Abstract
Converging lines of evidence suggest that individuals with comorbid post-traumatic stress disorder (PTSD) and alcohol use disorder (AUD) may be characterized by heightened defensive reactivity, which serves to maintain drinking behaviors and anxiety/hyperarousal symptoms. Notably, however, very few studies have directly tested whether individuals with PTSD and AUD exhibit greater defensive reactivity compared with individuals with PTSD without AUD. The aim of the current study was to therefore test this emerging hypothesis by examining individual differences in error related negativity (ERN), an event-related component that is larger among anxious individuals and is thought to reflect defensive reactivity to errors. Participants were sixty-six military veterans who completed a well-validated flanker task known to robustly elicit the ERN. Veterans were comprised of three groups: controls (i.e., no PTSD or AUD), PTSD-AUD (i.e., current PTSD but no AUD), and PTSD+AUD (i.e., current comorbid PTSD and AUD). Results indicated that in general, individuals with PTSD and controls did not differ in ERN amplitude. However, among individuals with PTSD, those with comorbid AUD had significantly larger ERNs than those without AUD. These findings suggest that PTSD+AUD is a neurobiologically unique subtype of PTSD and the comorbidity of AUD may enhance defensive reactivity to errors in individuals with PTSD.
Keywords: alcohol use, defensive reactivity, threat, error-related negativity, errors
Introduction
Post-traumatic stress disorder (PTSD) is a debilitating disorder characterized by unwanted intrusive thoughts, avoidance of trauma-related cues, sustained anxiety and hyperarousal (APA, 2015). Although the origin of onset is well-defined (i.e., a trauma), PTSD is heterogeneous with varying symptom profiles and comorbid psychopathologies. Traditionally, heightened defensive reactivity was considered a hallmark feature of PTSD and accordingly numerous studies have demonstrated that compared to healthy controls, individuals with PTSD exhibit greater aversive responding to trauma and non-trauma related negative stimuli (Fani et al., 2012; Liberzon et al., 1999; Grillon et al., 2009; Shin et al., 2004). However, studies have also indicated no association between PTSD and defensive responding (Lanius et al., 2001; Rabinak et al., 2013) or in contrast, dampened defensive responding (Britton et al., 2005; MacNamara, Post, Kennedy, Rabinak, & Phan, 2013). This together highlights the varied nature of the disorder and implies that heightened defensive reactivity may characterize only a subset of individuals with PTSD. Given that psychosocial and pharmacological treatments targeting defensive reactivity (e.g., anxiolytics) may be uniquely effective for specific subtypes (or groups), it is important to delineate the phenotypic, behavioral and biological factors that distinguish groups and contribute to their core dysfunction.
Converging lines of evidence suggest that one group characterized by heightened defensive reactivity may be individuals with PTSD and comorbid alcohol use disorder (AUD). Research suggests that some patients diagnosed with PTSD use alcohol as a means of avoiding aversive affective states and dampening defensive reactivity (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Stewart, 1997; Yeater, Ausin, Green, & Smith, 2010). Over time, this reliance on alcohol to regulate negative affect appears to drive excessive, continued use via negative reinforcement processes and contributes to the onset and worsening of AUD (Kassel, Jackson, & Unrod, 2000; Robinson, Sareen, Cox, & Bolton, 2011; Schroder & Perrine, 2007). Meanwhile, repeated exposure to alcohol is known to cause neuroadaptation, resulting in additional increases in anxiety, and persistent altered functioning of affective and motivational systems (Koob & LeMoal, 2001; Koob & Volkow, 2010). Therefore, heightened defensive reactivity in PTSD may play a role in the development and maintenance of comorbid AUD.
In support of this, several studies have shown that PTSD symptoms prospectively predict the onset of AUD (Kline et al., 2014), and that individuals with co-occurring PTSD and AUD evidence more PTSD symptoms and greater addiction severity than individuals with either disorder alone (Debell et al., 2014; Ouimette, Ahrens, Moos, & Finney, 1997). Remarkably, however, very few studies have tested whether individuals with comorbid PTSD and AUD display greater defensive reactivity than individuals with PTSD without AUD, as would be expected based on the broader alcohol and PTSD literatures. In other words, it is possible that heightened defensive reactivity is a vulnerability factor for trauma-related disorders and AUD comorbidity; however, this hypothesis requires further testing.
