Abstract
Although frank symptomatic biotin deficiency is rare, some evidence suggests that marginal biotin deficiency occurs spontaneously in a substantial proportion of women during normal human pregnancy and might confer an increased risk of birth defects. Herein I review 1) advances in assessing biotin status, including the relation between acylcarnitine excretion and biotin status; 2) recent studies of biotin status in pregnancy; 3) advances in understanding the role of biotin in gene expression and the potential roles of biotinylated proteins that are neither histones nor carboxylases; and 4) novel large-dose biotin supplementation as therapy for multiple sclerosis. The review concludes with a summary of recent studies that have reported potentially dangerous erroneous results in individuals consuming large amounts of biotin for measurements of various plasma hormones for common clinical assays that use streptavidin-biotin technology.
Keywords: biotin, nutritional supplements, interference, hormone assays, gene expression, multiple sclerosis
Introduction
Hamid Said, an eminent investigator in biotin nutrition and physiology, asked the following in the American Journal of Clinical Nutrition more than a decade ago: “Biotin bioavailability and adequate intake: why bother?” (1). This article reviews studies that have provided evidence that the bother is worthwhile and presents observations concerning cautions for individuals consuming large amounts of biotin. The reader is referred to several excellent reviews for a more thorough discussion of the traditional role of biotin as an essential cofactor for the 5 biotin-dependent carboxylases (2–7).
Historical Perspective
Frank symptomatic biotin deficiency is a rare occurrence. The only well-documented cases have occurred in association with total or near-total intravenous feeding without biotin supplementation during the chronic consumption of undenatured egg white and with inborn errors of metabolism that lead to biotin wasting (2, 6). A single case that does not fit any of these established associations is that of an infant fed a rice-based formula that was presumably very low in biotin (6). However, some studies have suggested that the absence of overt biotin deficiency does not imply optimal biotin nutritional status (8–14).
Brief Review
Biotin-dependent carboxylases are initially synthesized as enzymatically inactive apocarboxylase proteins that become active holocarboxylases after the covalent attachment of biotin. The attachment of biotin is catalyzed by holocarboxylase synthetase (HLCS); an amide bond is formed between the carboxyl group of the valeric acid side chain of biotin and the ε-amino group of a specific Lys residue in each of the 5 apocarboxylases. These regions contain sequences of amino acids (e.g., Met-Lys-Met at the attachment site) that are highly conserved for the individual carboxylases, and both the N and C terminus in holocarboxylase synthetase are important for the recognition of the apocarboxylases (15). In mammals, biotin serves as an essential cofactor for each of the 5 biotin-dependent carboxylases: acetyl-CoA carboxylase (ACC) 1, ACC2, methylcrotonyl-CoA carboxylase (MCC), pyruvate carboxylase, and propionyl-CoA carboxylase (PCC) (Figure 1). These roles have been extensively reviewed previously (2–7). Additional roles are being actively investigated as described in the following sections.
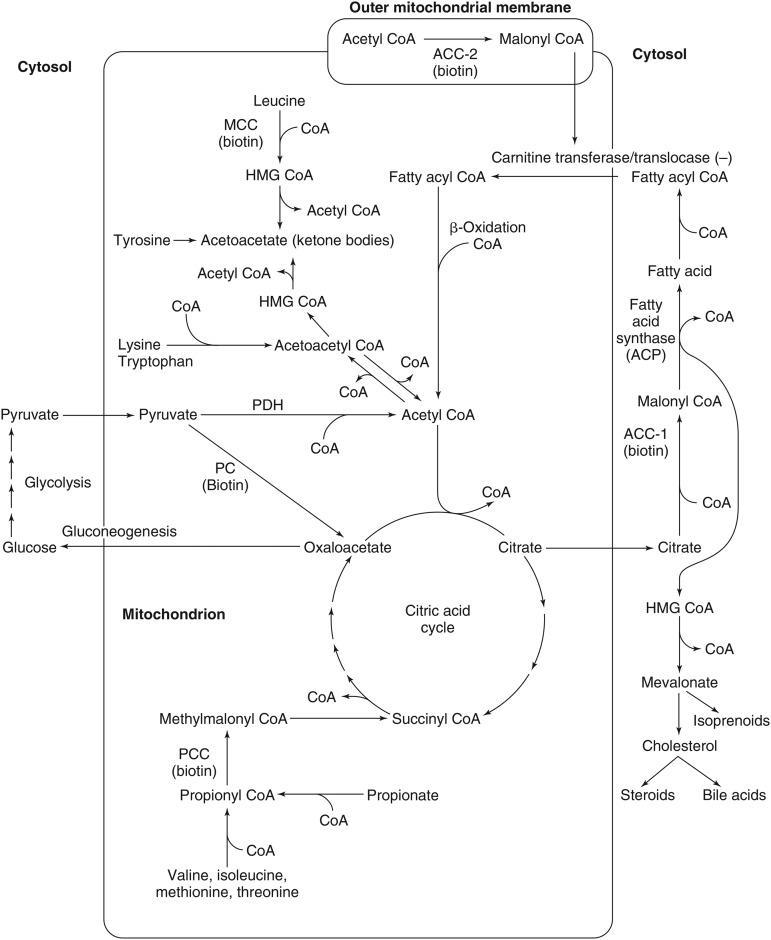
FIGURE 1.
