Abstract
Background: Almonds may increase circulating HDL cholesterol when substituted for a high-carbohydrate snack in an isocaloric diet, yet little is known about the effects on HDL biology and function.
Objective: The objective was to determine whether incorporating 43 g almonds/d in a cholesterol-lowering diet would improve HDL subspecies and function, which were secondary study outcomes.
Methods: In a randomized, 2-period, crossover, controlled-feeding study, a diet with 43 g almonds/d (percentage of total energy: 51% carbohydrate, 16% protein, and 32% total and 8% saturated fat) was compared with a similar diet with an isocaloric muffin substitution (58% carbohydrate, 15% protein, and 26% total and 8% saturated fat) in men and women with elevated LDL cholesterol. Plasma HDL subspecies and cholesterol efflux from J774 macrophages to human serum were measured at baseline and after each diet period. Diet effects were examined in all participants (n = 48) and in normal-weight (body mass index: <25; n = 14) and overweight or obese (≥25; n = 34) participants by using linear mixed models.
Results: The almond diet, compared with the control diet, increased α-1 HDL [mean ± SEM: 26.7 ± 1.5 compared with 24.3 ± 1.3 mg apolipoprotein A-I (apoA-I)/dL; P = 0.001]. In normal-weight participants, the almond diet, relative to the control diet, increased α-1 HDL (33.7 ± 3.2 compared with 28.4 ± 2.6 mg apoA-I/dL), the α-1 to pre–β-1 ratio [geometric mean (95% CI): 4.3 (3.3, 5.7) compared with 3.1 (2.4, 4.0)], and non–ATP-binding cassette transporter A1 cholesterol efflux (8.3% ± 0.4% compared with 7.8% ± 0.3%) and decreased pre–β-2 (3.8 ± 0.4 compared with 4.6 ± 0.4 mg apoA-I/dL) and α-3 (23.5 ± 0.9 compared with 26.9 ± 1.1 mg apoA-I/dL) HDL (P < 0.05). No diet effects were observed in the overweight or obese group.
Conclusions: Substituting almonds for a carbohydrate-rich snack within a lower-saturated-fat diet may be a simple strategy to maintain a favorable circulating HDL subpopulation distribution and improve cholesterol efflux in normal-weight individuals with elevated LDL cholesterol. This trial was registered at clinicaltrials.gov as NCT01101230.
Keywords: HDL metabolism, ATP-binding cassette transporter A1, cholesterol efflux, nutrition, almonds
Introduction
Contemporary dietary guidance includes food-based dietary pattern guidelines together with recommendations to decrease saturated fat, sodium, and added sugar (1, 2). Reduced saturated fat intake has been associated with a decreased risk of cardiovascular events (3). In addition, reducing dietary saturated fat decreases cardiovascular disease (CVD) risk factors, including total cholesterol and LDL cholesterol (2). This dietary change also results in lower HDL-cholesterol concentrations (4). Optimal macronutrient distribution remains controversial, especially concerning the replacement of saturated fat (5, 6). Evidence from the OmniHeart Trial, a randomized, controlled-feeding intervention comparing carbohydrate (58% carbohydrate, 15% protein, and 21% MUFAs and PUFAs), protein (48% carbohydrate, 25% protein, and 21% MUFAs and PUFAs), and unsaturated-fat (48% carbohydrate, 15% protein, and 31% MUFAs and PUFAs) diets matched for saturated fat (6%), showed that the unsaturated-fat diet prevented decreases in HDL cholesterol compared with the carbohydrate and protein diets, whereas all 3 diets elicited the same LDL-cholesterol-lowering effect (7).
We previously showed that a cholesterol-lowering diet with almonds (43 g/d) improves HDL cholesterol (51 ± 2 compared with 49 ± 2 mg/dL; P < 0.01) compared with a similar diet containing a high-carbohydrate snack isocalorically substituted for almonds (8). The almond diet, relative to the control diet, also increased HDL2 and HDL3, HDL subfractions determined by ultracentrifugation (HDL2: 11.3 ± 0.7 compared with 10.6 ± 0.6 mg/dL; P = 0.02; HDL3: 38.8 ± 1.3 compared with 37.4 ± 1.1 mg/dL; P = 0.01) (8).
Current evidence suggests that the cardioprotective effects of HDL may be more dependent on subpopulation distribution and function than on absolute HDL-cholesterol concentrations (9, 10). Therefore, on the basis of this and our preliminary findings, we hypothesized that incorporating 43 g almonds/d in a cholesterol-lowering diet would improve HDL function (i.e., cholesterol efflux to apoB-depleted serum) and HDL subspecies compared with a traditional cholesterol-lowering, lower-fat diet. We also sought to evaluate the effects of weight status and body composition on HDL function in response to the treatment and control diets because adiposity has been shown to affect HDL cholesterol (11).
Methods
Subjects.
Stored samples from a previous study (8) were used for the current analysis. Detailed methods and participant characteristics were described previously (8). Briefly, men and women (ages 30–65 y) with a BMI (in kg/m2) of 20–35 and LDL cholesterol ≥121–190 mg/dL for women and 128–194 mg/dL for men (50th–95th percentile based on NHANES 1999–2000) were eligible for the study. Exclusion criteria included the following: tobacco use; alcohol consumption of ≥14 drinks/wk (i.e., ≥196 g ethanol/wk); chronic illness; refusal to stop vitamin or mineral, lipid‐lowering, or other supplements; use of prescription cholesterol‐lowering medications; vegetarian diet; weight gain or loss of ≥10% within the previous 6 mo; and pregnant, lactating, or wanting to become pregnant before or during the study. Each participant signed a written informed consent to participate. The study protocol was approved by the Institutional Review Board of The Pennsylvania State University. This trial was registered at clinicaltrials.gov as NCT01101230.
Intervention.
