Abstract
Background: Epidemiologic, clinical, and experimental studies have suggested that fish oil (FO), a rich source of n–3 (ω-3) polyunsaturated fatty acids, protects against colon cancer. However, this message is confounded by the FDA’s warning that the consumption of certain types of fish should be restricted because of contamination with persistent organic pollutants (POPs), such as polychlorinated biphenyls (PCBs) and organochlorine pesticides.
Objective: We examined FO contaminated with POPs (PCBs, dichlorodiphenyltrichloroethane, and chlordane) compared with unmodified FO on the risk factors of colon cancer development.
Methods: Male Sprague-Dawley rats aged 28 d (n = 30) were allocated into 3 groups and fed 15% corn oil (CO), FO, or POP-contaminated FO for 9 wk with a subcutaneous injection of colon carcinogen azoxymethane at weeks 3 and 4. Colonic aberrant crypt foci (ACF) and cell proliferation were enumerated, and the gene expression of inflammation, antioxidant enzymes, and repair enzymes were determined with the use of real-time quantitative polymerase chain reaction analysis.
Results: FO-fed rats had a lower number of ACF (mean ± SE: 29 ± 4.0 for FO compared with 53 ± 8.4 for CO and 44 ± 4.6 for POP FO) and higher-multiplicity ACF than the CO and POP FO groups (4.7 ± 0.9 for FO compared with 11 ± 1.5 for CO and 9.6 ± 1.8 for POP FO) (P < 0.05). FO feeding lowered the proliferation index compared with the CO and POP FO feeding groups (18% ± 1.1% for FO compared with 25% ± 1.6% for CO and 23% ± 0.7% for POP FO) (P = 0.009). Superoxide dismutase [2.4 ± 0.6 relative quantification (RQ) for FO compared with 1.2 ± 0.2 RQ for CO and 1.3 ± 0.3 RQ for POP FO] and catalase gene expression (10 ± 2.0 RQ for FO compared with 5.4 ± 1.1 RQ for CO and 6.6 ± 1.5 RQ for POP FO) were higher in the FO group than in the CO and POP FO groups (P < 0.05). There were no differences between CO and POP FO on the variables.
Conclusion: These results indicate that POPs in FO reduce the preventive effects of FO on colon carcinogenesis by increasing preneoplastic lesion formation through the downregulation of antioxidant enzyme expression and increasing cell proliferation in rats.
Keywords: fish oil, persistent organic pollutants, aberrant crypt foci, colon, antioxidant enzyme
Introduction
Cancer is the second leading cause of death in the United States, and colorectal cancer (CRC) is the third most common type (1). Fatty fish and fish oil (FO) rich in n–3 FAs, specifically EPA (20:5n–3) and DHA (22:6n–3), have been documented in numerous studies to have chemopreventive and chemosensitizing effects on CRC (2–5). The pathogenesis of CRC is initiated through DNA damage followed by the mutation of oncogenes and tumor suppressor genes involved in vital processes, including cell proliferation and apoptosis, thus disturbing the homeostatic balance between cell production at the base and cell death at the surface of colonic crypts (2, 3).
The onset of CRC is promoted and exacerbated largely by chronic inflammation (3, 4, 6). This inflammation is associated with the gene expression of cyclooxygenase enzymes that regulate the synthesis of arachidonic acid–derived eicosanoids (3, 4). The competitive inhibition of cyclooxygenase 2 (Cox-2) by n–3 FAs leads not only to a decrease in inflammatory products but may also lead to the activation of apoptosis (3). Cox-2 also serves as a mediator for activating NF-κB, which can repress the transcription of the phosphatase and tensin homolog gene (2, 4). Previous studies have linked increased n–3 FA status to the decreased activity of NF-κB in mice (6). Thus, the mechanism by which n–3 FAs are chemopreventive in CRC is multifactorial and involves the suppression of cell proliferation, activation of colonocyte apoptosis, and suppression of chronic inflammation.
Because mammals are deficient for the gene that encodes n–3 desaturase, the availability of n–3 FAs depends primarily on dietary consumption (3). This consumption, however, allows anthropogenic persistent organic pollutants (POPs), including polychlorinated biphenyls (PCBs) and organochlorine pesticides that biomagnify in the marine food chain, to enter the human food chain (7–9). Clinical manifestations of PCB and organochlorine toxicity include endocrine, neurobehavioral, and developmental disruption, and PCB poses a considerable carcinogenic threat (9–11). A case-control study by Howsam et al. (11) showed that higher serum concentrations of mono-ortho PCB congeners were associated with elevated CRC risk. Although the risks and benefits of fish and FO consumption have been examined in several studies (12, 13), to our knowledge, the effects of FO contaminated with POPs on CRC have not been examined.
Given these concerns, we sought to investigate the effects of POP exposure through FO consumption on the anticarcinogenic properties of FO with the use of the azoxymethane-induced rat model of CRC. We investigated the effects of POP contamination in FO on colonocyte proliferation and apoptosis; gene expression of inflammatory biomarkers, DNA-repair enzymes, and antioxidant enzymes; and the number and multiplicity of aberrant crypt foci (ACF), which are considered the earliest neoplastic lesions in CRC progression and reliable biomarkers for colonic carcinogenesis (14–16). In addition, 8-hydroxy-2′-deoxyguanosine (8-OHdG) DNA adducts in serum were determined as a biomarker of DNA damage. We hypothesized that FO contaminated with POPs would reduce the protective capacity of FO against CRC by decreasing the expression of antioxidant enzymes, increasing the number and multiplicity of ACF, modulating 8-OHdG via expression of inflammation and repair enzymes, increasing proliferation and decreasing apoptosis in colon tissue of rats fed POP FO compared with rats fed uncontaminated FO.