Roles of the 5 biotin-dependent carboxylases of CoA and ACP within the cell. Shown is an overview of the metabolic pathways of ACC1 (cytosolic) and ACC2 (outer mitochondrial membrane) and the 3 mitochondrial carboxylases PCC, MCC, and PC. ACC1, acetyl-CoA carboxylase 1; ACC2, acetyl-CoA carboxylase 2; ACP, acyl carrier protein; HMG, 3-hydroxy-3-methylglutaryl; MCC, methylcrotonyl-CoA carboxylase; PC, pyruvate carboxylase; PCC, propionyl-CoA carboxylase; PDH, pyruvate dehydrogenase. Adapted from reference 16 with permission.
Does Biotin Deficiency Occur in Pregnancy?
Despite the rarity of symptomatic biotin deficiency, pregnancy is a clinical condition of particular concern with respect to biotin status. A post hoc analysis of data from a large multivitamin supplementation study (17) provided interesting, although indirect, evidence that the marginal degree of biotin deficiency that occurs spontaneously in normal human gestation may be teratogenic (8). Moreover, studies in several animal species, including mice, hamsters, chickens, and turkeys, have shown that biotin deficiency is teratogenic (8). For example, fetuses of marginally biotin-deficient mouse dams have been shown to have a high incidence of skeletal malformations, including >50% incidences of cleft palate, micrognathia, microglossia, and fore- and hind-limb shortening (9), yet these mouse dams showed only metabolic abnormalities. The dams gained weight normally and showed no physical signs of biotin deficiency (8, 9); neither reproductive efficiency nor fetal weight gain was affected. Moreover, this mouse model of biotin deficiency is relevant to human gestation. The timing and magnitude of the increase in urinary excretion of 3-hydroxyisovaleric acid (3HIA), which reflects the reduced activity of the biotin-dependent enzyme MCC, was similar to the 3HIA increase that occurred spontaneously during the first trimester of human pregnancy (8, 18). Increased urinary excretion of 3HIA tends to persist throughout pregnancy and will normalize (or at least return to near normal) within 2 wk of supplementation with 300 μg biotin/d (10), which is 10 times the current adequate intake (AI) for biotin.
The apparent disconnect between a modest reduction in murine maternal biotin status and the major effects on fetal skeletal development might result from the mouse fetus being a notably poor parasite for biotin (8, 9, 11, 18). Consistent with this observation, the placental transport of biotin is likely inadequate both in human pregnancies and in those of mice (6, 8).
In this context, the first human pregnancy study that controlled dietary biotin intake and quantitated the biotin content of the diet provided evidence that third-trimester pregnant women who consumed controlled intakes of ∼60 μg biotin/d (2 times the recommended AI) excreted more urinary 3HIA, a metabolite that accumulates when MCC activity is decreased, than control women (19). This study both confirmed previous reports that marginal biotin deficiency occurs spontaneously in a substantial proportion of women during normal human pregnancy (8) and suggested that a biotin intake ≥2–3 times the AI is likely needed to meet the requirement of pregnancy (19).