A randomized, crossover, 2-period (6 wk/period), controlled-feeding study (October 2009 to February 2012) was conducted to investigate the effects of almonds (43 g/d) within the context of a cholesterol-lowering diet on cardiometabolic risk factors. The almond and control diets were identical with the exception of the snack that was provided: 42.5 g (1.5 ounces) unsalted, whole, natural almonds with skins (253 kcal/d) or 106 g banana muffin + 2.7 g butter (273 kcal/d), respectively. All of the meals and snacks were provided to participants. The nutrient compositions of both the almond diet (percentage of total energy: 51% carbohydrate, 16% protein, and 32% total and 8% saturated fat) and the control diet (percentage of total energy: 58% carbohydrate, 15% protein, and 26% total and 8% saturated fat) were reported previously (8). At the beginning of the study (baseline) and at the end of each diet period, on 2 consecutive days participants underwent a series of clinical and physical assessments. On test mornings, participants arrived in the fasting state (12 h with only water, 48 h without alcohol, and 12 h without vigorous exercise) to the General Clinical Research Center. Trained research staff measured their height, weight, and body composition and obtained a fasting blood sample (∼30 mL on each day). Whole blood was drawn into either serum separator tubes and allowed to clot or into EDTA-containing tubes. Blood was centrifuged at 1500 × g and 4°C for 15 min, and aliquots of serum and plasma were stored in a −80°C freezer and not thawed until further analyses were conducted.
Serum lipids, lipoproteins, and apolipoproteins.
Serum lipids, lipoproteins, and apolipoproteins were measured by both standard enzymatic and spectrophotometric methods and the vertical auto profile technique described previously (8).
Plasma HDL subpopulations.
Plasma apoA-I–containing HDL subpopulations were determined by 2-dimensional (nondenaturing agarose-polyacrylamide) gel electrophoresis, immunoblotting, and image analysis (Boston Heart Diagnostics) as described (12, 13).
Cholesterol efflux to serum.
Cholesterol efflux was determined by using J774 macrophages (Vascular Strategies LLC) as previously described (14). Macrophages were labeled with 3H cholesterol for 24 h and allowed to equilibrate overnight with or without cAMP for the assessment of global cholesterol efflux and ATP-binding cassette transporter (ABC) A1 efflux, respectively. Polyethylene glycol was used to precipitate apoB-containing lipoproteins from serum samples to isolate the HDL fraction as previously described (15). The efflux acceptor medium (i.e., apoB-depleted serum) contained HDL in the supernatant at a concentration of 2.8% vol:vol. Acceptor medium for assay controls contained 20 μg human apoA-I (lipid-free) or 2% human serum/mL. Acceptor media was added to cells for 4 h; medium and cell monolayers were analyzed to determine the percentage of cholesterol released from cells. Results from multiple assays were normalized by using the human serum control in each assay.
Body-composition distribution.
Body composition was measured by using DXA (QDR‐4500W; Hologic Corp) as previously described (8).
Statistical analysis.
Statistical analyses were performed by using SAS (version 9.3; SAS Institute). One‐factor ANOVA was used to determine significant differences between pre- and postmenopausal women and men at baseline. Normality was assessed by using univariate analysis to quantitatively evaluate skewness and to visually inspect box and probability plots. Treatment effects were tested per protocol (participants completing the entire study were included in the analysis) and with the use of a linear mixed model, with treatment, visit (first or second), and their interaction as fixed effects and subject as a random effect. In addition, a linear mixed model was used for subgroup analyses to investigate whether participant baseline characteristics (i.e., BMI or sex) modified the effects of treatment on outcome variables. Baseline characteristics were stratified into categories on the basis of established cutoffs (i.e., BMI <25 or ≥25). For this secondary analysis, treatment, visit (first or second), group (BMI or sex), treatment-by-visit, and treatment-by-group were considered as fixed effects and subject as the random effect. The Bonferroni correction was used to adjust for multiple comparisons. Pearson correlations were used to evaluate associations between both baseline variables and change values for each diet period. Significance was set at P < 0.05. A sample size of 45 was determined on the basis of earlier studies (16, 17), which detected significant changes in LDL cholesterol, our primary outcome, and abdominal adiposity, a secondary outcome, with an α set to 0.05 and power set to 0.90.
Results
As previously reported (8), participants (n = 48; 26 women, 22 men) were middle-aged (50 ± 9 y) and overweight (BMI: 26 ± 3) with elevated LDL cholesterol (149 ± 20 mg/dL) and normal HDL cholesterol (55 ± 16 mg/dL). Baseline measures of apoA-I–containing HDL subpopulations and global and transporter-specific cholesterol efflux in pre- and postmenopausal women and men are presented in Table 1.
TABLE 1.
HDL subspecies and function at baseline for pre- and postmenopausal women and men with elevated serum LDL cholesterol1
| Women |
||||
| Variable | Premenopausal (n = 10) | Postmenopausal (n = 16) | Men (n = 22) | P |
| Age, y | 50 ± 3a,b | 57 ± 6a | 45 ± 10b | <0.001 |
| BMI, kg/m2 | 25 ± 2 | 27 ± 4 | 27 ± 2 | 0.16 |
| Serum lipids, lipoproteins, apoliproteins, mg/dL | ||||
| TGs | 84 (73–96)a | 110 (88–136)a,b | 135 (117–155)b | 0.002 |
| Total cholesterol | 239 ± 19 | 235 ± 24 | 218 ± 24 | 0.030 |
| LDL cholesterol | 158 ± 19 | 148 ± 22 | 144 ± 18 | 0.18 |
| HDL cholesterol | 63 ± 13a | 62 ± 19a | 46 ± 8b | <0.001 |
| apoA-I | 166 ± 20a | 168 ± 30a | 143 ± 14b | 0.002 |
| apoB | 115 ± 15 | 111 ± 16 | 114 ± 13 | 0.82 |
| Plasma HDL subspecies, mg apoA-I/dL | ||||
| Pre–β-1 | 9.0 ± 3.3 | 8.9 ± 2.5 | 7.8 ± 2.6 | 0.40 |
| Pre–β-2 | 3.9 ± 1.2 | 4.4 ± 1.0 | 4.0 ± 1.3 | 0.51 |
| α-1 | 35.4 ± 11.6a | 34.0 ± 12.1a | 21.5 ± 6.1b | <0.001 |
| α-2 | 66.0 ± 5.4a,b | 68.8 ± 14.6a | 58.6 ± 7.6b | 0.011 |
| α-3 | 25.6 ± 2.1 | 25.8 ± 3.6 | 26.9 ± 3.8 | 0.50 |
| α-4 | 13.1 ± 3.3 | 12.7 ± 1.8 | 12.9 ± 2.4 | 0.91 |
| Pre–α-1 | 2.9 (2.1, 4.1)a | 2.7 (2.0, 3.7)a | 1.7 (1.5, 2.0)b | 0.003 |
| Pre–α-2 | 5.8 (4.6, 7.2) | 5.9 (4.8, 7.2) | 5.1 (4.6, 5.7) | 0.35 |
| Pre–α-3 | 2.4 ± 0.8 | 2.5 ± 1.0 | 2.9 ± 0.8 | 0.23 |
| Pre–α-4 | 1.1 ± 0.3 | 1.2 ± 0.5 | 1.4 ± 0.4 | 0.19 |
| α-1:pre–β-1 | 4.0 (2.9, 5.4) | 3.7 (2.9, 4.8) | 2.8 (2.1, 3.5) | 0.10 |
| Cholesterol efflux to serum, % | ||||
| Global | 11.5 (10.0, 13.2) | 11.3 (10.1, 12.5) | 10.3 (9.8, 10.9) | 0.16 |
| ABCA1 | 3.3 ± 1.5 | 3.2 ± 1.8 | 3.3 ± 1.0 | 0.96 |
| Non-ABCA1 | 8.3 (7.4, 9.4)a | 8.1 (7.4, 9.0)a | 7.0 (6.7, 7.4)b | 0.003 |
Values are means ± SDs or geometric means (95% confidence limits for the mean) for non–normally distributed variables. Different letters within variables indicate differences between groups and were determined by using 1-factor ANOVA adjusted for multiple comparisons with the Bonferroni correction in SAS (version 9.3; SAS Institute, Inc.). ABCA1, ATP binding cassette transporter A1.
