Abstract
Background: Two indexes exist to describe dietary inflammatory potential: an empirical dietary inflammatory pattern (EDIP) composed of food groups as reported on a food-frequency questionnaire (FFQ) and a literature-derived dietary inflammatory index (DII) composed mainly of nutrients.
Objective: We compared the ability of the 2 indexes to predict concentrations of inflammatory markers and hypothesized that the EDIP would be more predictive because it was derived on the basis of circulating inflammatory markers.
Methods: Both EDIP and DII scores were calculated from FFQ data reported by 5826 women in the Nurses’ Health Study II and 5227 men in the Health Professionals Follow-Up Study. We used multivariable-adjusted linear regression analyses to calculate relative differences in concentrations of 4 plasma inflammatory markers—C-reactive protein (CRP; milligrams per liter), interleukin 6 (IL-6; picograms per milliliter), tumor necrosis factor α receptor 2 (TNFαR2; picograms per milliliter), and adiponectin (nanograms per milliliter)—in quintiles of the dietary indexes.
Results: Spearman correlations between the EDIP and DII scores were modest (r = 0.29 and 0.21 for women and men, respectively; all P < 0.0001). Higher scores on both dietary indexes were associated with higher concentrations of inflammatory markers, although they were associated with lower adiponectin concentrations and there was no association between the DII and adiponectin in men. For example, percentage differences in concentrations of biomarkers in quintile 5 generally were higher (lower for adiponectin) than in quintile 1 (for the EDIP and DII, respectively—women: CRP, +60% and +49%; IL-6, +23% and +21%; TNFαR2, +7% and +4%; adiponectin, −21% and −14%; men: CRP, +38% and +29%; IL-6, +14% and +24%; TNFαR2, +9% and +5%; adiponectin, −16% and −4%.)
Conclusion: Despite design differences, the EDIP and DII both assess dietary inflammatory potential in men and women, with the EDIP showing a greater ability to predict concentrations of plasma inflammatory markers.
Keywords: inflammation, inflammatory markers, dietary quality, dietary indexes, dietary patterns
Introduction
Dietary patterns or dietary indexes that account for multiple dietary factors may provide a more comprehensive assessment of diet, and may thus be more predictive of diet-disease associations than the approach of using single foods or nutrients (1, 2). Chronic inflammation plays an important role in the development of many chronic diseases (3–5), and diet, a modifiable factor, has been implicated in the etiology of these diseases. In addition, some dietary patterns have been shown to influence inflammation (6–9). Index-based or a priori–defined dietary patterns, such as the Mediterranean dietary pattern or the DASH (Dietary Approaches to Stop Hypertension) meal plan, have been inversely associated with concentrations of inflammatory markers (6, 7). The evidence is inconsistent for patterns defined by using data-driven or a posteriori methods (e.g., Western or prudent patterns) (8, 9).
With the use of data from peer-reviewed research publications, a literature-derived dietary inflammatory index (DII) was developed to summarize the association between dietary factors and inflammatory markers (10). The DII is based on 45 dietary factors known to predict concentrations of 6 inflammatory markers according to peer-reviewed publications through 2010 (10). The DII has been evaluated for validity and was found to predict inflammatory markers in several studies (11–13). More recently, an empirically derived dietary inflammatory pattern (EDIP) score based exclusively on food groups was developed in a US-based prospective cohort (14) and its validity evaluated by using data from 2 independent US-based prospective cohorts of health professionals (14). Methodologic differences between the EDIP and DII scores are summarized in Table 1.
TABLE 1.
Methodologic differences between the EDIP and DII scores1
| Dietary index |
||
| EDIP | DII | |
| Derivation approach | Empirical hypothesis–oriented | A priori |
| Statistical methods | Reduced rank regression, stepwise linear regression | Standardization, normalization |
| Predictors | Foods and food groups exclusively | Mainly nutrients |
| Outcomes | 3 circulating inflammatory markers (CRP, TNFαR2, IL-6) | 6 inflammatory markers reported in the literature (CRP, TNF-α, IL-6, IL-1β, IL-4, IL-10) |
| Number of components | 18 components (9 anti-inflammatory, 9 proinflammatory) | 45 components (36 anti-inflammatory, 9 proinflammatory) |
| Weights | Derived from regression analyses | Derived from the literature |
| Final score | 18 components, weighted and summed to derive the score for each individual | 45 components, weighted and summed to derive the score for each individual |
| Influenced by supplement use | No | Yes |
| Score interpretation | Higher scores indicate proinflammatory diets; lower scores indicate anti-inflammatory diets | Higher scores indicate proinflammatory diets; lower scores indicate anti-inflammatory diets |
CRP, C-reactive protein; DII, dietary inflammatory index; EDIP, empirical dietary inflammatory pattern; TNFαR2, TNF-α receptor 2.
Although the EDIP and DII both assess the inflammatory potential of diet, the 2 dietary indexes differ in conception and design (Table 1). The DII is an a priori index (i.e., developed on the basis of the prevailing scientific evidence on the association between dietary factors and inflammation), whereas the EDIP is a hypothesis-driven a posteriori index (i.e., its development is data-driven but focused on identifying a dietary pattern predictive of biological markers of inflammation). The DII is mainly nutrient-based (i.e., 35 of its 45 components are nutrients) and assesses dietary inflammatory potential as the net effect of anti- and proinflammatory nutrients in whole diets. In contrast, the EDIP is based exclusively on food groups and assesses the inflammatory potential of diet as the net effect of the anti- and proinflammatory foods in whole diets (Table 1). In this cross-sectional study, we used data from 2 large cohorts of women and men to compare the inflammation-predictive ability of the EDIP, DII, and a composite dietary inflammatory index (CDII) derived by combining EDIP and DII scores. We hypothesized that the EDIP would be more predictive than the DII because the EDIP was derived on the basis of circulating inflammatory markers. In addition, the CDII combines potentially independent information in the EDIP and DII scores and may therefore be more predictive than either of the 2 indexes alone.
Methods
Study populations.
The Nurses’ Health Study II (NHS-II) and the Health Professionals Follow-Up Study (HPFS) are ongoing prospective cohorts established in 1989 and 1986, respectively. The NHS-II (n = 116,430) enrolled female registered nurses aged 25–42 y (15), whereas the HPFS (n = 51,529) enrolled male health professionals aged 40–75 y. Blood samples were collected from subpopulations of the NHS-II (n = 29,611) in 1996–1999 and the HPFS (n = 18,225) from 1993 to 1994 (16). Blood collection was conducted by using similar protocols for both cohorts, and participants who donated blood samples were free of diagnosed chronic diseases such as cancer, cardiovascular disease, diabetes, and Alzheimer disease. The procedures, including blood collection, handling, and storage, have been summarized previously (17). In the current study, we used data from previous matched case-control studies nested within each of the 2 cohorts that measured C-reactive protein (CRP), IL-6, TNF-α receptor 2 (TNFαR2), and adiponectin. We excluded participants with missing dietary data (described below) and covariate data (n = 400 for NHS-II and n = 720 for HPFS). We therefore retained a total of 5826 women and 5227 men for the final analyses. The characteristics of participants excluded from the study were similar to those of the included participants. Biomarker sample sizes were different for each biomarker in each cohort (in the NHS-II: n = 3288 for CRP, n = 3000 for IL-6, n = 3258 for TNFαR2, and n = 3985 for adiponectin; in the HPFS: n = 4883 for CRP, n = 2923 for IL-6, n = 3912 for TNFαR2, and n = 4176 for adiponectin). The institutional review boards at Brigham and Women’s Hospital and at Harvard T.H. Chan School of Public Health approved this study.
Biomarker assessment.
Procedures for the measurement of plasma inflammatory markers (CRP, IL-6, TNFαR2, and adiponectin) in the NHS-II and HPFS have been described previously (16, 18, 19). IL-6 and TNFαR2 were measured by using ELISAs (R&D Systems). CRP was measured by using a high-sensitivity immunoturbidimetric assay with reagents and calibrators from Denka Seiken Co. Adiponectin was measured by using a competitive RIA (Linco Research). Thirty-one nested case-control studies assessed ≥1 inflammatory marker between August 1997 and December 2014, and participants donated blood samples before disease development. The CVs from blinded quality-control samples ranged from 2.9% to 12.8% for IL-6, 1.0% to 9.1% for CRP, 4.0% to 10.0% for TNFαR2, and 8.1% to 11.1% for adiponectin across batches. Quality-control samples were randomly interspersed among the case-control samples, and laboratory personnel were blinded to quality-control and case-control status for all assays. Batch calibration was performed to adjust for potential batch variability. A batch correction factor was calculated separately for each cohort by using linear regression analyses to model the association between assay batch and ln-transformed values of each biomarker. All of the values were recalibrated by using the batch correction factor to the value of an “average batch” (20).
Assessment of dietary and nondietary data.