Relation between Acylcarnitine Excretion and Biotin Status
Whether caused by genetic defects or biotin deficiency (2, 6), reduced MCC activity leads to the accumulation of its substrate 3-methylcrotonyl-CoA. Because acyl-CoA compounds are compartmentalized within the mitochondria, the accumulation of 3-methylcrotonyl-CoA and 3-hydroxyisovaleryl-CoA would lead to a disruption of the esterified CoA:free CoA ratios and, ultimately, to potentially lethal mitochondrial toxicity (20, 21). To prevent this, 3HIA-CoA is detoxified by carnitine transesterification to 3-hydroxyisovalerylcarnitine (3HIAc). The reaction is catalyzed by carnitine acetyltransferase, which is one of a family of enzymes with varying chain-length specificity and organelle and organ distribution; these enzymes act in concert with the transfer of the acylcarnitine out of the mitochondria by carnitine-acylcarnitine translocase to defend the CoA ratios (21). Accumulating 3HIAc is transferred across the inner mitochondrial membrane by the translocase, leading to increased plasma and urinary 3HIAc and 3HIA (12, 13) and potentially to secondary carnitine deficiency (22). 3HIA likely arises preferentially from the hydrolysis of 3HIAc in the cytosol.
Indicators of Biotin Status
Urinary 3HIA and 3HIAc are sensitive indicators of marginal biotin deficiency in healthy adults in whom biotin deficiency is induced experimentally (12). Interestingly, the urinary excretion of 3HIAc was actually lower among pregnant women than among nonpregnant control women in Perry et al. (19), indicating that urinary 3HIAc is not a reliable indicator of marginal biotin deficiency in pregnancy. Our group has observed the same unreliability of urinary 3HIA as an indicator of biotin deficiency in pregnancy (A Bogusiewicz, G Boysen, DM Mock, University of Arkansas for Medical Sciences, unpublished results, 2017) and, in a hepatocyte culture, has confirmed the metabolic pathogenesis presented previously by inducing separate and combined biotin and carnitine deficiency (23); the results were consistent with Perry et al. (19) in that urinary 3HIAc did not increase in parallel with urinary 3HIA because of the functional deficiency of hepatic carnitine. This observation also raises the possibility that this functional carnitine deficiency might impair this important cellular detoxification mechanism in pregnancy.
Biotin Catabolism
Perry et al. (19) also offered intriguing observations concerning biotin catabolism. Lactating women excreted substantially more of the inactive catabolite bisnorbiotin, which is created by the oxidation of the valeric acid side chain, than did control women. This observation suggests that lactation accelerates biotin turnover and loss. Indeed, the accelerated biotransformation of biotin to bisnorbiotin has been reported in early pregnancy (24); however, in that study, bisnorbiotin excretion returned to normal by late pregnancy.
Utility of Indicators of Biotin Status
In the context of these seemingly conflicting observations, Eng et al. (25) reported that the abundance of holo-PCC and holo-MCC in peripheral blood lymphocytes determined by gel densitometry and fluorescent-labeled streptavidin seem to be the best indicators of marginal biotin deficiency. Theoretically, the activity of PCC and MCC (2 of the 5 biotin-dependent carboxylases) in peripheral blood lymphocytes should be as sensitive and specific as the abundance of holo-PCC and holo-MCC. The assay quantitates the catalysis by PCC or MCC of 14C-bicarbonate incorporation into acid-precipitable material (i.e., methylmalonyl-CoA and methylglutaconyl-CoA, respectively) (26). Unfortunately, this assay is technically demanding, and the blood samples require special handling and storage, making gel densitometry abundance measurement more practical for field studies. Likewise, the urinary excretion of 3HIA and other organic acids characteristic of multiple carboxylase deficiencies as well as the related acylcarnitines seem to be less robust as indicators of biotin status in field studies (25), perhaps because of the variation in the dietary intake of precursors (e.g., Leu) in the hours before the collection of the urine sample (14, 27).
Role of Biotin in Gene Regulation
Hymes and Wolf (28) suggested that the posttranslational biotinylation of histones might play a role as a covalent modifier in the epigenetic code that regulates DNA transcription. However, subsequent work showed that <0.001% of human histones (primarily H3 and H4) are biotinylated, suggesting that the abundance is too low to elicit biological effects in vivo (29, 30). However, HLCS is located prominently in the nucleus (31), and the knockout of HLCS in drosophila as well as in human and other mammalian cells in culture produces distinct phenotypes, including the derepression of long terminal repeats and chromosomal instability; aspects of this HLCS knockout phenotype have been attributed to the effects on gene expression rather than reduced activities of the biotin-dependent carboxylases (2).