There were no baseline differences between pre- and postmenopausal women; thus, all women were combined for analyses. Men had significantly less α-1 HDL and cholesterol efflux from non-ABCA1 transporters than did pre- and postmenopausal women. Men also had reduced α-2 HDL compared with postmenopausal women. Despite these differences at baseline, no significant interactions of treatment by sex were observed (data not shown).
Baseline correlations.
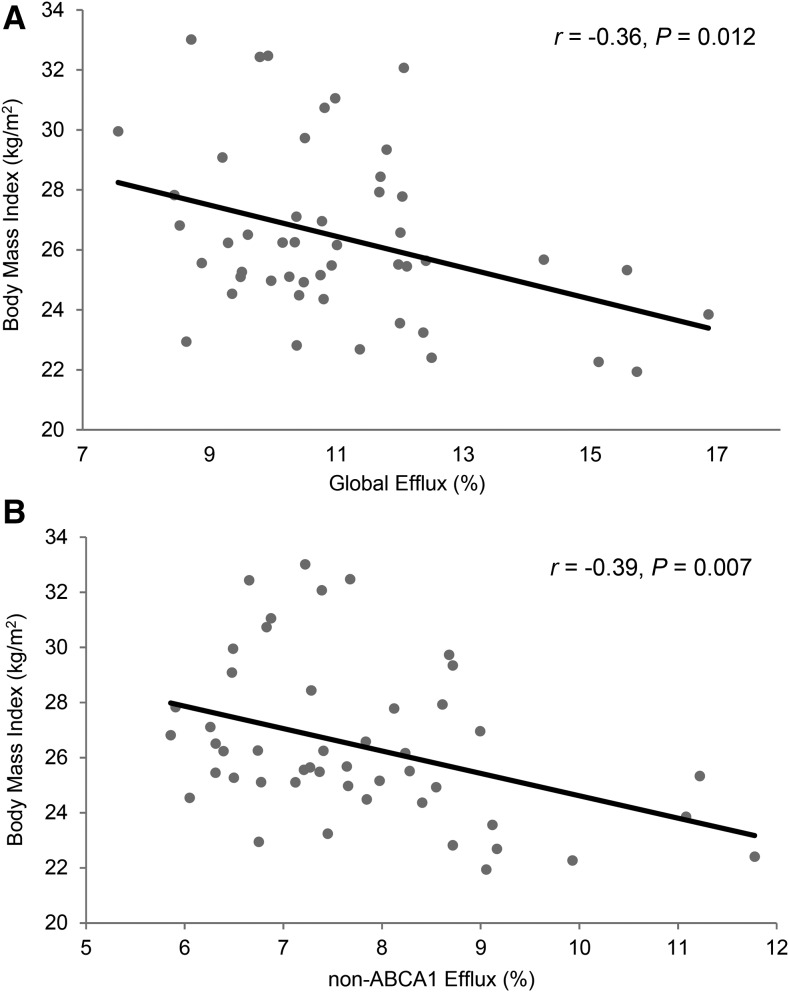
Correlations for baseline measures of HDL cholesterol, apoA-I, HDL subspecies, cholesterol efflux, BMI, abdominal fat, and leg fat are presented in Table 2 and Figure 1. HDL cholesterol and apoA-I were highly correlated (P < 0.0001) and similarly associated with pre–β-2 HDL, α-1 HDL, α-2 HDL, global cholesterol efflux, and non-ABCA1 cholesterol efflux (P < 0.05 for all). HDL subspecies α-1 and α-2 were also highly correlated (P < 0.0001), and both were associated with global efflux and non-ABCA1 efflux (P < 0.01 for all). BMI was inversely correlated with HDL cholesterol, apoA-I, α-1 HDL, α-2 HDL, global efflux, and non-ABCA1 efflux (P < 0.05 for all). In addition, abdominal fat was inversely associated with α-1 HDL (P < 0.05) and leg fat was positively associated with HDL cholesterol, apoA-I, and α-1 HDL (P < 0.05 for all). Correlations for outcome changes from baseline for each treatment period are presented in Supplemental Tables 1 and 2.
TABLE 2.