Methods
Animals, diets, and study design
Thirty male Sprague-Dawley rats aged 28 d were purchased from Harlan and individually housed in a temperature- and humidity-controlled environment with a 12-h light-dark cycle. The animal use protocol was approved by the San Diego State University Institutional Animal Care and Use Committee.
Rats were randomly assigned to 1 of 3 groups (n = 10/group) that received a diet of either corn oil (CO) as the control, FO, or FO contaminated with POPs. Diet composition was based on an AIN 93G diet with some modification of fat content and POP contaminants. Each diet contained 54% carbohydrates, 20% protein, and 15% fat by weight (17). CO contained 15% CO, FO contained 11.5% by weight of fat from unmodified FO with 3.5% CO, and POP FO contained 11.5% by weight of fat from FO contaminated with POPs and 3.5% CO.
Rats had ad libitum access to drinking water and food. Body weights were measured weekly, and 48-h food and water intake were measured throughout the study.
Oils from anchovies and sardines were the sources of FO (Nordic Naturals, Inc.), which contained 1725 mg n–3 FAs in 5 g FO and was purified by molecular distillation to remove PCBs, heavy metals, and pesticides according to the manufacturer’s instructions. The FO was analyzed for PCBs, dichlorodiphenyltrichloroethanes, and other organochlorine pesticides, and the concentrations were close to the detection limit or not detectable (PCBs: 0.0033 μg/g; dichlorodiphenyltrichloroethanes: 0.0056 μg/g; and chlordanes: <0.00001 μg/g, respectively) (17). For the POP FO diet, the FO was added with standards of the 7 most common PCB congeners (PCB28, PCB52, PCB101, PCB118, PCB138, PCB153, and PCB180), dichlorodiphenyltrichloroethanes (p,p′-dichlorodiphenyldichloroethylene, p,p′-dichlorodiphenyldichloroethane, and p,p′-dichlorodiphenyltrichloroethane), and chlordanes (γ-chlordane, trans-nonachlor, and cis-nonachlor). Final concentrations of PCB, dichlorodiphenyltrichloroethane, and chlordane were 2.40, 1.89, and 1.91 μg/g, respectively, in FO (17). The PCB concentration was within the range reported in the literature for fish (0.119–2.68 μg lipid weight/g); the dichlorodiphenyltrichloroethane and chlordane concentrations were above the literature ranges (0.180–0.446 and 0.255–0.698 μg lipid weight/g, respectively) (18–20).
All rats received the assigned diets for 9 wk. After 3 wk, all rats were subcutaneously injected with azoxymethane (15 mg body weight/kg) (Sigma-Aldrich) 1 time/wk at weeks 3 and 4. Six weeks after the second azoxymethane injection, rats were killed with the use of CO2.
Sample collection
After the rats were killed, blood samples were collected, and the serum was separated and stored at −80°C. The entire colon was harvested, resected, and cleaned with RNase-free PBS solution. One centimeter of the most distal colon closest to the rectum was removed and fixed in 4% paraformaldehyde solution for histology. The remaining colon was divided into 2 longitudinal pieces. One half was fixed in 70% ethanol for ACF enumeration, and the other half was used for gene expression analysis.
ACF enumeration
For the ACF measurements, colon sections were stained with 0.5% methylene blue. ACF formations stand out as darker, thicker, and larger than normal crypts (21). The number of ACFs and high-multiplicity ACFs (HMACFs) [foci with ≥4 apoptotic cells (ACs)] were counted under a light microscope (Axioimager; Zeiss) at 50× magnification (21).
Serum 8-OHdG
8-OHdG DNA adducts in serum samples were measured with the use of an 8-OHdG ELISA kit (Trevigen) according to the manufacturer’s protocol. Anti-8-OHdG monoclonal mouse antibody and HRP–conjugated antibody were used as primary and secondary antibodies, respectively. The TACS-Sapphire colorimetric substrate was added to visualize the formed complex, and the color absorbance was measured at 450 nm.
In vivo cell proliferation measurement
Colonic cell proliferation was determined by measuring Ki-67 with the use of immunohistochemical analysis. Mouse anti-human Ki-67 (BD Biosciences) and biotinylated rabbit anti-mouse IgG (Dako) were used as primary and secondary antibodies, respectively. The complex was visualized with the use of an avidin biotin complex (Vector Laboratories) followed by incubation with 3,3′-diaminobenzidine. At least 20 well-oriented crypt columns/rat were quantified. The proliferation index (PI) and proliferative zone (PZ) were calculated as previously described (21, 22). The crypt height in number of cells was divided into 3 compartments: bottom, middle, and top. The PI in each compartment was also calculated.
In vivo cell apoptosis measurement
A terminal deoxynucleotidyltransferase assay (EMD Millipore) was used to detect targeted cell apoptosis. Incubation with DNase I was used as a positive control, and the omission of the deoxynucleotidyltransferase enzyme was used as a negative control. Stained cells that exhibited apoptotic morphologic features were counted in 25 colonic crypt columns. A proportion of ACs in a crypt column was calculated (21, 22).
Gene expression
Colonic mucosa was scraped and total RNA was extracted with the use of the Trizol method (Invitrogen). Reverse transcription was conducted with the use of Superscript III (Invitrogen) with oligo(dT)12–18 primers (21). RNA was quantified by measuring the optical density at 260 nm. The mRNA levels of prostaglandin endoperoxide synthase 2 (Ptgs2), component of NF-κB (Rela), superoxide dismutase (Sod), catalase, O6-methylguanine DNA methyltransferase (Mgmt), and 8-oxoguanine glycosylase (Ogg1) were analyzed with the use of quantitative real-time PCR (ViiA7; Applied Biosystems). Ptgs2 is involved in the production of PGs, the inflammatory processes, and CRC development. Rela is involved in NF-κB heterodimer formation and activation (the dysregulation of which can lead to inflammation), aberrant cell proliferation, and protection against apoptosis. Sod and catalase are 2 major antioxidant enzymes responsible for the quenching of intracellular reactive oxygen species (ROS) and protection against CRC. Mgmt and Ogg1 are DNA-repair enzymes necessary for maintaining genome stability. Mgmt catalyzes the conversion of mutagenic O6-methylguanine back to guanine, whereas Ogg1 excises the mutagenic base 8-oxoguanine, a product of ROS-induced DNA damage. The gene expression was normalized to r18S expression.