HLCS Exerts Some of Its Roles in Gene Regulation through the Formation of a Multiprotein Gene-Repression Complex in Human Chromatin
Zempleni and colleagues (32, 33) have proposed that the biological effects of biotin on gene expression are caused by a multiprotein complex, including proteins involved in DNA methylation, histone methylation, and histone deacetylation. They proposed that the docking of HLCS in chromatin causes an occasional biotinylation of histones (“marks”) near the various HLCS-binding sites. Their studies provide evidence that HLCS enters the nuclear compartment and is recruited to chromatin through physical interactions with DNA methyltransferase 1 and methyl CpG-binding protein 2. Chromatin-bound HLCS has been shown to recruit the eukaryotic histone H3 methyltransferase euchromatic histone lysine N-methyltransferase 1, which creates abundant Lys9-methylated histone H3 gene repression marks. In addition, HLCS interacts with nuclear receptor corepressor, a protein known to facilitate the binding of histone deacetylases (HDACs) in chromatin. HDACs remove histone acetylation marks and thus play a critical role in gene repression. Overall, emerging data suggest histone biotinylation marks are a side effect of HLCS being in close physical proximity to histones and play no direct role in gene repression, despite contributing toward chromatin condensation (32).
Biotinylated Proteins Other Than Carboxylases and Histones: Evidence That the Number of Proteins Containing Covalently Bound Biotin Is Larger Than Previously Appreciated
A recent study (34) used MS to identify 108 novel biotinylation sites in the human embryonic kidney cells; members of the heat-shock protein (HSP) superfamily were overrepresented among the novel biotinylated proteins. Approximately 50% of the biotinylated proteins displayed increased Met oxidation; for biotinylated HSPs, Met sulfoxidation approached 100%. Protein structure analysis suggested that Met sulfoxides localized in close physical proximity to the biotinylated Lys residues on the protein surface and that the likelihood of Met sulfoxidation is greater when one of the adjacent Lys residues is biotinylated. HSP60 knockdown and biotin-depletion experiments supported a synergistic role of biotinylation of Lys residues in the Met defense against reactive oxygen species and further observed that high concentrations of Met sulfoxidation coincided with cell-cycle arrest in biotin-depleted cells.
High-Dose Biotin Therapy in Profound Neurologic Diseases
Biotin-thiamin–responsive basal ganglia disease (BTBGD) is a genetic disorder that affects the nervous system, including a group of structures in the brain termed the basal ganglia that help control movement. BTBGD is caused by a defect in the solute carrier family 19 member 3 gene that encodes for thiamin transporter 2 (35, 36) and is characterized by subacute episodes of encephalopathy often triggered by febrile illness and characterized by confusion, epilepsy, external ophthalmoplegia, dysphagia, and generalized stiffness; this disease eventually results in coma and death (37). Before elucidating the genetic pathogenesis, high doses of biotin alone (5–10 mg ⋅ kg−1 ⋅ d−1, which is ∼10,000-fold greater than the AIs for children and adults) had been used in the successful treatment of this disease; the addition of thiamin improved the clinical prognosis for some patients (38). To our knowledge, the therapeutic mechanism for high-dose biotin in BTBGD remains unknown, but interesting clues are emerging from observations in multiple sclerosis.
High-Dose Biotin May Slow the Advancement of Progressive Multiple Sclerosis
In 2011, Sedel et al. (39) reported on 5 patients who were suffering from optic neuropathies and leukoencephalopathy. Encouraged by the success of high-dose biotin in BTBGD, biotin therapy was instituted; all responded clinically. Thereafter, 1 of the 5 patients was diagnosed as actually having secondary progressive multiple sclerosis (40). High doses of biotin (100–300 mg/d) were then tested in 23 additional patients with primary or secondary progressive multiple sclerosis with the use of an open-label design; improved clinical outcomes were reported in nearly all participants (40). In 4 patients with prominent visual impairment related to optic nerve injury, visual acuity improved considerably, and visually evoked potentials improved in 2 patients. Proton magnetic resonance spectroscopy in 1 patient showed an improved choline:creatine ratio. One patient with left-sided blindness for both eyes steadily improved from 2 to 16 mo of biotin supplementation. Of the 18 patients with prominent spinal cord involvement, 16 improved; a blinded review of a videotaped clinical examination was possible for 9 patients and confirmed improvement in all of them. Preliminary results from multicenter double-blind placebo-controlled trials in Europe and the United States are encouraging (41).