Pearson correlations between HDL cholesterol, apoA-I, HDL subspecies, cholesterol efflux, and body composition at baseline in adults with elevated serum LDL cholesterol1
| HDL | apoA-I | Pre–β-1 | Pre–β-2 | α-1 | α-2 | α-3 | α-4 | Global | ABCA1 | Non-ABCA1 | BMI | Leg fat | |
| apoA-I | 0.98*** | ||||||||||||
| Pre–β-1 | 0.23 | 0.31* | |||||||||||
| Pre–β-2 | 0.29* | 0.33* | 0.11 | ||||||||||
| α-1 | 0.94*** | 0.92*** | 0.13 | 0.29* | |||||||||
| α-2 | 0.88*** | 0.88*** | 0.02 | 0.32* | 0.80*** | ||||||||
| α-3 | −0.01 | 0.07 | 0.40** | −0.13 | −0.14 | −0.15 | |||||||
| α-4 | 0.06 | 0.11 | 0.24 | −0.14 | −0.00 | −0.11 | 0.32* | ||||||
| Global | 0.56*** | 0.58*** | 0.50** | 0.36* | 0.48** | 0.46** | 0.25 | −0.00 | |||||
| ABCA1 | −0.06 | −0.03 | 0.46** | 0.13 | −0.10 | −0.13 | 0.27 | 0.03 | 0.72*** | ||||
| Non-ABCA1 | 0.87*** | 0.87*** | 0.25 | 0.40** | 0.81*** | 0.79*** | 0.08 | −0.04 | 0.72*** | 0.04 | |||
| BMI | −0.37** | −0.37* | −0.10 | −0.11 | −0.43** | −0.33* | 0.03 | 0.06 | −0.36* | −0.13 | −0.39** | ||
| Leg fat | 0.33* | 0.31* | 0.12 | 0.17 | 0.32* | 0.27 | −0.20 | −0.01 | −0.02 | −0.24 | 0.21 | 0.47** | |
| Abdominal fat | −0.26 | −0.27 | 0.04 | 0.03 | −0.31* | −0.27 | −0.03 | −0.05 | −0.19 | −0.03 | −0.25 | 0.75*** | 0.59*** |
n = 48. *P < 0.05, **P < 0.01, ***P < 0.0001. ABCA1, ATP binding cassette transporter A1.
FIGURE 1.
Association between BMI and global cholesterol efflux (A) and non-ABCA1 cholesterol efflux (B) at baseline in adults with elevated serum LDL cholesterol. Each participant is denoted by a solid black circle. The linear regression trend line is denoted by the solid black line. ABCA1, ATP binding cassette transporter A1.
apoA-I-containing HDL subspecies.
The almond diet improved α-1 HDL compared with the control diet (Table 3). There was a treatment-by-visit interaction (P = 0.04) for the α-1 to pre–β-1 ratio, with a significant post hoc difference between the almond and control diets at diet period 2 [geometric mean (95% CI): 3.7 (3.1, 4.4) compared with 2.5 (2.0, 3.1); P = 0.04]. There were no treatment effects for the remaining apoA-I–containing HDL subspecies.
TABLE 3.
Effects of almond consumption (43 g/d) on HDL subspecies and function in adults with elevated serum LDL cholesterol1
| P | ||||||
| Variable | Baseline (n = 48) | Almond (n = 48) | Control (n = 48) | Treatment | Visit | Treatment × visit |
| HDL cholesterol,2 mg/dL | 55 ± 2 | 51 ± 2a | 49 ± 2b | 0.004 | 0.17 | 0.87 |
| apoA-I,2 mg/dL | 156 ± 4 | 150 ± 3 | 148 ± 3 | 0.059 | 0.10 | 0.68 |
| Plasma HDL subspecies, mg apoA-I/dL | ||||||
| Pre–β-1 | 8.4 ± 0.4 | 8.0 ± 0.4 | 8.5 ± 0.4 | 0.12 | 0.57 | 0.09 |
| Pre–β-2 | 4.1 ± 0.2 | 3.9 ± 0.2 | 4.0 ± 0.2 | 0.26 | 0.74 | 0.99 |
| α-1 | 28.6 ± 1.7 | 26.7 ± 1.5a | 24.3 ± 1.3b | 0.001 | 0.93 | 0.70 |
| α-2 | 63.5 ± 1.6 | 61.8 ± 1.5 | 61.1 ± 1.3 | 0.31 | 0.13 | 0.97 |
| α-3 | 26.2 ± 0.5 | 25.6 ± 0.5 | 26.6 ± 0.5 | 0.065 | 0.36 | 0.10 |
| α-4 | 12.9 ± 0.3 | 11.9 ± 0.3 | 11.7 ± 0.4 | 0.55 | 0.59 | 0.12 |
| Pre–α-1 | 2.3 (1.9, 2.6) | 2.1 (1.8, 2.5) | 1.9 (1.7, 2.3) | 0.069 | 0.73 | 0.86 |
| Pre–α-2 | 5.5 (5.0, 6.0) | 5.3 (4.8, 5.9) | 5.2 (4.7, 5.7) | 0.41 | 0.09 | 0.86 |
| Pre–α-3 | 2.7 ± 0.1 | 2.6 ± 0.1 | 2.5 ± 0.1 | 0.86 | 0.77 | 0.25 |
| Pre–α-4 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.0 | 0.058 | 0.40 | 0.23 |
| α-1:pre–β-1 | 3.3 (2.8, 3.8) | 3.2 (2.8, 3.7)a | 2.9 (2.5, 3.3)b | 0.020 | 0.62 | 0.04 |
| Cholesterol efflux to serum, % | ||||||
| Global | 11.0 ± 0.3 | 10.2 ± 0.3 | 10.0 ± 0.2 | 0.33 | 0.64 | 0.68 |
| ABCA1 | 3.3 ± 0.2 | 2.8 ± 0.2 | 2.7 ± 0.2 | 0.82 | 0.22 | 0.65 |
| Non-ABCA1 | 7.8 ± 0.2 | 7.4 ± 0.2 | 7.3 ± 0.2 | 0.18 | 0.09 | 0.96 |
Values are means ± SEMs for normally distributed data or geometric means (95% confidence limits) for non–normally distributed variables. Linear mixed models (SAS, version 9.3; SAS Institute, Inc.) were used to determine the main effects of treatment, visit, and their interaction. Baseline values are included in the table for descriptive purposes and were not included in the linear mixed model. Labeled Almond and Control period means in a row without a common superscript letter differ, P ≤ 0.05. ABCA1, ATP binding cassette transporter A1.