Defensive reactivity may be triggered by several forms of aversive stimuli, including errors which are motivationally-salient events that signal the potential for harm and require attention and corrective action (Hajcak & Foti, 2008; Hajcak et al. 2005; Olvet & Hajcak, 2008). In this sense, errors are aversive, though individuals differ in their affective, behavioral and neurobiological responses to errors, all of which can be measured in the laboratory with behavioral and neural indices. The error-related negativity (ERN) is one such neurobiological measure which has consistently been shown to be elevated in anxious individuals and is thought to reflect individual differences in sensitivity to sustained threats (see Weinberg, Dieterich, & Riesel, 2015 for a review). Specifically, the ERN is a fronto-centrally maximal event-related potential (ERP) component that appears as a negative-going deflection in the waveform between 0 and 100 ms following the commission of an error (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993). Studies have suggested that the ERN reflects neural activation of an error detection system as it is generated by activity in the anterior cingulate cortex (ACC) (Dehaene, Posner, & Tucker, 1994; van Veen & Carter, 2002). Importantly, the ERN is reliable (Weinberg & Hajcak, 2011), relatively easy to record and is observed across various stimuli, response modalities, age ranges, and even species (Endrass, Klawohn, Gruetzmann, Ischebeck, & Kathmann, 2012; Hoffmann & Falkenstein, 2010; Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001; Riesel, Weinberg, Endrass, Meyer, & Hajcak, 2013).
Although the ERN has been examined in relation to multiple forms of psychopathology, especially anxiety disorders (Weinberg, Dieterich, & Riesel, 2015), only two prior studies have investigated the association between the ERN and PTSD. Rabinak and colleagues (2013) assessed the ERN in returning military veterans with and without PTSD and in a sample of healthy, non-traumatized college students. Findings indicated that there were no differences in ERN amplitude between individuals with PTSD and healthy controls, suggesting that the two groups did not differ in defensive reactivity to errors. Consistent with this, Swick, Honzel and Turken (2015) also found that there were no differences in the ERN between controls and veterans with PTSD, but that those with PTSD did make significantly more errors during a response inhibition task. It is important to highlight that neither of these prior studies examined the impact of comorbid AUD. In addition, Swick et al. (2015) noted that most participants in the sample had prior traumatic brain injuries (TBIs), raising concerns about confounding effects of brain trauma and injury-related changes in neural functioning. As such, there is a need to replicate prior findings and further clarify whether PTSD in-and-of-itself is associated with heightened threat responding measured via the ERN.
Although only a few studies have examined the ERN in AUD specifically, some have found a significant association. Consistent with the self-medication literature, Padilla et al. (2011) reported that relative to healthy controls, individuals previously diagnosed with an AUD who were currently abstinent exhibited larger ERNs. Most relevant to the current study, Schellekens et al. (2010) investigated the ERN in three groups: healthy controls, individuals with AUD-only, and individuals with AUD and a comorbid anxiety disorder. The comorbid group included individuals with panic disorder, social phobia, or generalized anxiety disorder, but none with PTSD. Results indicated that like Padilla et al. (2011), AUD-only individuals displayed a greater ERN compared with healthy controls. Moreover, individuals with AUD plus an anxiety disorder displayed an even greater ERN relative to both the AUD-only and control participants. The authors therefore concluded that AUD+anxiety disorder individuals represent a clinical subgroup that is characterized by heightened defensive reactivity to threat and that this excessive reactivity may contribute to patterns of problematic alcohol abuse. Indeed, as would be expected, several studies have demonstrated that acute alcohol administration dampens the ERN and alleviates reactivity to errors (Easdon, Izenberg, Armilio, Yu, & Alain, 2005; Ridderinkhof et al., 2002). Moreover, one study has specifically shown that alcohol dampens the ERN by reducing negative affect (Bartholow et al., 2012).
The current study was therefore designed to address a gap in the existing literature by examining the impact of comorbid PTSD and AUD on the ERN. All participants were U.S. military veterans returning from recent conflicts in Iraq and Afghanistan who often have PTSD and AUD and completed a well-validated flanker task known to robustly elicit the ERN. Veterans were divided into three groups: controls (i.e., no PTSD or AUD), PTSD-AUD (i.e., current PTSD but no AUD), and PTSD+AUD (i.e., current comorbid PTSD and AUD). We hypothesized that within those with PTSD, individuals with a comorbid AUD would exhibit larger ERNs than individuals without a comorbid AUD. Given the limited existing literature, we did not have specific hypotheses about whether those with PTSD and controls would differ from one another.