Thus, high-dose biotin supplementation may represent a therapeutic option in progressive multiple sclerosis; the mechanism(s) remains to be elucidated (42). The gradual worsening of neurologic disability in patients with progressive multiple sclerosis is caused by progressive axonal loss or damage. The triggers for axonal loss in multiple sclerosis likely include both inflammatory demyelination of the myelin sheath and primary neurodegeneration caused by a state of virtual hypoxia within the neuron. High-dose biotin might increase myelin production by increasing the generation of long-chain FAs (e.g., via effects on ACC1), increasing energy production via the tricarboxylic acid cycle in neuronal cells, or both (42). In the context that the tricarboxylic acid cycle is heavily dependent on acyl-CoA intermediates, reports that chickens fed a diet deficient in pantothenic acid (a required precursor of CoA) developed spinal nerve damage associated with the degeneration of the myelin sheath are intriguing (43). Early results from high-dose biotin treatment in animal models of multiple sclerosis favor increased myelin production.
Biotin Supplementation Can Interfere with Clinical Laboratory Assays That Use Streptavidin Technology
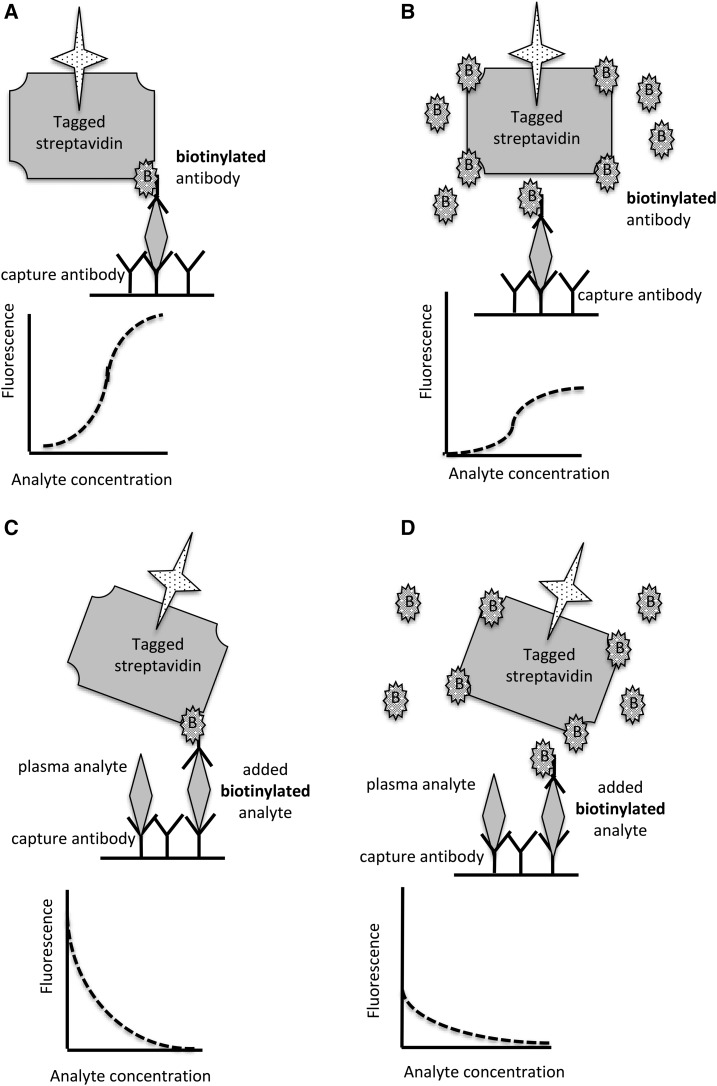
In both adults and children, pharmacologic doses of biotin are being used with increasing frequency for the legitimate indications discussed previously as well as for off-label conditions (44–47). This trend is likely to continue; the chances of interference with clinical diagnostic tests that depend on streptavidin-biotin technology will likely increase as well. Immunoassays that use streptavidin-biotin technology are now commonly used for hormone measurements and are common in high-throughput analytical platforms. Reports of erroneous analytical results are appearing with increasing frequency; most are encountered in individuals who received biotin supplementation. Pretreating the plasma sample with streptavidin microbeads normalizes assay results (47). However, a few cases were caused by anti-streptavidin antibody (45). For those receiving biotin therapy, the interference may induce either falsely increased or falsely decreased results, depending on the assay design. As depicted in Figure 2A, B, excess plasma biotin in competition assays characteristically lead to an overestimation of the analyte; as depicted in Figure 2C, D, excess plasma biotin in double-epitope “sandwich” detection and capture immunoassays cause underestimation. Unfortunately, the artifacts acting on 2 hormone assays in the same sample can simulate a seemingly coherent hormonal profile; e.g., falsely increased free thyroxin and falsely decreased thyroid-stimulating hormone in the same plasma sample suggests hyperthyroidism (45). Nutritionists, physicians, nurses, and other healthcare providers should include biotin in the nutritional history in patient groups that might have instituted biotin therapy, such as those with multiple sclerosis.