Values were reported previously (8).
Cholesterol efflux.
There were no treatment effects for global or transporter-specific cholesterol efflux to apoB-depleted serum (Table 3).
Diet comparisons in normal-weight and overweight or obese participants.
There were no treatment differences for total body weight or BMI (data not shown). Subgroup analysis showed that baseline BMI (<25 compared with ≥25) influences HDL cholesterol, pre–β-2 HDL, α-1 HDL, α-3 HDL, pre–α-1 HDL, pre–α-2 HDL, the α-1 to pre–β-1 ratio, and non-ABCA1 cholesterol efflux responses to diet (treatment-by-baseline BMI group interaction; Table 4). In normal-weight participants (BMI: 24 ± 1; range: 22–25; n = 14), the almond diet improved HDL cholesterol, α-1 HDL, the α-1 to pre–β-1 ratio, and cholesterol efflux via non-ABCA1 transporters compared with the control diet. In addition, the almond diet decreased pre–β-2 and α-3 HDL compared with the control diet in normal-weight individuals. Overweight and obese participants (BMI: 28 ± 2; range: 25–33; n = 34) did not experience any within-group effects of the almond diet on HDL subspecies or cholesterol efflux outcomes.
TABLE 4.
Effects of almond consumption (43 g/d) on HDL subspecies and function by BMI category in adults with elevated serum LDL cholesterol1
| BMI <25 (n = 14) |
BMI ≥25 (n = 34) |
P |
|||||||
| Variable | Baseline | Almond | Control | Baseline | Almond | Control | Treatment | Group | Treatment × group |
| HDL cholesterol, mg/dL | 64 ± 5 | 58 ± 4a | 55 ± 4b | 51 ± 2 | 47 ± 2b | 46 ± 2b | 0.001 | 0.02 | 0.06 |
| apoA-I, mg/dL | 170 ± 7 | 160 ± 7 | 157 ± 6 | 150 ± 4 | 146 ± 3 | 144 ± 3 | 0.06 | 0.04 | 0.66 |
| Plasma HDL subspecies, mg apoA-I/dL | |||||||||
| Pre–β-1 | 8.6 ± 0.6 | 7.7 ± 0.8 | 8.9 ± 0.6 | 8.3 ± 0.5 | 8.2 ± 0.4 | 8.3 ± 0.6 | 0.03 | 0.89 | 0.08 |
| Pre–β-2 | 4.3 ± 0.4 | 3.8 ± 0.4a | 4.6 ± 0.4b | 4.0 ± 0.2 | 3.9 ± 0.2a,b | 3.8 ± 0.2a,b | 0.01 | 0.44 | 0.002 |
| α-1 | 35.9 ± 3.2 | 33.7 ± 3.2a | 28.4 ± 2.6b | 25.5 ± 1.7 | 23.8 ± 1.5b | 22.6 ± 1.3b | <0.001 | 0.009 | 0.008 |
| α-2 | 68.9 ± 3.2 | 66.8 ± 3.0 | 65.3 ± 2.4 | 61.3 ± 1.7 | 59.8 ± 1.7 | 59.4 ± 1.5 | 0.25 | 0.03 | 0.57 |
| α-3 | 26.5 ± 0.7 | 23.5 ± 0.9a | 26.9 ± 1.1b | 26.1 ± 0.6 | 26.4 ± 0.6a,b | 26.5 ± 0.5a,b | 0.002 | 0.22 | 0.002 |
| α-4 | 12.8 ± 0.8 | 11.5 ± 0.8 | 11.4 ± 0.7 | 13.0 ± 0.4 | 12.0 ± 0.4 | 11.8 ± 0.4 | 0.63 | 0.57 | 0.88 |
| Pre–α-1 | 2.8 (2.1, 3.9) | 2.7 (1.9, 3.8)a | 2.1 (1.4, 3.1)b | 2.1 (1.7, 2.4) | 1.9 (1.6, 2.3)a,b | 1.9 (1.6, 2.2)a,b | 0.004 | 0.20 | 0.005 |
| Pre–α-2 | 5.7 (4.7, 6.9) | 6.2 (5.2, 7.4) | 5.1 (4.1, 6.5) | 5.4 (4.9, 6.0) | 5.0 (4.4, 5.6) | 5.2 (4.7, 5.7) | 0.06 | 0.27 | 0.01 |
| Pre–α-3 | 2.4 ± 0.2 | 2.4 ± 0.2 | 2.3 ± 0.2 | 2.7 ± 0.2 | 2.6 ± 0.2 | 2.6 ± 0.1 | 0.83 | 0.29 | 0.89 |
| Pre–α-4 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 0.07 | 0.16 | 0.77 |
| α-1:pre–β-1 | 4.1 (3.0, 5.4) | 4.3 (3.3, 5.7)a | 3.1 (2.4, 4.0)b | 3.0 (2.5, 3.6) | 2.9 (2.4, 3.3)b | 2.8 (2.3, 3.3)b | 0.001 | 0.08 | 0.003 |
| Cholesterol efflux to serum, % | |||||||||
| Global | 11.9 ± 0.7 | 10.9 ± 0.7 | 10.6 ± 0.5 | 10.7 ± 0.3 | 9.9 ± 0.2 | 9.8 ± 0.3 | 0.21 | 0.10 | 0.36 |
| ABCA1 | 3.2 ± 0.5 | 2.6 ± 0.5 | 2.7 ± 0.3 | 3.3 ± 0.2 | 2.8 ± 0.2 | 2.7 ± 0.2 | 0.84 | 0.79 | 0.33 |
| Non-ABCA1 | 8.7 ± 0.4 | 8.3 ± 0.4a | 7.8 ± 0.3b | 7.4 ± 0.2 | 7.0 ± 0.2b | 7.1 ± 0.2b | 0.03 | 0.005 | 0.02 |
Values are means ± SEMs for normally distributed data or geometric means (95% confidence limits) for non–normally distributed variables. Linear mixed models (SAS, version 9.3; SAS Institute, Inc.) were used to determine the main effects of treatment, visit, BMI group (in kg/m2), and treatment-by-visit and treatment-by-BMI group interactions. Baseline values are included in the table for descriptive purposes and were not included in the linear mixed model. Labeled Almond and Control period means in a row without a common superscript letter differ, P ≤ 0.05 (adjusted for multiple comparisons by using the Bonferroni correction). ABCA1, ATP binding cassette transporter A1.
