivation in high-glucose medium increases their glycolytic capacity together with reduced respiratory flux [15–19].
In addition to intercellular differences, there are also intracellular rearrangements resulting from tumor formation. The functional units within cells are often macromolecular complexes rather than single species [20]. In case of OXPHOS, it has been shown that complexes of the respiratory chain can form assemblies—supercomplexes—that lead to kinetic and possibly homeostatic advantages [21]. Therefore, pure genome or transcriptome data are not sufficient for describing the final in situ modifications and the final outcomes of a pathway or cellular processes are defined by actual activities of their separate proteins—or their assemblies—together with the respective regulatory mechanisms. More specifically, previous studies have shown that in cardiac and yeast cells, a large protein supercomplex is centrally positioned in regulation of mitochondrial respiration and mitochondrial energy fluxes. The supercomplex consists of ATP synthasome, mitochondrial creatine kinase (MtCK) or hexokinase (HK), voltage-dependent anion channel (VDAC), and some regulatory proteins expectedly coordinate the selective permeability of it. This complex is known as mitochondrial interactosome (MI) [22], and it is located in the contact sites of outer and inner mitochondrial membranes. This unit also includes supercomplexes formed by the respiratory chain [23, 24]. Changes in the content of ATP synthasome and respiratory chain supercomplexes in pathological conditions are still poorly studied. Inhibiting key respiratory enzymes or avoiding restructuring of mitochondrial supercomplexes in tumors has potential to disrupt disease progression without affecting normal cells, thus, providing a powerful new approach for developing novel therapeutic targets. Specifically, Rohlenova et al. recently demonstrated that breast cancer cells expressing HER2 oncogene develop specific RC supercomplexes which make complex I in these susceptible to treatment with chemically altered tamoxifen called MitoTam [25]. MitoTam is taken to a phase I clinical study [25], and there are other clinical studies undergoing that target OXPHOS in different cancer types (e.g., trial numbers NCT01957735 and NCT02650804). Therefore, despite the assumed glycolytic nature of human tumors, inhibition of oxidative respiration is proving to be a viable therapeutic strategy and further studies are needed to define differences between cancer types but also individual patients in regard to such treatment.
We have previously shown on clinical samples that both human breast cancer (HBC) and human colorectal cancer (HCC) are not purely glycolytic, but these tumors have sustained OXPHOS as a substantial provider of ATP [26–28]. Here, we extend our studies by comparing bioenergetics of HBC and HCC using kinetic methods.
2. Materials and Methods
2.1. Chemicals
All chemicals were purchased from Sigma-Aldrich (USA) and were of the highest purity available (>98%).
2.2. Clinical Materials
The tissue samples were provided by the Oncology and Haematology Clinic at the North Estonia Medical Centre (Tallinn). All the samples were analyzed immediately after surgery. Only primary tumors were examined and information from respective pathology reports was provided by the North Estonia Medical Centre for all the analyzed samples. Informed consent was obtained from all the patients and coded identity protection was applied. All investigations were approved by the Tallinn Medical Research Ethics Committee and were in accordance with the Helsinki Declaration and Convention of the Council of Europe on Human Rights and Biomedicine. The entire group consisted of 34 patients with breast cancer and 55 with colorectal cancer.
2.3. Cell Cultures
MDA-MB-231 and MCF-7 cells were grown as adherent monolayers in low glucose (1.0 g/L) Dulbecco's modified Eagle's medium (DMEM) with stable L-glutamine and sodium pyruvate (from Capricorn Scientific GmbH) supplemented with 10% heat-inactivated fetal bovine serum, 10 μg/mL human recombinant Zn insulin, and antibiotics: penicillin (100 U/mL), streptomycin (100 μg/mL), and gentamicin at a final concentration of 50 μg/mL. Cells were grown at 37°C in a humidified incubator containing 5% CO2 in air and were subcultured at 2-3-day intervals.
2.4. Mitochondrial Respiration in Saponin-Permeabilized Tissue Samples
Numerous studies have demonstrated that isolated mitochondria behave differently from mitochondria in situ [29–32]. We therefore have investigated respiratory activity of tumor and control tissues in situ using the skinned sample technique [26, 28, 29, 33]. This method allows analysis of the function of mitochondria in cells in their natural environment and leaves links between cytoskeletal structures and mitochondrial outer membranes intact [34–37]. Cytochrome c test was used to confirm integrity of the mitochondrial outer membrane (MOM) [22, 26, 28, 33]; mitochondrial inner membrane quality was checked using a carboxyatractyloside (CAT) test as the last procedure in every experiment [22, 26, 28, 33]. Rates of O2 consumption were assayed at 25°C using Oxygraph-2k high-resolution respirometer (Oroboros Instruments, Innsbruck, Austria) loaded with pre-equilibrated respiration buffer medium B [26]. Activity of the respiratory chain was measured by substrate-inhibitor titration as described earlier [26, 38]. The solubility of oxygen at 25°C was taken as 240 nM/mL [39]. The solubility of oxygen is much lower at 37 than at 25°C, but also, the skinned samples from malignant clinical material are more stable at 25°C. All rates of respiration (V) are expressed in nM O2/min per mg dry tissue weight for solid tumors and in nM O2/min per million cells for cell cultures.
2.5. Metabolic Control Analysis
Metabolic control analysis (MCA) is a method for studying regulatory mechanisms in complex metabolic systems [40–42]. Flux control coefficient (FCC) is defined as the ratio of fractional change in a system variable to fractional change in a biochemical activity that caused the change in the given system [42]. FCC or CviJ is the extent to which an enzyme in a pathway controls the flux (J); it corresponds to the percentage decrease in flux caused by a 1% decrease in the activity (𝑣i) of that enzyme [41, 43]:
| (1) |
This method shows how the control is shared between the enzymes and the transporters of the pathway and enables to identify the steps that could be modified to achieve successful alteration of the flux or metabolite concentration in the pathway. But it also permits the identification of system components that are crucial in the regulation of energy transfer and regulatory networks [40–42, 44–46].
MCA has previously been applied in our lab to human breast and colorectal cancer skinned samples to determine the FCCs for respiratory chain complexes. The flux was measured as the rate of O2 consumption in permeabilized tissues derived from HCC patients when all components of the OXPHOS system were titrated with specific irreversible or pseudoirreversible inhibitors to stepwise decrease selected respiratory chain complex activities according to a previously published method [26, 27, 47, 48].
2.6. Western Blot Analysis of the Level of Mitochondrial RC Complexes Expression
Postoperative human tissue samples (70–100 mg) were crushed in liquid nitrogen and homogenized in 20 volumes of RIPA lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 2 mM EDTA, 0.5% sodium deoxycholate, 0.1% SDS, 0.1% Triton X-100, and complete protease inhibitor cocktail (Roche)) by Retsch Mixer Mill at 25 Hz for 2 min. After homogenization, samples were incubated for 30 min on ice and centrifuged at 12,000 rpm for 20 min at 4°C. The proteins in the supernatants were precipitated using acetone/TCA to remove nonprotein contaminants. Briefly, supernatants were mixed with 8 volumes of ice-cold acetone and 1 volume of 100% TCA, kept at −20°C for 1 h and then pelleted at 11500 rpm for 15 min at 4°C. The pellets were washed twice with acetone and resuspended in 1x Laemmli sample buffer.
Proteins were separated by polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride (PVDF) membrane, and subjected to immunoblotting with the total OXPHOS antibody cocktail (ab110411). Then, the membranes were incubated with corresponding horseradish peroxidase-conjugated secondary antibody and visualized using an enhanced chemiluminescence system (ECL; Pierce, Thermo Fisher Scientific). After chemiluminescence reaction, the PVDF membranes were stained with Coomassie brilliant blue R250 to measure the total protein amount. The complexes I–V signal intensities were calculated by ImageJ software and normalized to total protein intensities.
Expression levels of complex I in HCC and normal tissues were additionally estimated using anti-NDUFA9 antibody that corresponds to NADH dehydrogenase 1α subcomplex 9 (SAB1100073). The samples were incubated and visualized as described above. Levels of NDUFA9 encoding protein were normalized to total protein content.
2.7. Citrate Synthase Activity
Activity of citrate synthase in tissue homogenates was measured as described by Srere [49]. Reactions were performed in 96-well plates containing 100 mM Tris-HCl pH 8.1, 0.3 mM AcCoA, 0.5 mM oxaloacetate, and 0.1 mM DTNB using FLUOstar Omega plate reader spectrophotometer (BMG Labtech).
2.8. Data Analysis
Data in the text, tables, and figures are presented as mean ± standard error (SEM). Results were analyzed by the Student t-test; p values <0.05 were considered statistically significant.
3. Results and Discussion
3.1. Respiratory Chain Analysis and Presence of Supercomplexes
Suppression of mitochondrial electron transport chain function is widespread in cancer, and this is closely connected to apoptosis resistance [50–54]. However, studies are often conducted on cell cultures and therefore little is known about respiratory chain (RC) function in clinical human breast and colorectal carcinomas in situ. To reveal possible disturbances, we conducted comparative quantitative analysis on the respiration rates for different RC complexes in permeabilized HBC and HCC and their adjacent normal tissue samples. Data for healthy breast tissue has been left out from most of the following calculations due to very low ADP-dependent oxygen consumption in this tissue type as it is not sufficient to assess inhibitory effects of antimycin A or rotenone or compare these results to other studied samples.