FIGURE 2.
Sandwich assay with the use of fluorescent-tagged streptavidin detection (A, B). (A) Normal conditions: bound antibody captures the analyte, which then binds a biotinylated antibody raised against a second epitope on the analyte. The biotinylated secondary antibody binds to fluorescent-tagged streptavidin. The greater the concentration of plasma analyte, the greater the fluorescent signal. (B) Excess plasma biotin blocks streptavidin binding to the biotinylated antibody, leading to an underestimation of the analyte. (C, D) Competition assay with the use of fluorescent-tagged streptavidin detection. (C) Normal conditions: plasma analyte competes with the added biotinylated analyte. The greater the concentration of the analyte, the less streptavidin is bound and the smaller the signal. (D) Excess biotin in plasma blocks streptavidin binding to the biotinylated analyte, leading to an overestimation of the plasma analyte.
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Footnotes
Abbreviations used: ACC, acetyl-CoA carboxylase; AI, adequate intake; BTBGD, biotin-thiamin–responsive basal ganglia disease; HLCS, holocarboxylase synthetase; HSP, heat-shock protein; MCC, methylcrotonyl-CoA carboxylase; PCC, propionyl-CoA carboxylase; 3HIA, 3-hydroxyisovaleric acid; 3HIAc, 3-hydroxyisovalerylcarnitine.
References
- 1.Said HM. Biotin bioavailability and estimated average requirement: why bother? Am J Clin Nutr 1999;69:352–3. [DOI] [PubMed] [Google Scholar]
- 2.Zempleni J. Biotin In: Erdman J, Macdonald I, Zeisel S, editors. Present knowledge in nutrition. Vol. 10, 10th ed. Hoboken (NJ): John Wiley & Sons; 2012. p. 359–74. [Google Scholar]
- 3.Zempleni J, Kuroishi T. Biotin. Adv Nutr 2012;3:213–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Said HM. Biotin: biochemical, physiological and clinical aspects. Subcell Biochem 2012;56:1–19. [DOI] [PubMed] [Google Scholar]
- 5.Mock DM. Biotin In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, editors. Modern nutrition in health and disease. 11th ed. Baltimore (MD): Lippincott Williams & Wilkens; 2012. p. 390–8. [Google Scholar]
- 6.Mock DM. Biotin In: Zempleni J, Suttie JW, Gregory JF, Stover PJ, editors. Handbook of vitamins. 5th ed. Boca Raton (FL): CRC Press; 2013. p. 397–420. [Google Scholar]
- 7.Mock DM. Biotin In: Caballero BA, Allen LH, Prentice A, editors. Encyclopedia of human nutrition. 3rd ed. Kidlington (United Kingdom): Elsevier; 2013. p. 182–90. [Google Scholar]
- 8.Mock DM. Marginal biotin deficiency is common in normal human pregnancy and is highly teratogenic in the mouse. J Nutr 2009;139:154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mock DM, Mock NI, Stewart CW, LaBorde JB, Hansen DK. Marginal biotin deficiency is teratogenic in ICR mice. J Nutr 2003;133:2519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mock DM, Quirk JG, Mock NI. Marginal biotin deficiency during normal pregnancy. Am J Clin Nutr 2002;75:295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sealey WM, Stratton SL, Mock DM, Hansen DK. Marginal maternal biotin deficiency in CD-1 mice reduces fetal mass of biotin-dependent carboxylases. J Nutr 2005;135:973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stratton SL, Horvath TD, Bogusiewicz A, Matthews NI, Henrich CL, Spencer HJ, Moran JH, Mock DM. Urinary excretion of 3-hydroxyisovaleryl carnitine is an early and sensitive indicator of marginal biotin deficiency in humans. J Nutr 2011;141:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stratton SL, Horvath TD, Bogusiewicz A, Matthews NI, Henrich CL, Spencer HJ, Moran JH, Mock DM. Plasma concentration of 3-hydroxyisovaleryl carnitine is an early and sensitive indicator of marginal biotin deficiency in humans. Am J Clin Nutr 2010;92:1399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mock