Dietary data are updated every 4 y in the NHS-II (since 1991) and in the HPFS (since 1986) with a self-administered semiquantitative FFQ that has been evaluated for validity in several studies (21–23). We used dietary data from the questionnaires closest to the blood draw (i.e., the 1999 FFQ for NHS-II and the 1994 FFQ for HPFS). Participants with excessive missing items (≥70) on the FFQ, implausibly low or high energy intakes (<600 or >3500 kcal/d for women and <800 or >4200 kcal/d for men) were excluded (24).
Both cohorts collected nondietary data (e.g., medical history and health practices) with the use of self-administered questionnaires. We calculated participants’ BMI (in kg/m2) by using height (meters) reported at baseline for each cohort and weight (kilograms) reported in 1999 (NHS-II) or 1994 (HPFS). Participants reported smoking status (never, former, or current), and we calculated physical activity by summing the average metabolic equivalent task hours per week for the following activities: tennis, squash, or racquetball; rowing; calisthenics; walking; jogging; running; bicycling; and swimming. The validity of the physical activity questionnaire has been evaluated (25, 26). Regular use of aspirin or other nonsteroidal anti-inflammatory drug was defined as the use of ≥2 standard tablets (325 mg) of aspirin or ≥2 tablets of a nonsteroidal anti-inflammatory drug/wk.
Description of the EDIP score.
The EDIP was developed in a sample of 5230 participants in the Nurses’ Health Study (NHS; the NHS is an independent cohort established 15 y before the NHS-II, the cohort used in the current study). The NHS is an ongoing prospective cohort of 121,701 female registered nurses aged 30–55 y at enrollment in 1976 (15). Blood samples were collected from subpopulations of NHS participants (n = 32,826) in 1989–1990 (16). The goal of the EDIP was to empirically create an overall score to assess the inflammatory potential of whole diets defined by using food groups (14). The investigators calculated intakes of 39 previously defined food groups (24) and applied them in reduced rank regression (RRR) models to derive a dietary pattern predictive of 3 inflammatory markers that included CRP, IL-6, and TNFαR2 with the use of the 39 food groups as predictors. The first factor obtained by RRR underwent further data reduction in stepwise linear regression analyses to identify the most important component food groups of the RRR dietary pattern, with the RRR dietary pattern as the dependent variable, the 39 food groups as independent variables, and a significance level of P = 0.05 for entry into, and retention in, the model. Regression coefficients from the final step of the regression analyses were used as component weights (14).
The stepwise linear regression analyses identified 18 food groups that were weighted and summed for each participant to constitute the EDIP scores. The component food groups comprising the EDIP are as follows: intakes of processed meat, red meat, organ meat, fish (other than dark-meat fish), other vegetables (i.e., vegetables other than green-leafy vegetables and dark-yellow vegetables), refined grains, high-energy beverages (cola and other carbonated beverages with sugar, fruit drinks), low-energy beverages (low-energy cola and other low-energy carbonated beverages), and tomatoes were positively related to concentrations of the inflammatory markers. Intakes of beer, wine, tea, coffee, dark-yellow vegetables (comprising carrots, yellow squash, and sweet potatoes), green-leafy vegetables, snacks, fruit juice, and pizza were inversely related to concentrations of the inflammatory markers. EDIP food group components are described in detail in the footnote to Table 2. The validity of EDIP scores was evaluated in 2 independent US-based cohorts of women and men (14).
TABLE 2.
Participant characteristics across quintiles of the EDIP and the DII scores in women and men1
| EDIP2 |
DII3 |
|||||
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| NHS-II (n = 5826) | ||||||
| Score range4 | −3.34 to <−0.35 | −0.14 to <0.01 | 0.20 to 2.81 | −4.78 to <−0.26 | 0.87 to <1.97 | 3.27 to 6.55 |
| n | 1165 | 1173 | 1150 | 1169 | 1196 | 1224 |
| Inflammatory markers | ||||||
| Plasma CRP, mg/L | 0.6 ± 1.7 | 0.8 ± 1.6 | 1.1 ± 1.7 | 0.7 ± 1.7 | 0.7 ± 1.7 | 1.0 ± 1.7 |
| Plasma IL-6, pg/mL | 1.0 ± 1.1 | 1.0 ± 1.0 | 1.2 ± 1.0 | 1.0 ± 1.0 | 1.1 ± 1.0 | 1.2 ± 1.0 |
| Plasma TNFαR2, pg/mL | 2.1 ± 0.5 | 2.2 ± 0.5 | 2.3 ± 0.5 | 2.2 ± 0.6 | 2.2 ± 0.5 | 2.3 ± 0.5 |
| Plasma adiponectin, ng/mL | 7.1 ± 0.8 | 6.2 ± 0.8 | 5.3 ± 0.9 | 6.6 ± 0.9 | 6.1 ± 0.8 | 5.6 ± 0.8 |
| Alternative Healthy Eating Index–2010 | 57.6 ± 11.8 | 54.0 ± 10.4 | 45.4 ± 10.1 | 63.2 ± 10.4 | 51.7 ± 9.8 | 44.2 ± 8.8 |
| BMI, kg/m2 | 25.1 ± 5.3 | 26.4 ± 6.1 | 29.3 ± 7.5 | 25.5 ± 5.6 | 26.6 ± 6.2 | 28.0 ± 7.2 |
| Overweight/obese (BMI ≥25), % | 36.9 | 46.6 | 66.3 | 41.6 | 48.4 | 57.8 |
| Age, y | 45.0 ± 4.2 | 44.5 ± 4.5 | 44.1 ± 4.6 | 45.4 ± 4.2 | 44.4 ± 4.5 | 43.8 ± 4.5 |
| Physical activity, MET-h/wk | 21.8 ± 24.8 | 17.4 ± 19.7 | 15.0 ± 19.5 | 24.9 ± 25.8 | 17.4 ± 19.6 | 13.0 ± 17.2 |
| Current smokers, % | 11.2 | 6.7 | 8.5 | 6.3 | 7.1 | 13.9 |
| Regular aspirin/NSAID user, % | 20.5 | 19.1 | 20.2 | 22.9 | 17.7 | 19.9 |
| Chronic disease/conditions comorbidity score5 | ||||||
| No chronic disease/condition | 72.1 | 68.7 | 61.1 | 68.2 | 66.3 | 67.4 |
| 1 chronic disease/condition | 21.8 | 23.3 | 26.9 | 24.6 | 25.3 | 22.6 |
| 2 chronic diseases/conditions | 5.3 | 6.3 | 9.2 | 6.0 | 6.7 | 6.8 |
| ≥3 chronic diseases/conditions | 0.8 | 1.7 | 2.8 | 1.3 | 1.8 | 3.2 |
| Postmenopausal women, % | 21.0 | 24.2 | 25.3 | 26.3 | 21.6 | 20.6 |
| Postmenopausal hormone users, % | 35.0 | 37.0 | 38.9 | 41.3 | 34.6 | 34.5 |
| HPFS (n = 5227) | ||||||
| Score range4 | −1.22 to <−0.19 | −0.08 to <−0.01 | 0.08 to 1.42 | −4.49 to <−1.25 | −0.28 to <0.71 | 1.98 to 5.93 |
| n | 1057 | 1031 | 1051 | 1027 | 1006 | 1081 |
| Inflammatory markers | ||||||
| Plasma CRP, mg/L | 0.7 ± 1.4 | 0.8 ± 1.3 | 1.0 ± 1.3 | 0.8 ± 1.3 | 0.8 ± 1.4 | 1.0 ± 1.3 |
| Plasma IL-6, pg/mL | 1.3 ± 1.0 | 1.4 ± 1.1 | 1.5 ± 1.1 | 1.3 ± 1.0 | 1.4 ± 1.1 | 1.5 ± 1.1 |
| Plasma TNFαR2, pg/mL | 2.5 ± 0.5 | 2.7 ± 0.6 | 2.8 ± 0.6 | 2.6 ± 0.6 | 2.7 ± 0.6 | 2.7 ± 0.5 |
| Plasma adiponectin, ng/mL | 6.7 ± 0.8 | 6.3 ± 0.8 | 5.8 ± 0.8 | 6.5 ± 0.8 | 6.1 ± 0.8 | 6.1 ± 0.8 |
| Alternative Healthy Eating Index–2010 | 72.2 ± 10.0 | 69.5 ± 10.3 | 65.5 ± 11.1 | 76.5 ± 7.4 | 70.1 ± 9.1 | 60.0 ± 10.6 |
| BMI, kg/m2 | 25.7 ± 3.2 | 26.1 ± 3.7 | 27.0 ± 4.0 | 25.7 ± 3.6 | 26.2 ± 3.6 | 26.7 ± 3.8 |
| Overweight/obese (BMI ≥25), % | 54.4 | 58.1 | 68.8 | 51.3 | 60.3 | 65.7 |
| Physical activity, MET-h/wk | 36.2 ± 36.0 | 40.1 ± 45.3 | 33.2 ± 38.2 | 41.7 ± 39.5 | 37.3 ± 40.9 | 29.5 ± 36.2 |
| Age at blood draw, y | 61.8 ± 8.5 | 63.7 ± 8.5 | 63.2 ± 8.8 | 64.1 ± 8.2 | 63.4 ± 8.7 | 61.2 ± 8.8 |
| Current smokers, % | 8.0 | 5.9 | 6.6 | 2.9 | 5.5 | 14.6 |
| Regular aspirin/NSAID user, % | 16.2 | 12.6 | 12.6 | 10.8 | 14.5 | 13.7 |
| Chronic disease/conditions comorbidity score5 | ||||||
| No chronic disease/condition | 40.9 | 38.3 | 33.9 | 33.6 | 33.5 | 45.1 |
| 1 chronic disease/condition | 33.3 | 33.5 | 33.2 | 32.7 | 34.6 | 32.9 |
| 2 chronic diseases/conditions | 16.3 | 17.6 | 19.6 | 19.6 | 19.9 | 14.1 |
| ≥3 chronic diseases/conditions | 9.6 | 10.7 | 13.3 | 14.1 | 12.0 | 7.9 |
For all 4 inflammatory markers, values are geometric means ± CVs; other values are means ± SDs or percentages as indicated. The Quan-Zhang formula (29), CV = (eSD − 1)1/2, was used to calculate CVs. Biomarker sample sizes were different for each biomarker in each cohort, as follows—in the NHS-II: n = 3288 for CRP, n = 3000 for IL-6, n = 3258 for TNFαR2, and n = 3985 for adiponectin; in the HPFS: n = 4883 for CRP, n = 2923 for IL-6, n = 3912 for TNFαR2, and n = 4176 for adiponectin. CRP, C-reactive protein; DII, dietary inflammatory index; EDIP, empirical dietary inflammatory pattern; HPFS, Health Professionals Follow-Up Study; MET-h, metabolic equivalent task hour; NHS-II, Nurses’ Health Study II; NSAID, nonsteroidal anti-inflammatory drug; Q, quintile; TNFαR2, TNF-α receptor 2.