Methods
Participants
Seventy-one participants, aged 18–55 years, were selected from a larger sample of U.S. military veterans returning from Operations Enduring Freedom, Iraqi Freedom and New Dawn (OEF/OIF/OND) and recruited at the VA Ann Arbor Healthcare System and the Jesse Brown VA Medical Center. All participants were administered the Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995) and semi-structured diagnostic interviews (see below), which were used to define the groups. Control participants (n = 24) were selected to have CAPS scores of 20 or less, reflecting the absence of PTSD, and could not meet criteria for alcohol or substance abuse. Participants in the PTSD group (n = 43) met DSM-IV criteria for PTSD and/or had CAPS scores of 40 or greater, reflecting moderate to severe PTSD symptoms and a threshold for clinically-relevant PTSD. Within the PTSD group, 18 met criteria for a current, comorbid AUD. Of those 18 with PTSD+AUD, 3 had comorbid cannabis abuse. No other participant had an active substance use disorder. Other psychiatric comorbidities were permitted in all groups, with the exception of lifetime psychosis. Exclusionary criteria for all participants included: presence of a clinically significant medical or neurological condition, presence of an organic mental syndrome, mental retardation or pervasive developmental disorder, and current substance abuse or suicidal ideation at a level that would interfere with the study protocol. In regards to substance abuse, the protocol specified that if any individual arrived to laboratory session visibly intoxicated and/or reported drug or alcohol use within the past 24-hours their session would be re-scheduled for a later date. If the subject felt that they were unable to abstain from substances for at least 24-hours, their participation would be terminated. These procedures resulted in a small number of participants being rescheduled and approximately three being excluded from the study. Five participants were excluded from data analysis because they made less than 6 errors on the task (Olvet & Hajcak, 2009), leaving a final sample of 66 participants for analysis (see Table 1). Ten participants in the control group and eight participants in the PTSD group were recruited and tested at the VA Ann Arbor Healthcare System, while the remainder were recruited and tested at the Jesse Brown VA Medical Center; however, EEG data were collected with identical equipment and parameters between the two sites and there were no significant differences in ERPs or behavioral measures between these sites.
Table 1.
Demographics and clinical characteristics
| Demographics | Controls (N=24) | PTSD-AUD (N=24) | PTSD+AUD (N=18) |
|---|---|---|---|
| Age (years) | 34.7 (8.3)a | 33.0 (7.5)a | 31.6 (4.9)a |
| Sex (% male) | 79.2%a | 100.0%a | 88.9%a |
| Education (years) | 16.0 (2.4)a | 13.8 (2.1)b | 14.4 (1.7)b |
| Race/Ethnicity | |||
| Caucasian | 58.3%a | 50.0%a | 27.8%b |
| African American | 29.2%a | 37.5%a | 33.3%a |
| Hispanic | 8.3%a | 8.3%a | 33.3%b |
| Asian | 4.2%a | 0.0%a | 0.0%a |
| Other | 0.0%a | 4.2%a | 5.6%a |
| Clinical Variables | |||
| AUDIT Total Score | 4.0 (2.3)a | 4.8 (4.8)a | 17.5 (9.0)b |
| DAST Total Score | 0.7 (0.7)a | 1.9 (2.5)a,b | 3.3 (3.9)b |
| PTSD Symptoms | 3.2 (3.9)a | 74.9 (24.3)b | 65.6 (15.5)b |
| Depressive Symptoms | 4.9 (5.3)a | 26.7 (11.9)b | 24.2 (11.2)b |
| Anxiety Symptoms | 5.4 (7.6)a | 24.3 (15.2)b | 20.5 (12.9)b |
| Taking Psychotropic Meds | 16.7%a | 58.3%b | 61.1%b |
| Task Variables | |||
| ERN (μV) | −0.68 (5.7)a | 1.88 (3.2)a | −1.93 (4.2)a |
| CRN (μV) | 6.8 (3.3)a | 7.0 (4.4)a | 5.7 (1.8)a |
| Error RT (ms) | 441.9 (196.0)a | 385.4 (56.9)a | 376.9 (67.0)a |
| Correct RT (ms) | 466.3 (71.9)a | 463.6 (41.3)a | 457.5 (52.1)a |
| Accuracy | 91.1 (9.3)%a | 88.6 (10.3)%a | 87.3 (7.7)%a |
Note. Means (and standard deviations) or percentages with different subscripts across rows were significantly different in pairwise comparisons (p < .05, chi-square test for categorical variables and Tukey’s honestly significant difference test for continuous variables). AUDIT = Alcohol Use Disorder Identification Test; DAST = The Drug Abuse Screening Test; PTSD symptoms assessed via the Clinician-Administered PTSD Scale (CAPS); Depressive and anxiety symptoms assessed via the Beck Depression Inventory-II and Beck Anxiety Inventory, respectively; ERN = error-related negativity; CRN = correct-related negativity; RT = reaction time.