Multiple substrate-inhibitor titration protocol was used for measuring respiratory capacities of different respiratory chain segments (Table 1) [30, 55]. All respiration rates corresponding to the activities of different RC complexes are increased in both investigated human cancers when compared to their adjacent normal tissue. The mean value of basal respiration (state 2, Vo) in skinned HCC samples is higher than that in normal tissue and depends on the used respiratory substrates. Specifically, in the presence of glutamate and malate, HCC and its control tissue fibers exhibit lower state 2 respiration rates than in the presence of glutamate, malate, and succinate; similar dependence was observed for the breast cancer samples (Figure 1). One possible reason for this difference can be succinate-dependent proton leak in tumor tissue [56–58]. Addition of 2 mM MgADP for studying complex I-based state 3 (in the presence of glutamate and malate without succinate) increased mitochondrial respiration rates in all tissue samples and following addition of complex I-specific inhibitor (rotenone) inhibited the respiration back to the initial state 2 levels (Table 1). Similarly, the function of complex II was quantified upon ADP-stimulated respiration in the presence of rotenone and succinate; at these conditions, the complex I activity is inhibited and apparent respiration rate originates from complex II. Complex III in both HBC and HCC was confirmed to be fully functional as an addition of antimycin A inhibited the electron flow from complex III to mitochondrial complex IV (COX) (Table 1). The activation of mitochondrial complex IV (addition of 5 mM ascorbate and 1 mM tetramethyl-p-phenylenediamine) resulted in a remarkable increase in the rate of O2 consumption in all examined samples, both cancerous and normal, but the increase was nearly two times higher in cancer tissue.
Table 1.
Characterization of respiratory parameters of permeabilized tissue samples derived from patients with breast or colorectal cancer.
| Parameters | HBC patients, n = 7 [26] | HCC patients, n = 7 [28] | ||
|---|---|---|---|---|
| Tumor | Control | Tumor | Control | |
| Vo | 0.294 ± 0.024 | 0.004 ± 0.007 | 1.06 ± 0.14 | 0.82 ± 0.15 |
| VADP | 0.71 ± 0.06 | 0.055 ± 0.004 | 2.02 ± 0.21 | 1.39 ± 0.21 |
| Vrot | 0.34 ± 0.04 | 0.070 ± 0.015 | 0.91 ± 0.11 | 0.85 ± 0.14 |
| V Succ | 0.74 ± 0.10 | 0.076 ± 0.008 | 2.22 ± 0.26 | 1.33 ± 0.18 |
| VANM | 0.38 ± 0.04 | 0.071 ± 0.018 | 1.04 ± 0.09 | 0.69 ± 0.07 |
| V COX | 2.36 ± 0.33 | 1.23 ± 0.18 | 6.59 ± 0.71 | 3.84 ± 0.58 |
Note: here, each data point is the mean ± SEM of respiratory values. Vo: basal respiration without ADP or ATP; VADP: ADP-stimulated respiration (final concentration 2 mM) in the presence of 5 mM glutamate and 2 mM malate (indicating the function of the respiratory chain complex I); Vrot: rates of respiration after addition of 50 μM rotenone (an inhibitor of complex I); VSucc: ADP-stimulated respiration in the presence of rotenone and 10 mM succinate (to estimate the function of complex II); VANM: rates of respiration after addition 10 μM antimycin-A (an inhibitor of complex III); VCOX: rates of O2 consumption in the presence of complex IV substrates (5 mM ascorbate jointly with 1 mM tetramethyl-p-phenylenediamine).
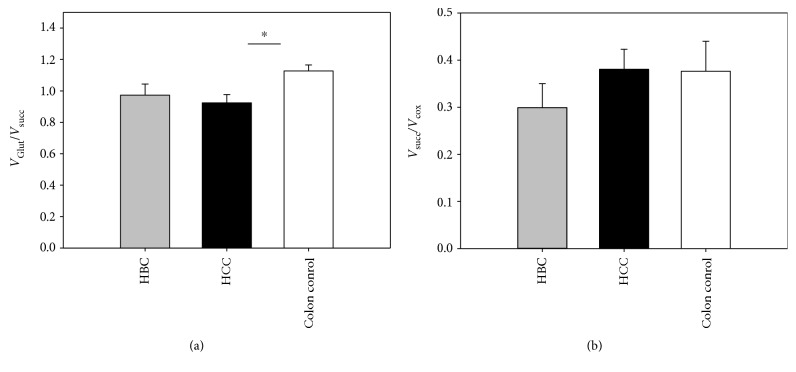
Figure 1.
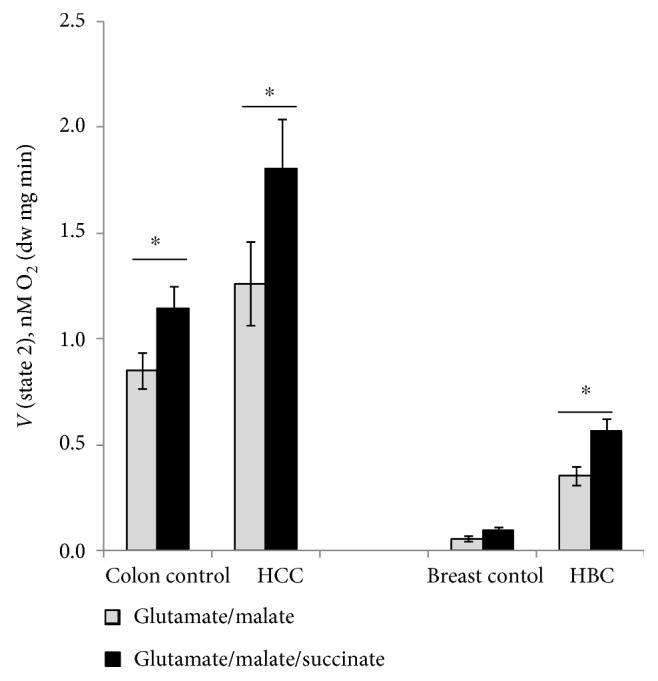
Assessment of state 2 respiration rates of the permeabilized HCC, HBC, and normal adjacent tissue samples in the presence of different combinations of respiratory substrates (5 mM glutamate, 2 mM malate, and 10 mM succinate). Bars are SEM, n = 8 for colon samples, and n = 12 for breast tissue samples, ∗p < 0.05.
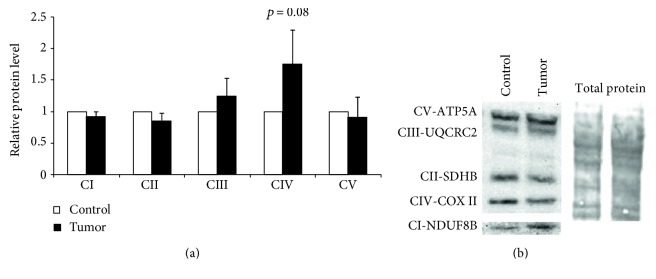
Complex I deficiency is the hallmark of multiple mitochondrial diseases and is generally considered to be an intrinsic property of some cancers [58–63]. Indeed, our experiments confirm that development of HCC results in reduced VGlut/VSucc ratio which indicates relative suppression of the complex I-dependent respiration [58]. Similar results have previously been described for gastric and ovarian cancer tissues but also in some cancer cell cultures [58, 63–66]. Deficiency of complex I in some tumors might be an early event causing an increase in mitochondrial biogenesis in an attempt to compensate for the reduction in OXPHOS function [63]. Computer modeling predicts that the mechanisms of this compensation can use multiple pathways like β-oxidation of fatty acids, mitochondrial folate metabolism, and others [67]. Our results showed that this suppression is pronounced on the functional level in HCC (Figure 2(a)), but to identify the changes on the protein expression level, we analyzed the RC complexes with total OXPHOS antibody cocktail (Figure 3). Based on this type of approach, the suppression of RC complex I was found to be absent if the results were normalized to total protein. The suppression of complex I in HCC was additionally studied by Western blot analysis with antibodies against only one complex I subunit—NDUFA9 (see Supplementary Fig 1 available online at https://doi.org/10.1155/2017/1372640). This result, however, confirmed the suppression of complex I. As seen from those experiments, analysis of RC, using the semiquantitative WB method, can be strongly dependent on experimental conditions: against what complex I subunit the antibodies were used and which normalization conditions are applied. Additionally, complex II in colon samples did not indicate possible suppression in that alternative pathway as differences in VSucc/VCOX ratios were not significant (Figure 2(b)) [68].
Figure 2.
(a) Oxygraphic analysis of the functioning of complex I in skinned tissues from patients with HBC or HCC; here, VGlut/VSucc is the ratio of ADP-stimulated respiration rate in the presence of 5 mM glutamate and 2 mM malate (activity of complex I) to ADP-stimulated respiration rate in the presence of 50 μM rotenone and 10 mM succinate (activity of complex II). (b) VSucc/VCOX is the ratio of complex II respiration rate to complex IV respiration rate. Data shown as mean ± SEM; n = 7 for colon [28] and breast tissue samples [26], ∗p < 0.05.
Figure 3.
Quantitative analysis of the expression levels of the respiratory chain complexes in HCC and normal tissue samples (a) along with a representative Western blot image (b). Protein levels were normalized to total protein staining by Coomassie blue; data shown as mean ± SEM of 5 independent experiments.
In contrast to HCC, mitochondrial respiration in HBC samples is not accompanied with suppression of complex I-dependent respiration (Figures 2(a) and 2(b)). Altogether, the relative complex I deficiency on the functional level in our oxygen consumption measurements is characteristic for HCC but not for HBC tissue.
Remarkable numbers of studies have shown that RC complexes can form protein assemblies (supercomplexes). These supramolecular structures provide kinetic advantage such as substrate channeling, increased efficiency in electron transport, prevention of destabilization, and degradation of respiratory enzyme complexes [21] and means to regulate ROS levels in the cell (most of the mitochondrial ROS originates from complexes I and III) [69] and hence, homeostasis. The RC complex I is considered to be the most important component in these assemblies, and it is a member of almost all known respirasomes [70–74]. In previous studies, complexes I, III, and IV were found to be assembled into supercomplexes in different configurations, but complex II was not confirmed to be a component of these RC supercomplexes and was assumed to move freely in the mitochondrial inner membrane [70, 75, 76]. Relative deficiency of the RC complex I on the functional level (as shown above) may be a result of changes in supercomplex composition as a part of malignant transformation.