Statistical analysis.
Differences in ACF, cell proliferation, cell apoptosis, gene expression, and serum 8-OHdG between groups were determined with the use of 1-factor ANOVA followed by the Student-Newman-Kauls multiple-comparison procedure. Correlations between variables were tested with the use of Spearman’s ρ. Levene tests were performed to account for unequal variance. If the variances were not equal between groups (Ptgs2 and Rela), Kruskal-Wallis 1-factor ANOVA was used for analysis. All statistical analyses were performed with the use of SPSS (version 22; IBM). All data are expressed as means ± SEs, and α < 0.05 was considered statistically significant.
Results
Body weight and food intake.
No significant differences were found in initial body weight, final body weight, body weight gain, and food intake between any of the experimental groups (data not shown).
ACF.
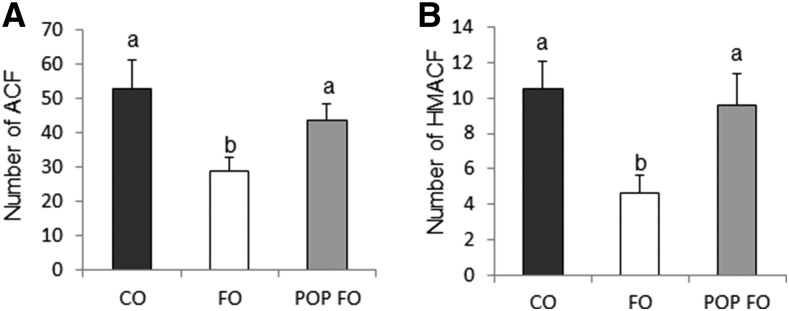
Significant differences were found in the number of ACF for the FO group compared with the CO and POP FO groups. The FO group had 48% and 36% fewer ACFs (Figure 1A) and 56% and 51% fewer HMACFs (Figure 1B) than the CO and POP FO groups (P < 0.05), which did not differ from one another.
FIGURE 1.
ACF (A) and HMACF (B) in male rats fed the CO, FO, or POP FO diet for 9 wk. Values are means ± SEs (n = 10). Means without a common letter differ, P < 0.05. ACF, aberrant crypt foci; CO, corn oil; FO, fish oil; HMACF, high-multiplicity ACF; POP, persistent organic pollutant.
Serum 8-OHdG.
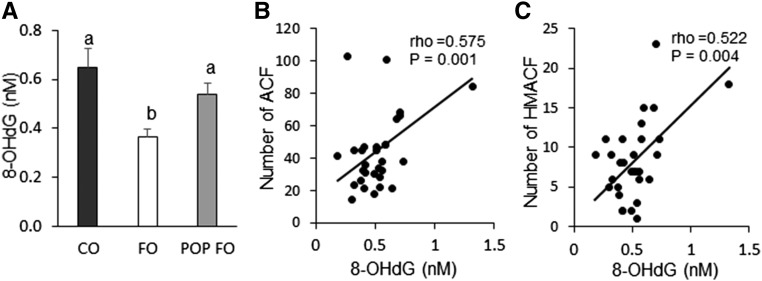
Serum 8-OHdG concentrations were lower in the FO group than the CO and POP FO groups (P < 0.05) (Figure 2A). The FO group had 43.7% and 32.4% fewer 8-OHdG DNA adducts than the CO and POP FO groups, respectively. There was no significant difference in 8-OHdG concentrations between the CO and POP FO groups. There were strong correlations between 8-OHdG DNA adducts and ACF (P = 0.001) and HMACF (P = 0.004) (Figure 2B, C). Rats with greater DNA damage showed higher numbers of ACF and HMACF.
FIGURE 2.
Serum 8-OHdG (A) and Spearman correlations between 8-OHdG and colon ACFs (B) and HMACFs (C) in male rats fed the CO, FO, or POP FO diet for 9 wk. Values in panel A are means ± SEs (n = 10). Means without a common letter differ, P < 0.05. ACF, aberrant crypt foci; CO, corn oil; FO, fish oil; HMACF, high-multiplicity ACF; POP, persistent organic pollutant; 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
Cell proliferation and apoptosis.
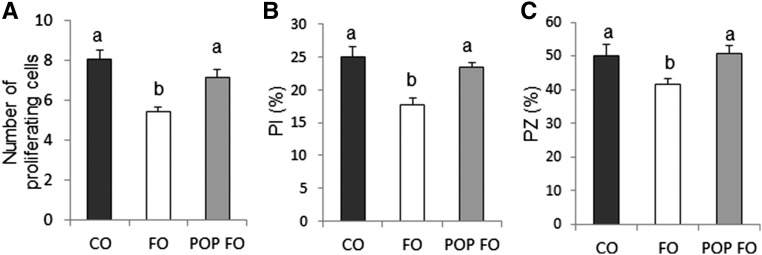
Rats fed FO had fewer proliferating cells per crypt column and a lower percentage of proliferating cells per crypt column than rats that consumed CO or POP FO diets (P < 0.05) (Figure 3A, B). CO and POP FO caused an expansion of the PZ that did not occur in the rats fed FO (P < 0.05) (Figure 3C). There were no significant differences between any of the experimental groups on apoptosis (data not shown).
FIGURE 3.
Number of proliferating cells (A), PI (B), and PZ (C) in male rats fed the CO, FO, or POP FO diet for 9 wk. Values are means ± SEs (n = 10). Means without a common letter differ, P < 0.05. CO, corn oil; FO, fish oil; PI, proliferation index; POP, persistent organic pollutant; PZ, proliferative zone.
