Abstract
Background: Cholecystokinin (CCK) is an important satiety factor, acting at type 1 receptors (CCK1Rs) on vagal afferent neurons; however, CCK agonists have failed clinical trials for obesity. We postulated that CCK1R function might be defective in such patients due to abnormal membrane composition, such as that observed in cholesterol gallstone disease.
Objective: Due to the challenges in directly studying CCK1Rs relevant to appetite control, our goal was to develop and apply a method to determine the impact of a patient’s own cellular environment on CCK stimulus-activity coupling and to determine whether CCK sensitivity correlated with the metabolic phenotype of a high-risk population.
Design: Wild-type CCK1Rs were expressed on leukocytes from 112 Hispanic patients by using adenoviral transduction and 24-h culture, with quantitation of cholesterol composition and intracellular calcium responses to CCK. Results were correlated with clinical, biochemical, and morphometric characteristics.
Results: Broad ranges of cellular cholesterol and CCK responsiveness were observed, with elevated cholesterol correlated with reduced CCK sensitivity. This was prominent with increasing degrees of obesity and the presence of diabetes, particularly when poorly controlled. No single standard clinical metric correlated directly with CCK responsiveness. Reduced CCK sensitivity best correlated with elevated serum triglycerides in normal-weight participants and with low HDL concentrations and elevated glycated hemoglobin in obese and diabetic patients.
Conclusions: CCK responsiveness varies widely across the population, with reduced signaling in patients with obesity and diabetes. This could explain the failure of CCK agonists in previous clinical trials and supports the rationale to develop corrective modulators to reverse this defective servomechanism for appetite control. This trial was registered at www.clinicaltrials.gov as NCT03121755.
Keywords: cholecystokinin, obesity drug development, hormonal responsiveness, population study, cholesterol, epidemiology
See corresponding editorial on page 437.
INTRODUCTION
Obesity has reached epidemic proportions and is associated with similar increases in type 2 diabetes with its comorbidities, resulting in immense personal and societal burdens. Lifestyle modification, although acutely effective, is not durable, and bariatric surgery, although effective in morbidly obese patients, is not a scalable solution for this cohort. Antiobesity drugs have had limited efficacy, substantial side effects, and poor long-term adherence, limiting their usefulness as a pharmacologic solution. Therefore, substantial interest exists in the use of gastrointestinal regulators of appetite that might contribute to the effectiveness of bariatric surgery, as single agents or in rational combinations (1–3). This has resulted in the recent approval of a glucagon-like peptide 1 agonist, used also for diabetes, as a satiety agent (4). However, the long-term safety and efficacy for this class of drugs in nondiabetic obese individuals have not been established (5, 6).
The earliest and most prominent gastrointestinal hormone recognized as a satiety factor is cholecystokinin (CCK) (7, 8), which also plays key roles in the regulation of digestive events (9). The role of CCK in appetite control is mediated via the type 1 CCK receptor (CCK1R), which is present on vagal afferent neurons (10, 11). These observations were instrumental in stimulating efforts to develop potent CCK1R agonists (12–15); however, in clinical trials for obesity, none of these reached the primary endpoints of effecting weight reduction more than acute dieting (16). We now postulate that abnormal CCK stimulus-activity coupling with reduced responsiveness to CCK agonists in the study populations could explain these failed trials.
Although CCK1R dysfunction was reported in a rare patient with abnormal processing of the CCK1R gene (17), no hormone or CCK1R variants have been described as being common enough to play a substantial role in the obesity epidemic. In contrast, we know that this receptor is sensitive to its microenvironment and to the composition of the membrane (18). Excess cholesterol in gallbladder myocytes in patients with cholesterol gallstones negatively affects CCK stimulus-activity coupling and CCK responsiveness (19–21). Although this has been assumed to be limited to cells in close contact with lithogenic bile, where cholesterol can be directly transferred, little is known about the microenvironment of CCK1Rs in the periphery in the general population, which could be of a scale relevant to having an impact on the role of CCK in appetite regulation.
Unfortunately, the only source of endogenous CCK1Rs accessible for functional testing in humans is excised gallbladders, where excess cholesterol and CCK1R dysfunction have already been recognized (19–21). It is quite challenging to directly study CCK1Rs on the target relevant to the effect of this hormone on appetite, vagal afferent neurons in the gut wall (10, 11). Because of the possibility that these CCK1Rs would be similar to those in other peripheral cells, we developed a method to introduce wild-type CCK1Rs ex vivo into circulating leukocytes where the impact of the individual’s own cells on receptor function could be quantified. After establishing and validating this technique, we applied it to participants undergoing metabolic phenotyping at a Southwest community medical center where the predominantly Hispanic population has a high prevalence of obesity.
METHODS
Study participants
This study was approved by the Mayo Clinic Institutional Review Board and registered at www.clinicaltrials.gov as NCT03121755, with all volunteers prospectively providing written informed consent. Study participants came from the Sangre Por Salud Biobank, which includes Hispanic adults between the ages of 18 and 85 y who are not pregnant and who have not had a diagnosis of cancer within 3 y, and who are being seen at a federally qualified community health center in Phoenix, Arizona. In this setting, most patients are of Mexican-American descent, representing an underserved minority population with a disproportionate prevalence of obesity and diabetes. Participants had complete medical histories, physical examinations, and a wide variety of common laboratory analyses, including a lipid profile and cardiometabolic risk factors, as well as an oral-glucose-tolerance test (unless they already had a diagnosis of diabetes mellitus) and determination of insulin sensitivity. For the current study, which was added on to the large, ongoing Sangre Por Salud study, participants were entered in a random, unselected manner, recognizing the need for freshly acquired blood samples and the temporal limitations for cell manipulations and analysis. Biospecimens and clinical and laboratory data were provided for this study in a coded, deidentified manner, with the code maintained by the general study coordinator.
Study design
Freshly drawn blood samples (∼5 mL) collected in EDTA-coated tubes were delivered to the laboratory, where buffy coat cells were prepared and analyzed for total cholesterol and protein. CCK responsiveness was studied 24 h after viral transduction. This provided direct analysis of the cholesterol content of the participants’ cells, as well as quantitation of CCK stimulus-activity coupling at the wild-type CCK1R expressed in these cells, which reflects the natural membrane microenvironment for each participant. Experimental data were fully analyzed before the clinical, biochemical, and morphometric data collected from the same participants were shared with investigators for the evaluation of correlations. The optimized procedures routinely used for sample handling are described below.
Cellular protein and cholesterol determinations
Buffy coat cells were isolated by using LymphoPrep (Axis-Shield) and washed before analysis for protein and cholesterol composition. Cells were sonicated and solubilized in phosphate-buffered saline containing 0.5% Triton X-100. Cholesterol was extracted from this preparation by using the method of Bligh and Dyer (22). Cholesterol content was quantified by using the Amplex Red Cholesterol Assay Kit (Invitrogen) according to the manufacturer’s protocol. The protein concentration from the same solubilized cell preparation was determined by using the Pierce BCA Protein Assay Kit (Thermo Scientific) with bovine serum albumin as the standard.
