Abstract
Background: Sugar-sweetened beverages (SSBs) have been associated with an increased risk of diabetes mellitus (DM), whereas the association with artificially sweetened beverages (ASBs) is unclear.
Objective: We aimed to evaluate the associations of ASB and SSB consumption with the risk of developing DM and the potential benefit of replacing SSBs with ASBs or water.
Design: The national Women’s Health Initiative recruited a large prospective cohort of postmenopausal women between 1993 and 1998. ASB, SSB, and water consumption was measured by lifestyle questionnaires, and DM was self-reported.
Results: Of 64,850 women, 4675 developed diabetes over an average of 8.4 y of follow-up. ASBs and SSBs were both associated with an increased risk of DM with an HR of 1.21 (95% CI: 1.08, 1.36) comparing ASB consumption of ≥2 serving/d to never or <3 serving/mo, and an HR of 1.43 (95% CI: 1.17, 1.75) comparing SSB consumption of ≥2 serving/d to <1 serving/wk (1 serving = one 12-ounce can or 355 mL). Subgroup analysis found an increased risk of DM associated with ASBs only in the obese group. Modeling the substitution of SSBs with an equal amount of ASBs did not significantly reduce the risk of developing DM. However, statistically substituting 1 serving of ASBs with water was associated with a significant risk reduction of 5% (HR: 0.95; 95% CI: 0.91, 0.99), whereas substituting 1 serving of SSBs with water was associated with a risk reduction of 10% (HR: 0.90; 95% CI: 0.85, 0.95).
Conclusions: ASBs were associated with a 21% increased risk of developing DM, approximately half the magnitude of SSBs (associated with a 43% increased risk). Replacing ASBs and SSBs with water could potentially reduce the risk. However, caution should be taken in interpreting these results as causal because both residual confounding and reverse causation could explain these results.
Keywords: diabetes mellitus, sweetened beverages, diet soda, sweetening agents, nonnutritive sweeteners
INTRODUCTION
Diabetes mellitus (DM) is a prevalent and costly disease whose burden is increasing. According to the CDC, DM affects ∼29.1 million people or 9.3% of the US population (1). The total estimated cost of diagnosed diabetes in 2012 was $245 billion, which included $176 billion in direct medical expenditures and $69 billion in reduced productivity (2). The American Diabetes Association recommends avoiding excess energy intake as a preventive measure for DM (3). Sugar-sweetened beverages (SSBs) are a leading source of added dietary sugar (4, 5) and excess energy, with most research linking SSBs to an increased risk of diabetes (6–17). SSB consumption is accompanied by weight gain and obesity (8), which are known DM risk factors (18). American Diabetes Association recommendations therefore suggest that nonnutritive sweeteners could replace added sugar within a structured diet to maintain a healthy weight and minimize risk; however, the evidence is inconclusive on the risks and benefits of nonnutritive sweeteners, including artificially sweetened beverages (ASBs) (19).
The number of studies evaluating the association of ASB consumption and the development of DM are somewhat limited compared with SSBs, and the results have been mixed (11–17). Findings from a US cohort (20), a European cohort (13), a Japanese cohort (17), and a meta-analysis of cohort studies (15) suggested an elevated DM risk related to a higher consumption of ASBs, whereas other studies found no association (11) or attenuated association when adjusting for adiposity and/or energy intake (12, 16). Interestingly, one analysis in both sexes identified a significantly higher risk of DM associated with caffeine-free ASBs only within women (14).
The primary objective of this study was to investigate the prospective association of ASBs and SSBs, individually and in combination, with DM in a well-characterized cohort of postmenopausal women. We hypothesized that both will be associated with a higher risk of DM, so we further modeled the possible impact of replacing SSBs with ASBs or plain water to prevent or delay the development of DM.
METHODS
Study participants
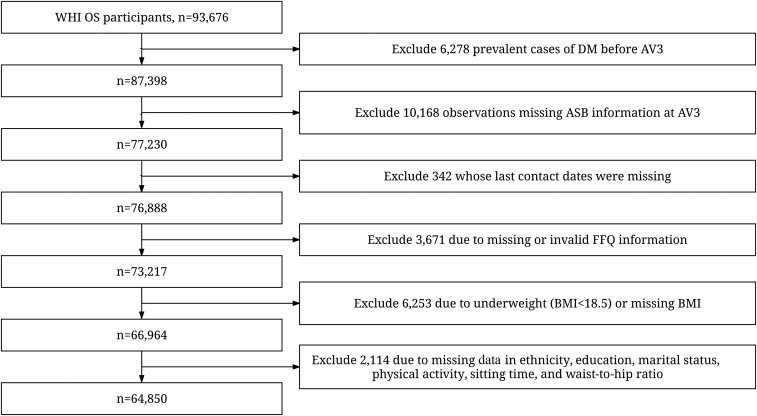
The Women’s Health Initiative (WHI) recruited a cohort of 93,676 women aged 50–79 y at 40 clinical centers across the United States between 1993 and 1998 in a prospective observational study (OS) (21). Data were collected through physical examination, demographics, medical history and outcomes, and lifestyle and dietary intake questionnaires. Details of the WHI OS design have been published elsewhere (21). Diet soda and diet fruit drink (ASB) consumption was first assessed by using a questionnaire separate from the WHI food-frequency questionnaire (FFQ) during the year 3 annual visit [(AV3) origin of our analysis]. Information on SSBs and other dietary intake was collected by using the FFQ at year 3, at the same time that ASB consumption was assessed. All outcomes related to DM were collected through 2010 as part of the WHI Extension Study. There were 64,850 women who were part of the current analysis based on our exclusion criteria: prevalent DM cases at baseline and before or at AV3 (6278 excluded), ASB consumption not measured at the AV3 (10,168 further excluded), follow-up length not available (342 further excluded), implausible dietary data (energy intake <600 or >5000 kcal/d) (3671 further excluded), underweight [BMI (in kg/m2) <18.5] or missing BMI (6253 further excluded), and missing important covariates (2114 further excluded) (Figure 1). The WHI protocol and consent forms were approved by the institutional review boards for each participating institution and the clinical coordinating center.
FIGURE 1.