DM, Stratton SL, Horvath TD, Bogusiewicz A, Matthews NI, Henrich CL, Dawson AM, Spencer HJ, Owen SN, Boysen G, et al. . Urinary excretion of 3-hydroxyisovaleric acid and 3-hydroxyisovaleryl carnitine increases in response to a leucine challenge in marginally biotin-deficient humans. J Nutr 2011;141:1925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan YI, Moriyama H, Olsen LJ, Bi X, Zempleni J. N- and C-terminal domains in human holocarboxylase synthetase participate in substrate recognition. Mol Genet Metab 2009;96:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mock D, Matthews N. Biotin and pantothenic acid In: Stipanuk MH, Caudill MA, editors. Biochemical, physiological and molecular aspects of human nutrition. 3rd ed Amsterdam (Netherlands): Elsevier; 2012. p. 610–25. [Google Scholar]
- 17.Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832–5. [DOI] [PubMed] [Google Scholar]
- 18.Mock DM. Adequate intake of biotin in pregnancy: why bother? J Nutr 2014;144:1885–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry CA, West AA, Gayle A, Lucas LK, Yan J, Jiang X, Malysheva O, Caudill MA. Pregnancy and lactation alter biomarkers of biotin metabolism in women consuming a controlled diet. J Nutr 2014;144:1977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasquali M, Monsen G, Richardson L, Alston M, Longo N. Biochemical findings in common inborn errors of metabolism. Am J Med Genet C Semin Med Genet 2006;142C:64–76. [DOI] [PubMed] [Google Scholar]
- 21.Zammit VA, Ramsay RR, Bonomini M, Arduini A. Carnitine, mitochondrial function and therapy. Adv Drug Deliv Rev 2009;61:1353–62. [DOI] [PubMed] [Google Scholar]
- 22.Shigematsu Y, Bykov IL, Liu YY, Nakai A, Kikawa Y, Sudo M, Fujioka M. Acylcarnitine profile in tissues and body fluids of biotin-deficient rats with and without L-carnitine supplementation. J Inherit Metab Dis 1994;17:678–90. [DOI] [PubMed] [Google Scholar]
- 23.Bogusiewicz A, Boysen G, Mock DM. In HepG2 cells, coexisting carnitine deficiency masks important indicators of marginal biotin deficiency. J Nutr 2015;145:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mock DM, Stadler DD, Stratton SL, Mock NI. Biotin status assessed longitudinally in pregnant women. J Nutr 1997;127:710–6. [DOI] [PubMed] [Google Scholar]
- 25.Eng WK, Giraud D, Schlegel VL, Wang D, Lee BH, Zempleni J. Identification and assessment of markers of biotin status in healthy adults. Br J Nutr 2013;110:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mock D, Henrich C, Carnell N, Mock N, Swift L. Lymphocyte propionyl-CoA carboxylase and accumulation of odd-chain fatty acid in plasma and erythrocytes are useful indicators of marginal biotin deficiency. J Nutr Biochem 2002;13:462–70. [DOI] [PubMed] [Google Scholar]
- 27.Mock DM, Henrich CL, Carnell N, Mock NI. Indicators of marginal biotin deficiency and repletion in humans: validation of 3-hydroxyisovaleric acid excretion and a leucine challenge. Am J Clin Nutr 2002;76:1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hymes J, Wolf B. Human biotinidase isn’t just for recycling biotin. J Nutr 1999;129:485S–9S. [DOI] [PubMed] [Google Scholar]
- 29.Bailey LM, Ivanov RA, Wallace JC, Polyak SW. Artifactual detection of biotin on histones by streptavidin. Anal Biochem 2008;373:71–7. [DOI] [PubMed] [Google Scholar]
- 30.Healy S, Perez-Cadahia B, Jia D, McDonald M, Davie J, Gravel R. Biotin is not a natural histone modification. Biochim Biophys Acta 2009;1789:719–33. [DOI] [PubMed] [Google Scholar]
- 31.Narang MA, Dumas R, Ayer LM, Gravel RA. Reduced histone biotinylation in multiple carboxylase deficiency patients: a nuclear role for holocarboxylase synthetase. Hum Mol Genet 2004;13:15–23. [DOI] [PubMed] [Google Scholar]