CCK1R-expressing adenovirus preparation and cell transduction
Wild-type human CCK1R complementary DNA was inserted into an adenoviral shuttle plasmid vector, dual-CCM(–), with sequence verified by direct DNA sequencing. This construct was then integrated into the arginine-glycine-aspartic acid sequence (RGD)–modified, E1/E3-deleted, enhanced green fluorescent protein (eGFP)–containing Ad5 genome by homologous recombination (Vector Biolabs), followed by transduction of human embryonic kidney (HEK)–293 cells for viral packaging and amplification. The reagent used for our studies contained ∼2 × 1010 plaque forming units/milliliter.
Buffy coat cells were mixed with Ad5(RGD-GFP)-human CCK1R at a multiplicity of infection of 100 in serum-free Roswell Park Memorial Institute–1640 medium containing 5 μg polybrene/mL. The mixture was then dispensed into poly-l-lysine–coated, clear-bottom, black, 96-well tissue culture plates at a density of 100,000 cells/well and incubated at 37°C in the presence of 5% CO2 for 3–4 h before the addition of complete Roswell Park Memorial Institute–1640 medium. Cells from each sample were distributed into 34 wells, supporting two 8-point dose-response curves set up in duplicate, as well as 2 wells with no virus, which were included as controls for background fluorescence.
CCK stimulus-activity coupling determination
Leukocytes transduced with CCK1R were studied for the ability of CCK to stimulate intracellular calcium responses. On the day after transduction, plates were centrifuged at room temperature at 400 × g for 5 min, and culture medium was carefully aspirated. Cells were incubated with 80 μL of 4.5 μM Cal-520/well in the presence of 1X Screen Quest 10X calcium assay buffer with Phenol Red Plus (AAT Bioquest) diluted in Krebs-Ringer-HEPES medium (25 mmol HEPES/L, pH 7.4; 104 mmol NaCl, 5 mmol KCl, 1.5 mmol CaCl2, 1.0 mmol KH2PO4, 1.2 mmol MgSO4, and 1.2 mmol MgCl2/L) containing 0.2% bovine serum albumin for 1 h at 37°C in the dark. After this, 80 μL Krebs-Ringer-HEPES medium containing 0.2% bovine serum albumin and 1 mmol probenecid/L was added, and the plates were centrifuged at 300 × g for 2 min. The intracellular calcium assay was performed in a Flexstation 3.0 plate reader (Molecular Devices) by using robotic addition of 40 μL various CCK-8 (Peninsula Laboratories) concentrations. Intracellular calcium responses were measured at 37°C by quantifying the fluorescence emission intensity at 525 nm after exciting the samples at 485 nm, with data collection every 4 s over a 120-s period. Data were analyzed by using a sigmoidal dose-response equation in Prism 5 (GraphPad) to determine bottom and peak responses and EC50 values (concentration stimulating one-half of the maximal response).
Inclusion criteria
Rigorous quality-control measures were established to ensure sample health and reliability of the data to be included in the study. These were applied independently of knowledge of any other assay results or phenotypic data. Samples with high visible hemolysis or contamination of the buffy coat with red blood cells were excluded (4 of 155 samples). Two full and reliable CCK concentration-response curves for intracellular calcium responses were required for data inclusion. Reasons for the elimination of samples included instrument failure, such as injector errors or tube impaction (16 of 155 samples), or errant data points interfering with unambiguous EC50 determination from full curves (8 of 155 samples). The first 15 of the 155 samples that were acquired were used for assay development and validation and were not used in the study. This left 112 samples for full analysis in the study.
Statistical analysis
Study group characteristics were analyzed by using t tests to compare means where data were normally distributed, Wilcoxon’s Rank Sum tests to compare medians where data were insufficiently normal, and chi-square tests to compare categories. Footnotes in Table 1 specify the specific statistical tests that were used for each variable. The t test was used to compare the mean age and weight within the subgroup of patients categorized according to sex, presence or absence of diabetes, and degree of CCK sensitivity; chi-square test was used to compare frequencies of sex, degrees of obesity based on BMI, and waist circumference above or below the threshold for metabolic syndrome within the subgroups; Wilcoxon’s test was used to compare the medians of BMI, waist circumference, fasting glucose (patients without diabetes), serum cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and systolic and diastolic blood pressure within the subgroups. Table 1 also compares the clinical, biochemical, and morphometric variables for participants in the top and bottom 20% of values for CCK sensitivity (EC50) in an effort to identify possible correlations. Means are reported with SDs and medians with IQRs where data were insufficiently normal.
TABLE 1.
Characteristics of the study population and its subsets1
| Characteristics |
||||||||||
| By sex |
Based on presence of diabetes |
Based on CCK sensitivity (upper 20% and lower 20%) |
||||||||
| Overall (total n = 112) | Female (n = 69) | Male (n = 43) | P | Diabetic (n = 35) | Nondiabetic (n = 77) | P | Higher sensitivity (n = 23) | Lower sensitivity (n = 23) | P | |
| Age | 0.20442 | <0.00012 | 0.84752 | |||||||