Flowchart for exclusion from the OS participants. ASB, artificially sweetened beverage; AV3, year 3 annual visit; DM, diabetes mellitus; FFQ, food-frequency questionnaire; OS, observational study; WHI, Women’s Health Initiative.
ASB consumption
Participants were asked about the frequency of ASB consumption during the past 3 mo on the AV3 OS follow-up questionnaire. Nine options were given, including “never or less than 1 per month,” “1–3 per month,” “1 per week,” “2–4 per week,” “5–6 per week,” “1 per day,” “2–3 per day,” “4–5 per day,” and “6 or more per day.” The unit of measurement was one 12-ounce can (355 mL). Because sample sizes in some categories were small, we collapsed these categories into 4 for analytic purposes: “never or less than 3 per month,” “1–6 per week,” “1 per day,” and “2 or more per day.” In addition, we treated the ASB consumption as continuous by taking the midpoint of the 9 categories to create a continuous variable.
SSB consumption
Participants were asked on the FFQ how often they consumed regular soft drinks (not diet) during the past 3 mo in number of medium servings (12 ounces or 1 can or 355 mL). The 9 options were the same as for the ASB question. Participants could check their serving size as small, medium, or large. Additionally, participants were asked how often they drank “orange juice and grapefruit juice,” “other fruit juices such as apple, grape,” and “Tang, Kool-Aid, Hi-C, and other fruit drinks” during the past 3 mo in number of 6-ounce glasses (177 mL). For these 3 questions, the response options were “never or less than once per month,” “1 per month,” “2–3 per month,” “1 per week,” “2 per week,” “3–4 per week,” “5–6 per week,” “1 per day,” and “2 or more per day”. These were converted into continuous variables by WHI, the sum of which taken as the continuous variable for SSB consumption in number of 12-ounce (355 mL) cans, after adjusting for serving size. We also analyzed SSBs in 4 categories comparable to ASB consumption: <1 serving/wk, 1 to <7 servings/wk, 1 to <2 servings/d, and ≥2 servings/d.
Water consumption
Participants were asked about the frequency of consumption of tap and bottled water, separately, during the past 3 mo, on the AV3 OS follow-up questionnaire. The same 9 frequency options as for the ASB question were given, except that the serving size was an 8-ounce glass (237 mL). We treated both tap water and bottled water consumption as continuous by taking the midpoint of the 9 categories to create continuous variables. Both variables were converted into the same serving size as ASBs and SSBs (measured in 12-ounce or 355-mL cans). Total water consumption was the sum of tap and bottled water.
DM
Prevalent and incident DM was assessed via questionnaires at enrollment and each annual follow-up. Participants were asked if “a doctor prescribed for the first time any of the following pills or treatments: pills for diabetes or insulin shots for diabetes” since their last medical update. They were classified as self-reported diabetes cases if they answered “yes” to this question. Because the incident diabetes cases were diagnosed in postmenopausal women >50 y of age, they are mostly likely to be type 2 diabetes cases.
Statistical analysis
Descriptive statistics were generated for participants’ characteristics at AV3 within categories of ASB consumption to evaluate their bivariate association. Specifically, means ± SDs were generated as descriptive statistics for each continuous covariate, whereas frequency (percentages) were generated for each categorical covariate. Potential confounders or mediators were determined based on previous knowledge and prior literature. Potential confounders measured at AV3 were included in the multivariable Cox proportional hazards models if the covariate was determined to be not on the causal pathway. The full model included ASB consumption (never or <3 servings/mo, 1–6 servings/wk, 1 serving/d, or ≥2 servings/d), SSB consumption (<1 serving/wk, 1 to <7 servings/wk, 1 to <2 servings/d, or ≥2 servings/d), age (continuous), race (non-Hispanic white, African American, Hispanic, or other), marital status (partnered or not partnered), family income (<$20k, $20k to <$50k, ≥$50k, or missing), education (more than high school, high school, some college or associate degree, college graduate or above), family history of diabetes (yes, no, or missing), BMI (continuous), change in BMI (continuous), waist-to-hip ratio (WHR; continuous), systolic blood pressure (continuous), insurance status (yes, no, or missing), antihypertensive use (yes or no), antihyperlipidemic use (yes or no), hormone replacement therapy use (never, past, current, or missing), calibrated energy (continuous, calibrated based on age, BMI, and ethnicity by using doubly labeled water) (22), glycemic load (GL) based on available carbohydrates (continuous), glycemic index (GI) based on available carbohydrates (continuous), Alternate Healthy Eating Index (continuous) (23), cardiovascular history (yes or no), hysterectomy history (yes or no), smoking status (never, past, current, or missing), physical activity (>20, 8.4–20, 1.8 to <8.4, or <1.8 metabolic equivalent h/wk), sitting time (≤5, 5 to <10, or ≥10 h/d), and alcohol consumption (continuous). An interaction term for SSB and ASB intake tested their multiplicative interaction.
Follow-up duration in person-years was calculated as the interval between AV3 and the earliest of any of the following: 1) date of annual medical history update when new DM was reported, 2) date of last data collection from the main study if the participant did not enter the Extension Study, 3) date of last data collection from the Extension Study, or 4) date of reported death. Adjusted HRs were computed for the relation between beverage consumption and the incidence of diabetes from Cox proportional hazards models. We visually examined the proportionality assumptions of the hazards models across ASB categories by using the log (log (survival probability) versus log (person-years) plot, and no violation was found. The analyses were also performed stratifying on BMI categories (normal weight, overweight, and obese) and simultaneously controlling for continuous BMI within each stratum.
We also broke down SSBs into 3 components: fruit juice, regular soda, and fruit drinks. They were included in the models simultaneously in place of SSBs, categorized as follows: <1 serving/wk (reference), 1 serving/wk to <1 serving/d, and ≥1 serving/d. The highest 2 categories used for SSB consumption were collapsed to achieve larger sample sizes and numbers of diabetes cases.
To model the potential impact of the replacement of ASBs and SSBs with water, we performed substitution analysis to examine whether there was a beneficial effect on the risk of developing DM. We included ASBs, SSBs, and water as continuous variables in the same model and used the differences in their β coefficients to estimate the HRs for substituting ASBs with water, substituting SSBs with water, and substituting SSBs with ASBs. The 95% CIs were computed by using their variance and covariance matrices (24, 25).