Clinical Measures
Psychiatric diagnoses
For participants recruited from the VA Ann Arbor Healthcare System, psychiatric disorders were assessed using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders for DSM-IV (SCID-NP; First, Spitzer, Gibbon, & Williams, 2002). For participants recruited from the Jesse Brown VA Medical Center, these diagnoses were established using the Mini International Neuropsychiatric Interview 6.0 (M.I.N.I) (Sheehan et al., 1998). Both the SCID and the MINI are well-validated, semi-structured clinical interviews for the assessment of DSM-IV defined Axis I lifetime diagnoses.
PTSD-related symptoms
Participants completed the CAPS (Blake et al., 1995), an interview-based measure designed to capture DSM-IV criteria for PTSD. The CAPS includes 17 PTSD symptom dimensions that are rated on both frequency and intensity using a 5-point (0 to 4) scale. Ratings are summed to compute a severity score for each symptom, and a composite PTSD severity score (ranging from 0 to 136). Exposure to war-time stressors was measured using the 7-item, self-report Combat Exposure Scale (CES; Keane et al., 1989). Items are rated on 5-point frequency, 5-point duration, 4-point frequency, or 4-point degree of loss scales. Total CES scores range from 0 to 41 and are derived by summing each weighted item.
Substance use
Current alcohol use was measured via the Alcohol Use Disorders Identification Test (AUDIT; Babor, de la Fuente, Saunders, & Grant, 1992), a widely-used 10-item self-report measure designed to screen for patterns of harmful and hazardous alcohol use. Each item is given a score from 0 to 4 with higher scores indicating greater levels of hazardous use. Total scores range from 0 to 40. Illicit drug problems were assessed using the Drug Abuse Screening Test (DAST; Skinner, 1982), a 28-item self-report scale capturing consequences or problems related to drug use. Each item is scored in a binary (yes/no) format and responses are summed to produce a total score ranging from 0 to 28.
Internalizing symptoms
Participants completed the Beck Depression Inventory (BDI-II; Beck, Steer, Ball, & Ranieri, 1996), a well-validated, 21-item self-report measure of depressive symptoms in the past two weeks. Each item on the BDI-II is scored on a 4-point scale (0 to 3), with a range from 0 to 63. Participants also completed the Beck Anxiety Inventory (BAI; Beck & Sheer, 1990), which includes 21-items assessing anxious symptomology within the past two weeks. Like the BDI-II, each item on the BAI is scored on a 4-point scale (0 to 3) with total scores ranging from 0 to 63.
Task
Participants completed a version of the flanker task (Eriksen & Eriksen, 1974). On each trial, participants viewed five horizontally aligned arrowheads. On half of the trials, arrows were compatible (“≫≫>” or “≪≪<”) and on the other half of trials, the arrows were incompatible (“≫<≫” or “≪>≪”); the order of these trials was random. Participants’ job was to respond as quickly and as accurately as possible to indicate the direction of the center arrow. Participants pressed the right mouse button if the center arrow faced toward the right side of the screen, or the left mouse button if the center arrow faced toward the left side of the screen. Each string of arrowheads occupied approximately 1.3 degrees of visual angle vertically and 9.2 degrees of visual angle horizontally. Stimuli were presented for 200 ms, followed by a white fixation cross centrally presented on a black background. Participants were given up to 1800 ms after the offset of the arrows to respond; this was followed by an intertrial interval that varied randomly between 1000 to 2000 ms, during which participants again viewed a white fixation cross presented on a black background. The task was administered on a PentiumD class computer with a 19-in. (48.3 cm) monitor, using Presentation software (Neurobehavioral Systems, Inc., Albany, CA). Participants were seated at a viewing distance of approximately 24 in (61 cm) from the monitor.