The food groups (including serving size per day for specific foods) in the EDIP were defined as follows: other fish [3–5 ounces (70–117 g) of canned tuna, shrimp, lobster, scallops, fish, and seafood other than dark-meat fish); tomatoes [1 fresh tomato, 1 small glass of tomato juice, 0.5 cup (115 g) tomato sauce]; high-energy beverages (1 glass, 1 bottle, or 1 can of cola with sugar; other carbonated beverages with sugar; fruit punch drinks); red meat [4–6 ounces (113–170 g) beef, pork, or lamb; 1 patty of hamburger]; low-energy beverages (1 glass, 1 bottle, or 1 can of low-energy cola; other low-energy carbonated beverages); refined grains [1 slice of white bread, 1 English muffin, 1 bagel or roll, 1 muffin or biscuit, 1 cup (250 g) white rice, 1 cup (140 g) pasta, 1 serving of pancakes or waffles]; organ meat [4 ounces (113 g) beef, calf, or pork liver; 1 ounce (28.3 g) chicken or turkey liver]; 2 slices pizza; wine [4-ounce (113 g) glass of red wine or white wine]; green-leafy vegetables (0.5 cup spinach, serving of iceberg or head lettuce, serving of romaine or leaf lettuce); dark-yellow vegetables [0.5 cup carrots, 0.5 cup yellow (winter) squash, 0.5 cup (100 g) yams, sweet potatoes]; beer (1 bottle, 1 glass, 1 can); 1 cup coffee; fruit juices (1 small glass of apple juice or cider, orange juice, grapefruit juice, or other fruit juice); snacks [1 small bag or 1 ounce (28.3 g) potato chips, corn chips, or popcorn; 1 cracker]; 1 cup tea (not herbal); processed meat (1 piece or 1 slice of processed meat, 2 slices bacon, 1 hot dog); other vegetables [4-inch (10.2 cm) stick of celery; 1 fresh, cooked, or canned mushroom; one-half of a green pepper; 1 ear or 0.5 cup (90 g) frozen or canned corn; 0.5 cup (75 g) mixed vegetables; 1 eggplant; 0.5 cup (90 g) zucchini; 0.5 cup (16 g) alfalfa sprouts; one-quarter of a cucumber].
The DII components available in the NHS-II and the HPFS FFQs are as follows: alcohol, vitamin B-6, vitamin B-12, β-carotene, protein, carbohydrates, cholesterol, total energy, saturated fat, total fat, dietary fiber, folic acid, selenium, thiamin, iron, trans fats, monounsaturated fat, polyunsaturated fat, omega-3 fat, omega-6 fat, riboflavin, niacin, caffeine, magnesium, zinc, isoflavones, flavan-3-ol, flavones, flavonols, flavonones, anthocyanidins, green and black tea, garlic, onion, and vitamins A, C, D, and E. The following components that are not available in the NHS-II and the HPFS FFQs were not included in the DII calculation: turmeric, thyme or oregano, hot pepper, rosemary, eugenol, ginger, and saffron.
For the index quintiles, lower (more negative) EDIP and DII scores indicate anti-inflammatory diets, whereas higher (more positive) scores indicate proinflammatory diets.
Chronic diseases/conditions included in the score (presence = 1, absence = 0) were as follows: hypercholesterolemia, cancer, diabetes, high blood pressure, heart disease, and rheumatoid or other arthritis.
Description of the DII score.
The DII was developed with the goal of creating a score for the inflammatory potential of the overall diet on the basis of dietary factors that have been shown to influence concentrations of 6 inflammatory markers: IL-1β, IL-4, IL-6, IL-10, TNF-α, and CRP. Details of the development (10) and validation (11–13) of the DII are described elsewhere. Briefly, a systematic review of the literature on the relation between diet and inflammatory markers was conducted, and 1943 articles were identified and scored. The search found 45 dietary factors to be associated with ≥1 of the 6 inflammatory markers (10, 27). One of three possible values was assigned to each article on the basis of the effect of the particular dietary factor on an inflammatory marker: +1 if proinflammatory, 0 if no change in inflammatory marker, and −1 if anti-inflammatory. Articles were weighted by study design, with intervention studies assigned the highest weight of 10 and cell culture studies the lowest weight of 3. The weighted number of pro- and anti-inflammatory articles was divided by the total weighted number of articles, and an inflammatory effect score for each DII component was calculated by subtracting the anti-inflammatory fraction from the proinflammatory fraction, and then relating this to a composite database derived from 11 dietary data sets from different parts of the world. The mean and SD of each DII component from this database are used to standardize actual dietary intake data (10).
The 45 components of the DII are as follows—anti-inflammatory components: alcohol, β-carotene, caffeine, dietary fiber, folic acid, magnesium, thiamin, riboflavin, niacin, zinc, monounsaturated fat, polyunsaturated fat, omega-3 fat, omega-6 fat, selenium, isoflavones, flavan-3-ol, flavones, flavonols, flavonones, anthocyanidins, green or black tea, garlic, onion, and vitamins B-6, A, C, D, and E; proinflammatory components: vitamin B-12, iron, trans fat, carbohydrates, cholesterol, total energy intake, protein, saturated fat, and total fat. The following 7 components (all anti-inflammatory)—turmeric, thyme and oregano, hot pepper, rosemary, eugenol, ginger, and saffron—were not available in the NHS-II and HPFS FFQs and were not included. We therefore computed the DII on the basis of 38 of the 45 available dietary intake components (i.e., excluding supplemental forms of the available nutrients). We adjusted nutrients for energy intake by using the residual method (28) and used data on the mean, SD, and inflammatory effect scores from Shivappa et al. (10) to calculate the DII scores as follows: 1) we standardized dietary intake data by using the mean and SD of each DII component from Shivappa et al. (10) to calculate z scores and converted them to centered percentiles to normalize their distributions; 2) we then multiplied (i.e., weighted) the standardized dietary data by the corresponding inflammatory effect scores (i.e., the literature-derived weights) to obtain scores for each DII component; and 3) finally, we summed all 38 component scores to obtain the overall DII score for each individual (10).
CDII score.
Given that the DII is mainly nutrient-based and the EDIP is based exclusively on foods, both indexes may potentially contain independent information not available in the other; therefore, we created a composite index (CDII). We constructed the CDII as follows: participants were ranked on the basis of their continuous EDIP or DII scores separately. The 2 ranks for each participant were then summed to create the CDII as a continuous variable. The continuous CDII was then categorized into quintiles and used in analyses. As an alternative to ranks, we calculated z scores for each EDIP and DII score. The 2 z scores were summed for each participant to constitute the CDII score as a continuous variable, which was then categorized into quintiles.
Statistical analysis.
Spearman correlation coefficients among the dietary indexes and biomarkers were calculated. We described participants’ characteristics by using means ± SDs for continuous variables or geometric means ± CVs (29–31) for ln-transformed inflammatory markers, and frequencies (percentages) for categorical variables across quintiles of EDIP and DII scores. Biomarker concentrations were back-transformed (ex, where x is the ln-transformed biomarker concentration) to their original units (29, 30).