- 32.Zempleni J, Liu D, Camara DT, Cordonier EL. Novel roles of holocarboxylase synthetase in gene regulation and intermediary metabolism. Nutr Rev 2014;72:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romagnolo DF, Zempleni J, Selmin OI. Nuclear receptors and epigenetic regulation: opportunities for nutritional targeting and disease prevention. Adv Nutr 2014;5:373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Malkaram SA, Zhou J, Zempleni J. Lysine biotinylation and methionine oxidation in the heat shock protein HSP60 synergize in the elimination of reactive oxygen species in human cell cultures. J Nutr Biochem 2014;25:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng WQ, Al-Yamani E, Acierno JS Jr, Slaugenhaupt S, Gillis T, Macdonald ME, Ozand PT, Gusella JF. Biotin-responsive basal ganglia disease maps to 2q36.3 and is due to mutations in SLC19A3. Am J Hum Genet 2005;77:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramanian VS, Marchant JS, Said HM. Biotin-responsive basal ganglia disease-linked mutations inhibit thiamine transport via hTHTR2: biotin is not a substrate for hTHTR2. Am J Physiol Cell Physiol 2006;291:C851–9. [DOI] [PubMed] [Google Scholar]
- 37.Ozand PT, Gascon GG, Al Essa M, Joshi S, Al Jishi E, Bakheet S, Al Watban J, Al-Kawi MZ, Dabbagh O. Biotin-responsive basal ganglia disease: a novel entity. Brain 1998;121:1267–79. [DOI] [PubMed] [Google Scholar]
- 38.Alfadhel M, Almuntashri M, Jadah RH, Bashiri FA, Al Rifai MT, Al Shalaan H, Al Balwi M, Al Rumayan A, Eyaid W, Al-Twaijri W. Biotin-responsive basal ganglia disease should be renamed biotin-thiamine-responsive basal ganglia disease: a retrospective review of the clinical, radiological and molecular findings of 18 new cases. Orphanet J Rare Dis 2013;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sedel F, Challe G, Vignal C, Assouad R, Bellanger A, Galanaud D. A novel biotin-sensitive leukodystrophy. J Inherit Metab Dis 2011;34:S267. [Google Scholar]
- 40.Sedel F, Papeix C, Bellanger A, Touitou V, Lebrun-Frenay C, Galanaud D, Gout O, Lyon-Caen O, Tourbah A. High doses of biotin in chronic progressive multiple sclerosis: a pilot study. Mult Scler Relat Disord 2015;4:159–69. [DOI] [PubMed] [Google Scholar]
- 41.Tourbah A, Lebrun-Frenay C, Edan G, Clanet M, Papeix C, Vukusic S, De Seze J, Debouverie M, Gout O, Clavelou P, et al. . MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: a randomised, double-blind, placebo-controlled study. Mult Scler 2016;22:1719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sedel F, Bernard D, Mock DM, Tourbah A. Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis. Neuropharmacology 2016;110:644–53. [DOI] [PubMed] [Google Scholar]
- 43.Gries CL, Scott ML. The pathology of thiamin, riboflavin, pantothenic acid and niacin deficiencies in the chick. J Nutr 1972;102:1269–85. [DOI] [PubMed] [Google Scholar]
- 44.Elston MS, Sehgal S, Du Toit S, Yarndley T, Conaglen JV. Factitious Graves’ disease due to biotin immunoassay interference—a case and review of the literature. J Clin Endocrinol Metab 2016;101:3251–5. [DOI] [PubMed] [Google Scholar]
- 45.Piketty ML, Polak M, Flechtner I, Gonzales-Briceño L, Souberbielle JC. False biochemical diagnosis of hyperthyroidism in streptavidin-biotin-based immunoassays: the problem of biotin intake and related interferences. Clin Chem Lab Med 2017;55:780–8. [DOI] [PubMed] [Google Scholar]
- 46.Kummer S, Hermsen D, Distelmaier F. Biotin treatment mimicking Graves’ disease. N Engl J Med 2016;375:704–6. [DOI] [PubMed] [Google Scholar]
- 47.Piketty ML, Prie D, Sedel F, Bernard D, Hercend C, Chanson P, Souberbielle JC. High-dose biotin therapy leading to false biochemical endocrine profiles: validation of a simple method to overcome biotin interference. Clin Chem Lab Med 2017;55:817–25. [DOI] [PubMed] [Google Scholar]