Because a substantial proportion (11.5%) of the participants was missing ASB information at AV3 and missing was significantly associated with BMI, we were concerned about missing data potentially biasing our results. We therefore performed inverse probability weighting (IPW) analysis as a sensitivity analysis (26). A prediction model was first built for the probability of not missing ASB information, and the reciprocals of the probabilities were used as weights in the Cox proportional hazards models to evaluate the association between ASB consumption and DM risk. We also performed lag analysis in which the diabetes cases that developed within the first 2, 3, or 4 y of follow-up were excluded, because of concerns of undetected prediabetes cases at AV3. All statistical analyses were conducted in SAS version 9.4 (SAS Institute).
RESULTS
Of the 93,676 women who participated in the OS, 64,850 were part of the current analysis. There was a total of 4675 incident DM cases, and the average length of follow-up was 8.4 y. The baseline characteristics according to the category of consumption of ASBs are presented in Table 1. Approximately 65.2% of the participants reported consuming <3 ASB servings/mo, 22.5% reported consuming 1–6 ASB servings/wk, 7.6% reported consuming 1 ASB serving/d, and 4.7% reported consuming >1 ASB servings/d. Women consuming >1 ASB servings/d were younger, were more likely to be former or current smokers, were less physically active, were more likely to be overweight or obese, had a higher proportion of WHR >0.85, were more likely to be on lipid-lowering drugs, were more likely to have a family history of DM, had greater energy intake, and had diets with higher GI and GL, although the differences in GI and GL may not be clinically meaningful.
TABLE 1.
Descriptive characteristics (at the year 3 annual visit unless otherwise specified) by ASB consumption categories1
| ASB consumption categories (n = 64,850) |
||||
| Never or <3 servings/mo (n = 42,257) | 1–6 servings/wk (n = 14,602) | 1 serving/d (n = 4961) | ≥2 servings/d (n = 3030) | |
| Age at screening, y | 64.1 ± 7.3 | 62.8 ± 7.1 | 61.5 ± 7.1 | 60.1 ± 6.8 |
| Race/ethnicity, n (%) | ||||
| White, non-Hispanic | 36,524 (86.4) | 12,974 (88.9) | 4432 (89.3) | 2738 (90.4) |
| African American | 2579 (6.1) | 773 (5.3) | 235 (4.7) | 148 (4.9) |
| Hispanic/Latino | 1179 (2.8) | 401 (2.8) | 141 (2.8) | 81 (2.7) |
| Other | 1975 (4.7) | 454 (3.1) | 153 (3.1) | 63 (2.1) |
| Education level, n (%) | ||||
| Less than high school | 1425 (3.4) | 525 (3.6) | 194 (3.9) | 117 (3.9) |
| High school | 10,203 (24.2) | 3710 (25.4) | 1212 (24.4) | 752 (24.8) |
| Some college or associate degree | 11,098 (26.3) | 3908 (26.8) | 1353 (27.3) | 877 (28.9) |
| College graduate or higher | 19,531 (46.2) | 6459 (44.2) | 2202 (44.4) | 1284 (42.4) |
| Family income, n (%) | ||||
| <$20,000 | 5034 (11.9) | 1433 (9.8) | 476 (9.6) | 344 (11.3) |
| $20,000 to <$50,000 | 16,448 (38.9) | 5566 (38.1) | 1753 (35.3) | 1114 (36.8) |
| ≥$50,000 | 18,267 (43.2) | 6783 (46.5) | 2451 (49.4) | 1429 (47.2) |
| Missing | 2508 (5.9) | 820 (5.6) | 281 (5.7) | 146 (4.8) |
| BMI (continuous), kg/m2 | 26.5 ± 5.3 | 28.0 ± 5.4 | 28.6 ± 5.8 | 29.8 ± 6.4 |
| BMI, n (%) | ||||
| 18.5 to <25.0 | 19,225 (45.5) | 4798 (32.9) | 1439 (29.0) | 701 (23.1) |
| 25.0 to <30.0 | 14,492 (34.3) | 5553 (38.0) | 1873 (37.8) | 1076 (35.5) |
| ≥30.0 | 8540 (20.2) | 4251 (29.1) | 1649 (33.2) | 1253 (41.4) |
| Change in BMI between enrollment and year 3 | 0.3 ± 2.9 | 0.4 ± 2.9 | 0.4 ± 3.2 | 0.6 ± 3.3 |
| Waist-to-hip ratio | 0.80 ± 0.08 | 0.81 ± 0.08 | 0.81 ± 0.08 | 0.82 ± 0.08 |
| Systolic blood pressure, mm Hg | 125.9 ± 17.4 | 125.8 ± 16.9 | 124.8 ± 16.6 | 124.9 ± 16.7 |
| Diastolic blood pressure, mm Hg | 73.2 ± 9.3 | 73.6 ± 9.0 | 73.8 ± 9.1 | 74.1 ± 9.1 |
| Has health insurance | ||||
| No | 825 (2.0) | 235 (1.6) | 89 (1.8) | 92 (3.0) |
| Yes | 41,087 (97.2) | 14,238 (97.5) | 4821 (97.2) | 2910 (96.0) |
| Missing | 345 (0.8) | 129 (0.9) | 51 (1.0) | 28 (0.9) |
| Calibrated total energy intake,2 kcal/d | 2217.7 | 2293.3 | 2344.6 | 2420.8 |
| Glycemic index based on available carbohydrates | 51.2 ± 4.0 | 51.3 ± 4.0 | 51.4 ± 4.5 | 51.8 ± 5.0 |
| Glycemic load based on available carbohydrates | 89.2 ± 35.6 | 89.5 ± 36.0 | 89.8 ± 37.3 | 93.9 ± 44.7 |
| Smoking status, n (%) | ||||