We took the following steps to compare the ability of the dietary indexes (EDIP, DII, and CDII) to predict inflammation. First, in age-adjusted and multivariable-adjusted linear regression analyses, we modeled the natural log of the inflammatory markers as the outcome and then back-transformed the result to obtain an estimate of the relative difference (as percentage change) in biomarker concentration between higher dietary index quintiles and the lowest quintile as the reference; separately for EDIP, DII, and CDII. Second, for each inflammatory marker, we included the EDIP and DII in the same model to determine associations of each dietary index with that marker, independent of the effects of the other index. Third, we used R2 to estimate the proportion of total variance in biomarker concentration explained by the separate models for the EDIP, DII, and CDII for each of the 4 biomarkers. All of the multivariable models were adjusted for the following potential confounding variables: age at blood draw, physical activity, smoking status (never, former, or current), total energy intake, case-control status, an inflammation-related chronic disease comorbidity score, and menopausal status and hormone use in women. We did not adjust for BMI in the primary analyses because BMI has been shown to mediate the association between diet quality and circulating inflammatory markers on the basis of its inflammatory potential (14). However, we conducted sensitivity analyses stratified by BMI categories (<25 and ≥25) to examine associations between the dietary indexes and inflammatory markers, limiting potential confounding or mediation by BMI. Alcohol is an anti-inflammatory component in both the EDIP and DII, and given that alcohol and smoking are positively correlated (32, 33), an anti-inflammatory dietary group may be contaminated by current smokers. Therefore, in another sensitivity analysis, we excluded current smokers from the models.
For analyses of linear trend, we entered each index as a continuous variable into the multivariable-adjusted models and interpreted the P value of the continuous dietary index as the P value for linear trend. All of the analyses were conducted by using SAS version 9.4 for UNIX (SAS Institute). All of the tests were 2-sided, and 95% CIs not including 0 were considered to indicate significant findings.
Results
Participant characteristics by EDIP and DII quintiles are shown in Table 2. Lower EDIP and DII scores indicate anti-inflammatory (higher quality) diets, whereas higher scores indicate more proinflammatory (lower quality) diets. Women and men who consumed the most anti-inflammatory diets (quintile 1 of the EDIP and DII) reported higher physical activity levels, lower BMI, and lower (higher for adiponectin) concentrations of plasma inflammatory markers than did those who consumed the most proinflammatory diets (quintile 5). The proportion of current smokers increased monotonically across DII quintiles in both women and men, whereas the proportion of current smokers was high in the lowest EDIP quintile in both women and men. Although the proportions of overweight or obese women and men increased across quintiles of both indexes, approximately two-thirds of participants in the highest EDIP and DII quintiles were overweight or obese (BMI ≥25) (Table 2). Spearman correlations between the EDIP and DII were modest (r = 0.29 in women and 0.21 in men; all P < 0.0001), but both indexes were highly correlated with the CDII (r > 0.75) (Table 3).
TABLE 3.
Spearman correlation coefficients between the dietary inflammatory indexes and plasma inflammatory markers in women and men1
| EDIP | DII | CDII | |
| NHS-II (n = 5826) | |||
| EDIP | 1 | 0.29 | 0.79 |
| DII | 0.29 | 1 | 0.81 |
| CDII | 0.79 | 0.81 | 1 |
| CRP | 0.14 | 0.09 | 0.14 |
| IL-6 | 0.14 | 0.09 | 0.14 |
| TNFαR2 | 0.11 | 0.07 | 0.11 |
| Adiponectin | −0.20 | −0.10 | −0.18 |
| HPFS (n = 5227) | |||
| EDIP | 1 | 0.21 | 0.76 |
| DII | 0.21 | 1 | 0.79 |
| CDII | 0.76 | 0.79 | 1 |
| CRP | 0.11 | 0.08 | 0.11 |
| IL-6 | 0.09 | 0.07 | 0.10 |
| TNFαR2 | 0.12 | 0.02* | 0.09 |
| Adiponectin | −0.11 | −0.05 | −0.10 |
Biomarker sample sizes were different for each biomarker in each cohort, as follows—in the NHS-II: n = 3288 for CRP, n = 3000 for IL-6, n = 3258 for TNFαR2, and n = 3985 for adiponectin; in the HPFS: n = 4883 for CRP, n = 2923 for IL-6, n = 3912 for TNFαR2, and n = 4176 for adiponectin. *P > 0.05. CDII, composite dietary inflammatory index derived by summing ranks of EDIP and DII for each participant; CRP, C-reactive protein; DII, literature-derived dietary inflammatory index; EDIP, empirical dietary inflammatory pattern; HPFS, Health Professionals Follow-Up Study; NHS-II, Nurses’ Health Study II; TNFαR2, TNF-α receptor 2.
In both women and men, higher index scores generally were associated with higher concentrations of inflammatory markers, although they were associated with lower adiponectin concentrations and there was no association between the DII and plasma adiponectin in men (Tables 4 and 5). For example, the percentage changes (95% CIs) in concentrations of CRP among women in index quintile 5 relative to quintile 1 were higher by 60% (35%, 91%; P-trend < 0.0001) for the EDIP, by 49% (25%, 77%; P-trend < 0.0001) for the DII, and by 78% (49%, 111%; P-trend < 0.0001) for the CDII (Table 4). Corresponding results in men were as follows: 38% (24%, 55%; P-trend < 0.0001), 29% (15%, 44%; P-trend < 0.0001), and 46% (31%, 63%; P-trend < 0.0001) for the EDIP, DII, and CDII, respectively (Table 5). In both women and men, associations between the CDII and all 4 biomarkers were of higher magnitudes than the associations between the DII and biomarkers, although the differences were more pronounced among women. The CDII also showed higher magnitudes of associations than the EDIP in the CRP and IL-6 models. For example, the percentage changes in concentrations of IL-6 in CDII quintile 5 compared with quintile 1 were 5.4% higher in women and 10.2% higher in men when compared with the EDIP models and 6.9% higher in women and 2.4% higher in men when compared with the DII models. Associations were of similar magnitude for the CDII and EDIP in the TNFαR2 models in women and men and adiponectin models in women, but the magnitude of the association between the EDIP and adiponectin was higher than for the CDII in men. In addition, the magnitude of the associations between the EDIP and biomarkers was higher than for DII in all biomarker models, with the exception of the IL-6 model in men. For example, the percentage changes in concentrations of biomarkers in quintile 5 compared with quintile 1 were as follows—for the EDIP and DII, respectively: CRP, +60% and +49%; IL-6, +23% and +21%; TNFαR2, +7% and +4%; and adiponectin, −21% and −14% in women (Table 4); and CRP, +38% and +29%; IL-6, +14% and +24%; TNFαR2, +9% and +5%; and adiponectin, −16% and −4% in men (Table 5). Results for the CDII with the use of z scores were not materially different than for the version created by using ranks (data not shown).
TABLE 4.