The task consisted of 11 blocks of 30 trials (330 trials in total), interspersed with self-timed breaks. To encourage both fast and accurate responding, participants received performance-based feedback at the end of each block. If accuracy was 75% correct or lower, the message “Please try to be more accurate” was presented; if accuracy was greater than 90%, the message, “Please try to respond faster” was displayed; in all other cases, participants saw the message, “You’re doing a great job”. There were 30 practice trials prior to beginning the task.
Data Recording
Continuous EEG was recorded throughout the task, using an elastic cap and the ActiveTwo BioSemi system (BioSemi, Amsterdam, Netherlands). Thirty-four electrode sites (standard 32 channel setup, as well as FCz and Iz) were used, based on the 10/20 system; in addition, one electrode was placed on each of the left and right mastoids. The electrooculogram (EOG) generated from eyeblinks and eye movements was recorded from four facial electrodes: vertical eye movements and blinks were measured with two electrodes placed approximately 1 cm above and below the right eye; horizontal eye movements were measured using two electrodes placed approximately 1 cm beyond the outer edge of each eye. The EEG signal was pre-amplified at the electrode to improve the signal-to-noise ratio. The data were digitized at 24-bit resolution with a Least Significant Bit (LSB) value of 31.25 nV and a sampling rate of 1024 Hz, using a low-pass fifth order sinc filter with a −3dB cutoff point at 204.8 Hz. The voltage from each active electrode was referenced online with respect to a common mode sense active electrode producing a monopolar (non-differential) channel.
Off-line analyses were performed using Brain Vision Analyzer 2 software (Brain Products, Gilching, Germany). Data were re-referenced to the average of the two mastoids and band-pass filtered with high-pass and low-pass filters of 0.1 and 30 Hz, respectively. Eye blink and ocular corrections used the method developed by Miller, Gratton & Yee (1988). Data were segmented beginning 500 ms before each response onset and continuing for 1500 ms (i.e., for 1000 ms following the response). Artifact analysis was used to identify a voltage step of more than 50.0 μV between sample points, a voltage difference of 300.0 μV within a trial, and a maximum voltage difference of less than 0.50 μV within 100 ms intervals. Trials were also inspected visually for any remaining artifacts, and data from individual channels containing artifacts were rejected on a trial-to-trial basis. Baseline correction for each trial was performed using the 500 to 300 ms prior to response onset. The ERN and CRN (i.e., correct-related negativity) were scored as the average activity on error and correct trials, respectively, from 0 to 100 ms after response at electrode Cz (Gehring et al., 1993; Rabinak et al., 2013), where amplitudes were maximal (Fig. 1). The Pe (i.e., error positivity; Falkenstein et al., 1991) was scored as the average activity on error and correct trials from 200 to 400 ms after response at electrode Pz (Rabinak et al., 2013).
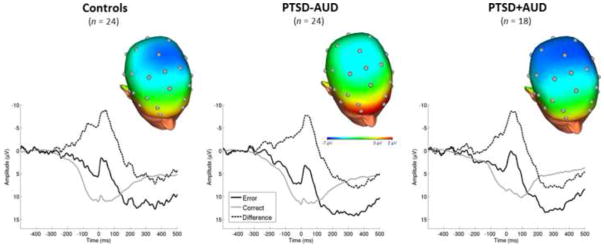
Fig. 1.
(top) Scalp topographies depicting the error minus correct amplitude difference from 0–100 ms post-response, shown separately for Controls (left), PTSD-AUD (middle) and PTSD+AUD (right) groups. (bottom) Response-locked ERP waveforms at Cz showing error and correct trial (and difference) waveforms for Controls (left), PTSD-AUD (middle) and PTSD+AUD (right) groups. For each panel, response onset occurred at 0 ms and negative amplitude is plotted up.
Accuracy data were computed as the percentage of correct trials. Reaction time was computed as the amount of time it took participants to respond from stimulus onset, separately for error and correct trials.