In addition to RC supercomplexes, along with respirasomes, one more molecular transmembrane protein supercomplex (which is known as ATP synthasome; [77]) was identified as the component of the OXPHOS system. The ATP synthasome complex consists of ATP synthase, inorganic phosphate carrier, and adenine nucleotide translocator (ANT) [48]. The current model of mitochondrial interactosome (MI) considers the ATP synthasome and RC complexes together with voltage-dependent anion channel (VDAC) and mitochondrial creatine kinase (MtCK) as components of intracellular energetic units [22]. Even though MI is proven in striated muscles, the functional role of it, together with MtCK, in malignant samples remains controversial [78], but it indicates that both ATP synthasome and RC complexes can form even more complex functional structures.
In addition to steady-state proteome studies, kinetic testing of metabolic fluxes using MCA can provide preliminary information about supramolecular organization in the energy transfer system and enables to quantify the flux exerted by the different RC and the ATP synthasome complexes [27, 28, 79]. MCA can discriminate between two prevailing models: the former model, based on the assumption that each enzyme can be rate controlling to a different extent, and a subsequent model, where whole metabolic pathway can behave as a single channel and inhibition of any of its components would give the same flux control [80]. Bianchi et al. proposed that both complexes I and III are highly rate controlling in NADH oxidation, suggesting the existence of functional association between these two complexes [80]. To confirm the formation of supercomplexes in HCC- and HBC-skinned samples using MCA, we investigated the flux control coefficients (FCCs) for the complexes involved in aerobic NADH oxidation (I, III, and IV), in succinate oxidation (II, III, and IV), and for components of the ATP synthasome. For this purpose, cancerous and normal tissue samples were titrated with increasing concentrations of specific inhibitors against all of the ATP synthasome and RC complexes. Figure 4 summarizes the data analyzed in three different ways: by a graphical model [40, 41, 81], according to Small [82], and the Gellerich model [44]. The obtained FCC values did not depend on which exact method was used for calculations. The main problem in these calculations is high heterogeneity of the clinical material, which from the one hand originates from cancer molecular subtypes (e.g., Lumianal A/B, HER2 or triple negative in HBC; unknown subtypes in HCC) but on the other hand originates from heterogeneity of tumor cells within each tumor [14] or irregular stromal burden. Therefore, the obtained coefficient values do not only depend on which patients were included to the study, but the results may also depend on which particular tumor region was used from each patient sample. This can be considered as an inevitable part in analyzing clinical samples.
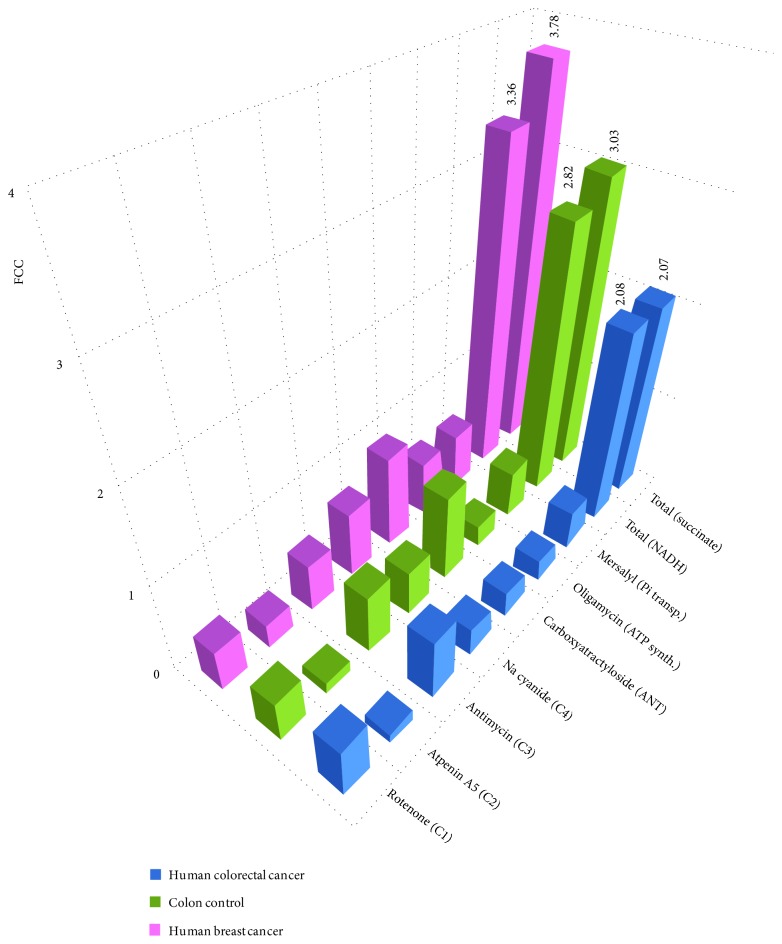
Figure 4.
FCCs for ATP synthasome and RC complexes as determined by MCA. Two ways of electron transfer were examined: NADH-dependent and succinate-dependent electron transfers, and respective sums of FCCs are calculated as the last bars. Data for HBC is published before in [26], except for complex II with atpenin A5. Isolated mucosal tissue was used for colon control.
Previous work has shown that the main respiratory rate-controlling steps in HBC cells are complex IV (FCC = 0.74) and adenine nucleotide transporter (ANT, FCC = 1.02) [26]. Similar control distribution was not observed within HCC ATP synthasome complex as FCCs for ANT were found to be significantly lower when HCC was compared to the results of healthy colon mucosa (FCC = 0.284 for HCC and FCC = 0.970 for healthy colon). These results show that ANT exerts high flux control in healthy colon tissue (and in HBC), but ANT seems to lose its limiting role in HCC. Ramsay et al. believe that hexokinase-voltage-dependent anion channel-ANT complex, which spans across the outer and inner mitochondrial membranes, is critical in cancer cells as this complex is the link between glycolysis, oxidative phosphorylation, and mitochondrial-mediated apoptosis [83]. Therefore, the difference between HBC and HCC, in regard to ANT-exerted flux control, indicates to distinct difference in energy metabolism between these two tumor types (Table 2; Figure 4). In addition, HBC is showing equal FCCs for ATP synthase and inorganic phosphate transporter (Pi) in ATP synthasome, but this phenomenon is not characteristic neither for healthy colon mucosa nor for colorectal cancer. These alterations could be related to mitochondrial permeability transition pore (mtPTP) and apoptosis. Bernardi et al. studied the key regulatory features of the mtPTP [84–87], and the same group of authors has pointed to the fact that ANT can modulate the mtPTP, possibly through its effects on the surface potential, but it is not a mandatory component of this channel.
Table 2.
FCCs for different components in mitochondrial Interactosome and ranges of the concentrations of inhibitors.
| MI component | Inhibitor | Range of inhibitor concentration | FCC | ||
|---|---|---|---|---|---|
| HCC | Control colon tissue (mucosa) | HBC | |||
| Complex I | Rotenone | 1–100 nM | 0.56 | 0.45 | 0.46∗ |
| Complex II | Atpenin A5 | 0.1–6 μM | 0.12 | 0.13 | 0.28 |
| Complex III | Antimycin | 1–200 nM | 0.68 | 0.66 | 0.54∗ |
| Complex IV | Na cyanide | 0.1–40 μM | 0.31 | 0.50 | 0.74∗ |
| ANT | Carboxyatractyloside | 1–200 nM | 0.28 | 0.97 | 1.02∗ |
| ATP synthase | Oligomycin | 1–600 nM | 0.25 | 0.24 | 0.61∗ |
| Pi transporter | Mersalyl | 1–200 μM | 0.43 | 0.53 | 0.60∗ |
| Sum 1, 3–7 | Total (NADH) | 2.08 | 2.82 | 3.36 | |
| Sum 2–7 | Total (succinate) | 2.07 | 3.03 | 3.78 |
Note: ∗from [26].
FCCs within the RC system in HBC do not differ significantly and the flux control is distributed almost uniformly throughout the different complexes (Table 2, Figure 4). Such condition is an indication of possible presence of protein supercomplexes (approximately equal values of FCCs for RC complexes I and III—0.46 versus 0.54, resp.). On the other hand, the flux distribution for normal colon tissue, when compared to HCC, showed slight difference for that for complex IV (FCC 0.50 versus 0.31), but flux control coefficients with close values were calculated for complex I (FCC 0.45 versus 0.56) and complex III (FCC 0.66 versus 0.68). Similarity in FCCs for complex I and complex III for both HCC and healthy colon tissue enables to propose that in healthy conditions, complex III is attached to complex I (possibly together with multiple copies of complex IV), but during carcinogenesis, the supercomplex assembly changes and even though complex I and complex III seem to stay linked, the participation of complex IV in this assembly becomes uncertain. Functional assembly of complexes I and III together with their rate-limiting roles will lead to sum of FCCs being greater than 1 [73] (see below).
Role of complex IV is multifaceted as three populations of it have previously been suggested: population assembled with complex I and complex III, population assembled with complex III alone and a non-interacting population [74]. Several data show that the absence of functional supercomplex assembly factor I (SCAF1) may be involved in distribution of complex IV [74, 75, 88]. As outlined in the review article by Enrìquez, total cell respiration (glucose, pyruvate, and glutamine as substrates) was significantly higher in cells lacking functional SCAF1 [74]. High total cell respiration was registered also for both cancer types described in this paper, but presence or absence of functional SCAF1 was not investigated.
The sums of the determined FCCs within cancerous and healthy sample groups were calculated to be in the range from 2.07 to 3.78. In theory, sum of FCCs in a linear system is 1 [5, 40, 42–44, 89, 90], but the value of it can increase if the system includes enzyme–enzyme interactions, direct channeling, and/or recycling within multienzyme complexes (i.e., system becomes nonlinear) [79, 80, 91, 92]. The higher sum of FCCs from our tests is not a result of diffusion restrictions because the concentration ranges for all of the inhibitors in various samples were similar and did not depend on the nature of the samples [26, 28, 47, 48].