Percentage changes (95% CIs) in relative concentrations of plasma inflammatory markers across quintiles of dietary indexes in NHS-II participants1
| Index quintile |
||||||
| 1 | 2 | 3 | 4 | 5 | P-trend2 | |
| EDIP | ||||||
| Plasma CRP (n = 3258), n | 657 | 658 | 658 | 658 | 657 | |
| Age-adjusted | 0 | +10% (−7%, +31%) | +25% (+5%, +48%) | +54% (+30%, +84%) | +72% (+44%, +104%) | <0.0001 |
| Multivariable-adjusted | 0 | +12% (−6%, +33%) | +28% (+8%, +52%) | +52% (+28%, +80%) | +60% (+35%, +91%) | <0.0001 |
| Plasma IL-6 (n = 3000), n | 600 | 600 | 600 | 600 | 600 | |
| Age-adjusted | 0 | +4% (−6%, +15%) | +6% (−4%, +17%) | +21% (+10%, +34%) | +26% (+14%, +39%) | <0.0001 |
| Multivariable-adjusted | 0 | +6% (−4%, +17%) | +8% (−2%, +19%) | +21% (+9%, +34%) | +23% (+12%, +37%) | <0.0001 |
| Plasma TNFαR2 (n = 3288), n | 651 | 652 | 652 | 652 | 651 | |
| Age-adjusted | 0 | +2% (−2%, +5%) | +1% (−2%, +5%) | +6% (+5%, +12%) | +8% (+2%, +9%) | <0.0001 |
| Multivariable-adjusted | 0 | +2% (−2%, +5%) | +2% (−2%, +5%) | +5% (+2%, +9%) | +7% (+3%, +10%) | <0.0001 |
| Plasma adiponectin (n = 3985), n | 797 | 797 | 797 | 797 | 797 | |
| Age-adjusted | 0 | −5% (−11%, +2%) | −12% (−18%, −6%) | −21% (−26%, −15%) | −25% (−29%, −19%) | <0.0001 |
| Multivariable-adjusted | 0 | −5% (−10%, +2%) | −12% (−18%, −6%) | −19% (−24%, −14%) | −21% (−26%, −16%) | <0.0001 |
| DII | ||||||
| Plasma CRP (n = 3288), n | 657 | 658 | 658 | 658 | 657 | |
| Age-adjusted | 0 | +16% (−3%, +38%) | +8% (−9%, +29%) | +29% (+8%, +53%) | +56% (+31%, +86%) | <0.0001 |
| Multivariable-adjusted | 0 | +14% (−3%, +36%) | +8% (−9%, +28%) | +25% (+5%, +48%) | +49% (+25%, +77%) | <0.0001 |
| Plasma IL-6 (n = 3000), n | 600 | 600 | 600 | 600 | 600 | |
| Age-adjusted | 0 | +3% (−6%, +15%) | +8% (−2%, +19%) | +9% (−1%, +21%) | +24% (+12%, +37%) | <0.0001 |
| Multivariable-adjusted | 0 | +3% (−6%, +14%) | +7% (−4%, +18%) | +7% (−3%, +19%) | +21% (+9%, +33%) | <0.0001 |
| Plasma TNFαR2 (n = 3258), n | 651 | 652 | 652 | 652 | 651 | |
| Age-adjusted | 0 | +1% (−3%, +4%) | 1% (−2%, +5%) | +2% (−1%, 6%) | +5% (+2%, +9%) | <0.0001 |
| Multivariable-adjusted | 0 | 0% (−3%, +4%) | 1% (−2%, +4%) | +2% (−2%, 5%) | +4% (+1%, +8%) | 0.0002 |
| Plasma adiponectin (n = 3985), n | 797 | 797 | 797 | 797 | 797 | |
| Age-adjusted | 0 | −4% (−10%, +3%) | −8% (−14%, −2%) | −9% (−15%, −2%) | −14% (−19%, −8%) | <0.0001 |
| Multivariable-adjusted | 0 | −2% (−8%, +4%) | −7% (−13%, −1%) | −7% (−13%, 0%) | −10% (−10%, −4%) | <0.0001 |
| CDII3 | ||||||
| Plasma CRP (n = 3288), n | 657 | 658 | 658 | 658 | 657 | |
| Age-adjusted | 0 | +19% (0%, +41%) | +37% (+15%, +63%) | +49% (+26%, +78%) | +91% (+61%, 128%) | <0.0001 |
| Multivariable-adjusted | 0 | +18% (0%, +40%) | +33% (+13%, +58%) | +44% (+21%, +70%) | +78% (+49%, 111%) | <0.0001 |
| Plasma IL-6 (n = 3000), n | 600 | 600 | 600 | 600 | 600 | |
| Age-adjusted | 0 | +7% (−3%, +19%) | +17% (+6%, +29%) | +18% (+7%, +30%) | +34% (+21%, +48%) | <0.0001 |
| Multivariable-adjusted | 0 | +7% (−3%, +18%) | +15% (+4%, +27%) | +16% (+5%, +28%) | +30% (+17%, +43%) | <0.0001 |
| Plasma TNFαR2 (n = 3258), n | 651 | 652 | 653 | 650 | 652 | |
| Age-adjusted | 0 | +3% (0%, +6%) | +5% (+2%, +9%) | +6% (+2%, +9%) | +10% (+6%, +13%) | <0.0001 |
| Multivariable-adjusted | 0 | +3% (0%, +6%) | +5% (+1%, +9%) | +5% (+1%, +8%) | +8% (+5% to +12%) | <0.0001 |
| Plasma adiponectin (n = 3985), n | 796 | 798 | 797 | 797 | 797 | |
| Age-adjusted | 0 | −10% (−15%, −3%) | −15% (−20%, −9%) | −20% (−25%, −14%) | −25% (−30% to −20%) | <0.0001 |
| Multivariable-adjusted | 0 | −8% (−14%, −2%) | −12% (−18%, −6%) | −17% (−23%, −12%) | −21% (−26% to −16%) | <0.0001 |
Values are percentage changes in concentrations of biomarkers (i.e., the relative differences in biomarker concentrations between higher dietary index quintiles relative to quintile 1 as the reference; e.g., biomarker concentration in quintile 5 minus the concentration in quintile 1) unless otherwise indicated; n = 5826. All values were back-transformed (ex), because biomarker data were ln-transformed before analyses. For the index quintiles, lower (more negative) EDIP and DII scores indicate anti-inflammatory diets, whereas higher (more positive) scores indicate proinflammatory diets. All multivariable-adjusted models were adjusted for age at blood draw, physical activity, smoking status, case-control status, regular aspirin/nonsteroidal anti-inflammatory drug use, menopausal status, postmenopausal hormone use, and an inflammation-related chronic disease comorbidity score. Chronic diseases/conditions included in the score (presence = 1, absence = 0) were hypercholesterolemia, cancer, diabetes, high blood pressure, heart disease, and rheumatoid or other arthritis. CDII, composite dietary inflammatory index derived by summing ranks of EDIP and DII for each participant; CRP, C-reactive protein; DII, literature-derived dietary inflammatory index; EDIP, empirical dietary inflammatory pattern; NHS-II, Nurses’ Health Study II; TNFαR2, TNF-α receptor 2.
P-trend values represent the P values of the dietary index as a continuous variable adjusted for all covariates listed in footnote 1.
The CDII was constructed by creating ranks for each dietary index and summing the ranks of both indexes for each individual into a composite score that was then categorized into quintiles.
TABLE 5.
Percentage changes (95% CIs) in concentrations of plasma inflammatory markers across quintiles of dietary indexes in HPFS participants1
| Index quintile |
||||||
| 1 | 2 | 3 | 4 | 5 | P-trend2 | |
| EDIP | ||||||
| Plasma CRP (n = 4883), n | 976 | 977 | 977 | 977 | 976 | |
| Age-adjusted | 0 | +5% (−6%, +18%) | +13% (+1%, +27%) | +15% (+3%, +29%) | +38% (+23%, +54%) | <0.0001 |
| Multivariable-adjusted | 0 | +5% (−6%, +18%) | +17% (+4%, +30%) | +18% (+6%, +32%) | +38% (+24%, +55%) | <0.0001 |
| Plasma IL-6 (n = 2923), n | 584 | 585 | 585 | 585 | 584 | |
| Age-adjusted | 0 | −4% (−14%, +6%) | +1% (−9%, +12%) | +8% (−3%, +20%) | +13% (+2%, +26%) | 0.0006 |
| Multivariable-adjusted | 0 | −4% (−14%, +6%) | +2% (−8%, +14%) | +9% (−2%, +21%) | +14% (+2%, +26%) | 0.0003 |
| Plasma TNFαR2 (n = 3912), n | 782 | 783 | 782 | 783 | 782 | |
| Age-adjusted | 0 | +2% (−1%, +6%) | +4% (0%, +7%) | +5% (+2%, +8%) | +9% (+6%, +13%) | <0.0001 |
| Multivariable-adjusted | 0 | +2% (−1%, +6%) | +4% (0%, +7%) | +5% (+2%, +8%) | +9% (+5%, +12%) | <0.0001 |
| Plasma adiponectin (n = 4176), n | 835 | 835 | 836 | 835 | 835 | |
| Age-adjusted | 0 | −6% (−11%, −1%) | −9% (−14%, −3%) | −10% (−15%, −5%) | −16% (−21%, −11%) | <0.0001 |
| Multivariable-adjusted | 0 | −6% (−11%, 0%) | −9% (−14%, −3%) | −10% (−15%, −5%) | −16% (−20%, −10%) | <0.0001 |
| DII | ||||||
| Plasma CRP (n = 4883), n | 976 | 977 | 977 | 977 | 976 | |
| Age-adjusted | 0 | +2% (−9%, +14%) | +15% (+3%, +29%) | +13% (+1%, +26%) | +33% (+19%, +49%) | <0.0001 |
| Multivariable-adjusted | 0 | +1% (−9%, +13%) | +13% (+1%, +26%) | +13% (+1%, +26%) | +29% (+15%, +44%) | <0.0001 |
| Plasma IL-6 (n = 2923), n | 584 | 585 | 585 | 585 | 584 | |
| Age-adjusted | 0 | +5% (−6%, +16%) | +14% (+2%, +26%) | +13% (+2%, +25%) | +28% (+15%, +42%) | <0.0001 |
| Multivariable-adjusted | 0 | +4% (−6%, +16%) | +11% (0%, +23%) | +12% (+1%, +25%) | +24% (+12%, +38%) | <0.0001 |
| Plasma TNFαR2 (n = 3912), n | 782 | 783 | 782 | 783 | 782 | |
| Age-adjusted | 0 | +1% (−2%, +5%) | +2% (−1%, +5%) | +4% (+1%, +8%) | +5% (+2%, +8%) | <0.0001 |
| Multivariable-adjusted | 0 | +1% (−2%, +5%) | +2% (−2%, +5%) | +4% (+1%, +8%) | +5% (+1%, +8%) | <0.0001 |
| Plasma adiponectin (n = 4176), n | 835 | 835 | 836 | 835 | 835 | |
| Age-adjusted | 0 | −3% (−8%, +3%) | −6% (−11%, 0%) | −4% (−9%, +2%) | −3% (−9%, +2%) | 0.23 |
| Multivariable-adjusted | 0 | −3% (−8%, +3%) | −6% (−11%, 0%) | −4% (−10%, +1%) | −4% (−9%, +2%) | 0.13 |
| CDII3 | ||||||
| Plasma CRP (n = 4883), n | 976 | 977 | 978 | 975 | 977 | |
| Age-adjusted | 0 | +13% (+1%, +27%) | +17% (+5%, +31%) | +24% (+11%, +39%) | +48% (+32%, +66%) | <0.0001 |
| Multivariable-adjusted | 0 | +12% (+1%, +25%) | +16% (+4%, +30%) | +24% (+11%, +38%) | +46% (+31%, +63%) | <0.0001 |
| Plasma IL-6 (n = 2923), n | 584 | 585 | 586 | 584 | 584 | |
| Age-adjusted | 0 | +7% (−3%, +19%) | +3% (−7%, +15%) | +16% (+4%, +29%) | +28% (+15%, +42%) | <0.0001 |
| Multivariable-adjusted | 0 | +7% (−4%, +18%) | +2% (−8%, +13%) | +15% (+4%, +28%) | +27% (+14%, +41%) | <0.0001 |
| Plasma TNFαR2 (n = 3912), n | 782 | 783 | 782 | 783 | 782 | |
| Age-adjusted | 0 | +1% (−2%, +4%) | +4% (+1%, +7%) | +4% (+1%, +8%) | +9% (+6%, +12%) | <0.0001 |
| Multivariable-adjusted | 0 | +1% (−2%, +4%) | +4% (+1%, +7%) | +4% (+1%, +7%) | +9% (+5%, +12%) | <0.0001 |
| Plasma adiponectin (n = 4176), n | 835 | 835 | 836 | 835 | 835 | |
| Age-adjusted | 0 | −4% (−9%, +2%) | −5% (−10%, 0%) | −11% (−14%, −6%) | −10% (−15%, −4%) | <0.0001 |
| Multivariable-adjusted | 0 | −4% (−9%, +2%) | −5% (−11%, 0%) | −11% (−16%, −6%) | −10% (−15%, −4%) | <0.0001 |
Values are percentage changes in concentrations of biomarkers (i.e., the relative differences in biomarker concentrations between higher dietary index quintiles relative to quintile 1 as the reference; e.g., biomarker concentration in quintile 5 minus the concentration in quintile 1); n = 5227. All values were back-transformed (ex), because biomarker data were ln-transformed before analyses. For the index quintiles, lower (more negative) EDIP and DII scores indicate anti-inflammatory diets, whereas higher (more positive) scores indicate proinflammatory diets. All multivariable-adjusted models were adjusted for age at blood draw, physical activity, smoking status, case-control status, regular aspirin/nonsteroidal anti-inflammatory drug use, menopausal status, postmenopausal hormone use, and an inflammation-related chronic disease comorbidity score. Chronic diseases/conditions included in the score (presence = 1, absence = 0) were hypercholesterolemia, cancer, diabetes, high blood pressure, heart disease, and rheumatoid or other arthritis. CDII, composite dietary inflammatory index derived by summing ranks of EDIP and DII for each participant; CRP, C-reactive protein; DII, literature-derived dietary inflammatory index; EDIP, empirical dietary inflammatory pattern; HPFS, Health Professionals Follow-Up Study; TNFαR2, TNF-α receptor 2.
P-trend values represent the P values of the dietary index as a continuous variable adjusted for all covariates listed in footnote 1.
The CDII was constructed by creating ranks for each dietary index and summing the ranks of both indexes for each individual into a composite score that was then categorized into quintiles.
When adjusted for DII scores in women, the magnitudes of the associations between the EDIP and CRP and IL-6 were attenuated (compared with corresponding results in Table 4) by −6.3% and −3.3%, respectively, in EDIP quintile 5, whereas there was no important change in the magnitude of association in the TNFαR2 and adiponectin models (Table 6). When adjusted for EDIP scores, the percentages of attenuation in the relative concentrations of biomarkers in the DII models were as follows: CRP, −13.7%; IL-6, −5.2%; adiponectin, −6.3%; and no material change in TNFαR2 concentration (Table 6). After adjusting for DII in men, the magnitudes of the associations between EDIP scores and CRP and IL-6 concentrations were attenuated (compared with corresponding results in Table 4) by −3.6% and −3.5%, respectively, in EDIP quintile 5, whereas there was no major change in the magnitude of association in the TNFαR2 and adiponectin models (Table 6). The percentages of attenuation in the relative concentrations of biomarkers in the DII models when adjusting for EDIP were as follows: CRP, −7.5%; IL-6, −5.6%; adiponectin, −6.3%, and no material change in TNFαR2 concentration (Table 6).
TABLE 6.
Percentage changes (95% CIs) in the concentrations of plasma inflammatory markers in quintiles of the dietary indexes adjusted for the effects of the other index (EDIP or DII)1
| Index quintile |
||||||
| 1 | 2 | 3 | 4 | 5 | P-trend2 | |
| NHS-II (n = 5826) | ||||||
| EDIP | ||||||
| CRP | 0 | +11% (−6%, +32%) | +26% (+6%, +49%) | +46% (+22%, +73%) | +50% (+25%, +79%) | <0.0001 |
| IL-6 | 0 | +6% (−6%, +17%) | +7% (−3%, +19%) | +19% (+8%, +32%) | +19% (+8%, +32%) | <0.0001 |
| TNFαR2 | 0 | +2% (−2%, +5%) | +2 (−2%, +5%) | +5% (+2%, +9%) | +6% (+3%, +10%) | <0.0001 |
| Adiponectin | 0 | −4% (−10%, +2%) | −12% (−17%, −6%) | −19% (−24%, −13%) | −21% (−26%, −15%) | <0.0001 |
| DII | ||||||
| CRP | 0 | +10% (−7%, +30%) | +1% (−15%, +20%) | +15% (−3%, +36%) | +31% (+10%, +57%) | <0.0001 |
| IL-6 | 0 | +2% (−8%, +12%) | +4% (−6%, +14%) | +4% (−6%, +15%) | +15% (+3%, +27%) | 0.002 |
| TNFαR2 | 0 | 0% (−3%, +3%) | 0% (−3%, +3%) | 0% (−3%, +4%) | +3% (−1%, +6%) | 0.04 |
| Adiponectin | 0 | +1% (−6%, +7%) | −3% (−10%, +3) | −1% (−8%, +5%) | −4% (−10%, +3%) | 0.009 |
| HPFS (n = 5227) | ||||||
| EDIP | ||||||
| CRP | 0 | +4% (−6%, +17%) | +15% (+3%, +28%) | +15% (+3%, +29%) | +33% (+19%, +49%) | <0.0001 |
| IL-6 | 0 | −5% (−14%, +5%) | +1% (−9%, +12%) | +7% (−4%, +17%) | +10% (−1%, +22%) | 0.01 |
| TNFαR2 | 0 | +2% (−1%, +6%) | +3% (0%, +7%) | +4% (+1%, +8%) | +8% (+5%, +12%) | <0.0001 |
| Adiponectin | 0 | −6% (−11%, 0%) | −9% (−14%, −3%) | −10% (−15%, −5%) | −16% (−20%, −11%) | <0.0001 |
| DII | ||||||
| CRP | 0 | +3% (−8%, +15%) | +8% (−4%, +20%) | +13% (+1%, +26%) | +20% (+7%, +34%) | <0.0001 |
| IL-6 | 0 | +3% (−8%, +14%) | +9% (−1%, +21%) | +12% (+1%, +2%5) | +17% (+6%, +30%) | <0.0001 |
| TNFαR2 | 0 | +2% (−1%, +6%) | +2% (−1%, +5%) | +3% (0%, +6%) | +5% (+1%, +8%) | 0.002 |
| Adiponectin | 0 | +1% (−5%, +6%) | −1% (−7%, +5%) | −1% (−7%, +5%) | +2% (−4%, +8%) | 0.64 |
Values are percentage changes in concentrations of biomarkers (i.e., the relative differences in biomarker concentrations between higher dietary index quintiles relative to quintile 1 as the reference; e.g., biomarker concentration in quintile 5 minus the concentration in quintile 1). All values were back-transformed (ex), because biomarker data were ln-transformed before analyses. For the index quintiles, lower (more negative) EDIP and DII scores indicate anti-inflammatory diets, whereas higher (more positive) scores indicate proinflammatory diets. Biomarker sample sizes were different for each biomarker in each cohort, as follows—in the NHS-II: n = 3288 for CRP, n = 3000 for IL-6, n = 3258 for TNFαR2- and n = 3985 for adiponectin; in the HPFS: n = 4883 for CRP, n = 2923 for IL-6, n = 3912 for TNFαR2, and n = 4176 for adiponectin. All models were adjusted for EDIP, DII, age, physical activity, smoking status, case-control status, regular aspirin/nonsteroidal anti-inflammatory drug use, an inflammation-related chronic disease comorbidity score, and menopausal status and postmenopausal hormone use (women). Chronic diseases/conditions included in the score were hypercholesterolemia, cancer, diabetes, high blood pressure, heart disease, and rheumatoid or other arthritis. CDII, composite dietary inflammatory index derived by summing ranks of EDIP and DII for each participant; CRP, C-reactive protein; DII, literature-derived dietary inflammatory index; EDIP, empirical dietary inflammatory pattern; HPFS, Health Professionals Follow-Up Study; NHS-II, Nurses’ Health Study II; TNFαR2, TNF-α receptor 2.
P-trend values represent the P values of the dietary index as a continuous variable adjusted for all covariates listed in footnote 1.
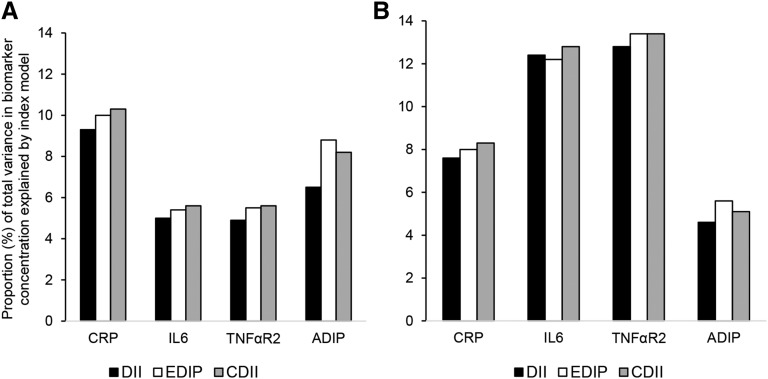
We qualitatively examined the proportions (percentages) of total variance in biomarker concentrations explained by the separate models for the EDIP, DII, and CDII, and the results closely reflected the patterns observed in the associations between the dietary indexes and biomarker concentrations described above in Tables 4 and 5. That is, the proportion of variance in biomarker concentrations explained by the CDII models was higher than for the DII in all 4 biomarker models and higher than the EDIP in the CRP and IL-6 models in both women and men and in the TNFαR2 model in women. Similarly, EDIP scores explained a higher proportion of variance in biomarker concentrations than did DII scores in both women and men, with the exception of the IL-6 model in men (Figure 1).
FIGURE 1.
The proportion of total variance in biomarker concentration explained by separate models for the food-based EDIP, the nutrient-based, literature-derived DII, and a CDII in women (A) and men (B). Models for the EDIP, DII, and CDII were adjusted for age at blood draw, physical activity, smoking status, case-control status, regular aspirin or nonsteroidal anti-inflammatory drug use, menopausal status, postmenopausal hormone use (in women), and an inflammation-related chronic disease comorbidity score. Chronic diseases or conditions included in the score (presence = 1, absence = 0) were hypercholesterolemia, cancer, diabetes, high blood pressure, heart disease, and rheumatoid or other arthritis. ADIP, adiponectin; CDII, composite dietary inflammatory index; CRP, C-reactive protein; DII, dietary inflammatory index; EDIP, empirical dietary inflammatory pattern; TNFαR2, TNF-α receptor 2.
In sensitivity analyses, there was no evidence of systematic differences in BMI strata in the associations for all 3 indexes (Supplemental Table 1). When current smokers were excluded, associations were stronger for CRP in the EDIP and CDII models in women; however, associations did not change materially for the other 3 biomarkers in women. In addition, there were no important changes in the associations for all 4 biomarkers in men (Supplemental Table 2).
Discussion
We calculated 2 indexes of dietary inflammatory potential—the EDIP, empirically derived and based exclusively on food groups, and the DII, which is literature-derived and based mainly on nutrients—and compared their inflammation-predictive ability with each other and with a composite index (CDII) derived by combining EDIP and DII scores. In both women and men, higher scores of all 3 dietary indexes were associated with higher (lower for adiponectin) concentrations of inflammatory markers (CRP, IL-6, TNFαR2, and adiponectin), with the exception of the null association between the DII and adiponectin in men. The magnitudes of the associations between the dietary indexes and biomarker concentrations for the CDII were higher than for the DII in all 4 biomarkers models and higher than the EDIP in the CRP and IL-6 models. Similarly, the associations between EDIP scores and biomarker concentrations were of higher magnitudes than DII scores in both women and men, with the exception of the IL-6 model in men.
The EDIP was recently developed and its validity evaluated in 2 independent cohorts of women and men who were different from the population in which the EDIP was developed (14). In the validation studies, the EDIP was found to significantly predict concentrations of inflammatory markers (14). The DII, in contrast, has been applied in many studies with inflammatory markers (11–13) and disease endpoints (34–36). Reasons for the differences in the associations of the EDIP and DII with IL-6 and adiponectin by sex are not clear, but previous DII studies also reported consistent and stronger associations between the DII and IL-6 compared with the DII’s association with CRP and TNFαR2 (11, 12, 37). In a study that used inflammatory marker data from ∼2600 women in the Women’s Health Initiative, higher DII scores were associated with significantly higher concentrations of IL-6 and TNFαR2 but not with concentrations of CRP after adjusting for multiple confounding factors (11). In another study that included ∼2500 participants, higher DII scores were associated with higher odds of high IL-6 concentrations (>1.6 pg/mL) in DII tertile 3 compared with tertile 1 but not with CRP concentrations (13). None of the previous studies that used the DII examined associations with adiponectin. Adiponectin is a hormone secreted by adipose tissue (38, 39), and concentrations of adiponectin are inversely associated with obesity and inflammation (38, 40). Although the DII was previously found to predict metabolic syndrome defined by standard criteria (37), the stronger associations of the EDIP with adiponectin, compared with the DII, may indicate that the EDIP may be more sensitive to dietary patterns predictive of hyperinsulinemia or insulin resistance in these study populations. However, neither the EDIP nor the DII was developed with the use of adiponectin.
Although the EDIP and DII both assess the inflammatory potential of diet, the modest correlation between the 2 indexes suggests that each index contains independent information not available in the other, and thus the CDII would be expected to have a higher predictive ability than the EDIP or DII examined individually. Although this was true for the DII in relation to concentrations of all 4 inflammatory markers, it was true for the EDIP in relation to concentrations of CRP and IL-6 only. The proportion of variance in biomarker concentrations and the magnitude of corresponding associations were the same for CDII and EDIP in TNFαR2 models and higher for the EDIP than the CDII in adiponectin models in both women and men. The differences in associations between the 3 dietary indexes and biomarker concentrations could support the multifactorial nature of chronic low-grade systemic inflammation in which different factors predict concentrations of different inflammatory markers, with few factors consistently predicting concentrations of most markers (41). The differences may also suggest that some dietary indexes may be better predictors of concentrations of some markers. In addition, the stronger association of the EDIP with inflammatory markers (especially adiponectin), even in BMI strata, suggests that the EDIP may be more sensitive (compared with the DII) to dietary factors or dietary patterns associated with obesity.
Our large population–based study is not without limitations. We had only 1 measurement of inflammatory markers, which may underestimate associations of the dietary indexes with the inflammatory markers (42). Some measurement error is inevitable in self-reported dietary intake, although previous studies in these cohorts that evaluated the relative validity of FFQ data have shown reasonably good correlations between FFQs and diet records, which suggests that dietary intake is generally well measured in our cohorts (21–23). The composition of food groups may not be uniform across studies, which would limit the ability to apply the EDIP across studies in a standardized manner, although investigators may be able to create unified food groups in pooled analyses of primary data or in multicenter studies (43, 44) and thus enhance the usefulness of this empirical hypothesis-oriented dietary index in large-scale epidemiologic research. In addition, calculation of the DII in the current study included 38 of the 45 original DII components. The 7 DII components missing from the FFQ, which included turmeric, thyme and oregano, hot pepper, rosemary, eugenol, ginger, and saffron (mainly food spices), are anti-inflammatory components of the DII. It is possible that they may contribute more to the DII in other populations, such as in South East Asia where these spices are consumed more frequently (45, 46). Other limitations may include the racial composition of study participants in both cohorts, which comprised mostly European-American health professionals, but the distributions of most participant characteristics are generally similar to those of the larger US multiracial/ethnic population (47). In addition, our biomarker data were pooled from both cases and controls; therefore, it is possible that case status may have influenced the results. However, the biomarker data were pooled from nested case-control studies in which participants donated blood before any disease development. Participants were also eligible for blood donation only if they were free of major chronic diseases such as cardiovascular disease, cancer, or diabetes. It is therefore unlikely that becoming a case for one of these diseases later during follow-up could have confounded associations between dietary exposures and biomarker concentrations. In addition, we adjusted for case-control status in the multivariable-adjusted models. We adjusted for a large number of potential confounding variables, but these variables were self-reported, thus allowing the possibility of residual confounding. However, results from the age-adjusted and multivariable-adjusted models were very similar in both women and men, which suggests that any confounding would have been minimal, although the possibility of confounding by unmeasured variables cannot be completely ruled out.
In summary, the human diet contains a highly complex mixture of chemicals, with some that are well characterized and that can be measured and others that are poorly understood or unknown and consequently not well measured or not measured at all (48). Capturing the complex interactions of nutrients and foods in whole diets is difficult, even with the increasing use of dietary patterns by researchers. In the current study, we used 2 different approaches to assess the inflammatory potential of diet—one focused mainly on nutrients identified from peer-reviewed articles on the basis of their biological activities in relation to inflammation (DII) and one that used RRR of food group intake and inflammatory markers (EDIP). Despite the differences in conception and design, the EDIP and DII both assess the inflammatory potential of the diet, because they both significantly predicted concentrations of inflammatory markers. Interestingly, the 2 dietary indexes were only modestly correlated, which suggests that they contain independent information not captured by the other, although the CDII combined this potential independent information yet did not perform better than the EDIP score, as we hypothesized. As such, both indexes may be useful in predicting inflammation-mediated diet-disease associations.
Acknowledgments
The authors’ responsibilities were as follows—FKT and ELG: designed the research; FKT: conducted the research, performed the statistical analysis, and wrote the manuscript; FKT, SAS-W, JEC, TTF, FBH, WCW, and ELG: analyzed and interpreted the data and provided critical input; ELG: provided the study oversight; and all authors: read and approved the final manuscript.
Footnotes
Abbreviations used: CDII, composite dietary inflammatory index; CRP, C-reactive protein; DII, literature-derived dietary inflammatory index; EDIP, empirical dietary inflammatory pattern; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHS-II, Nurses’ Health Study II; RRR, reduced rank regression; TNFαR2, TNF-α receptor 2.
References
- 1.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 2.Jacques PF, Tucker KL. Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr 2001;73:1–2. [DOI] [PubMed] [Google Scholar]
- 3.Krintus M, Kozinski M, Kubica J, Sypniewska G. Critical appraisal of inflammatory markers in cardiovascular risk stratification. Crit Rev Clin Lab Sci 2014;51:263–79. [DOI] [PubMed] [Google Scholar]
- 4.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107. [DOI] [PubMed] [Google Scholar]
- 5.Guina T, Biasi F, Calfapietra S, Nano M, Poli G. Inflammatory and redox reactions in colorectal carcinogenesis. Ann N Y Acad Sci 2015;1340:95–103. [DOI] [PubMed] [Google Scholar]
- 6.Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The Dietary Approaches to Stop Hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr 2011;141:1083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets 2014;14:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nanri A, Moore MA, Kono S. Impact of C-reactive protein on disease risk and its relation to dietary factors: literature review. Asian Pac J Cancer Prev 2007;8:167–77. [PubMed] [Google Scholar]
- 9.Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev 2013;71:511–27. [DOI] [PubMed] [Google Scholar]
- 10.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014;17:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, Hou L, Hurley TG, Hingle M, Jiao L, et al. . Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol 2015;25:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung FK, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr 2014;17:1825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivappa N, Hébert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Marcos A, Huybrechts I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr 2015;113:665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs SF, Hu FB, Chan AT, Willett WC, Giovannucci EL. Development and validation of an empirical index of dietary inflammatory potential. J Nutr 2016;146:1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer 2005;5:388–96. [DOI] [PubMed] [Google Scholar]
- 16.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, et al. . Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 2004;351:2599–610. [DOI] [PubMed] [Google Scholar]
- 17.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, Longcope C, Speizer FE. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst 1995;87:1297–302. [DOI] [PubMed] [Google Scholar]
- 18.Hankinson SE, Willett WC, Manson JE, Colditz GA, Hunter DJ, Spiegelman D, Barbieri RL, Speizer FE. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 1998;90:1292–9. [DOI] [PubMed] [Google Scholar]
- 19.Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer: does the association vary by a woman’s predicted breast cancer risk? J Clin Oncol 2006;24:1823–30. [DOI] [PubMed] [Google Scholar]
- 20.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol 2008;167:653–66. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 22.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 23.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26. [DOI] [PubMed] [Google Scholar]
- 24.Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food frequency questionnaire. Am J Clin Nutr 1999;69:243–9. [DOI] [PubMed] [Google Scholar]
- 25.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology 1996;7:81–6. [DOI] [PubMed] [Google Scholar]
- 26.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9. [DOI] [PubMed] [Google Scholar]
- 27.Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hébert JR. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr 2009;139:2365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 29.Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ 1996;312:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Statistics notes: logarithms. BMJ 1996;312:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan H, Zhang J. Estimate of standard deviation for a log-transformed variable using arithmetic means and standard deviations. Stat Med 2003;22:2723–36. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Wang K, Maisonet M, Wang L, Zheng S. Associations of lifestyle factors (smoking, alcohol consumption, diet and physical activity) with type 2 diabetes among American adults from National Health and Nutrition Examination Survey (NHANES) 2005–2014. J Diabetes 2016. Oct 17 (Epub ahead of print; DOI: 10.1111/1753–0407.12492). [DOI] [PubMed] [Google Scholar]
- 33.Tabung FK, Steck SE, Burch JB, Chen CF, Zhang H, Hurley TG, Cavicchia P, Alexander M, Shivappa N, Creek KE, et al. . A healthy lifestyle index is associated with reduced risk of colorectal adenomatous polyps among non-users of non-steroidal anti-inflammatory drugs. J Prim Prev 2015;36:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabung FK, Steck SE, Liese AD, Zhang J, Ma Y, Caan B, Chlebowski RT, Freudenheim JL, Hou L, Mossavar-Rahmani Y, et al. . Association between dietary inflammatory potential and breast cancer incidence and death: results from the Women’s Health Initiative. Br J Cancer 2016;114:1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabung FK, Steck SE, Ma Y, Liese AD, Zhang J, Caan B, Hou L, Johnson KC, Mossavar-Rahmani Y, Shivappa N, et al. . The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the Women’s Health Initiative. Cancer Causes Control 2015;26:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shivappa N, Bosetti C, Zucchetto A, Serraino D, La Vecchia C, Hébert JR. Dietary inflammatory index and risk of pancreatic cancer in an Italian case-control study. Br J Nutr 2015;113:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, Andrew ME, Hartley TA, Miller DB, Mnatsakanova A, et al. . Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med 2014;56:986–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010;316:129–39. [DOI] [PubMed] [Google Scholar]
- 39.Dyck DJ, Heigenhauser GJ, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf) 2006;186:5–16. [DOI] [PubMed] [Google Scholar]
- 40.Díez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol 2003;148:293–300. [DOI] [PubMed] [Google Scholar]
- 41.Navarro SL, Kantor ED, Song X, Milne GL, Lampe JW, Kratz M, White E. Factors associated with multiple biomarkers of systemic inflammation. Cancer Epidemiol Biomarkers Prev 2016;25:521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perrier F, Giorgis-Allemand Li, Slama R, Philippat C. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology 2016;27:378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith-Warner SA, Spiegelman D, Yaun SS, Adami HO, Beeson WL, van den Brandt PA, Folsom AR, Fraser GE, Freudenheim JL, Goldbohm RA, et al. . Intake of fruits and vegetables and risk of breast cancer: a pooled analysis of cohort studies. JAMA 2001;285:769–76. [DOI] [PubMed] [Google Scholar]
- 44.Wu K, Spiegelman D, Hou T, Albanes D, Allen NE, Berndt SI, van den Brandt PA, Giles GG, Giovannucci E, Alexandra Goldbohm R, et al. . Associations between unprocessed red and processed meat, poultry, seafood and egg intake and the risk of prostate cancer: a pooled analysis of 15 prospective cohort studies. Int J Cancer 2016;138:2368–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uma Pradeep K, Geervani P, Eggum BO. Common Indian spices: nutrient composition, consumption and contribution to dietary value. Plant Foods Hum Nutr 1993;44:137–48. [DOI] [PubMed] [Google Scholar]
- 46.Ferrucci LM, Daniel CR, Kapur K, Chadha P, Shetty H, Graubard BI, George PS, Osborne W, Yurgalevitch S, Devasenapathy N, et al. . Measurement of spices and seasonings in India: opportunities for cancer epidemiology and prevention. Asian Pac J Cancer Prev 2010;11:1621–9. [PMC free article] [PubMed] [Google Scholar]
- 47.Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N Engl J Med 2005;352:1611–3. [DOI] [PubMed] [Google Scholar]
- 48.Willett WC. Nutritional epidemiology. 2nd ed New York: Oxford University Press; 1998. [Google Scholar]