| n | 112 | 69 | 43 | 35 | 77 | 23 | 23 | |||
| Mean ± SD, y | 45.2 ± 13.6 | 46.5 ± 13.4 | 43.2 ± 13.7 | 55.4 ± 11.7 | 40.6 ± 11.8 | 45.7 ± 12.7 | 45.0 ± 12.3 | |||
| Sex, n (%) | 0.30693 | 0.35953 | ||||||||
| Female | 69 (61.6) | — | — | 24 (68.6) | 45 (58.4) | 16 (69.6) | 13 (56.5) | |||
| Male | 43 (38.4) | — | — | 11 (31.4) | 32 (41.6) | 7 (30.4) | 10 (43.5) | |||
| Weight | 0.00012 | 0.99442 | 0.45242 | |||||||
| n | 112 | 69 | 43 | 35 | 77 | 23 | 23 | |||
| Mean ± SD, kg | 79.5 ± 16.8 | 74.7 ± 15.8 | 87.3 ± 15.5 | 79.6 ± 19.5 | 79.5 ± 15.6 | 81.3 ± 11.0 | 78.5 ± 13.7 | |||
| BMI | 0.96414 | 0.16224 | 0.21664 | |||||||
| n | 112 | 69 | 43 | 35 | 77 | 23 | 23 | |||
| Median, kg/m2 | 29.0 | 29.0 | 29.0 | 30.0 | 28.0 | 30.0 | 28.0 | |||
| IQR, kg/m2 | 26.0, 32.0 | 26.0, 32.0 | 25.0, 32.0 | 26.0, 33.0 | 26.0, 31.0 | 27.0, 32.0 | 26.0, 31.0 | |||
| BMI category | 0.84753 | 0.21523 | 0.68103 | |||||||
| Normal-weight (BMI in kg/m2: <25), n (%) | 19 (17.0) | 13 (18.8) | 6 (14.0) | 6 (17.1) | 13 (16.9) | 3 (13.0) | 4 (17.4) | |||
| Median, kg/m2 | 22.0 | — | — | — | — | — | — | |||
| IQR, kg/m2 | 21.0, 24.0 | |||||||||
| Overweight (BMI: 25–29.9), n (%) | 46 (41.1) | 28 (40.6) | 18 (41.9) | 10 (28.6) | 36 (46.8) | 8 (34.8) | 11 (47.8) | |||
| Median, kg/m2 | 27.0 | — | — | — | — | — | — | |||
| IQR, kg/m2 | 26.0, 29.0 | |||||||||
| Obese (BMI: 30–34.9), n (%) | 35 (31.3) | 20 (29.0) | 15 (34.9) | 13 (37.1) | 22 (28.6) | 11 (47.8) | 7 (30.4) | |||
| Median, kg/m2 | 32.0 | — | — | — | — | — | — | |||
| IQR, kg/m2 | 31.0, 32.0 | |||||||||
| Morbidly obese (BMI ≥35), n (%) | 12 (10.7) | 8 (11.6) | 4 (9.3) | 6 (17.1) | 6 (7.8) | 1 (4.3) | 1 (4.3) | |||
| Median, kg/m2 | 38.0 | — | — | — | — | — | — | |||
| IQR, kg/m2 | 35.0, 44.0 | |||||||||
| WC | 0.07024 | 0.00524 | 0.23504 | |||||||
| n | 112 | 69 | 43 | 35 | 77 | 23 | 23 | |||
| Median, cm | 98.5 | 96.0 | 105.0 | 106.0 | 95.0 | 103.0 | 98.0 | |||
| IQR, cm | 92.0, 109.0 | 91.0, 106.0 | 92.0, 112.0 | 94.0, 113.0 | 91.0, 107.0 | 95.0, 109.0 | 93.0, 107.0 | |||
| WC category,5 n (%) | 0.00183 | 0.01753 | 0.08393 | |||||||
| Above risk threshold | 79 (70.5) | 56 (81.2) | 23 (53.5) | 30 (85.7) | 49 (63.6) | 20 (87.0) | 15 (65.2) | |||
| Below risk threshold | 33 (29.5) | 13 (18.8) | 20 (46.5) | 5 (14.3) | 28 (36.4) | 3 (13.0) | 8 (34.8) | |||
| Fasting glucose (patients without diabetes)6 | 0.00474 | |||||||||
| n | 82 | 49 | 33 | — | — | — | — | — | ||
| Median, mg/dL | 93.0 | 89.0 | 96.0 | — | — | — | — | — | ||
| IQR, mg/dL | 88.0, 99.0 | 85.0, 97.0 | 92.0, 99.0 | — | — | — | — | — | ||
| Serum cholesterol | 0.38564 | 0.96744 | 0.48894 | |||||||
| n | 112 | 69 | 43 | 35 | 77 | 23 | 23 | |||
| Median, mg/dL | 174.0 | 171.0 | 184.0 | 181.0 | 174.0 | 181.0 | 171.0 | |||
| IQR, mg/dL | 153.0, 204.0 | 153.0, 199.0 | 160.0, 213.0 | 153.0, 203.0 | 153.0, 205.0 | 160.0, 206.0 | 148.0, 219.0 | |||
| LDL cholesterol | 0.32554 | 0.80664 | 0.62524 | |||||||
| n | 108 | 68 | 40 | 34 | 74 | 22 | 23 | |||
| Median, mg/dL | 97.0 | 95.5 | 102.0 | 97.5 | 95.5 | 96.5 | 97.0 | |||
| IQR, mg/dL | 78.0, 126.0 | 78.0, 122.0 | 85.0, 127.5 | 72.0, 124.0 | 79.0, 128.0 | 84.0, 129.0 | 76.0, 129.0 | |||
| HDL cholesterol | 0.00604 | 0.87274 | 0.58254 | |||||||
| n | 112 | 69 | 43 | 35 | 77 | 23 | 23 | |||
| Median, mg/dL | 45.5 | 49.0 | 41.0 | 44.0 | 46.0 | 50.0 | 46.0 | |||
| IQR, mg/dL | 39.0, 56.5 | 40.0, 60.0 | 35.0, 52.0 | 40.0, 58.0 | 38.0, 56.0 | 38.0, 62.0 | 40.0, 56.0 | |||
| Triglycerides | 0.07814 | 0.12024 | 0.94744 | |||||||
| n | 112 | 69 | 43 | 35 | 77 | 23 | 23 | |||
| Median, mg/dL | 121.0 | 116.0 | 142.0 | 134.0 | 117.0 | 118.0 | 111.0 | |||
| IQR, mg/dL | 94.0, 172.5 | 92.0, 142.0 | 97.0, 251.0 | 106.0, 176.0 | 88.0, 171.0 | 102.0, 142.0 | 94.0, 211.0 | |||
| Systolic blood pressure | 0.01544 | 0.01194 | 0.26234 | |||||||
| n | 112 | 69 | 43 | 35 | 77 | 23 | 23 | |||
| Median, mm Hg | 120.0 | 118.0 | 125.0 | 125.0 | 118.0 | 124.0 | 119.0 | |||
| IQR, mm Hg | 112.5, 132.5 | 110.0, 127.0 | 115.0, 138.0 | 115.0, 139.0 | 110.0, 127.0 | 115.0, 138.0 | 111.0, 127.0 | |||
| Diastolic blood pressure | 0.00264 | 0.24894 | 0.88634 | |||||||
| n | 112 | 69 | 43 | 35 | 77 | 23 | 23 | |||
| Median, mm Hg | 77.0 | 75.0 | 82.0 | 77.0 | 76.0 | 76.0 | 78.0 | |||
| IQR, mm Hg | 72.0, 83.5 | 70.0, 80.0 | 74.0, 89.0 | 73.0, 86.0 | 70.0, 83.0 | 73.0, 79.0 | 67.0, 84.0 | |||
CCK, cholecystokinin; EC50, concentration stimulating one-half of the maximal response; WC, waist circumference.
t Test was used to compare the mean age and weight within the subgroup of patients categorized according to sex, presence or absence of diabetes, and degree of CCK sensitivity.
Chi-square test was used to compare frequencies of sex, degrees of obesity based on BMI, and waist circumference above or below the threshold for metabolic syndrome within the subgroups.
Wilcoxon’s test was used to compare the medians of BMI, waist circumference, fasting glucose (patients without diabetes), serum cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and systolic and diastolic blood pressure within the subgroups. High CCK sensitivity was defined as EC50 values ≤−10.32 log M (EC50 values in the top 20%); low CCK sensitivity was defined as EC50 values ≥−9.63 log M (EC50 values in the bottom 20%).
WC risk threshold of ≥101.6 cm in men and 88.9 cm in women.
Patients with a previous diagnosis of diabetes were excluded.
Spearman correlation coefficients (r) were calculated for bivariate associations. Correlations were compared between groups by using Fisher’s r-to-z transformation method. To isolate any potential multivariate effect among standard clinical metrics on CCK sensitivity, multivariate regression with forward selection (entry criteria of P < 0.05 with hierarchical structure retained) among main effects and all pairwise interactions was used to model CCK sensitivity (EC50 value) within subgroups of participants. Standard clinical metrics included age, sex, waist size, BMI, the presence of diabetes mellitus, systolic and diastolic blood pressure, and concentrations of fasting glucose, HDL cholesterol, LDL cholesterol, and glycated hemoglobin (HbA1c). A broader entry criterion of P < 0.15 was set to develop an overall model for all participants including the standard clinical metrics as well as leukocyte cellular cholesterol composition. P values <0.05 were considered significant throughout. The software used for analyses was SAS, version 9 (SAS Institute).
RESULTS
Study population characteristics
Samples from 112 serial, unselected participants that were consistent with the rules of inclusion (described in Methods) were included. Characteristics of the cohort are shown in Table 1.
Assay development
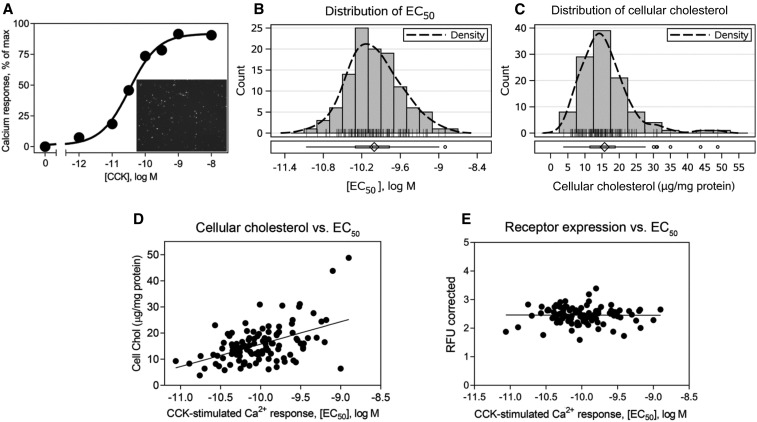
The goal was to achieve an adequate level of cell surface CCK1R expression in leukocytes in the shortest period of time possible, to ensure that the natural membrane composition would most closely reflect that present in cells at the time of harvesting. Optimal delivery and expression were achieved after only 24 h with an adenoviral construct (Figure 1), and intracellular calcium responses were quantified by using a method that was highly reproducible, with an interassay CV of 2.8%. Analysis of the cholesterol composition of these cells at the time of collection and after 24 h (following the standard protocol) showed that there was no significant change after this treatment (data not shown).
FIGURE 1.
Distribution of CCK sensitivity in the patient population and its correlation with cellular cholesterol. Shown is a typical CCK concentration-response curve generated by measuring increase in intracellular calcium concentrations in leukocytes 24 h after transduction (A). The inset in panel A shows a representative image of the efficiency CCK1R expression achieved within this time as measured by GFP fluorescence. Histograms of the CCK EC50 (B) and cellular cholesterol (C) distributions, with density curves for the total population included in the study (n = 112). Correlation plots between the CCK EC50 on the x axis and cellular cholesterol (r = 0.4051, P = 0.000009) (D) or CCK1R expression level as measured by GFP fluorescence (r = −0.005468, P = 0.9569) (E) on the y axis. Spearman’s correlation test was used to obtain the r and P values. CCK, cholecystokinin; CCK1R, type 1 cholecystokinin receptor; Chol, cholesterol; EC50, concentration stimulating one-half of the maximal response; GFP, green fluorescent protein; max, maximum; RFU, relative fluorescence unit.
CCK stimulus-activity coupling at CCK1R expressed ex vivo on leukocytes and cell cholesterol composition
A very broad range of CCK responsiveness in the ex vivo intracellular calcium assay was observed across the study population, with the 95% CI spanning 2.1 orders of magnitude (logs) response as measured in potency values (EC50) (Figure 1B). EC50 values followed an approximately normal distribution and ranged from −11.1 to −8.9 log M (mean ± SD: −10.0 ± 0.4). It is important to note that this variation in CCK sensitivity was not due to differences in receptor density at the time of study, as supported by data showing no correlation between values for CCK EC50 and cellular fluorescence representing cellular receptor expression levels (Figure 1E). There was a similar broad range in cellular cholesterol composition of these cells (Figure 1C). Cellular cholesterol values ranged from 3.8 to 48.8 μg/mg protein but appeared to be nonnormally distributed (median: 14.75 μg/mg protein; IQR: 11.5–18.9 μg/mg protein). There was a clear correlation between CCK responsiveness and cellular cholesterol composition in the study cohort, with cells reflecting the lowest responsiveness to CCK having the highest concentrations of cholesterol (r = 0.4051, P < 0.001) (Figure 1D).
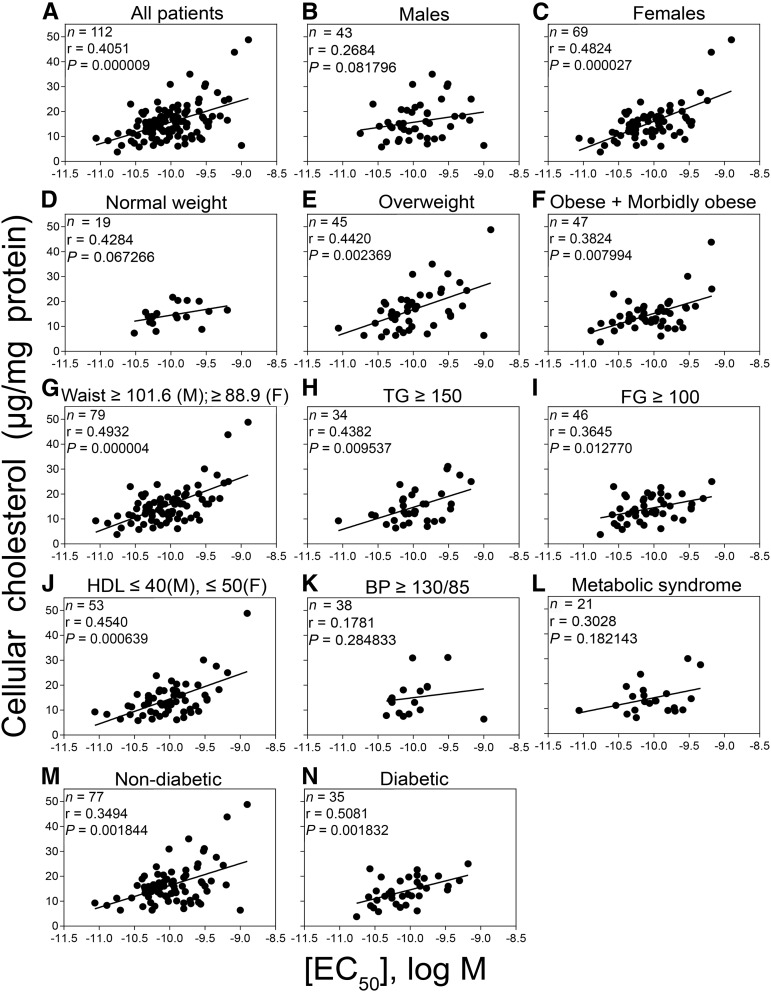
We studied the correlation between CCK EC50 and cellular cholesterol in different subgroups within this cohort that are likely to include candidates for treatment with a CCK-like drug. These include subjects with varied degrees of obesity, components of metabolic syndrome, and diabetes. This correlation trended in the same direction for all of these groups, although some failed to achieve significance, possibly due to small sample sizes. The overall correlation between EC50 and cellular cholesterol was driven predominantly by women, with an overall correlation of r = 0.4051 (P < 0.0001): women with an r = 0.4824 (P < 0.0001) and men with an r = 0.2684 (P = 0.08) (Figure 2B,). The possible impact of body mass was evaluated (Figure 2D–F). There was no significant correlation in the normal-weight patients (group comprising predominantly normal-weight individuals, with 2 of 19 participants being underweight); however, this increased with the incidence of obesity (r = 0.4420 for overweight, r = 0.3824 for obese and morbidly obese). We also examined participants who exhibited components of metabolic syndrome (who were not diabetic). Individual features were first evaluated for the association of EC50 with cellular cholesterol (Figure 2G–K). Sensitivity of CCK1R to cell cholesterol was observed in patients who presented with the following: large waist circumference (r = 0.4932), high triglycerides (r = 0.4382), elevated fasting glucose (patients without diabetes) (r = 0.3645), and low HDL cholesterol (r = 0.4540). In participants who satisfied the criteria for metabolic syndrome who were not yet diabetic, the correlation reached r = 0.3028 but did not achieve significance (Figure 2L). We also examined participants with and without diabetes mellitus [r = 0.5081 (P < 0.002) and r = 0.3494 (P < 0.002), respectively], with both groups showing significant correlations but not one stronger than the other (P = 0.3576) (Figure 2M, N).
FIGURE 2.
Correlations between CCK sensitivity and cellular cholesterol in different patient populations. Shown are the comparisons for the correlation between the potency of CCK responses (EC50) and cellular cholesterol along with the respective n values, Spearman correlation coefficients (r), and P values for all patients (A) and patients selected on the basis of sex (B and C), obesity (D–F), components of metabolic syndrome (in patients without diabetes) (G–K), metabolic syndrome (in patients without diabetes) (L), and the absence or presence of diabetes (M and N). Values significant at P < 0.05 include correlations in all patients in the cohort, women, and patients who were overweight or obese and morbidly obese, with a large waist size, with elevated triglycerides, with elevated FG, with reduced HDL cholesterol, and both nondiabetic and diabetic patients. There were 21 patients in the metabolic syndrome group who were nondiabetic and fulfilled the American Heart Association criteria for metabolic syndrome, including ≥3 of the following 5 criteria: waist circumference ≥101.6 cm in men and ≥88.9 cm in women, triglyceride concentration of ≥150 mg/dL, HDL concentrations ≤40 mg/dL in men and ≤50 mg/dL in women, FG ≥100 mg/dL, and BP ≥130/85 mm Hg. Spearman’s correlation test was used to obtain the r and P values. BP, blood pressure; CCK, cholecystokinin; EC50, concentration stimulating one-half of the maximal response; FG, fasting glucose; TG, triglycerides.
We compared the biochemical, clinical, and morphometric characteristics of participants in both extremes of CCK responsiveness in the study cohort (the lowest and highest 20%) to gain insights into what criteria might be most helpful in identifying those who might require medication to correct defective CCK stimulus-activity coupling (Table 1). None of these comparisons reached significance when evaluated alone, which supports the interpretation that a multifactorial approach will be necessary for the entire population, with the possibility that different factors may be most relevant for different groups.
We examined which variables might best correlate with CCK responsiveness along the clinical continuum of patients who might be candidates for drug therapy (Table 2). For normal-weight participants, the most informative variable with the highest correlation to CCK EC50 value was serum triglycerides, with cholesterol lipid values being less informative. In contrast, as participants became obese, lipid values related to cholesterol, as reflected by low HDL cholesterol, became most informative and the correlation with triglycerides was lost. The correlations for those participants who were diabetic continued to exhibit the correlation with low HDL concentrations present in obesity and had a correlation with degree of diabetic control as reflected in elevated HbA1c.
TABLE 2.
Correlations for CCK sensitivity and cellular cholesterol with serum biochemical markers in normal-weight, obese and morbidly obese, and diabetic patients1
| BMI (kg/m2) <25 (normal weight) |
BMI >30 (obese and morbidly obese) |
Diabetic |
|||||||
| Serum markers | r | P | n | r | P | n | r | P | n |
| Correlations with CCK sensitivity (EC50 values) | |||||||||
| Triglycerides | 0.4765* | 0.0391* | 19 | 0.0320 | 0.8311 | 47 | 0.1207 | 0.4899 | 35 |
| Serum cholesterol | 0.1496 | 0.5411 | 19 | −0.0717 | 0.6318 | 47 | −0.0423 | 0.8094 | 35 |
| LDL cholesterol | 0.0031 | 0.9900 | 19 | 0.0647 | 0.6765 | 47 | 0.0624 | 0.7258 | 34 |
| HDL cholesterol | −0.2253 | 0.3537 | 19 | −0.3441* | 0.0179* | 47 | −0.3307#x2020 | 0.0523#x2020 | 35 |
| HbA1c | 0.1351 | 0.5813 | 19 | −0.1636 | 0.2717 | 47 | 0.3320#x2020 | 0.0514#x2020 | 35 |
| Correlations with cellular cholesterol | |||||||||
| Triglycerides | 0.7571* | 0.0002* | 19 | −0.0318 | 0.8317 | 47 | 0.0880 | 0.6154 | 35 |
| Serum cholesterol | 0.1898 | 0.4364 | 19 | 0.2099 | 0.1568 | 47 | 0.2795 | 0.1040 | 35 |
| LDL cholesterol | 0.1509 | 0.5375 | 19 | 0.3339* | 0.0267* | 44 | 0.3476* | 0.0440* | 34 |
| HDL cholesterol | −0.4756* | 0.0396* | 19 | 0.0204 | 0.8918 | 47 | −0.0855 | 0.6255 | 35 |
| HbA1c | 0.1370 | 0.5759 | 19 | −0.1109 | 0.4580 | 47 | 0.3410* | 0.0450* | 35 |
The numbers of patients studied (n), Spearman correlation coefficients (r), and P values are shown. *Significant at P < 0.05 (for correlations with CCK sensitivity, these represent triglyceride concentrations in normal-weight participants and HDL-cholesterol concentrations in obese and morbidly obese participants; for correlations with cellular cholesterol, these represent triglyceride concentrations in normal-weight participants, LDL-cholesterol concentrations in obese and morbidly obese participants and in patients with diabetes, HDL-cholesterol concentrations in normal-weight participants, and HbA1c concentrations in patients with diabetes). †Trend toward significance (P value between 0.05 and 0.10) with limited sample sizes (for correlations with CCK sensitivity, these represent HDL-cholesterol and HbA1c concentrations in patients with diabetes). Spearman’s correlation test was used to obtain the r and P values. CCK, cholecystokinin; EC50, concentration stimulating one-half of the maximal response; HbA1c, glycated hemoglobin.
We also looked at whether the same variables correlated with the cellular cholesterol measurements in the same groups (Table 2, bottom rows). Although there were similar trends in the most informative variables among these groups as seen with CCK responsiveness (Table 2, top rows), there were also differences.
Development of a predictive model for abnormal CCK responsiveness
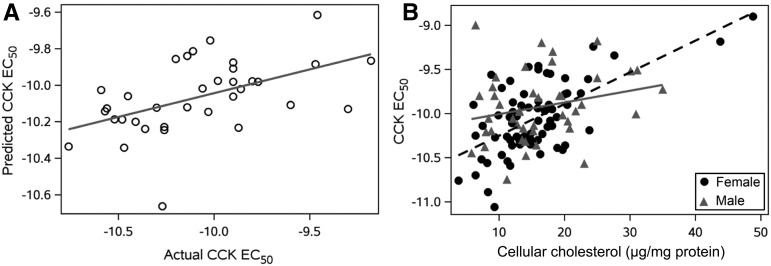
We initially attempted to develop a coherent multifactorial prediction model for the entire study cohort with the use of routine, clinically available measurements. Select subgroups of participants were also evaluated for the effects of these metrics on CCK responsiveness by using a multivariate model selected on the basis of forward regression, including participants with diabetes mellitus as well as BMI groupings (normal weight, overweight, and obese and morbidly obese combined). Among participants with diabetes (n = 35), the final model included HDL cholesterol (coefficient: −0.01; P = 0.04) and HbA1c (coefficient: 0.06; P = 0.01) as significant predictors of CCK sensitivity (intercept term of −10.17 and r2 = 0.26). The actual EC50 values compared with the model-predicted values for diabetic participants are shown in Figure 3A. Model selection for both normal-weight (n = 19) and overweight (n = 46) BMI groups did not result in any significant findings. The combined group of obese and morbidly obese participants (n = 47) resulted in a final model that included HDL cholesterol (coefficient: −0.01; P = 0.02) with an intercept term of −9.56 and r2 = 0.12.
FIGURE 3.
Multivariate models to predict CCK EC50 values in patient populations. (A) Actual and predicted CCK sensitivity (EC50 values) among diabetic participants based on a model that included HDL cholesterol and glycated hemoglobin as predictor variables (line represents the unity line where the actual CCK EC50 equals the predicted CCK EC50; n = 35; r2 = 0.26, P < 0.01). (B) Actual CCK sensitivity data by leukocyte cellular cholesterol values with plotting symbols for men (n = 43) and women (n = 69) in the entire population (lines represent predicted CCK EC50 based on a model that included sex, leukocyte cellular cholesterol, and sex-by-leukocyte cellular cholesterol interaction). The overall r2 value for the model was 0.29 (P < 0.001). Sex subgroupings without a cell cholesterol-by-sex interaction term included estimates of r2 = 0.40 (P < 0.001) for women and r2 = 0.06 (P = 0.12) for men. CCK, cholecystokinin; EC50, concentration stimulating one-half of the maximal response.
We were more successful in developing a predictive model applicable to the entire cohort when we used a nonstandard metric, representing leukocyte cellular cholesterol composition, along with sex. Along with male sex (coefficient: 0.47; P = 0.01) and cellular cholesterol (coefficient: 0.04; P < 0.001), a male sex-by-cell cholesterol interaction (coefficient: −0.02; P = 0.02) remained in the final model as a significant predictor of CCK sensitivity (intercept term of −10.6 and r2 = 0.29). Sex subgroupings without a cell cholesterol-by-sex interaction term included estimates of r2 = 0.40 (P < 0.001) for women and r2 = 0.06 (P = 0.12) for men, which is shown in Figure 3B.
DISCUSSION
The gastrointestinal tract is known to play an important role in nutrient assimilation and weight management. Contributing to this are networks of neurohormonal regulators and receptors that help to coordinate transit of chyme, digestion, and absorption, as well as communicating with the brain to regulate appetite. The first gastrointestinal hormone recognized to have satiety effects was CCK (23), released from intestinal I cells in response to fat and protein. It mediates a critical servomechanism regulating postprandial appetite control (8, 24–26).
Disruption of this servomechanism could contribute to obesity. However, although there is a report of a patient with dysfunctional CCK1R resulting from mRNA missplicing who had early-onset gallstones and morbid obesity (17), genetic variation or abnormalities in CCK or CCK1R are extremely rare and not a common contributor to obesity. In contrast, the possibility exists for the membrane to exert a quantitatively important negative impact on this servomechanism in a substantial fraction of the population. This relates to the well-recognized negative impact of excess cholesterol on CCK1R function (18).
Numerous lines of evidence support the impact of cholesterol on CCK1R function. This was first recognized as playing a key role in the pathogenesis of cholesterol gallstone disease (27), with elevated cholesterol in bile being transferred to muscularis myocytes where it reduced CCK stimulus-activity coupling (20). Reversal by extraction of excess cholesterol provided definitive proof of the importance of this lipid (19). This phenomenon could also be reproduced in animal models (28) and in vitro in model cell systems (18). The latter have shown a direct impact of cholesterol on a motif in transmembrane 3 of CCK1R (29, 30), with this sterol-binding site also shown to be relevant to regulatory interactions of bile acids and phytosterols (31, 32).
It is not possible to extrapolate from the gallbladder CCK1R experience to propose similar dysfunction at this receptor in other sites where cells do not come in contact with cholesterol-rich bile. However, isolated reports have described elevated cholesterol in leukocytes in patients with hypercholesterolemia or metabolic syndrome, resulting in abnormal function of formyl-Met-Leu-Phe and angiotensin receptors (33–35).
The approach we have taken to examine impact of a patient’s natural membrane environment on receptor function is, to our knowledge, unique, and potentially applicable to many receptors. This was necessary due to the inability to acquire adequate cells naturally expressing CCK1R, other than those in resected diseased gallbladders. There is now recognition that many membrane receptors can be functionally affected by their membrane microenvironment (18, 36, 37).
It was important to develop a method that provided cell surface receptor expression relatively quickly, to allow functional characterization without significant changes in membrane composition (P < 0.05). By using an adenoviral CCK1R construct (38), we were able to generate reproducible CCK concentration-response curves to determine hormone responsiveness in <24 h, when natural concentrations of cholesterol were retained.
Of note, there was a broad range of CCK responsiveness in these cells across the population. We know that all of these receptors had the same wild-type sequence and primary structure and that levels of receptor expression were not different across this cohort. Similarly, there was a range of cholesterol compositions directly measured for these samples and a clear and significant correlation between this variable and CCK responsiveness, with those with high concentrations of cholesterol being least responsive to CCK. This is consistent with previous cell model studies (19, 29). It is quite unlikely that cholesterol is the only relevant determinant of CCK responsiveness at this receptor, but the correlation supports its having an effect. It was quite interesting that this correlation was present in all of the groups who might be candidates for appetite modulation therapy. This includes participants who were obese, those with components of metabolic syndrome, and those with diabetes mellitus, although small numbers of particpiants in some groups provided inadequate power to reach significance. The correlation was stronger in women than in men, and greater degrees of obesity resulted in stronger correlations. Of interest, the degree of diabetic control was also an important influence.
Clinical and biochemical variables that best correlated with CCK sensitivity varied among the different patient groups belonging to the different stages of the spectrum of metabolic disease. One key finding is that the CCK sensitivity best correlated with different types of lipids in the normal-weight and obese groups. Hypertriglyceridemia was most predictive of aberrant CCK responsiveness in normal-weight participants, whereas cholesterol variables were most predictive of CCK responsiveness in obese and diabetic patients. The strongest indicator for defective CCK responsiveness in patients with diabetes was poor disease control, as reflected by elevated HbA1c. It is important to not interpret these observations as being directly causative for CCK responsiveness in any given group. They support the interpretation that there is no single factor, such as cholesterol, that is fully responsible for an individual’s CCK sensitivity. These observations may support a hypothesis that different groups of patients with CCK dysfunction respond best to different modes of therapy directed toward different abnormalities of lipids, glucose, or both.
Because different clinical metrics were most predictive for different patient groups, and due to the small size of some subgroups, no single multifactorial model successfully fit the whole group. We were successful, however, in developing a model that used the nonroutine measurement of leukocyte cholesterol composition along with sex. This may represent a useful way to predict CCK responsiveness in an individual, if this were critical for drug choice.
These observations have very important implications and may explain why previous clinical trials that used full agonists of CCK1R for weight loss (16, 39) did not reach their primary endpoints. The design of those studies that compared acutely administered CCK1R agonist with lifestyle modification is already challenging. If some patients being studied also exhibited low responsiveness to CCK due to this lateral allosteric modulation, it could have blunted or eliminated the satiety responses. There has been recent recognition of the value of allosteric modulators to modify the effects of a natural agonist acting at a receptor (40, 41). The first such agents acting at a G protein–coupled receptor (GPCR) have been approved and shown to have outstanding clinical efficacy (42). Theoretically, it is possible to identify a “corrective” positive allosteric modulator (PAM) of the CCK1R to correct this defect in CCK1R function (1, 43). To our knowledge, no such drugs have yet been described. There is also the possibility to identify PAMs or corrective PAMs that do not possess intrinsic agonist activity, as recently proposed as a novel strategy to more safely and effectively increase ability of CCK to elucidate satiety at a physiologically relevant and finite time point when meal size could be best regulated (1). This would have the theoretical advantage of not prolonging its effect and avoiding side effects and toxicity, as well as receptor downregulation.
The current observations provide important justification to develop drugs effective not only at wild-type CCK1R in a normal conformation but also this receptor in an abnormal conformation achieved as a result of its membrane microenvironment. This study also provides a powerful new approach to examine the possible impact of the membrane environment present in individual patients on various plasma membrane receptors. This could become an important theme for the era of individualized medicine. In addition, these data emphasize the diversity of responsiveness to a particular hormone by a single molecular form of its receptor. Endogenous allosteric regulators are being increasingly recognized (40, 41) and provide additional levels of complexity to be aware of in designing therapeutics and in administering drugs.
Acknowledgments
We thank Lawrence J Mandarino, now at the University of Arizona, who was Principal Investigator of the Sangre Por Salud Biobank, and Chang-Xin Shi for help with viral construct preparation.
The authors’ responsibilities were as follows—LJM, AJD, and MD: conceived and designed the study and interpreted the data; AJD and MD: acquired and analyzed the data; BTL and ACD: performed the statistical analysis; and all authors: contributed to writing the manuscript, and read and approved the final manuscript. The authors declared that they have no competing interests.
Footnotes
Abbreviations used: CCK, cholecystokinin; CCK1R, type 1 cholecystokinin receptor; EC50, concentration stimulating one-half of the maximal response; HbA1c, glycated hemoglobin; PAM, positive allosteric modulator.
REFERENCES
- 1.Miller LJ, Desai AJ. Metabolic actions of the type 1 cholecystokinin receptor: its potential as a therapeutic target. Trends Endocrinol Metab 2016;27:609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trevaskis JL, Turek VF, Griffin PS, Wittmer C, Parkes DG, Roth JD. Multi-hormonal weight loss combinations in diet-induced obese rats: therapeutic potential of cholecystokinin? Physiol Behav 2010;100:187–95. [DOI] [PubMed] [Google Scholar]
- 3.Young AA. Brainstem sensing of meal-related signals in energy homeostasis. Neuropharmacology 2012;63:31–45. [DOI] [PubMed] [Google Scholar]
- 4.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11–22. [DOI] [PubMed] [Google Scholar]
- 5.Holmes D. Diabetes: concerns about long-term use of GLP-1 analogues. Nat Rev Endocrinol 2016;12:186. [DOI] [PubMed] [Google Scholar]
- 6.Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 2013;36:2118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith GP, Gibbs J. The satiety effect of cholecystokinin: recent progress and current problems. Ann N Y Acad Sci 1985;448:417–23. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhri OB, Salem V, Murphy KG, Bloom SR. Gastrointestinal satiety signals. Annu Rev Physiol 2008;70:239–55. [DOI] [PubMed] [Google Scholar]
- 9.Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes 2007;14:63–7. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Owyang C. Endogenous cholecystokinin stimulates pancreatic enzyme secretion via vagal afferent pathway in rats. Gastroenterology 1994;107:525–31. [DOI] [PubMed] [Google Scholar]
- 11.Sternini C, Wong H, Pham T, De Giorgio R, Miller LJ, Kuntz SM, Reeve JR, Walsh JH, Raybould HE. Expression of cholecystokinin A receptors in neurons innervating the rat stomach and intestine. Gastroenterology 1999;117:1136–46. [DOI] [PubMed] [Google Scholar]
- 12.Bignon E, Bachy A, Boigegrain R, Brodin R, Cottineau M, Gully D, Herbert JM, Keane P, Labie C, Molimard JC, et al. SR146131: a new potent, orally active, and selective nonpeptide cholecystokinin subtype 1 receptor agonist. I. In vitro studies. J Pharmacol Exp Ther 1999;289:742–51. [PubMed] [Google Scholar]
- 13.Berger R, Zhu C, Hansen AR, Harper B, Chen Z, Holt TG, Hubert J, Lee SJ, Pan J, Qian S, et al. 2-Substituted piperazine-derived imidazole carboxamides as potent and selective CCK1R agonists for the treatment of obesity. Bioorg Med Chem Lett 2008;18:4833–7. [DOI] [PubMed] [Google Scholar]
- 14.Cameron KO, Beretta EE, Chen Y, Chu-Moyer M, Fernando D, Gao H, Kohrt J, Lavergne S, Jardine Pda S, Guzman-Perez A, et al. Discovery of new piperidine amide triazolobenzodiazepinones as intestinal-selective CCK1 receptor agonists. Bioorg Med Chem Lett 2012;22:2943–7. [DOI] [PubMed] [Google Scholar]
- 15.Castillo EJ, Delgado-Aros S, Camilleri M, Burton D, Stephens D, O’Connor-Semmes R, Walker A, Shachoy-Clark A, Zinsmeister AR. Effect of oral CCK-1 agonist GI181771X on fasting and postprandial gastric functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 2004;287:G363–9. [DOI] [PubMed] [Google Scholar]
- 16.Jordan J, Greenway FL, Leiter LA, Li Z, Jacobson P, Murphy K, Hill J, Kler L, Aftring RP. Stimulation of cholecystokinin-A receptors with GI181771X does not cause weight loss in overweight or obese patients. Clin Pharmacol Ther 2008;83:281–7. [DOI] [PubMed] [Google Scholar]
- 17.Miller LJ, Holicky EL, Ulrich CD, Wieben ED. Abnormal processing of the human cholecystokinin receptor gene in association with gallstones and obesity. Gastroenterology 1995;109:1375–80. [DOI] [PubMed] [Google Scholar]
- 18.Desai AJ, Miller LJ. Sensitivity of cholecystokinin receptors to membrane cholesterol content. Front Endocrinol (Lausanne) 2012;3:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao ZL, Chen Q, Amaral J, Biancani P, Jensen RT, Behar J. CCK receptor dysfunction in muscle membranes from human gallbladders with cholesterol stones. Am J Physiol 1999;276:G1401–7. [DOI] [PubMed] [Google Scholar]
- 20.Xiao ZL, Chen Q, Amaral J, Biancani P, Behar J. Defect of receptor-G protein coupling in human gallbladder with cholesterol stones. Am J Physiol Gastrointest Liver Physiol 2000;278:G251–8. [DOI] [PubMed] [Google Scholar]
- 21.Yu P, Chen Q, Harnett KM, Amaral J, Biancani P, Behar J. Direct G protein activation reverses impaired CCK signaling in human gallbladders with cholesterol stones. Am J Physiol 1995;269:G659–65. [DOI] [PubMed] [Google Scholar]
- 22.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911–7. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 1973;84:488–95. [DOI] [PubMed] [Google Scholar]
- 24.Ballinger AB, Clark ML. L-phenylalanine releases cholecystokinin (CCK) and is associated with reduced food intake in humans: evidence for a physiological role of CCK in control of eating. Metabolism 1994;43:735–8. [DOI] [PubMed] [Google Scholar]
- 25.Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr 1981;34:154–60. [DOI] [PubMed] [Google Scholar]
- 26.Overduin J, Gibbs J, Cummings DE, Reeve JR Jr. CCK-58 elicits both satiety and satiation in rats while CCK-8 elicits only satiation. Peptides 2014;54:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behar J, Lee KY, Thompson WR, Biancani P. Gallbladder contraction in patients with pigment and cholesterol stones. Gastroenterology 1989;97:1479–84. [DOI] [PubMed] [Google Scholar]
- 28.Yu P, Chen Q, Biancani P, Behar J. Membrane cholesterol alters gallbladder muscle contractility in prairie dogs. Am J Physiol 1996;271:G56–61. [DOI] [PubMed] [Google Scholar]
- 29.Potter RM, Harikumar KG, Wu SV, Miller LJ. Differential sensitivity of types 1 and 2 cholecystokinin receptors to membrane cholesterol. J Lipid Res 2012;53:137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai AJ, Harikumar KG, Miller LJ. A type 1 cholecystokinin receptor mutant that mimics the dysfunction observed for wild type receptor in a high cholesterol environment. J Biol Chem 2014;289:18314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desai AJ, Dong M, Harikumar KG, Miller LJ. Impact of ursodeoxycholic acid on a CCK1R cholesterol-binding site may contribute to its positive effects in digestive function. Am J Physiol Gastrointest Liver Physiol 2015;309:G377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai AJ, Dong M, Miller LJ. Beneficial effects of beta-sitosterol on type 1 cholecystokinin receptor dysfunction induced by elevated membrane cholesterol. Clin Nutr 2016;35:1374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paragh G, Kovacs E, Seres I, Keresztes T, Balogh Z, Szabo J, Teichmann F, Foris G. Altered signal pathway in granulocytes from patients with hypercholesterolemia. J Lipid Res 1999;40:1728–33. [PubMed] [Google Scholar]
- 34.Seres I, Foris G, Pall D, Kosztaczky B, Paragh G Jr., Varga Z, Paragh G. Angiotensin II-induced oxidative burst is fluvastatin sensitive in neutrophils of patients with hypercholesterolemia. Metabolism 2005;54:1147–54. [DOI] [PubMed] [Google Scholar]
- 35.Seres I, Foris G, Varga Z, Kosztaczky B, Kassai A, Balogh Z, Fulop P, Paragh G. The association between angiotensin II-induced free radical generation and membrane fluidity in neutrophils of patients with metabolic syndrome. J Membr Biol 2006;214:91–8. [DOI] [PubMed] [Google Scholar]
- 36.Dawaliby R, Trubbia C, Delporte C, Masureel M, Van Antwerpen P, Kobilka BK, Govaerts C. Allosteric regulation of G protein-coupled receptor activity by phospholipids. Nat Chem Biol 2016;12:35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paila YD, Tiwari S, Chattopadhyay A. Are specific nonannular cholesterol binding sites present in G-protein coupled receptors? Biochim Biophys Acta 2009;1788:295–302. [DOI] [PubMed] [Google Scholar]
- 38.Sengupta S, Ulasov IV, Thaci B, Ahmed AU, Lesniak MS. Enhanced transduction and replication of RGD-fiber modified adenovirus in primary T cells. PLoS One 2011;6:e18091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elliott RL, Cameron KO, Chin JE, Bartlett JA, Beretta EE, Chen Y, Jardine Pda S, Dubins JS, Gillaspy ML, Hargrove DM, et al. Discovery of N-benzyl-2-[(4S)-4-(1H-indol-3-ylmethyl)-5-oxo-1-phenyl-4,5-dihydro-6H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepin-6-yl]-N-isopropylacetamide, an orally active, gut-selective CCK1 receptor agonist for the potential treatment of obesity. Bioorg Med Chem Lett 2010;20:6797–801. [DOI] [PubMed] [Google Scholar]
- 40.Christopoulos A. Advances in G protein-coupled receptor allostery: from function to structure. Mol Pharmacol 2014;86:463–78. [DOI] [PubMed] [Google Scholar]
- 41.van der Westhuizen ET, Valant C, Sexton PM, Christopoulos A. Endogenous allosteric modulators of G protein-coupled receptors. J Pharmacol Exp Ther 2015;353:246–60. [DOI] [PubMed] [Google Scholar]
- 42.Davey AE, Leach K, Valant C, Conigrave AD, Sexton PM, Christopoulos A. Positive and negative allosteric modulators promote biased signaling at the calcium-sensing receptor. Endocrinology 2012;153:1232–41. [DOI] [PubMed] [Google Scholar]
- 43.Desai AJ, Henke BR, Miller LJ. Elimination of a cholecystokinin receptor agonist ‘trigger’ in an effort to develop positive allosteric modulators without intrinsic agonist activity. Bioorg Med Chem Lett 2015;25:1849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]