The organization of RC complexes in the mitochondrial inner membrane has been an object of intense debate and it is not studied systematically in human normal or cancerous tissues. Given the known theoretical framework, our results confirm the plasticity model and agree with the data from Bianchi et al. [80], but the distribution of complex IV remains unclear—both random distribution and association into I-III-IV supercomplex can be possible. Large FCC for complex II is not characteristic neither for HBC, for HCC, nor for healthy colon tissue, and therefore, our kinetic studies confirmed previous findings that this complex is not a part of RC supercomplexes.
The question about the changes in the composition and stoichiometry of protein supercomplexes, which result from carcinogenesis, needs further studies, and in addition, as mitochondria have other additional roles in cellular metabolism, it can be presumed that changes in RC are also affecting cataplerotic processes sprouting from the mitochondria, but such link has not yet been studied yet.
3.2. ADP-Regulated Mitochondrial Respiration in HBC and HCC Fibers
Table 3 summarizes ADP-regulated mitochondrial respiration parameters determined for skinned tissue samples taken from both patient groups. Differences in the rates of maximal ADP-activated respiration (Vmax) in colon tissue samples are corresponding to the differences in the content of mitochondria in these cells (the amount of mitochondria in HCC is 50% higher than that in healthy control tissue [28] (supplementary Table 1)). Our previous experiments have shown that HBC tissue, too, contains an increased number of mitochondria in comparison to its adjacent normal tissue [27, 93] (supplementary Table 1). As indicated above, ADP-dependent respiration in healthy human breast tissue is absent. Breast samples contain lot of fat tissue, but low Vmax values were evident even if clearly lobular/ductal structures were separated and tested. Low respiratory capacity can also be indicating to lowered metabolic activity in normal ductal/lobular tissue in older women (average age of HBC patients in this study was 63.4 years). In contrast to normal breast tissue, the colon control tissue samples have significantly higher respiration rates (Table 3). Specifically, respiratory capacity is higher in apparent mucosal/submucosal section of the normal colon tissue samples compared to that of the underlying smooth muscle part as we manually separated and tested these two layers in a selection of colon tissue samples (Figure 5(b)).
Table 3.
Apparent Km (appKm) and maximal rate of respiration (Vmax) values for ADP-dependent respiration calculated for HBC, HCC and their adjacent healthy tissue samples.
| Tissues | app K m, μM ± SEM | V max ± SEM |
|---|---|---|
| Human breast cancer tissue | 114.8 ± 13.6∗ | 1.09 ± 0.04∗ |
| Healthy adjacent breast control tissue | — | 0.02 ± 0.01∗ |
| Human colorectal cancer tissue | 93.6 ± 7.7∗∗ | 2.41 ± 0.32 |
| Healthy adjacent colon control tissue | 256∗∗ ± 34 | 0.71 ± 0.23 |
Note: ∗from [26] and ∗∗ [38]; Vmax values are presented as nM O2/min/mg dry tissue weight without proton leak rates. These Km and Vmax values for ADP were determined from corresponding titration curves by fitting experimental data to non-linear regression equation according to a Michaelis–Menten model. 35 patients used for analysis of HBC and 35 for HCC.
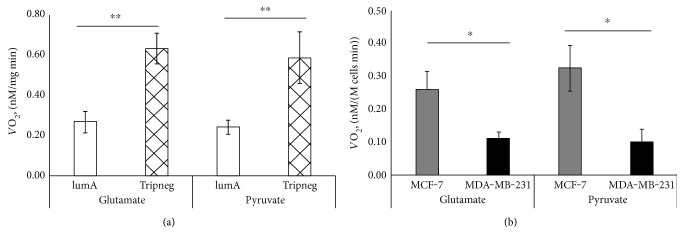
Figure 5.
(a) Respiration rates for clinical samples of luminal-A and triple negative HBC subtypes in the presence of 5 mM glutamate or 5 mM pyruvate; n = 13/12 for luminal-A and n = 7/8 for triple negative subtypes, respectively. (b) Respiratory rates for luminal-A type MCF-7 and triple negative MDA-MB-231 cells in the presence of 5 mM glutamate or 5 mM pyruvate; n = 3 for each measurement; ∗p < 0.05, ∗∗p < 0.005.
HBC arises from tissue with almost absent ADP-related respiration, but once formed, the mechanism of energy conversion seems to acquire a more complicated form and it can be associated with both increased mitochondrial biogenesis and interplay between cancer and stromal cells [26]. HBC can be classified into four clinically distinct and significant molecular subtypes: luminal-A, luminal-B, HER2 expressing, and triple negative. Clinically, luminal-A is considered the least and triple negative as the most aggressive subtype. Therefore, we expected to see clear differences when respiratory parameters of those two extreme subtypes were measured. Initially, respiration rates were analyzed in Luminal-A type MCF7 and triple negative MDA-MB-231 cell lines. When compared, respiration rates in presence of glutamate or pyruvate clearly showed that oxygen consumption in luminal-A subtype cells is remarkably higher (Figure 5(b)). But in contrast, the exact opposite was registered for the same parameters in clinical samples (Figure 5(a)) as the highest respiratory rates were registered for the most aggressive triple negative subtype. From the one hand, this contradicting result shows that cell cultures are not directly comparable to respective clinical counterparts and can lead to misguiding expectations. On the other hand, it proves that the role of OXPHOS becomes increasingly important in clinical samples as aggressiveness of the tumor increases, but it is not evident in the respective culture cells. In the present case, it is not a result of increased glucose availability in the growth medium, which could lead the cells to acquire glycolytic phenotype and explain the difference with clinical samples, because low-glucose media was used.
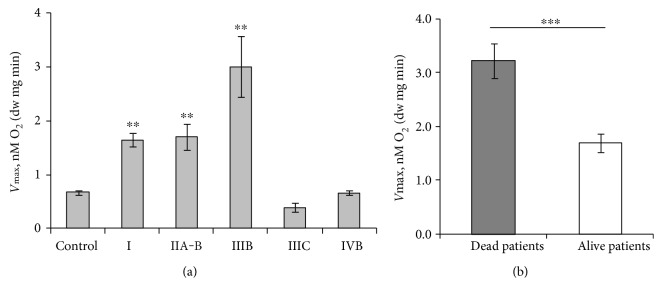
For HCC, which is without distinct clinical subtypes, we compared disease stage to average Vmax value for that stage (Figure 6(a)). Even though increase in Vmax in initial stages can be calculated in comparison to control sample, the decrease in Vmax for stages IIIC and IVB does not fit this increase in dependence. The disease stage at diagnosis itself is not a valid marker of aggressiveness and therefore such plotting can be debated. Therefore, we gathered initial longitudinal data on patient progression in our HCC cohort and confirmed that 7 out of 32 eligible patients had died (median follow-up time 47.3 ± 4.9 months). Vmax values in patients that succumbed to the disease were significantly higher than that in the currently not progressed group (Figure 6(b)). As was shown for HBC above, higher respiratory capacity was registered for the most aggressive triple negative subgroup. Therefore, it can be argued based on similarity that higher tumor respiratory parameters in the dead HCC patients were indicating to more aggressive disease. In addition, lower than expected respiratory rate in some triple negative tumors can therefore indicate that given patient, when compared to the average in the triple negative subgroup, has less aggressive disease than could be expected. To confirm this in larger cohorts and relate aggressiveness in HCC and HBC to Vmax value, additional longitudinal studies are necessary.
Figure 6.
(a) Dependence of maximal rate of mitochondrial respiration (Vmax) compared with the HCC at different stages. Stage I was calculated as the mean of 13 patients, IIA, IIB - 13 patients, IIIB-4 patients, IIIC-3 patients and IVB-1 patient. Control colon tissue is obtained from 34 patients. Maximal respiration rate Vmax is compared with that in control tissue. Bars are SEM; ∗∗p < 0.005. (b) Vmax in HCC patients based on disease state in follow-up setting. Seven patients out of 32 are confirmed to have succumbed to HCC (Vmax = 3.19 ± 0.34); 25 patients out of 32 stay in remission (Vmax = 1.70 ± 0.17), ∗∗∗p < 0.001.
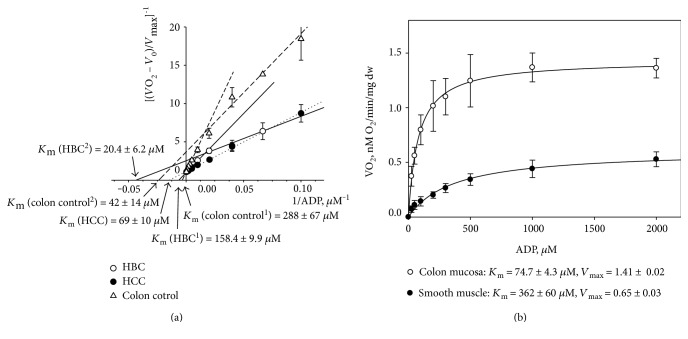
We next measured apparent Michaelis–Menten constants (Km) for ADP to characterize the affinity of mitochondria for exogenous ADP (i.e., permeability of mitochondrial outer membrane). Corresponding Km values for permeabilized tumor and nontumorous tissues were determined from titration experiments using exogenously added ADP. The obtained data were plotted as rates of O2 consumption versus ADP concentration and apparent Km values were calculated from these plots by nonlinear regression equation. Healthy colon tissue displayed low affinity for ADP (Km = 256 ± 3 μM), whereas that in HCC is significantly higher (Km = 93.6 ± 7.7 μM) [38]. The Km (ADP) value for HBC tissue samples (Km = 114.8 ± 13.6 μM) was similar to that for HCC [26].
According to the classical studies by Chance and Williams [94, 95] and the data of many other investigators [29, 30, 37], the apparent Km value for ADP for isolated mitochondria is low, about 15 μM, but the observed apparent Km values in our study for permeabilized clinical HBC and HCC samples were 6–8 times higher than this value (Table 3). Our previous studies have shown that sensitivity of the mitochondrial respiration for exogenous ADP for permeabilized NB HL-1 cells is also high as the apparent Km equaled to 25 ± 4 μM and was similar to that of isolated heart mitochondria [34, 96]. The similar low apparent Km values were also registered for undifferentiated and differentiated neuroblastoma culture cells, where the corresponding Km for ADP were measured as 20.3 ± 1.4 μM and 19.4 ± 3.2 μM, respectively [97]. The registered difference between culture cells and clinical samples, despite the used preparation method, again indicates to differences present in these two sample groups.
We treated permeabilized samples with incremental concentrations of ADP and the measured O2 consumption rates (normalized to Vmax) were analyzed against respective ADP concentration values as double reciprocal Lineweaver–Burk plots (Figures 7(a) and 7(b)) [29]. Figure 7(a) shows the results of the Lineweaver–Burk treatment of the experimental data linked with ADP-regulated mitochondrial respiration in skinned fibers of HCC, healthy colon, and HBC. Corresponding Vmax and Km values were calculated from the linearization approach. Saks and colleagues have previously shown that the presence of biphasic respiration regulation on the graph curve indicates the existence of two populations of mitochondria with different affinities for ADP [29]. Our results indicated such differences in colon control and HBC samples. Specifically, monophasic regulation of mitochondrial respiration is apparent in HCC tissue, but in healthy colon tissue, two populations of mitochondria with very different properties were found (Figure 7(a)). One population of mitochondria is characterized with lower Km (42 ± 14 μM), whereas the apparent Km (ADP) value for the second mitochondrial population is nearly seven times higher (288 ± 67 μM). We thereafter again separated mucosal and smooth muscle parts from the colon samples before additional Km measurements to characterize their isolated contributions. Apparent Km value for mucosal part was measured to be 74.7 ± 4.3 μM and the same value for colon smooth muscle tissues was found to be 362 ± 60 μM (Figure 7(b)). Therefore, results after separation explain the results from the initial experiment where the entire colon wall was analyzed and two separate groups of mitochondria were discovered. Additionally, we could also distinguish two differently regulated types of mitochondria in HBC samples: one with apparent Km value for MgADP of 20.4 ± 6.2 μM, but the same for the second mitochondrial population was nearly ten times higher, 158.5 ± 9.9 μM (Figure 4(a)). The phenomenon shown in Figure 7(a) can be associated, on the one hand, simply with elevated stromal content (in such case, similar results should have been also registered for HCC), but on the other hand, with possible two-compartment tumor metabolism in HBC, what states that tumor cells function as metabolic parasites and extract energy from supporting host cells such as fibroblasts [98–103]. In such case, the stromal part of the HBC samples can be characterized with glycolytic metabolism representing the low Km value due to high levels of autophagy, mitophagy, glycolysis, and lipolysis, while cancer cells have high mitochondrial mass, OXPHOS, and β-oxidation activity, which is represented by the mitochondrial population with the high Km (ADP) values. From the given comparison between HBC and HCC, the two subpopulations of mitochondria are specific only to HBC samples (confirmed in 32 cases out of the total 34) but not to HCC samples, and it indicates that tumor formation leads to distinct changes, which is related to the tissue type the tumor originates from.
Figure 7.
(a) Dependences of normalized respiration rate values for HCC (dotted line), HBC (solid line), and healthy colon tissue samples (dashed line); double reciprocal Lineweaver–Burk plots. Samples from 32 patients with breast cancer and 10 patients with colorectal cancer were examined. (b) ADP-dependent respiration in healthy colon mucosa and smooth muscle tissue samples (Michaelis–Menten curve, n = 8). Here, Vo and Vmax are rates of basal and maximal ADP-activated respiration, respectively.
Altogether, these results indicate the remarkable differences in the regulation of mitochondrial outer membrane (MOM) permeability between cultured tumor cells and clinical material (including between different tumor types and even between patients). Even further, the results can be contradictory as registered for respiration parameters. It can be estimated, based on the results from our lab, that low Km value for ADP can be a common characteristic for cancer cells grown in culture, but in in vivo tumor samples, the regulation of MOM permeability is more complicated and probably related to interplay between energy transfer pathways and changes in the phosphorylation state of VDAC channels [32, 104–107] and also with modulation of cytoskeleton or membrane potential as a result of tumor formation.
4. Concluding Remarks
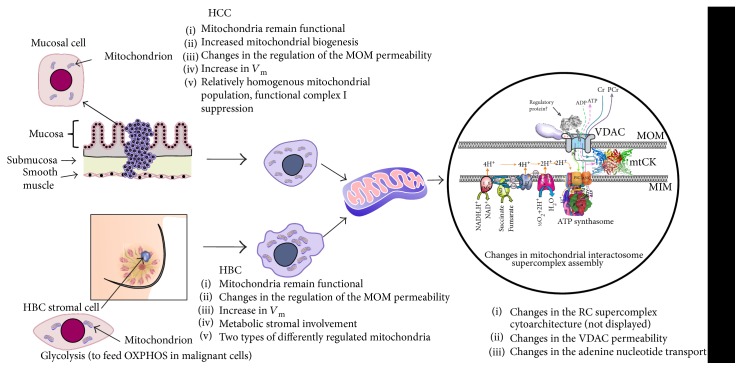
To understand the energy metabolism of tumors, it is necessary to detect bioenergetic fingerprints of each individual tumor type. Our results confirmed that respiratory capacity is preserved in both HBC and HCC as these both demonstrated substantial rates of oxidative phosphorylation, which contradicts with earlier widespread understanding that the metabolism of human breast and colorectal carcinomas is prevalently glycolytic. Studies on cell lines up to now have led to many lifesaving technologies and treatments in humans, but the scientific level might be nearing the end of readily transferrable results between the cell model and human physiology. Our results indicated that apparent glycolytic nature of some breast cancer types could be expected based on cell cultures, but this presumption was in sharp conflict when culture cell results were compared with these from respective clinical samples. In addition, when compared to their healthy adjacent tissue, both clinical cancer types showed increased respiratory capacity. Despite the increased respiratory capacity in HCC, relative deficiency of complex I was registered for it on the functional level Western blot analysis was not sufficient to confirm this deficiency on the protein level as two different antibody approaches gave conflicting results, but this result proves the necessity to measure pathways also on the functional level whenever possible to compare the function to steady-state markers like presence or abundance of certain enzymes. Our experiments indicate that the respiratory chain and ATP synthasome can form macromolecular assemblies (supercomplexes) with reorganized composition and/or stoichiometry while the changes are specific for different tumor types. This is in good agreement with recent studies from other laboratories [25] and the current work shows that equal results can be obtained using kinetic methods, but additional studies are warranted to include results from protein level studies using the blue native gel electrophoresis (BNGE) technique. Our Km measurements confirmed that two populations of mitochondria registered in healthy colon tissue can be categorized as different layers of the colon wall, but in HBC, the subgroups can be linked to two-compartment metabolism where tumor acts as a metabolic parasite on normal stromal cells. Mitochondria of HCC are homogenous in terms of regulation of the mitochondrial outer membrane permeability and MCA (Figure 8).
Figure 8.
Mitochondrial alterations in HCC and HBC tissue cells. Mitochondrial interactosome is a large supercomplex consisting of ATP synthasome, VDAC, mitochondrial kinases like adenylate kinase, hexokinase or mitochondrial creatine kinase (MtCK), and respiratory chain (super)complexes. Here, the octameric MtCK characteristic is shown for the striated muscles and also as the possible component of the MI in the healthy colon [108–110]. The complex of VDAC together with other proteins controls the exchange of adenine nucleotides and regulates energy fluxes between mitochondrial and cytosolic compartments. Changes in the structure and function of MI are the important parts of cancer mitochondrial metabolism.
Mitochondria are not only the centers of cellular energy conversion but are also the important part in biosynthetic metabolism and apoptosis. Therefore, direct detection of profound changes in the ATP synthasome components and in the architecture of the respiratory chain complexes, as shown in the current work, can support development of new predictive models or therapies.
Supplementary Material
Supplementary Table 1. Activity of citrate synthase as marker of mitochondrial mass [105], in human breast and colorectal cancers as well as in corresponding normal tissues. Enzymatic activity measured as mU/mg protein, mean ± SE. Supplementary Fig. 1. Quantification of Complex I subunit NDUFA9 expression levels using Western blot (WB) and anti-NDUFA9 antibody in HCC and healthy colon tissue samples (A); respective WB image of NDUFA9 protein levels (B). Levels of NDUFA9 were normalized to total protein content quantified by coomassie blue staining. Data are represented as mean values ± SEM from 4 independent experiments; ∗ p<0.05.
Acknowledgments
This work was supported by the institutional research funding IUT23-1 of the Estonian Ministry of Education.
Abbreviations
- ANT:
Adenine nucleotide translocator
- CAT:
Carboxyatractyloside
- COX:
Cytochrome c oxidase
- FCC:
Flux control coefficient
- HBC:
Human breast cancer
- HCC:
Human colorectal cancer
- HK:
Hexokinase
- MCA:
Metabolic control analysis
- MI:
Mitochondrial interactosome
- MOM:
Mitochondrial outer membrane
- MtCK:
Mitochondrial creatine kinase
- mtPTP:
Mitochondrial permeability transition pore
- OXPHOS:
Oxidative phosphorylation
- RC:
Respiratory chain
- SCAF1:
Supercomplex assembly factor I
- TME:
Tumor microenvironment
- VDAC:
Voltage-dependent anion channel.
Additional Points
Highlights. (1) Relative complex I functional deficiency is characteristic for HCC but not for HBC. (2) HBC respiratory capacity severely higher than in adjacent normal breast tissue. (3) Complexes I and III expectedly assembled in both tumorous and normal tissues. (4) Km for ADP shows distinct differences between cell cultures and clinical samples. (5) Two distinct mitochondrial populations present in HBC but not in HCC.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Rodriguez-Enriquez S., Hernández-Esquivel L., Marín-Hernández A., et al. Mitochondrial free fatty acid beta-oxidation supports oxidative phosphorylation and proliferation in cancer cells. The International Journal of Biochemistry & Cell Biology. 2015;65:209–221. doi: 10.1016/j.biocel.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Gomez L. S., Zancan P., Marcondes M. C., et al. Resveratrol decreases breast cancer cell viability and glucose metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie. 2013;95(6):1336–1343. doi: 10.1016/j.biochi.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Ma Y., Bai R. K., Trieu R., Wong L. J. Mitochondrial dysfunction in human breast cancer cells and their transmitochondrial cybrids. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2010;1797(1):29–37. doi: 10.1016/j.bbabio.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun R. C., Fadia M., Dahlstrom J. E., Parish C. R., Board P. G., Blackburn A. C. Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast Cancer Research and Treatment. 2010;120(1):253–260. doi: 10.1007/s10549-009-0435-9. [DOI] [PubMed] [Google Scholar]
- 5.Moreno-Sanchez R., Marín-Hernández A., Saavedra E., Pardo J. P., Ralph S. J., Rodríguez-Enríquez S. Who controls the ATP supply in cancer cells? Biochemistry lessons to understand cancer energy metabolism. The International Journal of Biochemistry & Cell Biology. 2014;50:10–23. doi: 10.1016/j.biocel.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Enriquez S., Gallardo-Pérez J. C., Marín-Hernández A., et al. Oxidative phosphorylation as a target to arrest malignant neoplasias. Current Medicinal Chemistry. 2011;18(21):3156–3167. doi: 10.2174/092986711796391561. [DOI] [PubMed] [Google Scholar]
- 7.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. [PubMed] [Google Scholar]
- 8.Lisanti M. P., Martinez-Outschoorn U. E., Sotgia F. Oncogenes induce the cancer-associated fibroblast phenotype: metabolic symbiosis and “fibroblast addiction” are new therapeutic targets for drug discovery. Cell Cycle. 2013;12(17):2740–2749. doi: 10.4161/cc.25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotgia F., Whitaker-Menezes D., Martinez-Outschoorn U. E., et al. Mitochondrial metabolism in cancer metastasis: visualizing tumor cell mitochondria and the “reverse Warburg effect” in positive lymph node tissue. Cell Cycle. 2012;11(7):1445–1454. doi: 10.4161/cc.19841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witkiewicz A. K., Whitaker-Menezes D., Dasgupta A., et al. Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle. 2012;11(6):1108–1117. doi: 10.4161/cc.11.6.19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smolkova K., Plecitá-Hlavatá L., Bellance N., Benard G., Rossignol R., Ježek P. Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells. The International Journal of Biochemistry & Cell Biology. 2011;43(7):950–968. doi: 10.1016/j.biocel.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Payen V. L., Porporato P. E., Baselet B., Sonveaux P. Metabolic changes associated with tumor metastasis, part 1: tumor pH, glycolysis and the pentose phosphate pathway. Cellular and Molecular Life Sciences. 2016;73(7):1333–1348. doi: 10.1007/s00018-015-2098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swartz M. A., Iida N., Roberts E. W., et al. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Research. 2012;72(10):2473–2480. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGranahan N., Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell. 2015;27(1):15–26. doi: 10.1016/j.ccell.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Swerdlow R. H., Lezi E., Aires D., Lu J. Glycolysis-respiration relationships in a neuroblastoma cell line. Biochimica et Biophysica Acta (BBA) - General Subjects. 2013;1830(4):2891–2898. doi: 10.1016/j.bbagen.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jose C., Rossignol R. Rationale for mitochondria-targeting strategies in cancer bioenergetic therapies. The International Journal of Biochemistry & Cell Biology. 2013;45(1):123–129. doi: 10.1016/j.biocel.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Gnaiger E., Kemp R. B. Anaerobic metabolism in aerobic mammalian cells: information from the ratio of calorimetric heat flux and respirometric oxygen flux. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1990;1016(3):328–332. doi: 10.1016/0005-2728(90)90164-Y. [DOI] [PubMed] [Google Scholar]
- 18.Gstraunthaler G., Seppi T., Pfaller W. Impact of culture conditions, culture media volumes, and glucose content on metabolic properties of renal epithelial cell cultures. Are renal cells in tissue culture hypoxic? Cellular Physiology and Biochemistry. 1999;9(3):150–172. doi: 10.1159/000016312. [DOI] [PubMed] [Google Scholar]
- 19.Sherr C. J., DePinho R. A. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102(4):407–410. doi: 10.1016/S0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 20.Moreno-Sanchez R., Saavedra E., Gallardo-Pérez J. C., Rumjanek F. D., Rodríguez-Enríquez S. Understanding the cancer cell phenotype beyond the limitations of current omics analyses. The FEBS Journal. 2016;283(1):54–73. doi: 10.1111/febs.13535. [DOI] [PubMed] [Google Scholar]
- 21.Lenaz G., Genova M. L. Kinetics of integrated electron transfer in the mitochondrial respiratory chain: random collisions vs. solid state electron channeling. American Journal of Physiology. Cell Physiology. 2007;292(4):C1221–C1239. doi: 10.1152/ajpcell.00263.2006. [DOI] [PubMed] [Google Scholar]
- 22.Timohhina N., Guzun R., Tepp K., et al. Direct measurement of energy fluxes from mitochondria into cytoplasm in permeabilized cardiac cells in situ: some evidence for mitochondrial interactosome. Journal of Bioenergetics and Biomembranes. 2009;41(3):259–275. doi: 10.1007/s10863-009-9224-8. [DOI] [PubMed] [Google Scholar]
- 23.Lenaz G., Genova M. L. Structural and functional organization of the mitochondrial respiratory chain: a dynamic super-assembly. The International Journal of Biochemistry & Cell Biology. 2009;41(10):1750–1772. doi: 10.1016/j.biocel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Vonck J., Schafer E. Supramolecular organization of protein complexes in the mitochondrial inner membrane. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2009;1793(1):117–124. doi: 10.1016/j.bbamcr.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Rohlenova K., Sachaphibulkij K., Stursa J., et al. Selective disruption of respiratory supercomplexes as a new strategy to suppress Her2high breast cancer. Antioxidants & Redox Signaling. 2017;26(2):84–103. doi: 10.1089/ars.2016.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaambre T., Chekulayev V., Shevchuk I., et al. Metabolic control analysis of cellular respiration in situ in intraoperational samples of human breast cancer. Journal of Bioenergetics and Biomembranes. 2012;44(5):539–558. doi: 10.1007/s10863-012-9457-9. [DOI] [PubMed] [Google Scholar]
- 27.Kaambre T., Chekulayev V., Shevchuk I., et al. Metabolic control analysis of respiration in human cancer tissue. Frontiers in Physiology. 2013;4, article 151:1–6. doi: 10.3389/fphys.2013.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaldma A., Klepinin A., Chekulayev V., et al. An in situ study of bioenergetic properties of human colorectal cancer: the regulation of mitochondrial respiration and distribution of flux control among the components of ATP synthasome. The International Journal of Biochemistry & Cell Biology. 2014;55:171–186. doi: 10.1016/j.biocel.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Saks V. A., Veksler V. I., Kuznetsov A. V., et al. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Molecular and Cellular Biochemistry. 1998;184(1-2):81–100. [PubMed] [Google Scholar]
- 30.Kuznetsov A. V., Strobl D., Ruttmann E., Königsrainer A., Margreiter R., Gnaiger E. Evaluation of mitochondrial respiratory function in small biopsies of liver. Analytical Biochemistry. 2002;305(2):186–194. doi: 10.1006/abio.2002.5658. [DOI] [PubMed] [Google Scholar]
- 31.Kuznetsov A. V. Structural organization and dynamics of mitochondria in the cells in vivo. In: Saks V., editor. Molecular System Bioenergetics: Energy for Life. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co KGaA; 2007. pp. 137–162. [Google Scholar]
- 32.Varikmaa M., Bagur R., Kaambre T., et al. Role of mitochondria-cytoskeleton interactions in respiration regulation and mitochondrial organization in striated muscles. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2014;1837(2):232–245. doi: 10.1016/j.bbabio.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Kuznetsov A. V., Veksler V., Gellerich F. N., Saks V., Margreiter R., Kunz W. S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nature Protocols. 2008;3(6):965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 34.Monge C., Beraud N., Tepp K., et al. Comparative analysis of the bioenergetics of adult cardiomyocytes and nonbeating HL-1 cells: respiratory chain activities, glycolytic enzyme profiles, and metabolic fluxes. Canadian Journal of Physiology and Pharmacology. 2009;87(4):318–326. doi: 10.1139/Y09-018. [DOI] [PubMed] [Google Scholar]
- 35.Appaix F., Kuznetsov A. V., Usson Y., et al. Possible role of cytoskeleton in intracellular arrangement and regulation of mitochondria. Experimental Physiology. 2003;88(1):175–190. doi: 10.1113/eph8802511. [DOI] [PubMed] [Google Scholar]
- 36.Kuznetsov A. V., Tiivel T., Sikk P., et al. Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. European Journal of Biochemistry. 1996;241(3):909–915. doi: 10.1111/j.1432-1033.1996.00909.x. [DOI] [PubMed] [Google Scholar]
- 37.Saks V. A., Kuznetsov A. V., Khuchua Z. A., et al. Control of cellular respiration in vivo by mitochondrial outer membrane and by creatine kinase. A new speculative hypothesis: possible involvement of mitochondrial-cytoskeleton interactions. Journal of Molecular and Cellular Cardiology. 1995;27(1):625–645. doi: 10.1016/S0022-2828(08)80056-9. [DOI] [PubMed] [Google Scholar]
- 38.Chekulayev V., Mado K., Shevchuk I., et al. Metabolic remodeling in human colorectal cancer and surrounding tissues: alterations in regulation of mitochondrial respiration and metabolic fluxes. Biochemistry and Biophysics Reports. 2015;4:111–125. doi: 10.1016/j.bbrep.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gnaiger E. Oxygen solubility in experimental media. OROBOROS Bioenerg. News. 2001;6(3):1–6. [Google Scholar]
- 40.Kacser H., Burns J. A. The control of flux. Symposia of the Society for Experimental Biology. 1973;27:65–104. [PubMed] [Google Scholar]
- 41.Fell D. Understanding the control of metabolism. In: Snell K., editor. Frontiers in Metabolism 2. xii. London, Miami: Portland Press; 1997. p. p. 301. [Google Scholar]
- 42.Fell D. Metabolic control analysis. In: Alberghina L., Westerhoff H. V., editors. Systems Biology. Berlin: Springer: Verlag; 2005. pp. 69–80. [Google Scholar]
- 43.Moreno-Sanchez R., Saavedra E., Rodríguez-Enríquez S., Olín-Sandoval V. Metabolic control analysis: a tool for designing strategies to manipulate metabolic pathways. Journal of Biomedicine & Biotechnology. 2008;2008:30. doi: 10.1155/2008/597913.597913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gellerich F. N., Kunz W. S., Bohnensack R. Estimation of flux control coefficients from inhibitor titrations by non-linear regression. FEBS Letters. 1990;274(1-2):167–170. doi: 10.1016/0014-5793(90)81355-r. [DOI] [PubMed] [Google Scholar]
- 45.Kuznetsov A. V., Clark J. F., Winkler K., Kunz W. S. Increase of flux control of cytochrome c oxidase in copper-deficient mottled brindled mice. The Journal of Biological Chemistry. 1996;271(1):283–288. doi: 10.1074/jbc.271.1.283. [DOI] [PubMed] [Google Scholar]
- 46.Kuznetsov A. V., Winkler K., Kirches E., Lins H., Feistner H., Kunz W. S. Application of inhibitor titrations for the detection of oxidative phosphorylation defects in saponin-skinned muscle fibers of patients with mitochondrial diseases. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1997;1360(2):142–150. doi: 10.1016/S0925-4439(96)00072-5. [DOI] [PubMed] [Google Scholar]
- 47.Tepp K., Shevchuk I., Chekulayev V., et al. High efficiency of energy flux controls within mitochondrial interactosome in cardiac intracellular energetic units. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2011;1807(12):1549–1561. doi: 10.1016/j.bbabio.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Tepp K., Timohhina N., Chekulayev V., Shevchuk I., Kaambre T., Saks V. Metabolic control analysis of integrated energy metabolism in permeabilized cardiomyocytes - experimental study. Acta Biochimica Polonica. 2010;57(4):421–430. [PubMed] [Google Scholar]
- 49.Srere P. A. [1] Citrate synthase. Methods in Enzymology. 1969;13:3–11. [Google Scholar]
- 50.Davoudi M., Kotarsky H., Hansson E., Fellman V. Complex I function and supercomplex formation are preserved in liver mitochondria despite progressive complex III deficiency. PloS One. 2014;9(1, article e86767) doi: 10.1371/journal.pone.0086767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castro-Gago M., Blanco-Barca M. O., Gómez-Lado C., Eirís-Puñal J., Campos-González Y., Arenas-Barbero J. Respiratory chain complex I deficiency in an infant with Ohtahara syndrome. Brain dev. 2009;31(4):322–325. doi: 10.1016/j.braindev.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Finsterer J., Kovacs G. G., Rauschka H., Ahting U. Adult, isolated respiratory chain complex IV deficiency with minimal manifestations. Folia Neuropathologica. 2015;53(2):153–157. doi: 10.5114/fn.2015.52412. [DOI] [PubMed] [Google Scholar]
- 53.Mowat D., Kirby D. M., Kamath K. R., Kan A., Thorburn D. R., Christodoulou J. Respiratory chain complex III [correction of complex] in deficiency with pruritus: a novel vitamin responsive clinical feature. The Journal of Pediatrics. 1999;134(3):352–354. doi: 10.1016/S0022-3476(99)70463-4. [DOI] [PubMed] [Google Scholar]
- 54.Lemarie A., Grimm S. Mitochondrial respiratory chain complexes: apoptosis sensors mutated in cancer? Oncogene. 2011;30(38):3985–4003. doi: 10.1038/onc.2011.167. [DOI] [PubMed] [Google Scholar]
- 55.Eimre M., Paju K., Pelloux S., et al. Distinct organization of energy metabolism in HL-1 cardiac cell line and cardiomyocytes. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2008;1777(6):514–524. doi: 10.1016/j.bbabio.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 56.Seppet E., Eimre M., Peet N., et al. Compartmentation of energy metabolism in atrial myocardium of patients undergoing cardiac surgery. Molecular and Cellular Biochemistry. 2005;270(1-2):49–61. doi: 10.1007/s11010-005-3780-y. [DOI] [PubMed] [Google Scholar]
- 57.Parker N., Vidal-Puig A., Brand M. D. Stimulation of mitochondrial proton conductance by hydroxynonenal requires a high membrane potential. Bioscience Reports. 2008;28(2):83–88. doi: 10.1042/BSR20080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puurand M., Peet N., Piirsoo A., et al. Deficiency of the complex I of the mitochondrial respiratory chain but improved adenylate control over succinate-dependent respiration are human gastric cancer-specific phenomena. Molecular and Cellular Biochemistry. 2012;370(1-2):69–78. doi: 10.1007/s11010-012-1399-3. [DOI] [PubMed] [Google Scholar]
- 59.Triepels R. H., Van Den Heuvel L. P., Trijbels J. M., Smeitink J. A. Respiratory chain complex I deficiency. American Journal of Medical Genetics. 2001;106(1):37–45. doi: 10.1002/ajmg.1397. [DOI] [PubMed] [Google Scholar]
- 60.Swalwell H., Kirby D. M., Blakely E. L., et al. Respiratory chain complex I deficiency caused by mitochondrial DNA mutations. European Journal of Human Genetics. 2011;19(7):769–775. doi: 10.1038/ejhg.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ostergaard E., Rodenburg R. J., van den Brand M., et al. Respiratory chain complex I deficiency due to NDUFA12 mutations as a new cause of Leigh syndrome. Journal of Medical Genetics. 2011;48(11):737–740. doi: 10.1136/jmg.2011.088856. [DOI] [PubMed] [Google Scholar]
- 62.Kirby D. M., Crawford M., Cleary M. A., Dahl H. H., Dennett X., Thorburn D. R. Respiratory chain complex I deficiency: an underdiagnosed energy generation disorder. Neurology. 1999;52(6):1255–1264. doi: 10.1212/WNL.52.6.1255. [DOI] [PubMed] [Google Scholar]
- 63.Simonnet H., Demont J., Pfeiffer K., et al. Mitochondrial complex I is deficient in renal oncocytomas. Carcinogenesis. 2003;24(9):1461–1466. doi: 10.1093/carcin/bgg109. [DOI] [PubMed] [Google Scholar]
- 64.Gruno M., Peet N., Tein A., et al. Atrophic gastritis: deficient complex I of the respiratory chain in the mitochondria of corpus mucosal cells. Journal of Gastroenterology. 2008;43(10):780–788. doi: 10.1007/s00535-008-2231-4. [DOI] [PubMed] [Google Scholar]
- 65.Lim H. Y., Ho Q. S., Low J., Choolani M., Wong K. P. Respiratory competent mitochondria in human ovarian and peritoneal cancer. Mitochondrion. 2011;11(3):437–443. doi: 10.1016/j.mito.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 66.Bonora E., Porcelli A. M., Gasparre G., et al. Defective oxidative phosphorylation in thyroid oncocytic carcinoma is associated with pathogenic mitochondrial DNA mutations affecting complexes I and III. Cancer Research. 2006;66(12):6087–6096. doi: 10.1158/0008-5472.CAN-06-0171. [DOI] [PubMed] [Google Scholar]
- 67.Zieliński Ł. P., Smith A. C., Smith A. G., Robinson A. J. Metabolic flexibility of mitochondrial respiratory chain disorders predicted by computer modelling. Mitochondrion. 2016;31:45–55. doi: 10.1016/j.mito.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Demine S., Reddy N., Renard P., Raes M., Arnould T. Unraveling biochemical pathways affected by mitochondrial dysfunctions using metabolomic approaches. Metabolites. 2014;4(3):831–878. doi: 10.3390/metabo4030831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Genova M. L., Lenaz G. The interplay between respiratory supercomplexes and ROS in aging. Antioxidants & Redox Signaling. 2015;23(3):208–238. doi: 10.1089/ars.2014.6214. [DOI] [PubMed] [Google Scholar]
- 70.Acin-Perez R., Enriquez J. A. The function of the respiratory supercomplexes: the plasticity model. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2014;1837(4):444–450. doi: 10.1016/j.bbabio.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Dudkina N. V., Kudryashev M., Stahlberg H., Boekema E. J. Interaction of complexes I, III, and IV within the bovine respirasome by single particle cryoelectron tomography. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(37):15196–15200. doi: 10.1073/pnas.1107819108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moreno-Lastres D., Fontanesi F., García-Consuegra I., et al. Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metabolism. 2012;15(3):324–335. doi: 10.1016/j.cmet.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lenaz G., Tioli G., Falasca A. I., Genova M. L. Complex I function in mitochondrial supercomplexes. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2016;1857(7):991–1000. doi: 10.1016/j.bbabio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 74.Enrìquez J. A. Supramolecular organization of respiratory complexes. Annual Review of Physiology. 2016;78:533–561. doi: 10.1146/annurev-physiol-021115-105031. [DOI] [PubMed] [Google Scholar]
- 75.Lapuente-Brun E., Moreno-Loshuertos R., Acín-Pérez R., et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science. 2013;340(6140):1567–1570. doi: 10.1126/science.1230381. [DOI] [PubMed] [Google Scholar]
- 76.Acín-Pérez R., Fernández-Silva P., Peleato M. L., Pérez-Martos A., Enriquez J. A. Respiratory active mitochondrial supercomplexes. Molecular Cell. 2008;32(4):529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 77.Pedersen P. L. Voltage dependent anion channels (VDACs): a brief introduction with a focus on the outer mitochondrial compartment's roles together with hexokinase-2 in the “Warburg effect” in cancer. Journal of Bioenergetics and Biomembranes. 2008;40(3):123–126. doi: 10.1007/s10863-008-9165-7. [DOI] [PubMed] [Google Scholar]
- 78.Qian X. L., Li Y. Q., Gu F., et al. Overexpression of ubiquitous mitochondrial creatine kinase (uMtCK) accelerates tumor growth by inhibiting apoptosis of breast cancer cells and is associated with a poor prognosis in breast cancer patients. Biochemical and Biophysical Research Communications. 2012;427(1):60–66. doi: 10.1016/j.bbrc.2012.08.147. [DOI] [PubMed] [Google Scholar]
- 79.Lenaz G., Genova M. L. Supramolecular organisation of the mitochondrial respiratory chain: a new challenge for the mechanism and control of oxidative phosphorylation. Advances in Experimental Medicine and Biology. 2012;748:107–144. doi: 10.1007/978-1-4614-3573-0_5. [DOI] [PubMed] [Google Scholar]
- 80.Bianchi C., Genova M. L., Castelli G. P., Lenaz G. The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. The Journal of Biological Chemistry. 2004;279(35):36562–36569. doi: 10.1074/jbc.M405135200. [DOI] [PubMed] [Google Scholar]
- 81.Groen A. K., Wanders R. J., Westerhoff H. V., Van der Meer R., Tager J. M. Quantification of the contribution of various steps to the control of mitochondrial respiration. The Journal of Biological Chemistry. 1982;257(6):2754–2757. [PubMed] [Google Scholar]
- 82.Small J. R. Flux control coefficients determined by inhibitor titration: the design and analysis of experiments to minimize errors. The Biochemical Journal. 1993;296(Part 2):423–433. doi: 10.1042/bj2960423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramsay E. E., Hogg P. J., Dilda P. J. Mitochondrial metabolism inhibitors for cancer therapy. Pharmaceutical Research. 2011;28(11):2731–2744. doi: 10.1007/s11095-011-0584-5. [DOI] [PubMed] [Google Scholar]
- 84.Bernardi P. The mitochondrial permeability transition pore: a mystery solved? Frontiers in Physiology. 2013;4, article 95:1–12. doi: 10.3389/fphys.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Di Lisa F., Fogolari F., Lippe G. From ATP to PTP and back: a dual function for the mitochondrial ATP synthase. Circulation Research. 2015;116(11):1850–1862. doi: 10.1161/CIRCRESAHA.115.306557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bernardi P., Rasola A., Forte M., Lippe G. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiological Reviews. 2015;95(4):1111–1155. doi: 10.1152/physrev.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rasola A., Bernardi P. The mitochondrial permeability transition pore and its adaptive responses in tumor cells. Cell Calcium. 2014;56(6):437–445. doi: 10.1016/j.ceca.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Althoff T., Mills D. J., Popot J. L., Kühlbrandt W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. The EMBO Journal. 2011;30(22):4652–4664. doi: 10.1038/emboj.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marín-Hernández A., López-Ramírez S. Y., Gallardo-Pérez J. C., Rodríguez-Enríquez S., Moreno-Sánchez R., Saavedra E. Systems biology approaches to cancer energy metabolism. In: Aon M. A., Saks V., Schlattner U., editors. Systems Biology of Metabolic and Signaling Networks. Berlin Heidelberg: Springer; 2014. pp. 213–239. [Google Scholar]
- 90.Kholodenko B. N., Westerhoff H. V. Metabolic channelling and control of the flux. FEBS Letters. 1993;320(1):71–74. doi: 10.1016/0014-5793(93)81660-R. [DOI] [PubMed] [Google Scholar]
- 91.Kholodenko B. N., Rohwer J. M., Cascante M., Westerhoff H. V. Subtleties in control by metabolic channelling and enzyme organization. Molecular and Cellular Biochemistry. 1998;184(1-2):311–320. [PubMed] [Google Scholar]
- 92.Kholodenko B. N., Demin O. V., Westerhoff H. V. ‘Channelled’ pathways can be more sensitive to specific regulatory signals. FEBS Letters. 1993;320(1):75–78. doi: 10.1016/0014-5793(93)81661-I. [DOI] [PubMed] [Google Scholar]
- 93.Cormio A., Guerra F., Cormio G., et al. Mitochondrial DNA content and mass increase in progression from normal to hyperplastic to cancer endometrium. BMC Research Notes. 2012;5(1):p. 279. doi: 10.1186/1756-0500-5-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chance B., Williams G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. The Journal of Biological Chemistry. 1955;217(1):383–393. [PubMed] [Google Scholar]
- 95.Chance B., Williams G. R. Respiratory enzymes in oxidative phosphorylation. VI. The effects of adenosine diphosphate on azide-treated mitochondria. The Journal of Biological Chemistry. 1956;221(1):477–489. [PubMed] [Google Scholar]
- 96.Anmann T., Guzun R., Beraud N., et al. Different kinetics of the regulation of respiration in permeabilized cardiomyocytes and in HL-1 cardiac cells. Importance of cell structure/organization for respiration regulation. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2006;1757(12):1597–1606. doi: 10.1016/j.bbabio.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 97.Klepinin A., Chekulayev V., Timohhina N., et al. Comparative analysis of some aspects of mitochondrial metabolism in differentiated and undifferentiated neuroblastoma cells. Journal of Bioenergetics and Biomembranes. 2014;46(1):17–31. doi: 10.1007/s10863-013-9529-5. [DOI] [PubMed] [Google Scholar]
- 98.Salem A. F., Whitaker-Menezes D., Lin Z., et al. Two-compartment tumor metabolism: autophagy in the tumor microenvironment and oxidative mitochondrial metabolism (OXPHOS) in cancer cells. Cell Cycle. 2012;11(13):2545–2556. doi: 10.4161/cc.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinez-Outschoorn U. E., Lisanti M. P., Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Seminars in Cancer Biology. 2014;25:47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Martinez-Outschoorn U., Sotgia F., Lisanti M. P. Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function. Seminars in Oncology. 2014;41(2):195–216. doi: 10.1053/j.seminoncol.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 101.Sotgia F., Whitaker-Menezes D., Martinez-Outschoorn U. E., et al. Mitochondria “fuel” breast cancer metabolism: fifteen markers of mitochondrial biogenesis label epithelial cancer cells, but are excluded from adjacent stromal cells. Cell Cycle. 2012;11(23):4390–4401. doi: 10.4161/cc.22777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lisanti M. P., Sotgia F., Pestell R. G., Howell A., Martinez-Outschoorn U. E. Stromal glycolysis and MCT4 are hallmarks of DCIS progression to invasive breast cancer. Cell Cycle. 2013;12(18):2935–2936. doi: 10.4161/cc.26280. [DOI] [PubMed] [Google Scholar]
- 103.Sotgia F., Martinez-Outschoorn U. E., Pavlides S., Howell A., Pestell R. G., Lisanti M. P. Understanding the Warburg effect and the prognostic value of stromal caveolin-1 as a marker of a lethal tumor microenvironment. Breast Cancer Research. 2011;13(4):p. 213. doi: 10.1186/bcr2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tepp K., Mado K., Varikmaa M., et al. The role of tubulin in the mitochondrial metabolism and arrangement in muscle cells. Journal of Bioenergetics and Biomembranes. 2014;46(5):421–434. doi: 10.1007/s10863-014-9579-3. [DOI] [PubMed] [Google Scholar]
- 105.Varikmaa M., Timohhina N., Tepp K., et al. Formation of highly organized intracellular structure and energy metabolism in cardiac muscle cells during postnatal development of rat heart. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2014;1837(8):1350–1361. doi: 10.1016/j.bbabio.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 106.Sheldon K. L., Maldonado E. N., Lemasters J. J., Rostovtseva T. K., Bezrukov S. M. Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin. PLoS One. 2011;6(10, article e25539) doi: 10.1371/journal.pone.0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rostovtseva T. K., Bezrukov S. M. VDAC inhibition by tubulin and its physiological implications. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2012;1818(6):1526–1535. doi: 10.1016/j.bbamem.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gruno M., Peet N., Seppet E., et al. Oxidative phosphorylation and its coupling to mitochondrial creatine and adenylate kinases in human gastric mucosa. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;291(4):R936–R946. doi: 10.1152/ajpregu.00162.2006. [DOI] [PubMed] [Google Scholar]
- 109.Ishida Y., Wyss M., Hemmer W., Wallimann T. Identification of creatine kinase isoenzymes in the guinea-pig. Presence of mitochondrial creatine kinase in smooth muscle. FEBS Letters. 1991;283(1):37–43. doi: 10.1016/0014-5793(91)80548-H. [DOI] [PubMed] [Google Scholar]
- 110.Kushmerick M. J. Energetics studies of muscles of different types. Basic Research in Cardiology. 1987;82(Supplement 2):17–30. doi: 10.1007/978-3-662-11289-2_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Activity of citrate synthase as marker of mitochondrial mass [105], in human breast and colorectal cancers as well as in corresponding normal tissues. Enzymatic activity measured as mU/mg protein, mean ± SE. Supplementary Fig. 1. Quantification of Complex I subunit NDUFA9 expression levels using Western blot (WB) and anti-NDUFA9 antibody in HCC and healthy colon tissue samples (A); respective WB image of NDUFA9 protein levels (B). Levels of NDUFA9 were normalized to total protein content quantified by coomassie blue staining. Data are represented as mean values ± SEM from 4 independent experiments; ∗ p<0.05.