Furthermore, BMI group comparisons showed that HDL cholesterol, α-1 HDL, the α-1 to pre–β-1 ratio, and cholesterol efflux via non-ABCA1 transporters were greater after almond consumption in the normal-weight group than after both the control and almond diets in the overweight or obese group (Table 4).
Discussion
This is the first study, to our knowledge, to evaluate the effects of almond consumption on HDL subspecies and HDL function as assessed by global and transporter-specific cholesterol efflux. We found that including almonds in a cholesterol-lowering diet (moderate in total fat due to the almonds; percentage of total energy: 32% total and 8% saturated fat, 16% protein, and 51% carbohydrate) prevented decreases in α-1 HDL that are observed with a traditional lower-fat diet (26% total and 8% saturated fat, 15% protein, and 58% carbohydrate). A previous study (18) in male participants showed that α-1 HDL was a better predictor of ischemic heart disease (IHD) than total HDL cholesterol. In this same cohort, each 1-mg apoA-I increase/dL in α-1 HDL was associated with a 26% decrease in the odds of IHD, whereas each 1-mg apoA-I increase/dL in α-3 HDL was associated with an 18% increase in the odds of IHD (18). Furthermore, in the current study, an overall improvement in the subpopulation profile was evidenced by the preservation of the α-1 to pre–β-1 ratio with almond consumption. Low concentrations of α-1 HDL and high concentrations of pre–β-1 HDL, individually or in combination, have been shown to increase CVD risk (18, 19). The α-1 to pre–β-1 ratio allows a proportional comparison of antiatherogenic α-1 HDL to immature, proatherogenic pre–β-1 HDL, providing additional information about HDL maturation and CVD risk.
The PREDIMED (Prevención con Dieta Mediterránea) trial found a reduction of ∼30% in major cardiovascular events in individuals who consumed a Mediterranean diet (MedDiet) supplemented with either 30 g nuts/d (walnuts, almonds, and hazelnuts) or 50 g (1 L ⋅ wk−1 ⋅ family−1) extra-virgin olive oil/d compared with individuals who were given advice to decrease their dietary fat intake (20). However, contrary to our findings, the PREDIMED trial found no changes in large, medium, or small HDL particle number, as measured by NMR spectroscopy, with the MedDiet supplemented with nuts compared with the MedDiet with olive oil or with the lower-fat control diet (21). This discrepancy could be due to differences in measurement technique, dietary intervention (both treatment and control diets were relatively high in total fat compared with the current study), or study population; participants in all 3 intervention groups were overweight [nut group: mean (95% CI) BMI = 29 (28, 30); olive oil group: BMI = 30 (29, 31); and control group: BMI = 30 (29, 31)] (21). When our population was classified by BMI group (<25 or ≥25) we also observed no treatment effects in the overweight or obese group; however, lean individuals had pronounced effects, including significantly higher α-1 HDL– and α-1–to–pre–β-1 ratios and lower pre–β-2 and α-3 HDL concentrations with the almond diet, relative to the control diet.
In the current study, we saw no treatment effects on global or transporter-specific cholesterol efflux; however, with both diets there were decreases in global, ABCA1, and non-ABCA1 efflux compared with baseline. Conversely, results from a previous study in our laboratory showed a 3.3% increase in postprandial (4 h) cholesterol efflux relative to fasting baseline after consumption of whole walnuts (85 g) (22). Inherent study design differences preclude direct comparison between studies but emphasize distinctions that may account for discrepancies in the literature. For example, our study on walnut consumption (22) was an acute-feeding exposure, measuring postprandial cholesterol efflux to whole serum and including all efflux pathways, whereas in the current study, we investigated the effects of chronic almond intake on fasting cholesterol efflux to apoB-depleted serum (HDL fraction) via global, ABCA1-mediated, and non-ABCA1–mediated routes.
Several comparable dietary interventions (23–25) also reported no treatment effects of a MUFA-rich diet on cholesterol efflux capacity when compared with carbohydrate (23), saturated fat (24), or linoleic acid (18:2n–6) (25)–rich diets, despite a reduction in both atherogenic lipoproteins (23, 24) and HDL oxidative modification (25) with the MUFA-rich diets. Blanco-Molina et al. (23) conducted a study investigating the effects of a National Cholesterol Education Program (NCEP) step 1 diet (28% total fat, 9% saturated fat, 14% MUFAs, 5% PUFAs, and 0.027 mg cholesterol/kJ) compared with a MUFA diet (39% total fat, 9% saturated fat, 25% MUFAs, 5% PUFAs, and 0.027 mg cholesterol/kJ) with or without added cholesterol (0.068 mg cholesterol/kJ) on cholesterol efflux to whole serum from rat hepatoma cells. Their results showed a benefit of the MUFA-rich diet on apoB, the total-cholesterol to HDL-cholesterol ratio, and the apoB to apoA-I ratio compared with the NCEP step 1 diet; however, the MUFA-rich diet had no effect on cholesterol efflux (23). Moreover, this group did observe an increase in cholesterol efflux with the consumption of the high-cholesterol NCEP step 1 diet compared with the low-cholesterol NCEP step 1 diet, suggesting that dietary cholesterol intake may have a regulatory effect on cholesterol efflux (23). This finding has been corroborated in a study in mice, which showed that a high-fat, high-cholesterol diet increases cholesterol efflux from macrophages compared with both a high-fat, low-cholesterol diet and a low-fat, low-cholesterol diet; increases in the expression of liver ABCG5 and ABCG8 with the high-fat, high-cholesterol diet may explain a mechanism by which dietary cholesterol influences efflux capacity (26). Therefore, in the current study, decreases in overall cholesterol efflux may be due to the low cholesterol content of the study diets.
Furthermore, we were interested to find a treatment effect on non-ABCA1 cholesterol efflux when participants were stratified by BMI, similar to previous findings that lean individuals respond more sensitively to dietary interventions aimed at lowering LDL cholesterol (27, 28). In normal-weight individuals, the almond diet prevented decreases in non-ABCA1 efflux that occurred with consumption of the control diet. Global efflux to apoB-depleted serum encompasses cholesterol efflux via passive diffusion, secretion of sterol 27-hydroxylase metabolites, the ABC transporters, particularly ABCA1 and ABCG1, and scavenger receptor class B type 1 (SR-B1) (29–31). ABCA1 efflux quantifies cholesterol removed by the ABCA1 transporter, and non-ABCA1 (global efflux minus ABCA1 cholesterol efflux) represents all of the aforementioned pathways except for the ABCA1 transporter. In the current study, baseline ABCA1 efflux was associated with lipid-poor pre–β-1, which has been shown previously in healthy men (15), whereas baseline non-ABCA1 efflux was associated with HDL cholesterol, apoA-I, pre–β-2 HDL, α-1 HDL, and α-2 HDL. In addition, changes in non-ABCA1 efflux were positively associated with changes in HDL cholesterol, apoA-I, pre–β-1 HDL, α-1 HDL, and α-2 HDL.
Absolute concentrations of HDL cholesterol are associated with a decreased risk of CVD in observational studies, and strategies to increase HDL cholesterol have been explored as a way to decrease CVD risk (32). However, current pharmacologic interventions with niacin and cholesteryl ester transfer protein inhibitors have not shown a benefit on CVD events or mortality, despite an increase in HDL-cholesterol concentrations (33, 34). Therefore, it is important to quantify functional and biological characteristics of HDL, in addition to measuring HDL-cholesterol and apoA-I concentrations.
A potential limitation of the current study, and all studies evaluating HDL biology and function, is a lack of method standardization across trials. HDL subclasses can be measured by a variety of methods, including ultracentrifugation, NMR spectrometry, and 1-dimensional gel electrophoresis. The method we chose uses 2-dimensional gel electrophoresis, which separates HDL subclasses by size and charge and then quantifies apoA-I in each subparticle, providing the most comprehensive measure of HDL subspecies distribution. In addition, cholesterol efflux methods can vary by cell line, use of whole serum or the HDL fraction, and global or transporter-specific outcomes. Furthermore, the sample size for this cohort was determined on the basis of the primary outcome measures, LDL cholesterol and abdominal adiposity (8); therefore, power to detect differences in secondary outcomes by BMI group may be limited. Post hoc power analyses, with the use of sample size and SDs from the current analysis, indicated that differences of 8–28% and 3–13% were detectable for HDL subspecies in the normal-weight and overweight or obese groups, respectively, whereas, differences of 7–23% and 3–15% were detectable for cholesterol efflux outcomes in the normal-weight and overweight or obese groups, respectively. Strengths of the current study include the controlled dietary intervention and novel HDL subpopulation and function measures.
In conclusion, incorporating almonds in a lower-saturated-fat diet improves HDL subspecies, specifically by preventing decreases in α-1 HDL caused by a traditional low-fat diet. Furthermore, almond consumption improves HDL subpopulation distribution and non-ABCA1–mediated cholesterol efflux, relative to the control diet, in normal-weight participants. Therefore, substituting almonds for a carbohydrate-rich snack within a lower-saturated-fat diet maintains favorable circulating HDL subpopulation distribution and function in normal-weight individuals with elevated LDL cholesterol.
Acknowledgments
The authors’ responsibilities were as follows—PMK-E, JAF, and CEB: designed the research; CEB and JAF: conducted the research; CEB: performed the statistical analysis; CEB and PMK-E: wrote the manuscript; PMK-E: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Footnotes
Abbreviations used: ABC, ATP-binding cassette transporter; CVD, cardiovascular disease; IHD, ischemic heart disease; MedDiet, Mediterranean diet; NCEP, National Cholesterol Education Program; PREDIMED, Prevención con Dieta Mediterránea.
References
- 1.US Department of Health and Human Services; USDA. 2015 – 2020 Dietary guidelines for Americans. 8th ed Washington (DC): US Government Printing Office; 2015. [Google Scholar]
- 2.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, et al. . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 2014;129:S76–99. [DOI] [PubMed] [Google Scholar]
- 3.Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, Moore HJ, Davey Smith G. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev 2012;5:CD002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katan MB. Effect of low-fat diets on plasma high-density lipoprotein concentrations. Am J Clin Nutr 1998;67(Suppl):573S–6S. [DOI] [PubMed] [Google Scholar]
- 5.Flock MR, Fleming JA, Kris-Etherton PM. Macronutrient replacement options for saturated fat: effects on cardiovascular health. Curr Opin Lipidol 2014;25:67–74. [DOI] [PubMed] [Google Scholar]
- 6.Astrup A, Dyerberg J, Elwood P, Hermansen K, Hu FB, Jakobsen MU, Kok FJ, Krauss RM, Lecerf JM, LeGrand P, et al. . The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: where does the evidence stand in 2010? Am J Clin Nutr 2011;93:684–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER III, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, et al. . Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005;294:2455–64. [DOI] [PubMed] [Google Scholar]
- 8.Berryman CE, West SG, Fleming JA, Bordi PL, Kris-Etherton PM. Effects of daily almond consumption on cardiometabolic risk and abdominal adiposity in healthy adults with elevated LDL-cholesterol: a randomized controlled trial. J Am Heart Assoc 2015;4:e000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med 2011;17:594–603. [DOI] [PubMed] [Google Scholar]
- 10.Rader DJ, Tall AR. The not-so-simple HDL story: is it time to revise the HDL cholesterol hypothesis? Nat Med 2012;18:1344–6. [DOI] [PubMed] [Google Scholar]
- 11.Bays HE, Toth PP, Kris-Etherton PM, Abate N, Aronne LJ, Brown WV, Gonzalez-Campoy JM, Jones SR, Kumar R, La Forge R, et al. . Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. J Clin Lipidol 2013;7:304–83. [DOI] [PubMed] [Google Scholar]
- 12.Asztalos BF, Roheim PS, Milani RL, Lefevre M, McNamara JR, Horvath KV, Schaefer EJ. Distribution of ApoA-I–containing HDL subpopulations in patients with coronary heart disease. Arterioscler Thromb Vasc Biol 2000;20:2670–6. [DOI] [PubMed] [Google Scholar]
- 13.Asztalos B, Lefevre M, Wong L, Foster TA, Tulley R, Windhauser M, Zhang W, Roheim PS. Differential response to low-fat diet between low and normal HDL-cholesterol subjects. J Lipid Res 2000;41:321–8. [PubMed] [Google Scholar]
- 14.Yancey PG, Asztalos BF, Stettler N, Piccoli D, Williams DL, Connelly MA, Rothblat GH. SR-BI- and ABCA1-mediated cholesterol efflux to serum from patients with Alagille syndrome. J Lipid Res 2004;45:1724–32. [DOI] [PubMed] [Google Scholar]
- 15.Asztalos BF, de la Llera-Moya M, Dallal GE, Horvath KV, Schaefer EJ, Rothblat GH. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J Lipid Res 2005;46:2246–53. [DOI] [PubMed] [Google Scholar]
- 16.Gebauer SK, West SG, Kay CD, Alaupovic P, Bagshaw D, Kris-Etherton PM. Effects of pistachios on cardiovascular disease risk factors and potential mechanisms of action: a dose-response study. Am J Clin Nutr 2008;88:651–9. [DOI] [PubMed] [Google Scholar]
- 17.Paniagua JA, de la Sacristana AG, Romero I, Vidal-Puig A, Latre JM, Sanchez E, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Monounsaturated fat–rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care 2007;30:1717–23. [DOI] [PubMed] [Google Scholar]
- 18.Asztalos BF, Cupples LA, Demissie S, Horvath KV, Cox CE, Batista MC, Schaefer EJ. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler Thromb Vasc Biol 2004;24:2181–7. [DOI] [PubMed] [Google Scholar]
- 19.Lamon-Fava S, Herrington DM, Reboussin DM, Sherman M, Horvath K, Schaefer EJ, Asztalos BF. Changes in remnant and high-density lipoproteins associated with hormone therapy and progression of coronary artery disease in postmenopausal women. Atherosclerosis 2009;205:325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, et al. . Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 21.Damasceno NRT, Sala-Vila A, Cofán M, Pérez-Heras AM, Fitó M, Ruiz-Gutiérrez V, Martínez-González M-Á, Corella D, Arós F, Estruch R, et al. . Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis 2013;230:347–53. [DOI] [PubMed] [Google Scholar]
- 22.Berryman CE, Grieger JA, West SG, Chen C-YO, Blumberg JB, Rothblat GH, Sankaranarayanan S, Kris-Etherton PM. Acute consumption of walnuts and walnut components differentially affect postprandial lipemia, endothelial function, oxidative stress, and cholesterol efflux in humans with mild hypercholesterolemia. J Nutr 2013;143:788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanco-Molina A, Castro G, Martín-Escalante D, Bravo D, López-Miranda J, Castro P, López-Segura F, Fruchart JC, Ordovás JM, Pérez-Jiménez F. Effects of different dietary cholesterol concentrations on lipoprotein plasma concentrations and on cholesterol efflux from Fu5AH cells. Am J Clin Nutr 1998;68:1028–33. [DOI] [PubMed] [Google Scholar]
- 24.Montoya MT, Porres A, Serrano S, Fruchart JC, Mata P, Gerique JAG, Castro GR. Fatty acid saturation of the diet and plasma lipid concentrations, lipoprotein particle concentrations, and cholesterol efflux capacity. Am J Clin Nutr 2002;75:484–91. [DOI] [PubMed] [Google Scholar]
- 25.Solà R, La Ville AE, Richard JL, Motta C, Bargalló MT, Girona J, Masana L, Jacotot B. Oleic acid rich diet protects against the oxidative modification of high density lipoprotein. Free Radic Biol Med 1997;22:1037–45. [DOI] [PubMed] [Google Scholar]
- 26.Escolà-Gil JC, Llaverias G, Julve J, Jauhiainen M, Méndez-González J, Blanco-Vaca F. The cholesterol content of Western diets plays a major role in the paradoxical increase in high-density lipoprotein cholesterol and upregulates the macrophage reverse cholesterol transport pathway. Arterioscler Thromb Vasc Biol 2011;31:2493–9. [DOI] [PubMed] [Google Scholar]
- 27.Lefevre M, Champagne CM, Tulley RT, Rood JC, Most MM. Individual variability in cardiovascular disease risk factor responses to low-fat and low-saturated-fat diets in men: body mass index, adiposity, and insulin resistance predict changes in LDL cholesterol. Am J Clin Nutr 2005;82:957–63. [DOI] [PubMed] [Google Scholar]
- 28.Jansen S, Lopez-Miranda J, Salas J, Castro P, Paniagua JA, Lopez-Segura F, Ordovas JM, Jimenez-Pereperez JA, Blanco A, Perez-Jimenez F. Plasma lipid response to hypolipidemic diets in young healthy non-obese men varies with body mass index. J Nutr 1998;128:1144–9. [DOI] [PubMed] [Google Scholar]
- 29.Babiker A, Andersson O, Lund E, Xiu R-J, Deeb S, Reshef A, Leitersdorf E, Diczfalusy U, Björkhem I. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism: comparison with high density lipoprotein-mediated reverse cholesterol transport. J Biol Chem 1997;272:26253–61. [DOI] [PubMed] [Google Scholar]
- 30.Westerterp M, Bochem AE, Yvan-Charvet L, Murphy AJ, Wang N, Tall AR. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ Res 2014;114:157–70. [DOI] [PubMed] [Google Scholar]
- 31.Rosenson RS, Brewer HB, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang X-C, Phillips MC, Rader DJ, et al. . Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012;125:1905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJP, Bittner V, Fruchart J-C. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007;357:1301–10. [DOI] [PubMed] [Google Scholar]
- 33.AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67. [DOI] [PubMed] [Google Scholar]
- 34.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJP, Komajda M, Lopez-Sendon J, Mosca L, Tardif J-C, Waters DD, et al. . Effects of Torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–22. [DOI] [PubMed] [Google Scholar]