Data Analysis
To test whether the three groups differed on the ERN, we conducted multilevel mixed models which allows for the use of all ERN responses as the dependent variable, estimates within- and between-subject variance and appropriately models missing trial-level data (Goldstein, 2011). Given that group was a 3-level variable, it was re-coded into two 2-level variables for use in the multilevel mixed models. We created one variable in which the controls were the referent group (i.e., controls vs. other two groups) and one variable in which individuals with PTSD+AUD were the referent group (i.e., PTSD+AUD vs. the other two groups). Notably, this approach allows for all participants to be included in each model. These group variables were then entered into two separate multilevel mixed models and specified as fixed effects. Age, total CES scores (i.e., level of combat exposure), total CAPS scores (i.e., level of PTSD severity), and psychotropic medication status (yes/no; dummy-coded) were included as covariates in both models. The CRN was also included as a covariate to test whether the groups differed in ERN when controlling for potential differences in neural response to correct trials, consistent with prior studies (e.g., Henderson et al., 2015). The multilevel models used restricted maximum likelihood (REML) estimation and an unstructured covariance matrix. Continuous variables were grand-mean centered while dichotomous variables were effects coded. Hedges’ g, an index of effect size that accounts for differences in the number of participants in each group (Hedges, 1982), is reported for significant group differences.
In addition to testing group differences in the ERN, we ran parallel analyses for the behavioral measures (i.e., accuracy and reaction time). Of note, because overall task accuracy is a composite variable (rather than a trial-level variable) we tested group differences in accuracy using a hierarchical linear regression analysis, rather than a multilevel mixed model. All covariates listed above were included in the analysis of the behavioral data. We additionally ran parallel analyses with the Pe but because there were no significant group differences, these results are not presented (all ps > 0.78).
Results
Demographics and Clinical Characteristics
The demographic and clinical characteristics of individuals within each group are presented in Table 1. The three groups were matched on age and biological sex. Controls completed more years of education than individuals in both PTSD groups (who did not differ from each other). As would be expected, the PTSD groups reported higher levels of depressive and anxiety symptoms relative to controls, and were more likely to be taking psychotropic medications. The PTSD+AUD group reported significantly higher scores on a self-report index of hazardous and problematic drinking (i.e., AUDIT) compared with the PTSD-AUD group and controls. There were importantly no differences in AUDIT scores between the PTSD-AUD and control groups.
Behavioral Performance
Behavioral data means and SDs by group are displayed in Table 1. Results of the statistical models testing whether there were group differences in task accuracy, error trial reaction times, and correct trial reaction times are displayed in Table 2. On average, participants committed 35.6 ± 24.9 errors across the task and correctly responded on 89.2 ± 8.9% of trials. There were no significant group differences in task accuracy. As expected, across participants, reaction times were faster for errors, M=403.6 ±129.5 ms, than for correct responses, M=462.9 ±56.2 ms; t(65) = −5.22, p< 0.01. The groups did not differ in reaction time for either response type.
Table 2.
Models Examining Group Differences in Task Accuracy and Reaction Times
| Accuracy | Error Reaction Time | Correct Reaction Time | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variable | β | t | p-value | b | t | p-value | b | t | p-value |
| Model 1 | |||||||||
| Age | 0.11 | 0.87 | 0.39 | 1.05 | 0.77 | 0.45 | 2.79* | 3.14 | <0.01 |
| Med Status | −0.20 | −1.34 | 0.19 | −23.85* | −2.51 | 0.02 | −21.79* | −3.19 | <0.01 |
| CES Total | 0.09 | 0.59 | 0.56 | −1.45 | −1.34 | 0.19 | −0.80 | −1.10 | 0.28 |
| CAPS Total | −0.17 | −0.56 | 0.58 | 1.02* | 2.11 | 0.04 | 0.47 | 1.31 | 0.20 |
| Controls vs Others | 0.06 | 0.21 | 0.83 | −48.20 | −1.93 | 0.07 | −3.11 | −0.24 | 0.81 |
| Model 2 | |||||||||
| Age | 0.11 | 0.82 | 0.42 | 1.24 | 0.68 | 0.50 | 3.00* | 3.28 | <0.01 |
| Med Status | −0.20 | −1.35 | 0.18 | −33.98* | −2.82 | 0.01 | −24.76* | −3.28 | <0.01 |
| CES Total | 0.08 | 0.51 | 0.61 | −1.65 | −1.24 | 0.23 | −0.49 | −0.63 | 0.53 |
| CAPS Total | −0.10 | −0.58 | 0.57 | 0.47 | 0.94 | 0.35 | 0.40 | 1.56 | 0.13 |
| PTSD+AUD vs Others | 0.04 | 0.26 | 0.79 | −16.16 | −1.18 | 0.25 | 1.13 | 0.15 | 0.88 |
Note.
p < 0.05;
CES = Combat Exposure Scale; CAPS = Clinician-Administered PTSD Scale; Med Status = dichotomous variable reflecting whether or not the individual is currently taking psychotropic medication.