Colonic gene expression.
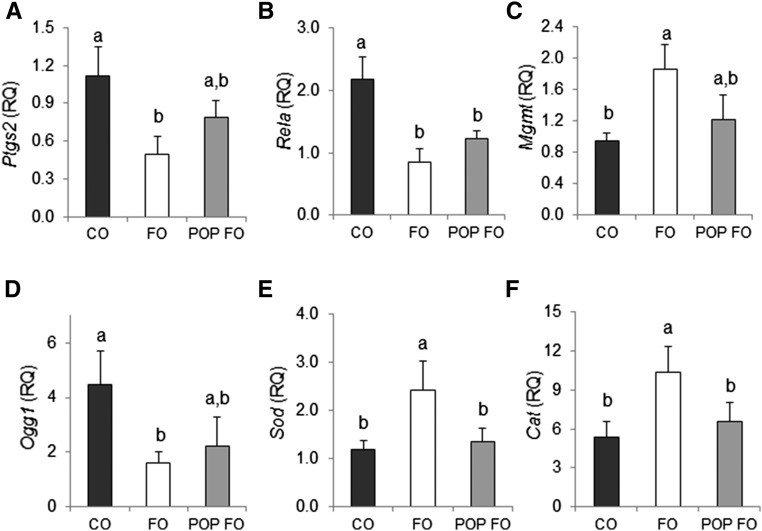
The FO group had 56% lower Ptgs2 transcription amounts than the CO group (P = 0.042) (Figure 4A). No significant differences in Ptgs2 expression were found for the POP FO group compared with the CO and FO groups. Both the FO and POP FO groups resulted in lower Rela expression than CO (by 63% and 58%, respectively) (P = 0.011) (Figure 4B). The FO group upregulated Mgmt 2-fold (P = 0.044) and downregulated Ogg1 by 64% (P = 0.047) compared with the CO group (Figure 4C, D). The POP FO group was not significantly different than the CO and FO groups for Mgmt and Ogg1 expression. The FO group resulted in >1.5-elevated amounts of Sod and catalase compared with the CO and POP FO groups (P < 0.05), whereas no differences in Sod and catalase amounts between the POP FO and CO groups were observed (Figure 4E, F).
FIGURE 4.
Colonic gene expression of Ptgs2 (A), Rela (B), Mgmt (C), Ogg1 (D), Sod (E), and Cat (F) in male rats fed the CO, FO, or POP FO diet for 9 wk. Values are means ± SEs (n = 10). Means without a common letter differ, P < 0.05. Cat, catalase; CO, corn oil; FO, fish oil; Mgmt, O6-methylguanine DNA methyltransferase; Ogg1, 8-oxoguanine glycosylase; POP, persistent organic pollutant; Ptgs2, prostaglandin endoperoxide synthase 2; Rela, component of NF-κB; RQ, relative quantification; Sod, superoxide dismutase.
Correlation between endpoints.
The correlations between outcome measures and ACF, HMACF, and serum 8-OHdG are shown in Table 1. ACF, HMACF, and serum 8-OHdG showed positive correlations with PI in the middle and top regions of the colonic crypts and the PZ (P < 0.05). A greater PI and extended PZ were correlated with a higher number of ACF and HMACF and a greater DNA adduct amount. Higher numbers of ACs were significantly correlated with serum DNA damage (P = 0.026). Positive trends were observed between AC and ACF (P = 0.085) and HMACF (P = 0.137). For Sod and catalase expression in the colon, there were inverse relations between ACF, HMACF, and serum 8-OHdG (P < 0.05). Rats with a higher gene expression of Sod and catalase had reduced ACF and HMACF formation and lower amounts of serum DNA damage. Inverse relations were also shown between the Mgmt repair enzyme and ACF (P = 0.050), HMACF (P = 0.011), and serum 8-OHdG concentrations (P = 0.007). A mild correlation was observed between the Ogg1 repair enzyme and serum DNA damage (P = 0.067) but not with ACF nor HMACF.
TABLE 1.
Spearman correlations between variables in male rats fed the CO, FO, or POP FO diet for 9 wk1
| ACF |
HMACF |
8-oxo-dG |
||||
| ρ | P value | ρ | P value | ρ | P value | |
| PI | 0.424 | 0.131 | 0.336 | 0.240 | 0.643 | 0.010 |
| PI (bottom 1/3) | −0.077 | 0.794 | −0.113 | 0.701 | 0.029 | 0.919 |
| PI (middle 1/3) | 0.499 | 0.035 | 0.402 | 0.077 | 0.621 | 0.013 |
| PI (top 1/3) | 0.595 | 0.025 | 0.626 | 0.017 | 0.706 | 0.003 |
| PZ | 0.520 | 0.027 | 0.455 | 0.029 | 0.645 | 0.002 |
| AC | 0.389 | 0.085 | 0.314 | 0.137 | 0.572 | 0.026 |
| Sod | −0.391 | 0.040 | −0.421 | 0.026 | −0.399 | 0.043 |
| Catalase | −0.338 | 0.053 | −0.374 | 0.036 | −0.344 | 0.040 |
| Mgmt | −0.330 | 0.050 | −0.492 | 0.011 | −0.496 | 0.007 |
| Ogg1 | 0.155 | 0.459 | 0.168 | 0.450 | 0.297 | 0.067 |
AC, apoptotic cell; ACF, aberrant crypt foci; CO, corn oil; FO, fish oil; HMACF, high-multiplicity ACF; Mgmt, O6-methylguanine DNA methyltransferase; Ogg1, 8-oxoguanine glycosylase; PI, proliferating index; POP, persistent organic pollutant; PZ, proliferative zone; Sod, superoxide dismutase; 8-oxo-dG, 8-oxodeoxyguanosine.
Discussion
Studies have examined cancer risk related to POPs, including PCB, organochlorine, and mercury; however, there has been limited reporting on the risk factors of exposure to POPs through an FO diet and the beneficial efficiency of FO related to CRC development. We investigated whether the anticarcinogenic effects of FO are attenuated when contaminated with POPs with the use of a rat model and treatment with azoxymethane to induce CRC. The POP doses administered were selected within the ranges found in fish and FO (18–20), which help strengthen the practical implications of our results. We found that ACF, neoplastic lesions found in early CRC progression and reliable biomarkers for colonic carcinogenesis (14–16), were suppressed by FO, but this protective effect was inhibited in the POP FO group. The oral administration of PCBs has been shown to decrease tight-junction protein (zonula occludens 1 and occludin) expression, which disrupts intestinal epithelial integrity and permeability (23). This mechanism may have contributed to the higher ACF found in the POP FO group.
Colon cancer cells are characterized by uncontrolled cell proliferation via the perturbation of the cell cycle. Supplementation with FO protects against colon cancer development by reducing uncontrolled cell proliferation via the decreased expression of the Ras protein, which is involved in signaling pathways that regulate colonic epithelial growth, differentiation, and tumor formation (5). However, our results indicate that treatment with POP FO may contribute to uncontrolled cell proliferation by increasing preneoplastic lesions, as evidenced by substantially higher PI and PZ in the POP FO group, whereas the uncontaminated FO provided beneficial effects. Further research is needed to determine whether increased cell proliferation in POP FO was via the modulation of cell-cycle mediators or Ras signaling.
Colonic cell homeostasis depends on the equilibrium of cell proliferation and apoptosis. An imbalance can exacerbate colon cancer development. FO has been shown to suppress CRC pathogenesis by increasing apoptosis in colonocytes. The mechanism involves upregulating the expression of phosphatase and the tensin homolog, an important regulator in the apoptotic signaling pathway (2). It has also been shown that FO enhances apoptosis by reducing antiapoptotic B-cell lymphoma 2 mediator expression (24–27). FO treatment did not increase apoptosis, as has been shown previously. It has been shown that FO with pectin or butyrate enhances apoptosis in the colon, whereas FO with cellulose did not increase apoptosis (28). Our study used cellulose as a fiber source, which may explain why there were no changes on apoptosis. Further investigations on the interactive effects of dietary fat and fiber are warranted. Our study did not find a significant difference in apoptosis between FO and POP FO treatments; however, higher serum DNA damage was correlated with increased apoptotic activity that removed damaged cells. Enhanced targeted apoptosis response to oxidative DNA damage was described in colon carcinogenesis models (27, 29).
Azoxymethane is metabolized by cytochrome P450 in the liver and leads to the formation of DNA adducts, of which the 8-OHdG DNA adduct is a major product of oxidative stress that has been used as a marker for DNA damage and inflammation (30). Elevated amounts of DNA adducts have been reported in gastric tumors compared with normal mucosa (31) and associated with poor survival in CRC patients (27). The use of azoxymethane induces highly mutagenic 8-OHdG DNA adducts that promote a GC → TA transition, which is frequently found in tumor suppressor gene mutations (32, 33). Studies have shown that an increased concentration of 8-OHdG in the blood is associated with the development of colorectal adenoma and cancer (34, 35). In this study, FO treatment decreased the azoxymethane-induced 8-OHdG in serum, whereas POP FO did not. Wen et al. (36) found that a higher exposure to PCBs was associated with increased amounts of 8-OHdG DNA adducts in humans. POPs induce ROS, leading to increased oxidative DNA damage that can initiate DNA mutations associated with tumor development (37). Our study supported previous results that showed that 8-OHdG was positively correlated with azoxymethane-induced ACF formation (38). Therefore, in the POP FO group, elevated 8-OHdG concentrations might be associated with the higher ACF formation. Investigation into whether adding POP to the CO diet would increase 8-OHdG and the determination of colonic 8-OHdG concentrations are underway in our next study.
DNA damage can be removed by repair enzymes, which play key roles in genome stability and preventing the onset of CRC. The repair enzyme Ogg1 is responsible for excising 8-OHdG DNA damage, and elevated amounts of Ogg1 have been reported in colonic tumors compared with controls (31, 39). Another repair enzyme, Mgmt, converts azoxymethane-induced O6-methylguanine DNA adducts back to guanine (40). Reduced Mgmt expression was found in tumor tissues compared with normal tissue in CRC patients (41). Methylation of the Mgmt promotor was associated with a loss of Mgmt expression in >40% of CRC cases (40). This study found that FO treatment upregulated colonic gene expression of Mgmt and downregulated Ogg1. These results were consistent with earlier work that reported that FO was associated with higher Mgmt and lower Ogg1 gene transcription, as well as reduced serum DNA damage (42, 43). However, these protective effects were not clearly observed in the POP FO group.
Chronic inflammation is a major risk factor for CRC development, with some studies suggesting that inflammatory cytokines are responsible for stimulating the uncontrolled cell growth of cancer cells (4, 44). Cyclooxygenase is involved in the production of PGs, which are related to inflammatory processes and colon cancer development (45). Dysregulation of the NF-κB pathway through constitutive activation has been shown to cause abnormal cell proliferation and apoptosis in CRC cells (46). Supplementation with FO inhibits the growth of colon tumors by suppressing inflammation through decreases in NF-κB (6) and Cox-2 (47, 48). Our results are supported by previous studies that showed that FO treatment substantially lowered Ptgs2 (Cox-2) and Rela expression, in which the latter was involved in NF-κB heterodimer formation and activation. Interestingly, POP contamination did not alter the anti-inflammatory effect of FO, as evidenced by the considerably reduced Rela expression for both FO groups, suggesting that POP toxicity does not interfere with the anti-inflammatory properties of FO.
A considerable amount of evidence suggests that antioxidants play a critical role in protecting against inflammation and CRC (49). In this study, only uncontaminated FO substantially increased colonic gene expression of the antioxidant enzymes Sod and catalase. For Sod and catalase, there were strong inverse correlations between ACF, HMACF, and serum 8-OHdG. This suggests that POP contamination reduced the capacity of FO to induce the expression of key protective enzymes. POPs exert their toxicity by binding to nuclear receptors, including the aryl hydrocarbon receptor. This binding increases intracellular ROS, which in turn can lead to the upregulation of NF-κB and production of proinflammatory proteins (50). Because Sod and catalase play a critical role in removing ROS, the downregulation of Sod and catalase can contribute to ROS accumulation and exert inflammation and aberrant crypt formation. It has been shown that low Sod activity is associated with higher cell proliferation in the colon (51). Further mechanistic research that includes ROS as an indicator of inflammation is in progress.
Overall, POP contamination reduced the protective effect of FO on risk factors related to CRC development. Of particular note, measured variables for the POP FO group either fell between FO and CO or were similar to CO but were not worse than CO. Some studies have shown the importance of the interaction between POPs and different dietary lipids (i.e., n–3 compared with n–6) (52–54). Future studies would benefit by adding a POP CO group to investigate any exacerbating effects of POPs in an n–6-rich environment. It should be noted that dioxin and dioxin-like PCBs, known carcinogens, were not tested in this study. Therefore, further research should also investigate the effects of the carcinogens and other POPs present in fish and FO on risk factors related to CRC development.
This study evaluated the integrative impact of n–3 FAs and POPs in FO. POP contamination in FO reduced the beneficial effects of FO on CRC development markers in rats. This was evidenced by multiple measures such as a substantially higher number of ACF, increased cell proliferation, and reduced antioxidant enzyme amounts. The results indicate the potential risk of exposure to POPs via fish or FO on colon cancer development. This study suggests that further investigations of the impact of POPs on the beneficial effects of FO are needed.
Acknowledgments
We thank Penelope Quintana for critically reading the manuscript and Nathan Dodder for editing the manuscript. The authors’ responsibilities were as follows—MYH and EH: designed the research and analyzed the data; MYH, BK, RD, JYK, and JL: conducted the research; MYH, EH, and JL: wrote the paper; MYH: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Footnotes
Abbreviations used: AC, apoptotic cell; ACF, aberrant crypt foci; CO, corn oil;Cox-2, cyclooxygenase 2; CRC, colorectal cancer; FO, fish oil; HMACF,high-multiplicity aberrant crypt foci;Mgmt, O6-methylguanine DNA methyltransferase; Ogg1, 8-oxoguanine glycosylase; PCB, polychlorinated biphenyl; PI, proliferation index; POP, persistent organic pollutant; Ptgs2, PG endoperoxide synthase 2; PZ, proliferative zone; Rela, component of NF-κB; ROS, reactive oxygen species; Sod, superoxide dismutase; 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Kansal S, Bhatnagar A, Agnihotri N. Fish oil suppresses cell growth and metastatic potential by regulating PTEN and NF-κB signaling in colorectal cancer. PLoS One 2014;9:e84627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skender B, Vaculova AH, Hofmanova J. Docosahexaenoic fatty acid (DHA) in the regulation of colon cell growth and cell death: a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2012;156:186–99. [DOI] [PubMed] [Google Scholar]
- 4.Hofmanova J, Strakova N, Vaculova AH, Tylichova Z, Safarikova B, Skender B, Kozubík A. Interaction of dietary fatty acids with tumor necrosis factor family cytokines during colon inflammation and cancer. Mediators Inflamm 2014;2014:848632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kansal S, Negi AK, Bhatnagar A, Agnihotri A. Ras signaling pathway in the chemopreventive action of different ratios of fish oil and corn oil in experimentally induced colon carcinogenesis. Nutr Cancer 2012;64:559–68. [DOI] [PubMed] [Google Scholar]
- 6.Nowak J, Weylandt KH, Habbel P, Wang J, Dignass A, Glickman JN, Kang JX. Colitis-associated colon tumorigenesis is suppressed in transgenic mice rich in endogenous n-3 fatty acids. Carcinogenesis 2007;28:1991–5. [DOI] [PubMed] [Google Scholar]
- 7.Hennig B, Ormsbee L, McClain CJ, Watkins BA, Blumberg B, Bachas LG, Sanderson W, Thompson C, Suk WA. Nutrition can modulate the toxicity of environmental pollutants: implications in risk assessment and human health. Environ Health Perspect 2012;120:771–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs DR Jr., Ruzin J, Lee DH. Environmental pollutants: downgrading the fish food stock affects chronic disease risk. J Intern Med 2014;276:240–2. [DOI] [PubMed] [Google Scholar]
- 9.Martí M, Ortiz X, Gasser M, Martí R, Montaña MJ, Díaz-Ferrero J. Persistent organic pollutants (PCDD/Fs, dioxin-like PCBs, marker PCBs, and PBDEs) in health supplements on the Spanish market. Chemosphere 2010;78:1256–62. [DOI] [PubMed] [Google Scholar]
- 10.Mrema EJ, Rubin FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 2013;307:74–88. [DOI] [PubMed] [Google Scholar]
- 11.Howsam M, Grimalt JO, Guinó E, Navarro M, Martí-Ragué J, Peinado MA, Capellá G, Moreno V. Organochlorine exposure and colorectal cancer risk. Environ Health Perspect 2004;112:1460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonijevic B, Matthys C, Sioen I, Bilau M, Van Camp J, Willems JL, De Henauw S. Simulated impact of a fish based shift in the population n-3 fatty acids intake on exposure to dioxins and dioxin-like compounds. Food Chem Toxicol 2007;45:2279–86. [DOI] [PubMed] [Google Scholar]
- 13.Domingo JL, Bocio A, Falcó G, Llobet JM. Benefits and risks of fish consumption Part 1. A quantitative analysis of intake of omega-3 fatty acids and chemical contaminants. Toxicology 2007;230:219–26. [DOI] [PubMed] [Google Scholar]
- 14.Orlando FA, Tan D, Baltodano JD, Khoury T, Gibbs JF, Hassid VJ, Ahmed BH, Alrawi SJ. Aberrant crypt foci as precursors in colorectal cancer progression. J Surg Oncol 2008;98:207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Ceron M, Pellise M. Biology and diagnosis of aberrant crypt foci. Colorectal Dis 2012;14:e157–64. [DOI] [PubMed] [Google Scholar]
- 16.Raju J. Azoxymethane-induced rat aberrant crypt foci: relevance in studying chemoprevention of colon cancer. World J Gastroenterol 2008;14:6632–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong MY, Lumibao J, Mistry P, Saleh R, Hoh E. Fish oil contaminated with persistent organic pollutants reduces antioxidant capacity and induces oxidative stress without affecting its capacity to lower lipid concentrations and systemic inflammation in rats. J Nutr 2015;145:939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baeyens W, Leermakers M, Eiskens M, Van Larebeke N, De Bont R, Vanderperren H, Fontaine A, Degroodt JM, Goeyens L, Hanot V, et al. . PCBs and PCDD/FS in fish and fish products and their impact on the human body burden in Belgium. Arch Environ Contam Toxicol 2007;52:563–71. [DOI] [PubMed] [Google Scholar]
- 19.Glasius M, Christensen JH, Platz J, Vorkamp K. Halogenated organic contaminants in marine fish and mussels from southern Greenland—pilot study on relations to trophic levels and local sources. J Environ Monit 2005;7:127–31. [DOI] [PubMed] [Google Scholar]
- 20.Szlinder-Richert J, Barska I, Mazerski J, Usydus Z. PCBs in fish from the southern Baltic Sea: levels, bioaccumulation features, and temporal trends during the period from 1997 to 2006. Mar Pollut Bull 2009;58:85–92. [DOI] [PubMed] [Google Scholar]
- 21.Hong MY, Nulton E, Shelechi M, Hernández LM, Nemoseck T. Effects of dark chocolate on azoxymethane-induced colonic aberrant crypt foci. Nutr Cancer 2013;65:677–85. [DOI] [PubMed] [Google Scholar]
- 22.Hong MY, Turner ND, Murphy ME, Carroll R, Chapkin RS, Lupton JR. In vivo regulation of colonic cell proliferation, differentiation, apoptosis and P27Kip1 by dietary fish oil and butyrate in rats. Cancer Prev Res (Phila) 2015;8:1076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi YJ, Seelbach MJ, Pu H, Eum SY, Chen L, Zhang B, Hennig B, Toborek M. Polychlorinated biphenyls disrupt intestinal integrity via NADPH oxidase-induced alterations of tight junction protein expression. Environ Health Perspect 2010;118:976–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crim KC, Sanders LM, Hong MY, Taddeo SS, Turner ND, Chapkin RS, Lupton JR. Upregulation of p21Waf1/Cip1 expression in vivo by butyrate administration can be chemoprotective or chemopromotive depending on the lipid component of the diet. Carcinogenesis 2008;29:1415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borghetti G, Yamaguchi AA, Aikawa J, Yamazaki RK, de Brito GA, Fernandes LC. Fish oil administration mediates apoptosis of Walker 256 tumor cells by modulation of p53, Bcl-2, caspase-7 and caspase-3 protein expression. Lipids Health Dis 2015;14:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho Y, Turner ND, Davidson LA, Chapkin RS, Carroll RJ, Lupton JR. A chemoprotective fish oil/pectin diet enhances apoptosis via Bcl-2 promoter methylation in rat azoxymethane-induced carcinomas. Exp Biol Med (Maywood) 2012;237:1387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong MY, Chapkin RS, Davidson LA, Turner ND, Morris JS, Carroll RJ, Lupton JR. Fish oil enhances targeted apoptosis during colon tumor initiation in part by downregulating Bcl-2. Nutr Cancer 2003;46:44–51. [DOI] [PubMed] [Google Scholar]
- 28.Chang WL, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. J Nutr 1998;128:491–7. [DOI] [PubMed] [Google Scholar]
- 29.Sheridan J, Wang LM, Tosetto M, Sheahan K, Hyland J, Fennelly D, O’Donoghue D, Mulcahy H, O’Sullivan J. Nuclear oxidative damage correlates with poor survival in colorectal cancer. Br J Cancer 2009;100:381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ock CY, Kim EH, Choi DJ, Lee HJ, Hahm KB, Chung MH. 8-Hydroxydeoxyguanosine: not mere biomarker for oxidative stress, but remedy for oxidative stress-implicated gastrointestinal diseases. World J Gastroenterol 2012;18:302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borrego S, Vazquez A, Dasi F, Cerda C, Iradi A, Tormos C, Sánchez JM, Bagán L, Boix J, Zaragoza C, et al. . Oxidative stress and DNA damage in human gastric carcinoma: 8-oxo-7′8-dihydro-2′-deoxyguanosine (8-oxo-dG) as a possible tumor marker. Int J Mol Sci 2013;14:3467–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez-González I, Garcia-Melo F, Vásquez-Garzón VR, Villa-Treviño S, Madrigal-Santillán EO, Morales-González JA, Madrigal-Bujaidar E. Evaluation of blueberry juice in mouse azoxymethane-induced aberrant crypts and oxidative damage. Evid Based Complement Alternat Med 2014;2014: 379890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tudek B, Winczura A, Janik J, Siomek A, Foksinski M, Oliński R. Involvement of oxidatively damaged DNA and repair in cancer development and aging. Am J Transl Res 2010;2:254–84. [PMC free article] [PubMed] [Google Scholar]
- 34.Chang D, Wang F, Zhao YS, Pan HZ. Evaluation of oxidative stress in colorectal cancer patients. Biomed Environ Sci 2008;21:286–9. [DOI] [PubMed] [Google Scholar]
- 35.Sato T, Takeda H, Otake S, Yokozawa J, Nishise S, Fujishima S, Orii T, Fukui T, Takano J, Sasaki Y, et al. . Increased plasma levels of 8-hydroxydeoxyguanosine are associated with development of colorectal tumors. J Clin Biochem Nutr 2010;47:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen S, Yang FX, Gong Y, Zhang XL, Hui Y, Li JG, Liu AL, Wu YN, Lu WQ, Xu Y. Elevated levels of urinary 8-hydroxy-2′-deoxyguanosine in male electrical and electronic equipment dismantling workers exposed to high concentrations of polychlorinated dibenzo-p-dioxins and dibenzofurans, polybrominated diphenyl ethers, and polychlorinated biphenyls. Environ Sci Technol 2008;42:4202–7. [DOI] [PubMed] [Google Scholar]
- 37.Ludewig G, Robertson LW. Polycholorinated biphenyls (PCBs) as initiating agents in hepatocellular carcinoma. Cancer Lett 2013;334:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaneko T, Tahara S, Takabayashi F. Inhibitory effect of natural coumarin compounds, esculetin and esculin, on oxidative DNA damage and formation of aberrant crypt foci and tumors induced by 1,2-dimethylhydrazine in rat colons. Biol Pharm Bull 2007;30:2052–7. [DOI] [PubMed] [Google Scholar]
- 39.Dziaman T, Ludwiczak H, Ciesla JM, Banaszkiewicz Z, Winczura A, Chmielarczyk M, Wisniewska E, Marszalek A, Tudek B, Olinski R. PARP-1 expression is increased in colon adenoma and carcinoma and correlates with OGG1. PLoS One 2014;9:e115558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minoo P. Toward a molecular classification of colorectal cancer: the role of MGMT. Front Oncol 2013;3:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordeiro AT, Silva CM, Bartchewsky Júnior W, Ribeiro ML, Martinez CA. Evaluation of the expression of the MGMT gene in normal and neoplastic tissue of patients with colorectal cancer. Rev Col Bras Cir 2012;39:48–53. [PubMed] [Google Scholar]
- 42.Bancroft LK, Lupton JR, Davidson LA, Taddeo SS, Murphy ME, Carroll RJ, Chapkin RS. Dietary fish oil reduces oxidative DNA damage in rat colonocytes. Free Radic Biol Med 2003;35:149–59. [DOI] [PubMed] [Google Scholar]
- 43.Hong MY, Lupton JR, Morris JS, Wang N, Carroll RJ, Davidson LA, Elder RH, Chapkin RS. Dietary fish oil reduces O6-methylguanine DNA adduct levels in rat colon in part by increasing apoptosis during tumor initiation. Cancer Epidemiol Biomarkers Prev 2000;9:819–26. [PubMed] [Google Scholar]
- 44.Janakiram NB, Rao CV. The role of inflammation in colon cancer. Adv Exp Med Biol 2014;816:25–52. [DOI] [PubMed] [Google Scholar]
- 45.Moreira L, Castells A. Cyclooxygenase as a target for colorectal cancer chemoprevention. Curr Drug Targets 2011;12:1888–94. [DOI] [PubMed] [Google Scholar]
- 46.Liu S, Sun X, Wang M, Hou Y, Zhan Y, Jiang Y, Liu Z, Cao X, Chen P, Liu Z, et al. . A microRNA 221-and 222-mediated feedback loop maintains constitutive activation of NF kappa B and STAT3 in colorectal cancer cells. Gastroenterology 2014;147:847–59.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song NY, Na HK, Baek JH, Surh YJ. Docosahexaenoic acid inhibits insulin-induced activation of sterol regulatory-element binding protein 1 and cyclooxygenase-2 expression through upregulation of SIRT1 in human colon epithelial cells. Biochem Pharmacol 2014;92:142–8. [DOI] [PubMed] [Google Scholar]
- 48.Kusmardi, Priosoeryanto BP, Harlina E, Cornain S. Inhibition activities of fish oil in iNOs, COX-2, and β-catenin expressions in colorectal preneoplasia of mice induced by azoxymetane and dextran sodium sulfate. J Appl Biotechnol 2014;2:91–101. [Google Scholar]
- 49.Stone WL, Krishnan K, Campbell SE, Palau VE. The role of antioxidants and pro-oxidants in colon cancer. World J Gastrointest Oncol 2014;6:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petriello MC, Newsome B, Hennig B. Influence of nutrition in PCB-induced vascular inflammation. Environ Sci Pollut Res Int 2014;21:6410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satomi A, Murakami S, Hashimoto T, Ishida K, Matsuki M, Sonoda M. Significance of superoxide dismutase (SOD) in human colorectal cancer tissue: correlation with malignant intensity. J Gastroenterol 1995;30:177–82. [DOI] [PubMed] [Google Scholar]
- 52.Hennig B, Reiterer G, Toborek M, Matveev SV, Daugherty A, Smart E, Robertson LW. Dietary fat interacts with PCBs to induce changes in lipid metabolism in mice deficient in low-density lipoprotein receptor. Environ Health Perspect 2005;113:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hennig B, Slim R, Toborek M, Robertson LW. Linoleic acid amplifies polychlorinated biphenyl-mediated dysfunction of endothelial cells. J Biochem Mol Toxicol 1999;13:83–91. [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Reiterer G, Toborek M, Hennig B. Changing ratios of omega-6 to omega-3 fatty acids can differentially modulate polychlorinated biphenyl toxicity in endothelial cells. Chem Biol Interact 2008;172:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]