| Never | 22,436 (53.1) | 7359 (50.4) | 2394 (48.3) | 1355 (44.7) |
| Former | 17,947 (42.5) | 6706 (45.9) | 2358 (47.5) | 1454 (48.0) |
| Current | 1851 (4.4) | 532 (3.6) | 207 (4.2) | 218 (7.2) |
| Missing | 23 (0.1) | 5 (0.03) | 2 (0.04) | 3 (0.1) |
| Physical activity, metabolic equivalent h/wk, n (%) | ||||
| >20 | 11,090 (26.2) | 3718 (25.5) | 1210 (24.4) | 652 (21.5) |
| 8.4–20 | 12,673 (30.0) | 4428 (30.3) | 1380 (27.8) | 751 (24.8) |
| 1.8 to <8.4 | 10,650 (25.2) | 3723 (25.5) | 1340 (27.0) | 799 (26.4) |
| <1.8 | 7844 (18.6) | 2733 (18.7) | 1031 (20.8) | 828 (27.3) |
| Sitting time, h/d, n (%) | ||||
| ≤5 | 16,281 (38.5) | 5471 (37.5) | 1722 (34.7) | 921 (30.4) |
| >5 to <10 | 18,050 (42.7) | 6357 (43.5) | 2110 (42.5) | 1221 (40.3) |
| ≥10 | 7926 (18.8) | 2774 (19.0) | 1129 (22.8) | 888 (29.3) |
| Alcohol consumption, g/d | 6.0 ± 11.7 | 5.7 ± 11.0 | 5.5 ± 10.9 | 4.6 ± 10.7 |
| Using antihypertensives, n (%) | 5843 (13.8) | 2173 (14.9) | 733 (14.8) | 441 (14.6) |
| Using antihyperlipidemics, n (%) | 5768 (13.7) | 2377 (16.3) | 846 (17.1) | 508 (16.8) |
| Family history of diabetes, n (%) | ||||
| No | 29,594 (67.7) | 9356 (64.1) | 3123 (63.0) | 1859 (61.4) |
| Yes | 11,833 (28.0) | 4635 (31.7) | 1650 (33.3) | 1023 (33.8) |
| Missing | 1830 (4.3) | 611 (4.2) | 188 (3.8) | 148 (4.9) |
| SSB consumption, servings/d | 0.5 ± 0.5 | 0.4 (0.4) | 0.5 (0.5) | 0.5 (1.0 |
| SSB consumption, categorical | ||||
| <1 serving/wk | 12,950 (30.7) | 4434 (30.4) | 1720 (34.7) | 1297 (42.8) |
| 1–6 servings/wk | 25,125 (59.5) | 9100 (62.3) | 2608 (52.6) | 1295 (42.7) |
| 1 to <2 servings/d | 3479 (8.2) | 923 (6.3) | 550 (11.1) | 221 (7.3) |
| ≥2 servings/d | 703 (1.7) | 145 (1.0) | 83 (1.7) | 217 (7.2) |
| Regular soda (servings/d) | 0.11 ± 0.4 | 0.10 ± 0.3 | 0.14 ± 0.4 | 0.26 ± 0.9 |
| Fruit juice (servings/d) | 0.32 ± 0.3 | 0.30 ± 0.3 | 0.29 ± 0.3 | 0.25 ± 0.3 |
| Fruit drinks (servings/d) | 0.02 ± 0.1 | 0.02 ± 0.1 | 0.02 ± 0.1 | 0.03 ± 0.2 |
| Incident DM, n (%) | 2751 (6.5) | 1108 (7.6) | 485 (9.8) | 331 (10.9) |
Values are means ± SDs unless otherwise indicated. ASB, artificially sweetened beverage; DM, diabetes mellitus; SSB, sugar-sweetened beverage.
Values are geometric means.
There was a significant dose-response relation between ASB consumption and diabetes after adjusting for SSB consumption, age, race, marital status, family income, education, family history of diabetes, BMI, change in BMI, WHR, systolic blood pressure, insurance status, usual care provider, timing of last medical visit, antihypertensive use, antihyperlipidemic use, hormone replacement therapy use, calibrated total energy intake (22), GL and GI based on available carbohydrates, Alternate Healthy Eating Index (23), cardiovascular disease history, hysterectomy history, smoking status, physical activity, sitting time, and alcohol consumption, with HRs of 1.03 (95% CI: 0.96, 1.11), 1.24 (95% CI: 1.13, 1.37), and 1.21 (95% CI: 1.08, 1.36) for ASB consumption of 1–6 servings/wk, 1 serving/d, and ≥2 servings/d, respectively, compared with never or <3 servings/mo (P-trend < 0.0001) (Table 2). From the same model, consumption of SSB also increased the risk of diabetes with HRs of 1.05 (95% CI: 0.98, 1.12), 1.09 (95% CI: 0.97, 1.23), and 1.43 (95% CI: 1.17, 1.75) for SSB consumption of 1 to <7 servings/wk, 1 to <2 servings/d, and ≥2 servings/d, respectively, compared with <1 serving/wk (P-trend = 0.0004) (1 serving = one 12-ounce can or 355 mL). The interaction term for SSB and ASB intake was not significant and thus dropped from the model.
TABLE 2.
Associations between ASB consumption and incident diabetes mellitus1
| ASB consumption categories (n = 64,850) |
|||||
| Never or <3 servings/mo | 1–6 servings/wk | 1 serving/d | ≥2 servings/d | P-trend | |
| n (%) | 42,257 (65.16) | 14,602 (22.52) | 4961 (7.65) | 3030 (4.67) | |
| Cases, n | 2751 | 1108 | 485 | 331 | |
| Unadjusted | Referent | 1.16 (1.08, 1.25) | 1.51 (1.37, 1.66) | 1.70 (1.51, 1.90) | <0.0001 |
| Age adjusted | Referent | 1.18 (1.10, 1.26) | 1.55 (1.40, 1.70) | 1.76 (1.57, 1.97) | <0.0001 |
| Age and race adjusted | Referent | 1.20 (1.12, 1.29) | 1.60 (1.45, 1.76) | 1.83 (1.63, 2.05) | <0.0001 |
| Fully adjusted2 | Referent | 1.03 (0.96, 1.11) | 1.24 (1.13, 1.37) | 1.21 (1.08, 1.36) | <0.0001 |
Values are HRs (95% CIs) estimated from Cox proportional hazards models. ASB, artificially sweetened beverage.
Model adjusted for age, race, marital status, family income, education, family history of diabetes, BMI, change in BMI, waist-to-hip ratio, systolic blood pressure, insurance status, antihypertensive use, antihyperlipidemic use, hormone replacement therapy use, calibrated energy, sugar-sweetened beverage consumption, glycemic load based on available carbohydrates, glycemic index based on available carbohydrates, Alternate Healthy Eating Index, cardiovascular history, hysterectomy history, smoking status, physical activity, sitting time, and alcohol consumption.
We further stratified the analytic sample based on BMI categories including normal weight (18.5 ≤ BMI < 25); overweight (25 ≤ BMI < 30), and obese (BMI ≥30), with adjustment for continuous BMI. Consumption of ASBs was most significantly associated with incident diabetes in obese women. This relation remained significant after adjustment for all previously described covariates. The HRs were 1.02 (95% CI: 0.92, 1.13), 1.24 (95% CI: 1.08, 1.41), and 1.26 (95% CI: 1.09, 1.46) for ASB consumption of 1–6 servings/wk, 1 serving/d, and ≥2 servings/d, respectively, compared with never or <3 servings/mo (P-trend = 0.0002) (Table 3).
TABLE 3.
Associations between ASB consumption and incident diabetes mellitus stratified by BMI categories1
| ASB consumption categories (n = 64,850) |
||||||
| BMI categories, kg/m2 | n | Never or <3 servings/mo | 1–6 servings/wk | 1 serving/d | ≥2 servings/d | P-trend |
| Normal weight (18.5 to <25.0) | 26,163 | |||||
| Unadjusted | Referent | 0.96 (0.81, 1.14) | 1.13 (0.87, 1.47) | 1.11 (0.76, 1.62) | 0.4277 | |
| Age and race adjusted | Referent | 1.05 (0.88, 1.24) | 1.35 (1.03, 1.76) | 1.38 (0.94, 2.02) | 0.0434 | |
| Full model2 | Referent | 0.98 (0.82, 1.16) | 1.13 (0.86, 1.49) | 1.21 (0.83, 1.78) | 0.2359 | |
| Overweight (25.0 to <30.0) | 22,994 | |||||
| Unadjusted | Referent | 1.01 (0.90, 1.14) | 1.22 (1.03, 1.45) | 1.05 (0.83, 1.33) | 0.3725 | |
| Age and race adjusted | Referent | 1.08 (0.96, 1.22) | 1.34 (1.12, 1.59) | 1.20 (0.95, 1.52) | 0.0417 | |
| Full model2 | Referent | 1.03 (0.91, 1.16) | 1.24 (1.05, 1.48) | 1.01 (0.80, 1.28) | 0.5649 | |
| Obese (≥30.0) | 15,693 | |||||
| Unadjusted | Referent | 1.00 (0.90, 1.11) | 1.27 (1.11, 1.45) | 1.38 (1.19, 1.59) | <0.0001 | |
| Age and race adjusted | Referent | 1.01 (0.92, 1.12) | 1.29 (1.13, 1.47) | 1.38 (1.20, 1.60) | <0.0001 | |
| Full model2 | Referent | 1.02 (0.92, 1.13) | 1.24 (1.08, 1.41) | 1.26 (1.09, 1.46) | 0.0002 | |
Values are HRs (95% CIs) estimated from Cox proportional hazards models. The interaction between ASB and BMI was not significant, but we were concerned about the possible reverse causality between BMI and ASB consumption, so we performed stratified analysis with respect to BMI categories. ASB, artificially sweetened beverages.
Full model adjusted for age, race, marital status, family income, education, family history of diabetes, BMI, change in BMI, waist-to-hip ratio, systolic blood pressure, insurance status, antihypertensive use, antihyperlipidemic use, hormone replacement therapy use, calibrated energy, sugar-sweetened beverages, glycemic load based on available carbohydrates, glycemic index based on available carbohydrates, Alternate Healthy Eating Index, cardiovascular history, hysterectomy history, smoking status, physical activity, sitting time, and alcohol consumption.
When breaking down SSBs into fruit juice, regular soda, and fruit drinks, compared with <1 serving/wk, consumption of fruit juice was associated with an HR of 1.04 (95% CI: 0.98, 1.11) and an HR of 1.24 (95% CI: 1.07, 1.44), respectively, for 1 serving/wk to <1 serving/d and ≥1 serving/d, whereas regular soda was also associated with a HR of 1.12 (95% CI: 1.02, 1.22) and an HR of 1.12 (95% CI: 0.97, 1.29), respectively, and fruit drinks with an HR of 0.99 (95% CI: 0.85, 1.15) and an HR of 1.33 (95% CI: 0.89, 1.98), respectively, adjusting for all other covariates in the full model.
Treating ASBs and SSBs as continuous variables, we found an increased risk for both ASBs and SSBs without significant interaction. The HR associated with every 12 ounces (355 mL) of increased ASB consumption was 1.07 (95% CI: 1.03, 1.11), and the HR associated with the same amount increase of SSB consumption was 1.13 (95% CI: 1.07, 1.20). Modeling the substitution of 1 serving ASB/d for water suggested a reduction in the risk of DM by 5% (HR: 0.95; 95% CI: 0.91, 0.99), whereas modeling the substitution of 1 serving SSB/d for water suggested a reduction in the risk of DM by 10% (HR: 0.90; 95% CI: 0.85, 0.95). We also modeled the substitution of SSBs with ASBs, which suggested a potential but nonsignificant reduction in the risk (HR: 0.94; 95% CI: 0.88, 1.01).
Sensitivity analysis with the use of IPW for missing ASB consumption information showed similar results (Supplemental Table 1). Because some individuals may change their dietary habits and/or body weights if they had prediabetes, we performed another sensitivity analysis excluding individuals who developed diabetes in the first 2–4 y after ASB assessment (Supplemental Table 2). The association remained significant for those who consumed 1 or ≥2 ASB servings/d compared with the lowest consumption category (never or <3 servings/mo). In addition, because the highest intake of ASBs was also associated with higher amounts of SSBs, we examined the association of ASBs with incident DM in those with <1 SSB serving/wk and found a similar graded response—HR: 1.15 (95% CI: 1.02, 1.32), HR: 1.20 (95% CI: 1.01, 1.44), and HR: 1.25 (95% CI: 1.03, 1.51) for ASB consumption of 1–6 servings/wk, 1 serving/d, and ≥2 servings/d, respectively, compared with never or <3 servings/mo.
DISCUSSION
In our analyses, ASBs and SSBs were both associated with an increased risk of DM independent of multiple known risk factors, including BMI, change in BMI, and total energy intake at the start of follow-up. As mentioned in the Introduction, numerous studies have associated a higher consumption of SSBs with an increased risk of diabetes (6–17), but evidence was inconsistent for ASBs. In some previous prospective cohort studies, ASBs were shown to have no association with an increased risk of type 2 diabetes (9, 11). The EPIC (European Prospective Investigation into Cancer and Nutrition)-Norfolk found an elevated risk of DM associated with ASBs, but adjustment for adiposity attenuated the association (16). However, the Multi-Ethnic Study of Atherosclerosis showed that ASB consumption was associated with type 2 diabetes after multivariate adjustment (20). Similarly, findings from the EPIC-French cohort (13) and a Japanese cohort of men (17) demonstrated positive associations between ASB intake and diabetes, when accounting for baseline BMI. A recent meta-analysis by Greenwood et al. (15), combining the results from multiple prospective cohorts, found an increased relative risk of diabetes of 1.20 for 330 mL SSB/d (P < 0.001) and a relative risk of 1.13 for 330 mL ASB/d (P = 0.02), which was attenuated by adjustment of BMI, but the authors cautioned that the interpretation may have been hampered by large degrees of heterogeneity and the possibility of reverse causality and lack of adjustment for residual confounding. Similar findings were obtained by a more recent meta-analysis, which nonetheless concluded that ASBs were unlikely to be healthy alternatives to SSBs for the prevention of type 2 diabetes (27). Both SSBs and ASBs appeared to be associated with an increased risk of metabolic syndrome development, a precursor to diabetes, in the Framingham Heart Study (28) and the Atherosclerosis Risk in Communities Study (29). Consistent with these findings, our results supported a detrimental role of both SSBs and ASBs in the development of DM. Evidence from randomized controlled trials was largely inconclusive. In a systematic review and meta-analysis, which compared noncaloric sweeteners and saccharides, 2-h glucose responses were not significantly different based on 3 trials identified, although another 3 trials examined longer-term outcomes and found a significant energy reduction in participants consuming the noncaloric sweetener, but inconsistent results for weight and BMI and nonsignificant differences in glycated hemoglobin, insulin resistance, and lipid profile (30). However, in a later subgroup analysis, a 10-wk diet high in sucrose resulted in significant elevations of postprandial glycemia, insulinemia, and lipidemia compared with a diet high in artificial sweeteners (31). Although these trials mostly failed to detect adverse effects of artificial sweeteners on glucose metabolism and risk factors of diabetes, they were all of relatively short duration and in comparison with caloric sweeteners rather than plain water.
Apart from the elevated energy intake and weight gain associated with SSB consumption, calories from the rapidly absorbed sugars in liquid form have been suggested to result in less satiety and an incomplete compensatory reduction in energy intake at subsequent meals (32). The moderate-to-high GI of regular soda, fruit drinks, or fruit juice could be an independent risk factor (33). Randomized short-term studies investigating the mechanism have also established causal association of SSBs with insulin resistance and chronic inflammation, both being biomarkers of diabetes mellitus (5). Although nonnutritive sweeteners have generally been considered metabolically inert, some data suggest that these sweeteners may have physiologic effects affecting hormone secretion, as well as intestinal absorption of glucose, that alter appetite and/or glucose metabolism (34). Egan and Margolskee (35) proposed that nonnutritive sweeteners binding to sweet-taste receptors in the intestines may lead to increased glucagon-like peptide 1 secretion, which in turn would lower blood glucose by increasing insulin secretion and thus increase appetite as well as induce weight gain. Corkey (36) raised a similar hypothesis in the 2011 Banting Lecture, speculating that nonnutritive sweeteners and other food additives might induce hypersecretion of the pancreatic islet-cell, leading to hepatic insulin resistance and increased fat accumulation, both key components of obesity and type 2 diabetes. In addition, a recent article by Suez et al. (37) demonstrated that artificial sweeteners induced glucose intolerance by altering gut microbiota in mice and that the induced glucose intolerance was abated by treatment with antibiotics. Additionally, they demonstrated in a small sample of human volunteers that artificial sweetener (5 mg saccharin/kg)–induced dysbiosis and glucose intolerance was transferable by fecal transplants to germ-free mice, suggesting a causal relation. Thus, there are several lines of evidence suggesting a plausible biologic mechanism through which ASBs affects the risk of DM. The caffeine content of both ASBs and SSBs could play a role, but the evidence regarding the relation between caffeine and diabetes appears to be mixed, in that caffeine in coffee has been suggested to be protective of the risk (38, 39), but acute doses of caffeine have also been suggested to impair insulin sensitivity (40).
In the face of a rapidly growing diabetes epidemic, individuals have turned to artificially sweetened foods and ASBs during the past 3 decades. Implicit and explicit messages from manufacturers and conventional wisdom have suggested that the use of artificially sweetened products would help in weight control and might help prevent diabetes as well as metabolic syndrome. However, our study and others (13, 17, 20) showed that ASB consumption is associated with an increased risk of DM independent of multiple confounding factors. Further research is needed to replicate our results and understand whether a causal relation exists between ASBs and DM, especially in light of the findings by Suez et al. (37). Of note, the increased risk of diabetes associated with both ASBs and SSBs could potentially be reduced with water replacement as suggested by our statistical substitution analyses. This is consistent with and somewhat more conservative than the substitution results from the EPIC-Norfolk cohort (16). This supports the notion that, on an individual and population level, switching to water may help control the well-documented increased risk of diabetes and metabolic syndrome with SSBs and perhaps the increased risk with ASBs found in our study.
One of the main strengths of our study is that it was based on a large, multiethnic, geographically dispersed cohort with many of the known DM risk factors measured. Other strengths are that we accounted for ∼8 y of follow-up and that the outcome of self-reported DM has been validated by medical record review and laboratory data in this cohort (41). We tried to account for many confounders that may affect the consumption of ASBs, as well as incidence of diabetes.
One major limitation of this study is its observational study design. There is the possibility that residual confounding might have biased our results, leading to false-positive associations. The possibility of reverse causality, where participants at a higher risk of diabetes or prediabetes choose to consume ASBs in an attempt to control weight, is a particular problem when interpreting these results. We have attempted to control for this in multiple sensitivity analyses including lag analysis, IPW for selection bias due to missing data, and adjustment for BMI, calibrated energy, and changes in BMI from baseline to AV3 when ASB consumption was ascertained. We also showed the strongest association between ASBs and DM in obese women (BMI ≥ 30) even after adjustment of continuous BMI within strata. However, despite our results showing an increased risk of diabetes, reverse causality or unmeasured confounding could still explain our results; therefore, caution is necessary when interpreting the results. Measurement error could always be an issue when assessing dietary intakes from an FFQ or questions similar to those in the FFQ. It has been reported that for most nutrients means estimated by the FFQ were within 10% of the food records or diet recalls, and the correlation between the FFQ and the records and recalls was similar to other FFQs (42). We adjusted for calibrated total energy in an attempt to control for both random and systematic measurement errors in the dietary intakes. The current analysis only included postmenopausal women, so results are not readily generalizable to other populations.
In summary, postmenopausal women with a higher ASB consumption had a dose-dependent increased risk of incident diabetes independent of known risk factors. A significant dose-response association remained after accounting for BMI and other known risk factors in obese women. The risk of developing diabetes associated with ASBs was of smaller magnitude than with SSBs but still elevated to a meaningful degree especially at the highest level of intake. The risk of incident diabetes may potentially be reduced by replacing ASBs and SSBs with water.
Acknowledgments
Program Office—National Heart, Lung, and Blood Institute, Bethesda, Maryland: Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center—Fred Hutchinson Cancer Research Center, Seattle, Washington: Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L Kooperberg, Ruth E Patterson, and Anne McTiernan; Medical Research Laboratories, Highland Heights, Kentucky: Evan Stein; and University of California at San Francisco, San Francisco, California: Steven Cummings. Clinical Centers—Albert Einstein College of Medicine, Bronx, New York: Sylvia Wassertheil-Smoller; Baylor College of Medicine, Houston, Texas: Aleksandar Rajkovic; Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts: JoAnn E Manson; Brown University, Providence, Rhode Island: Charles B Eaton; Emory University, Atlanta, Georgia; Lawrence Phillips; Fred Hutchinson Cancer Research Center, Seattle, Washington: Shirley Beresford; George Washington University Medical Center, Washington, DC: Lisa Martin; Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, California: Rowan Chlebowski; Kaiser Permanente Center for Health Research, Portland, Oregon: Yvonne Michael; Kaiser Permanente Division of Research, Oakland, California: Bette Caan; Medical College of Wisconsin, Milwaukee, Wisconsin: Jane Morley Kotchen; MedStar Research Institute/Howard University, Washington, DC: Barbara V Howard; Northwestern University, Chicago/Evanston, Illinois: Linda Van Horn; Rush Medical Center, Chicago, Illinois: Henry Black; Stanford Prevention Research Center, Stanford, California: Marcia L Stefanick; State University of New York at Stony Brook, Stony Brook, New York: Dorothy Lane; The Ohio State University, Columbus, Ohio: Rebecca Jackson; University of Alabama at Birmingham, Birmingham, Alabama: Cora E. Lewis; University of Arizona, Tucson/Phoenix, Arizona: Cynthia A Thomson; University at Buffalo, Buffalo, New York: Jean Wactawski-Wende; University of California at Davis, Sacramento, California: John Robbins; University of California at Irvine, California: F Allan Hubbell; University of California at Los Angeles, Los Angeles, California: Lauren Nathan; University of California at San Diego, La Jolla/Chula Vista, California: Robert D Langer; University of Cincinnati, Cincinnati, Ohio: Margery Gass; University of Florida, Gainesville/Jacksonville, Florida: Marian Limacher; University of Hawaii, Honolulu, Hawaii: J David Curb; University of Iowa, Iowa City/Davenport, Iowa: Robert Wallace; University of Massachusetts/Fallon Clinic, Worcester, Massachusetts: Judith Ockene; University of Medicine and Dentistry of New Jersey, Newark, New Jersey: Norman Lasser; University of Miami, Miami, Florida: Mary Jo O’Sullivan; University of Minnesota, Minneapolis, Minnesota: Karen Margolis; University of Nevada, Reno, Nevada: Robert Brunner; University of North Carolina, Chapel Hill, North Carolina: Gerardo Heiss; University of Pittsburgh, Pittsburgh, Pennsylvania: Lewis Kuller; University of Tennessee Health Science Center, Memphis, Tennessee: Karen C Johnson; University of Texas Health Science Center, San Antonio, Texas: Robert Brzyski; University of Wisconsin, Madison, Wisconsin: Gloria E Sarto; Wake Forest University School of Medicine, Winston-Salem, North Carolina: Mara Vitolins; and Wayne State University School of Medicine/Hutzel Hospital, Detroit, Michigan: Michael Simon. Women’s Health Initiative Memory Study—Wake Forest University School of Medicine, Winston-Salem, North Carolina: Sally Shumaker.
The authors’ responsibilities were as follows—MH: analyzed the data, interpreted the analysis results, and drafted and revised the manuscript; AQ: conceived the study and drafted the manuscript; LS: helped analyze the data and critically reviewed the manuscript; JMS: helped collect the data and critically reviewed the manuscript; BVH: helped collect the data and critically reviewed the manuscript; RMK: helped interpret the analysis results and critically reviewed the manuscript; BL: helped analyze the data and critically reviewed the manuscript; JEM: helped collect the data and critically reviewed the manuscript; CBE: conceived and designed the study and analysis plan, helped interpret the analysis results, and critically reviewed the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ASB, artificially sweetened beverage; AV3, year 3 annual visit; DM, diabetes mellitus; EPIC, European Prospective Investigation into Cancer and Nutrition; FFQ, food-frequency questionnaire; GI, glycemic index; GL, glycemic load; IPW, inverse probability weighting; OS, observational study; SSB, sugar-sweetened beverage; WHI, Women’s Health Initiative; WHR, waist-to-hip ratio.
REFERENCES
- 1.CDC. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta (GA): US Department of Health and Human Services; 2014. [Google Scholar]
- 2.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, et al. . Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31(Suppl 1):S61–78. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537–43. [DOI] [PubMed] [Google Scholar]
- 5.Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev 2013;14:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paynter NP, Yeh HC, Voutilainen S, Schmidt MI, Heiss G, Folsom AR, Brancati FL, Kao WH. Coffee and sweetened beverage consumption and the risk of type 2 diabetes mellitus: the atherosclerosis risk in communities study. Am J Epidemiol 2006;164:1075–84. [DOI] [PubMed] [Google Scholar]
- 7.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34. [DOI] [PubMed] [Google Scholar]
- 9.Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med 2008;168:1487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odegaard AO, Koh WP, Arakawa K, Yu MC, Pereira MA. Soft drink and juice consumption and risk of physician-diagnosed incident type 2 diabetes: the Singapore Chinese Health Study. Am J Epidemiol 2010;171:701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93:1321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romaguera D, Norat T, Wark PA, Vergnaud AC, Schulze MB, van Woudenbergh GJ, Drogan D, Amiano P, Molina-Montes E, Sanchez MJ, et al. . Consumption of sweet beverages and type 2 diabetes incidence in European adults: results from EPIC-InterAct. Diabetologia 2013;56:1520–30. [DOI] [PubMed] [Google Scholar]
- 13.Fagherazzi G, Vilier A, Saes Sartorelli D, Lajous M, Balkau B, Clavel-Chapelon F. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l’Education Nationale-European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr 2013;97:517–23. [DOI] [PubMed] [Google Scholar]
- 14.Bhupathiraju SN, Pan A, Malik VS, Manson JE, Willett WC, van Dam RM, Hu FB. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr 2013;97:155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenwood DC, Threapleton DE, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, Burley VJ. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr 2014;112:725–34. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor L, Imamura F, Lentjes MA, Khaw KT, Wareham NJ, Forouhi NG. Prospective associations and population impact of sweet beverage intake and type 2 diabetes, and effects of substitutions with alternative beverages. Diabetologia 2015;58:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakurai M, Nakamura K, Miura K, Takamura T, Yoshita K, Nagasawa SY, Morikawa Y, Ishizaki M, Kido T, Naruse Y, et al. . Sugar-sweetened beverage and diet soda consumption and the 7-year risk for type 2 diabetes mellitus in middle-aged Japanese men. Eur J Nutr 2014;53:251–8. [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–7. [DOI] [PubMed] [Google Scholar]
- 19.Gardner C, Wylie-Rosett J, Gidding SS, Steffen LM, Johnson RK, Reader D, Lichtenstein AH. Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2012;35:1798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR Jr. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009;32:688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Design of the women’s health initiative clinical trial and observational study. The women’s health initiative study group. Control Clin Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 22.Prentice RL, Mossavar-Rahmani Y, Huang Y, Van Horn L, Beresford SA, Caan B, Tinker L, Schoeller D, Bingham S, Eaton CB, et al. . Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am J Epidemiol 2011;174:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–71. [DOI] [PubMed] [Google Scholar]
- 24.Pan A, Malik VS, Schulze MB, Manson JE, Willett WC, Hu FB. Plain-water intake and risk of type 2 diabetes in young and middle-aged women. Am J Clin Nutr 2012;95:1454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40. [DOI] [PubMed] [Google Scholar]
- 26.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res 2013;22:278–95. [DOI] [PubMed] [Google Scholar]
- 27.Imamura F, O’Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015;351:h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D’Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007;116:480–8. [DOI] [PubMed] [Google Scholar]
- 29.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation 2008;117:754–61. [DOI] [PubMed] [Google Scholar]
- 30.Wiebe N, Padwal R, Field C, Marks S, Jacobs R, Tonelli M. A systematic review on the effect of sweeteners on glycemic response and clinically relevant outcomes. BMC Med 2011;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raben A, Moller BK, Flint A, Vasilaris TH, Christina Moller A, Juul Holst J, Astrup A. Increased postprandial glycaemia, insulinemia, and lipidemia after 10 weeks’ sucrose-rich diet compared to an artificially sweetened diet: a randomised controlled trial. Food Nutr Res 2011. Jul 20 [Epub ahead of print; DOI: 10.3402/fnr.v55i0.5961]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord 2000;24:794–800. [DOI] [PubMed] [Google Scholar]
- 33.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown RJ, Rother KI. Non-nutritive sweeteners and their role in the gastrointestinal tract. J Clin Endocrinol Metab 2012;97:2597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egan JM, Margolskee RF. Taste cells of the gut and gastrointestinal chemosensation. Mol Interv 2008;8:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corkey BE. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 2012;61:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, et al. . Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014;514:181–6. [DOI] [PubMed] [Google Scholar]
- 38.Jiang X, Zhang D, Jiang W. Coffee and caffeine intake and incidence of type 2 diabetes mellitus: a meta-analysis of prospective studies. Eur J Nutr 2014;53:25–38. [DOI] [PubMed] [Google Scholar]
- 39.Santos RM, Lima DR. Coffee consumption, obesity and type 2 diabetes: a mini-review. Eur J Nutr 2016;55:1345–58. [DOI] [PubMed] [Google Scholar]
- 40.Shearer J, Graham TE. Performance effects and metabolic consequences of caffeine and caffeinated energy drink consumption on glucose disposal. Nutr Rev 2014;72 Suppl 1:121–36. [DOI] [PubMed] [Google Scholar]
- 41.Jackson JM, DeFor TA, Crain AL, Kerby TJ, Strayer LS, Lewis CE, Whitlock EP, Williams SB, Vitolins MZ, Rodabough RJ, et al. . Validity of diabetes self-reports in the Women’s Health Initiative. Menopause 2014;21:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–87. [DOI] [PubMed] [Google Scholar]