ERN
ERN and CRN means and SDs are presented in Table 1. Results from the multilevel mixed models testing for group differences in the ERN are presented in Table 3. Figure 1 displays the topographical map for each of the three groups, which depicts differences in voltage (in μV) across the scalp for error minus correct responses (i.e., ERN – CRN; ΔERN) in the time window of the ERN (i.e., 0–100 ms post-response).
Table 3.
Multilevel Mixed Models Examining Group Differences in ERN
| Variable | b | SE | t | p-value |
|---|---|---|---|---|
| Model 1 | ||||
| Age | −0.07 | 0.13 | −0.50 | 0.62 |
| Med Status | −0.52 | 1.09 | −0.48 | 0.64 |
| CES Total | −0.10 | 0.11 | −0.89 | 0.38 |
| CAPS Total | 0.01 | 0.06 | 0.12 | 0.91 |
| CRN | −0.01 | 0.02 | −0.23 | 0.82 |
| Controls vs Others | 0.09 | 2.04 | 0.04 | 0.97 |
| Model 2 | ||||
| Age | −0.08 | 0.12 | −0.68 | 0.50 |
| Med Status | −0.56 | 1.04 | −0.54 | 0.59 |
| CES Total | −0.17 | 0.11 | −1.55 | 0.13 |
| CAPS Total | 0.04 | 0.03 | 1.23 | 0.23 |
| CRN | −0.01 | 0.02 | −0.24 | 0.81 |
| PTSD+AUD vs Others | −2.71* | 1.07 | −2.53 | 0.02 |
Note.
p < 0.05;
CES = Combat Exposure Scale; CAPS = Clinician-Administered PTSD Scale; CRN = correct-related negativity; Med Status = dichotomous variable reflecting whether or not the individual is currently taking psychotropic medication.
Results from the first model indicated that as expected, there were no significant differences in the ERN between controls and individuals with PTSD, both with and without AUD. However, results from the second model revealed that there was a significant effect of PTSD+AUD, suggesting that those with PTSD+AUD displayed larger ERNs relative to those with PTSD-AUD. To verify this pattern of results, we conducted follow-up paired comparisons which indicated that indeed, individuals with PTSD+AUD exhibited larger ERNs relative to PTSD-AUD (b = −6.39, SE =2.18, t = −2.94, p < 0.01; Hedges g = 0.34), but that there was no difference between individuals with PTSD+AUD and controls (b = −1.40, SE =4.11, t = −0.34, ns). There were no significant main effects of any of covariate.
Discussion
Converging lines of evidence suggest that individuals with comorbid PTSD and AUD may be characterized by heightened defensive reactivity, which could serve to maintain drinking behaviors and anxiety/hyperarousal symptoms. Notably, however, very few studies have directly tested whether individuals with PTSD and AUD (PTSD+AUD) exhibit greater defensive reactivity to errors compared with individuals with PTSD who do not have a comorbid AUD (PTSD-AUD). Here, we found that among individuals with PTSD, those with comorbid AUD had significantly larger ERNs than those without AUD, suggesting that PTSD+AUD may represent a neurobiologically distinct subgroup of individuals with PTSD, and that the comorbidity of AUD may be associated with enhanced defensive responding in PTSD individuals. Meanwhile, neither the PTSD+AUD group nor the PTSD-AUD group significantly differed from the veteran controls in the ERN highlighting that defensive reactivity to errors does not distinguish PTSD groups from non-PTSD groups.
The fact that individuals with and without PTSD do not differ in the ERN is consistent with the prior findings by Rabinak et al. (2013) and Swick et al. (2015) and together suggests that PTSD in-and-of-itself is not characterized by an increased ERN. In contrast, increased ERN has been previously observed in those with obsessive-compulsive disorder (OCD; Hajcak, Franklin, Foa, & Simons, 2008; Riesel, Endrass, Kaufmann, & Kathmann, 2011) and generalized anxiety disorder (GAD; Weinberg, Olvet, & Hajcak, 2010; Xiao et al. 2010), and individuals with high levels of trait anxiety (Meyer, Weinberg, