Abstract
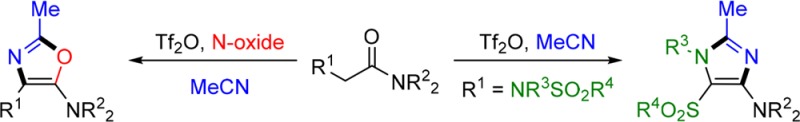
The preparation of substituted aminooxazoles and aminoimidazoles from α-arylamides and α-aminoamides through triflic anhydride-mediated amide activation is reported. These reactions proceed via the intermediacy of nitrilium adducts and feature N-oxide-promoted umpolung of the α-position of amides as well as a mechanistically intriguing sequence that results in sulfonyl migration from nitrogen to carbon. Quantum-chemical mechanistic analysis sheds light on the intricacies of the process.
Nitrogen-containing heterocycles are a nearly ubiquitous feature in nature. In particular, both oxazoles and imidazoles are core structural motifs of several pharmaceuticals and bioactive compounds (Figure 1).1 Oxazoles are known for their use as nonsteroidal anti-inflammatory drugs, PPAR modulators, hypoglycemics, and antibacterial agents,2 while imidazoles play important therapeutic roles, for example as antifungals, proton pump inhibitors, angiotensin inhibitors, and kinase inhibitors.3 As a result of this central role, a vast number of approaches and synthetic routes for the formation of both oxazoles and imidazoles have been reported.4−6
Figure 1.
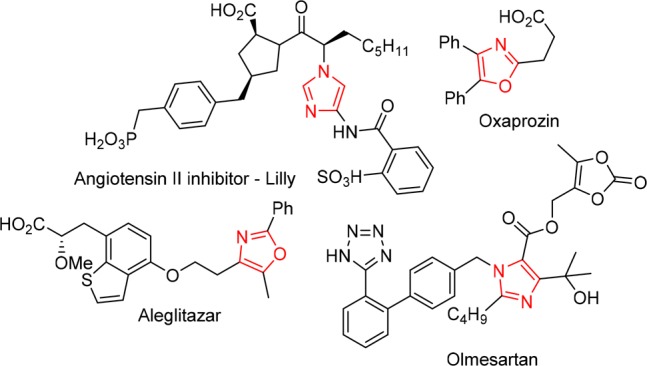
Pharmaceuticals and bioactive compounds containing oxazole and imidazole motifs.
Amide activation using triflic anhydride has shown considerable synthetic versatility and utility in organic synthesis.7 Since the pioneering work of Ghosez,8 a plethora of synthetic methods for the functionalization of amides have been developed.9 In particular, the preparation of heterocycles has received a great deal of attention in recent years, with the syntheses of pyrimidines,10 pyridines,11 lactones,12 and tetrazoles13 at the forefront of these developments.
Recently, our group disclosed the lutidine N-oxide (LNO)-mediated oxidative C–C coupling of arenes and alkenes with amides (Scheme 1a).14 Therein, the formation of a latent electrophilic enolonium species (I) enabled intramolecular nucleophilic attack on the α-position of a selectively activated amide, generating tetrahydroisoquinolinones.
Scheme 1. (a) Oxidative C–C Coupling at the α-Position of Amides; (b) Formation of Imidazoles and Oxazoles by Alternative Pathways.
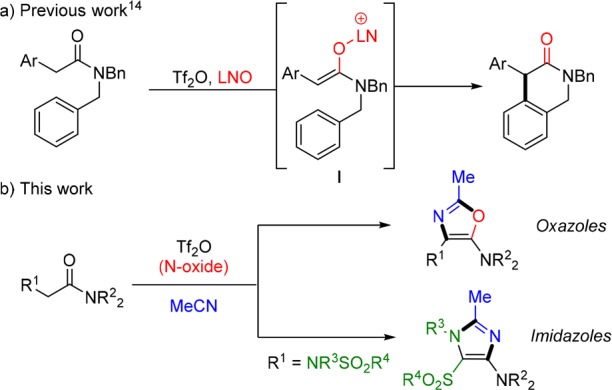
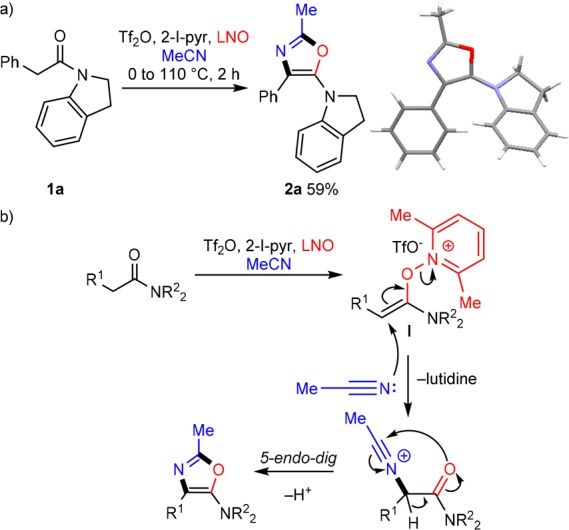
During the exploration of this transformation, we were intrigued by the side products obtained from premature interception of putative reactive intermediates with nucleophiles other than the aromatic rings (or olefins) employed in the bulk of the work (Scheme 1b). In the case of an α-arylamide with lowered capability of intramolecular cyclization (1a; Scheme 2a), we were surprised to isolate oxazole 2a as the exclusive reaction product, as confirmed by X-ray crystallography (CCDC 1537944; see the Supporting Information (SI) for further details). Mechanistically, this reaction outcome can be explained by the addition of LNO to the activated amide, forming α-electrophilic intermediate I,14 followed by intermolecular addition of acetonitrile (Scheme 2b). Oxazole formation is finalized via 5-endo-dig cyclization of the amide onto the transiently formed nitrilium ion.15
Scheme 2. (a) Unexpected Oxazole Formation; (b) Putative Reaction Mechanism.
Brief optimization (see the SI) identified milder conditions that, for simple substrates, reliably afforded the desired substituted oxazoles in moderate to good yields (Scheme 3). Varying substitution on nitrogen was tolerated, and the use of different nitriles similarly afforded the desired compounds (2a–h). We were additionally able to scale up the synthesis of 2b to 1.5 mmol with comparable isolated yield. It quickly became evident, however, that the possible substituent R1 was limited to aromatics (cf. 2i, for which R1 = Pr). We attributed this observation to increased LUMO stabilization of intermediate I by virtue of the aryl moiety.14
Scheme 3. Formation of Oxazoles from Simple Amides.
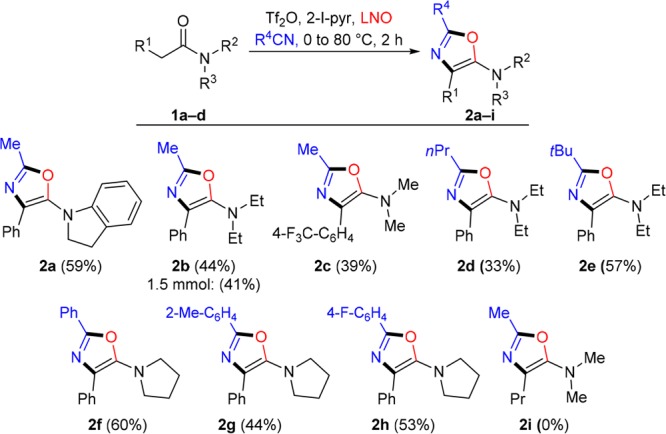
Reaction conditions: To amide 1 (0.2 mmol) and 2-I-pyr (0.4 mmol) in R4CN (2 mL) at 0 °C was added Tf2O (0.2 mmol). After 15 min, LNO (0.21 mmol) was added, followed by heating to 80 °C for 2 h. Isolated yields are shown.
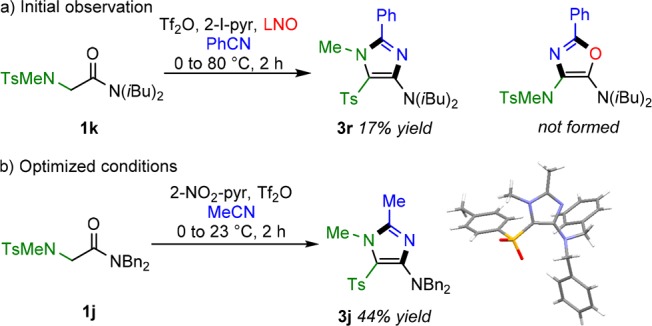
We thus logically attempted to widen the scope of R1 to heteroatoms, such as protected nitrogens (1k). To our surprise, however, reaction of 1k with benzonitrile under the standard conditions did not lead to the expected oxazole; in the event, a 1,2,4,5-substituted imidazole (3r) was observed as the only product (Scheme 4a). Particularly striking is the observation that in the course of the reaction, the tosyl substituent of substrate 1k appears to have migrated from nitrogen to carbon. Additionally, intermediate I—formed by attack of LNO (see Scheme 2b)—does not appear to be involved in this transformation. Indeed, optimization of the reaction conditions using 1j confirmed that LNO plays no role in the formation of the imidazole and also showed that the reaction could be run at room temperature (Scheme 4b; see the SI for details). Further optimization showed an added beneficial effect of using 2-nitropyridine (2-NO2-pyr), presumably due to decreased nucleophilicity (in comparison to 2-I-pyr) and therefore an increased fraction of keteniminium in solution (vide infra for a mechanistic discussion). Additionally, X-ray crystallographic analysis unambiguously confirmed the assigned structure of 3j as well as the tosyl migration (Scheme 4b, CCDC 1553624; see the SI for further details).
Scheme 4. Unexpected Imidazole Formation.
With the optimized conditions in hand, the scope of the reaction was explored (Scheme 5). While alkylnitriles gave low to moderate conversions throughout (3j–l), benzonitrile led to imidazole formation in good yield (3m). Other arylnitriles were also tolerated (3n–q), showcasing a trend where less nucleophilic (i.e., electron-poor) nitriles afforded lower yields (compare 3m and 3o). Simple alkyl substituents on the amide nitrogen, as in the case of diisobutylamide 1k, also allowed the formation of the corresponding 2-aminoimidazole (3r). Besides the tosyl protecting group, mesyl sulfonamides were also viable substrates (3s–u). The nosyl protecting group, however, led to a marked decrease in product yield (3v), presumably due to the much diminished O-nucleophilicity of the sulfonamide (vide infra). Nitriles containing electron-withdrawing groups or functionality that could interfere with triflic anhydride were not viable coupling partners in this reaction (3w–y).
Scheme 5. Scope and Limitations of Imidazole Formation with Various Nitriles.
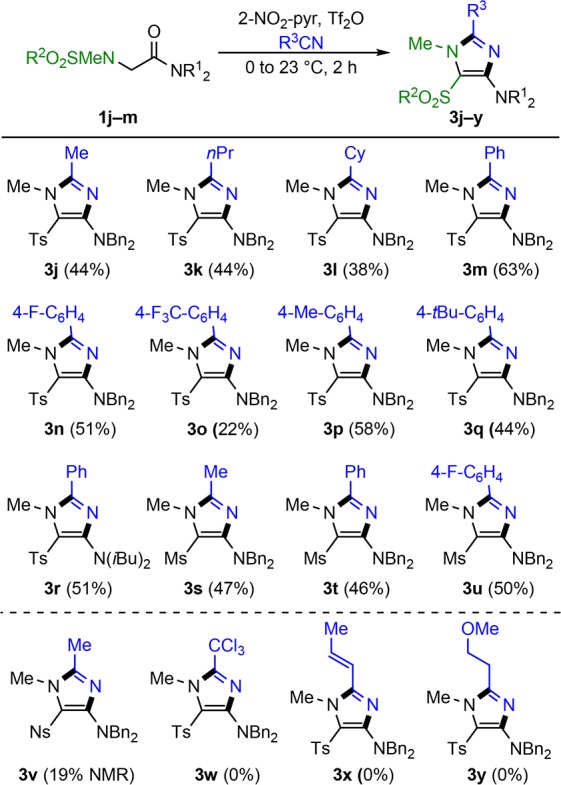
Reaction conditions: To amide 1 (0.2 mmol) and 2-NO2-pyr (0.4 mmol) in R3CN (2 mL) at 0 °C was added Tf2O (0.4 mmol). After 15 min, the reaction mixture was warmed to 23 °C for 2 h. Isolated yields are shown.
The imidazoles presented in Scheme 5 are products of a mechanistically interesting net N-to-C sulfonyl migration. In order to further elucidate the mechanism of this reaction, quantum-chemical calculations were performed. Scheme 6 depicts the mechanism of this transformation, as calculated for the simplified substrate S (see the SI for the computational details). The first two steps are the activation of the starting material S by virtue of triflic anhydride and the subsequent reaction of the keteniminium ion S′ with acetonitrile leading to intermediate A. Although similar steps are well-known,10,16 initially it was not clear how A could be converted into the final product, imidazole F. Scheme 6 and Figure 2 present the structures of the computed intermediates (A–F) and the corresponding transition states as well as the reaction profile.
Scheme 6. Mechanism of the Formation of Tetrasubstituted Imidazoles.
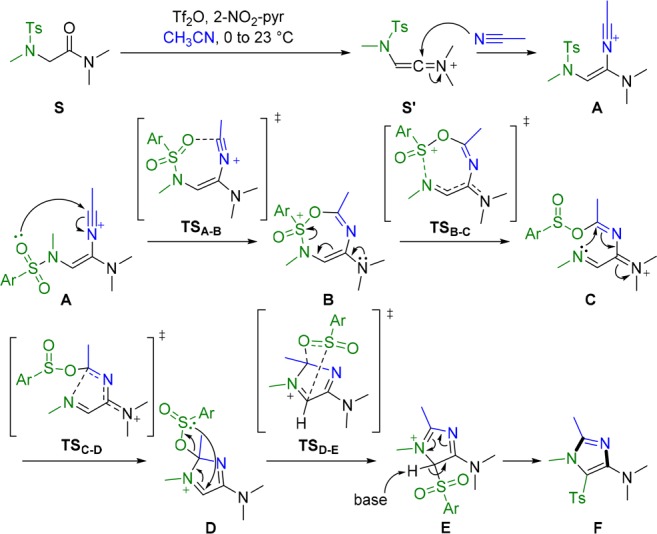
Figure 2.
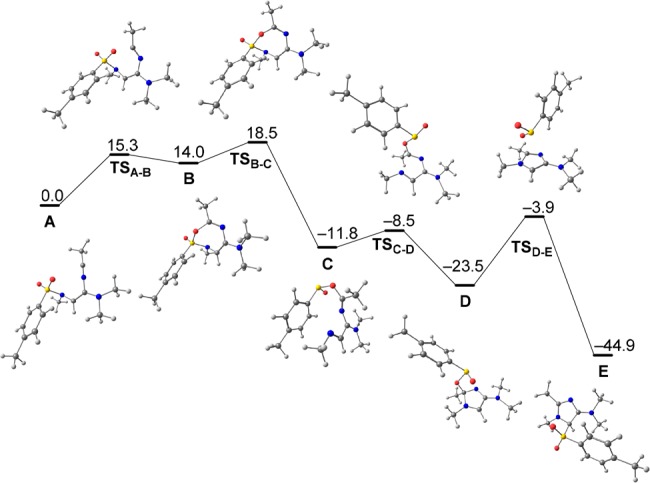
Computed reaction pathway (ΔG298,MeCN, kcal mol–1) for the conversion of A to E (see Scheme 6) and the optimized structures.
Intermediate A forms the seven-membered intermediate B via O-nucleophilic attack of the sulfonamide on the nitrilium (7-endo-dig), forming a new C–O bond. Although this step is endergonic (ΔG(A–B) = +14 kcal mol–1), it predetermines a substantial energetic stabilization in the next step, where the S–N bond breaks, forming intermediate C (ΔG(B–C) = −25.8 kcal mol–1). Additional thermodynamic stabilization is achieved via the second annulation step and TSC–D. The five-membered-ring intermediate D undergoes a strongly exergonic [2,3]-sigmatropic rearrangement (ΔG(D–E) = −21.4 kcal mol–1), affording intermediate E with the concerted formation of the C–S bond and breaking of the C–O bond. Finally, intermediate E is deprotonated by the base, generating the final product F through aromatization.
While the formation of intermediate A was to be expected, informed by similar attacks of nitriles on activated amides,10,15 the subsequent 7-endo-dig cyclization was initially less obvious. The experimental observation that electron-poor sulfonamides do not allow the transformation to take place, however, is in good agreement with the O-nucleophilicity necessary for going from A to B.
Following the cleavage of B to give sulfinate C and subsequent cyclization to form D, a [2,3]-sigmatropic rearrangement—reminiscent of a retro-Mislow–Evans-type reaction—constitutes the final peculiarity of this mechanism, driven to completion by final aromatization from E to F. This last step is evident and does not require a theoretical explanation; accordingly, it was excluded from the computational study. Although a mechanism involving addition of the sulfonamide nitrogen to nitrilium ion A might seem plausible at first glance, our computations argue against it, as the necessary intermediates could not be found using two different quantum-chemical approaches: density functional theory (DFT) and an even higher level, second-order Møller–Plesset perturbation theory (MP2).
In summary, we have herein reported the facile syntheses of fully substituted 5-aminooxazoles and 4-aminoimidazoles. The title products are formed upon electrophilic activation of simple amides with triflic anhydride in the presence of nitriles. Computational studies shed light on the unusual mechanistic pathway of imidazole formation, showcasing an intriguing N-to-C sulfonyl migration via a [2,3]-sigmatropic rearrangement of a sulfinate intermediate.
Acknowledgments
We thank the ERASMUS Program for a studentship to G.D.M., Dipl. Ing. (FH) A. Roller (U. Vienna) for X-ray analysis and Dr. H. Kählig (U. Vienna) for expert NMR analysis. This research was supported by the ERC (CoG VINCAT 682002), the FWF (P30226), and the University of Vienna. Calculations were partially performed at the Vienna Scientific Cluster (VSC). D.K. is a recipient of a DOC fellowship of the Austrian Academy of Sciences.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.7b01678.
Author Contributions
§ G.D.M. and B.M. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Selected reviews of natural products containing oxazoles, imidazoles, and other heterocycles:; a Lewis J. Nat. Prod. Rep. 2001, 18, 95. 10.1039/a909077k. [DOI] [PubMed] [Google Scholar]; b Yeh V. S. C. Tetrahedron 2004, 60, 11995. 10.1016/j.tet.2004.10.001. [DOI] [Google Scholar]; c Weinreb S. Nat. Prod. Rep. 2007, 24, 931. 10.1039/b700206h. [DOI] [PubMed] [Google Scholar]; d Jin Z. Nat. Prod. Rep. 2013, 30, 869. 10.1039/c3np70006b. [DOI] [PubMed] [Google Scholar]; e Jin Z. Nat. Prod. Rep. 2016, 33, 1268. 10.1039/C6NP00067C. [DOI] [PubMed] [Google Scholar]
- a Zhou X.-P.; Zhang M.-X.; Sun W.; Yang X. H.; Wang G.-S.; Sui D.-Y.; Yu X.-F.; Qu S.-C. Biol. Pharm. Bull. 2009, 32, 1986. 10.1248/bpb.32.1986. [DOI] [PubMed] [Google Scholar]; b Waites C. R.; Dominick M. A.; Sanderson T. P.; Schilling B. E. Toxicol. Sci. 2007, 100, 248. 10.1093/toxsci/kfm193. [DOI] [PubMed] [Google Scholar]; c Conti P.; Dallanoce C.; De Amici M. D.; De Micheli C.; Klotz K.-N. Bioorg. Med. Chem. 1998, 6, 401. 10.1016/S0968-0896(97)10051-7. [DOI] [PubMed] [Google Scholar]; d Kang Y. K.; Shin K. J.; Yoo K. H.; Seo K. J.; Hong C. Y.; Lee C.-S.; Park S. Y.; Kim D. J.; Park S. W. Bioorg. Med. Chem. Lett. 2000, 10, 95. 10.1016/S0960-894X(99)00646-0. [DOI] [PubMed] [Google Scholar]
- a Lackner T. E.; Clissold S. P. Drugs 1989, 38, 204. 10.2165/00003495-198938020-00004. [DOI] [PubMed] [Google Scholar]; b Walker K. A. M.; Braemer A. C.; Hitt S.; Jones R. E.; Matthews T. R. J. Med. Chem. 1978, 21, 840. 10.1021/jm00206a028. [DOI] [PubMed] [Google Scholar]; c Godefroi E. F.; Heeres J.; Van Cutsem J.; Janssen P. A. J. J. Med. Chem. 1969, 12, 784. 10.1021/jm00305a014. [DOI] [PubMed] [Google Scholar]; d Veraldi S. Mycoses 2013, 56, 3. 10.1111/myc.12054. [DOI] [PubMed] [Google Scholar]; e Kurup A.; Garg R.; Carini D. J.; Hansch C. Chem. Rev. 2001, 101, 2727. 10.1021/cr000025g. [DOI] [PubMed] [Google Scholar]; f Aulakh G. K.; Sodhi R. K.; Singh M. Life Sci. 2007, 81, 615. 10.1016/j.lfs.2007.06.007. [DOI] [PubMed] [Google Scholar]; g Manley P. W.; Stiefl N.; Cowan-Jacob S. W.; Kaufman S.; Mestan J.; Wartmann M.; Wiesmann M.; Woodman R.; Gallagher N. Bioorg. Med. Chem. 2010, 18, 6977. 10.1016/j.bmc.2010.08.026. [DOI] [PubMed] [Google Scholar]; h Francini C. M.; Fallacara A. L.; Artusi R.; Mennuni L.; Calgani A.; Angelucci A.; Schenone S.; Botta M. ChemMedChem 2015, 10, 2027. 10.1002/cmdc.201500428. [DOI] [PubMed] [Google Scholar]
- Selected examples of oxazole formation:; a Weintraub P. J. Med. Chem. 1972, 15, 419. 10.1021/jm00274a025. [DOI] [PubMed] [Google Scholar]; b Wipf P.; Miller C. J. Org. Chem. 1993, 58, 3604. 10.1021/jo00066a004. [DOI] [Google Scholar]; c Tavares F.; Meyers A. Tetrahedron Lett. 1994, 35, 6803. 10.1016/0040-4039(94)85009-7. [DOI] [Google Scholar]; d Graham T. Org. Lett. 2010, 12, 3614. 10.1021/ol101346w. [DOI] [PubMed] [Google Scholar]; e Zhang L.; Zhao X. Org. Lett. 2015, 17, 184. 10.1021/ol5030986. [DOI] [PubMed] [Google Scholar]; f Zhao Y.; Hu Y.; Wang C.; Li X.; Wan B. J. Org. Chem. 2017, 82, 3935. 10.1021/acs.joc.7b00076. [DOI] [PubMed] [Google Scholar]; g Yagyu T.; Takemoto Y.; Yoshimura A.; Zhdankin V. V.; Saito A. Org. Lett. 2017, 19, 2506. 10.1021/acs.orglett.7b00742. [DOI] [PubMed] [Google Scholar]; h Soeta T.; Matsumoto A.; Sakata Y.; Ukaji Y. J. Org. Chem. 2017, 82, 4930. 10.1021/acs.joc.7b00296. [DOI] [PubMed] [Google Scholar]
- Selected examples of imidazole formation:; a Iwashita Y.; Sakuraba M. J. Org. Chem. 1971, 36, 3927. 10.1021/jo00824a017. [DOI] [Google Scholar]; b Sorrell T. N.; Allen W. E. J. Org. Chem. 1994, 59, 1589. 10.1021/jo00085a056. [DOI] [Google Scholar]; c Katritzky A. R.; Qiu G. J. Org. Chem. 2001, 66, 2862. 10.1021/jo0016632. [DOI] [PubMed] [Google Scholar]; d Frantz D. E.; Morency L.; Soheili A.; Murry J. A.; Grabowski E. J. J.; Tillyer R. D. Org. Lett. 2004, 6, 843. 10.1021/ol0498803. [DOI] [PubMed] [Google Scholar]; e Horneff T.; Chuprakov S.; Chernyak N.; Gevorgyan V.; Fokin V. V. J. Am. Chem. Soc. 2008, 130, 14972. 10.1021/ja805079v. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Hu B.; Wang Z.; Ai N.; Zheng J.; Liu X.-H.; Shan S.; Wang Z. Org. Lett. 2011, 13, 6362. 10.1021/ol202650z. [DOI] [PubMed] [Google Scholar]; g Chen C.-Y.; Hu W.-P.; Yan P.-C.; Senadi G. C.; Wang J.-J. Org. Lett. 2013, 15, 6116. 10.1021/ol402892z. [DOI] [PubMed] [Google Scholar]; h Zeng Z.; Jin H.; Xie J.; Tian B.; Rudolph M.; Rominger F.; Hashmi A. S. K. Org. Lett. 2017, 19, 1020. 10.1021/acs.orglett.7b00001. [DOI] [PubMed] [Google Scholar]; i Xu W.; Wang G.; Sun N.; Liu Y. Org. Lett. 2017, 19, 3307. 10.1021/acs.orglett.7b01469. [DOI] [PubMed] [Google Scholar]
- Selected reviews of oxazole/imidazole formation:; a Zhang J.; Coqueron P.-Y.; Ciufolini M. A. Heterocycles 2011, 82, 949. 10.3987/REV-10-SR(E)3. [DOI] [Google Scholar]; b Gulevich A.; Dudnik A.; Chernyak N.; Gevorgyan V. Chem. Rev. 2013, 113, 3084. 10.1021/cr300333u. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Ibrar A.; Khan I.; Abbas N.; Farooq U.; Khan A. RSC Adv. 2016, 6, 93016. 10.1039/C6RA19324B. [DOI] [Google Scholar]
- a Baraznenok I. L.; Nenajdenko V. G.; Balenkova E. S. Tetrahedron 2000, 56, 3077. 10.1016/S0040-4020(00)00093-4. [DOI] [Google Scholar]; b Madelaine C.; Valerio V.; Maulide N. Chem. - Asian J. 2011, 6, 2224. 10.1002/asia.201100108. [DOI] [PubMed] [Google Scholar]; c Kaiser D.; Maulide N. J. Org. Chem. 2016, 81, 4421. 10.1021/acs.joc.6b00675. [DOI] [PubMed] [Google Scholar]
- a Falmagne J.-B.; Escudero J.; Taleb-Sahraoui S.; Ghosez L. Angew. Chem., Int. Ed. Engl. 1981, 20, 879. 10.1002/anie.198108791. [DOI] [Google Scholar]; b Markó I.; Ronsmans B.; Hesbain-Frisque A.-M.; Dumas S.; Ghosez L.; Ernst B.; Greuter H. J. Am. Chem. Soc. 1985, 107, 2192. 10.1021/ja00293a073. [DOI] [Google Scholar]
- Selected examples:; a Charette A. B.; Grenon M. Can. J. Chem. 2001, 79, 1694. 10.1139/v01-150. [DOI] [Google Scholar]; b Barbe G.; Charette A. B. J. Am. Chem. Soc. 2008, 130, 18. 10.1021/ja077463q. [DOI] [PubMed] [Google Scholar]; c Pelletier G.; Bechara W. S.; Charette A. B. J. Am. Chem. Soc. 2010, 132, 12817. 10.1021/ja105194s. [DOI] [PubMed] [Google Scholar]; d Bechara W. S.; Pelletier G.; Charette A. B. Nat. Chem. 2012, 4, 228. 10.1038/nchem.1268. [DOI] [PubMed] [Google Scholar]; e Xiao K.-J.; Wang A.-E.; Huang P.-Q. Angew. Chem., Int. Ed. 2012, 51, 8314. 10.1002/anie.201204098. [DOI] [PubMed] [Google Scholar]; f Peng B.; Geerdink D.; Maulide N. J. Am. Chem. Soc. 2013, 135, 14968. 10.1021/ja408856p. [DOI] [PubMed] [Google Scholar]; g Peng B.; Geerdink D.; Farès C.; Maulide N. Angew. Chem., Int. Ed. 2014, 53, 5462. 10.1002/anie.201402229. [DOI] [PubMed] [Google Scholar]; h Mewald M.; Medley J. W.; Movassaghi M. Angew. Chem., Int. Ed. 2014, 53, 11634. 10.1002/anie.201405609. [DOI] [PMC free article] [PubMed] [Google Scholar]; i White K. L.; Movassaghi M. J. Am. Chem. Soc. 2016, 138, 11383. 10.1021/jacs.6b07623. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Kolleth A.; Lumbroso A.; Tanriver G.; Catak S.; Sulzer-Mossé S.; De Mesmaeker A. Tetrahedron Lett. 2016, 57, 2697. 10.1016/j.tetlet.2016.04.092. [DOI] [Google Scholar]; k Huang P.-Q.; Huang Y.-H.; Xiao K.-J. J. Org. Chem. 2016, 81, 9020. 10.1021/acs.joc.6b01647. [DOI] [PubMed] [Google Scholar]; l Tona V.; de la Torre A.; Padmanaban M.; Ruider S.; González L.; Maulide N. J. Am. Chem. Soc. 2016, 138, 8348. 10.1021/jacs.6b04061. [DOI] [PMC free article] [PubMed] [Google Scholar]; m de la Torre A.; Kaiser D.; Maulide N. J. Am. Chem. Soc. 2017, 139, 6578. 10.1021/jacs.7b02983. [DOI] [PubMed] [Google Scholar]
- Movassaghi M.; Hill M. D. J. Am. Chem. Soc. 2006, 128, 14254. 10.1021/ja066405m. [DOI] [PubMed] [Google Scholar]
- a Movassaghi M.; Hill M. D.; Ahmad O. K. J. Am. Chem. Soc. 2007, 129, 10096. 10.1021/ja073912a. [DOI] [PubMed] [Google Scholar]; b Movassaghi M.; Hill M. D. Org. Lett. 2008, 10, 3485. 10.1021/ol801264u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Madelaine C.; Valerio V.; Maulide N. Angew. Chem., Int. Ed. 2010, 49, 1583. 10.1002/anie.200906416. [DOI] [PubMed] [Google Scholar]; b Valerio V.; Madelaine C.; Maulide N. Chem. - Eur. J. 2011, 17, 4742. 10.1002/chem.201003591. [DOI] [PubMed] [Google Scholar]; c Valerio V.; Petkova D.; Madelaine C.; Maulide N. Chem. - Eur. J. 2013, 19, 2606. 10.1002/chem.201203906. [DOI] [PubMed] [Google Scholar]
- Tona V.; Maryasin B.; de la Torre A.; Sprachmann J.; González L.; Maulide N. Org. Lett. 2017, 19, 2662. 10.1021/acs.orglett.7b01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D.; de la Torre A.; Shaaban S.; Maulide N. Angew. Chem., Int. Ed. 2017, 56, 5921. 10.1002/anie.201701538. [DOI] [PubMed] [Google Scholar]
- This mechanism is closely related to the formation of heterocycles via gold-catalyzed activation of alkynes in the presence of nitriles and N-oxides. For examples, see:; a He W.; Li C.; Zhang L. J. Am. Chem. Soc. 2011, 133, 8482. 10.1021/ja2029188. [DOI] [PubMed] [Google Scholar]; b Xiao Y.; Zhang L. Org. Lett. 2012, 14, 4662. 10.1021/ol302102h. [DOI] [PubMed] [Google Scholar]; c Luo Y.; Ji K.; Li Y.; Zhang L. J. Am. Chem. Soc. 2012, 134, 17412. 10.1021/ja307948m. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zhang L. Acc. Chem. Res. 2014, 47, 877. 10.1021/ar400181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Xie L.-G.; Niyomchon S.; Mota A. J.; González L.; Maulide N. Nat. Commun. 2016, 7, 10914. 10.1038/ncomms10914. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Xie L.-G.; Shaaban S.; Chen X.; Maulide N. Angew. Chem., Int. Ed. 2016, 55, 12864. 10.1002/anie.201606604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


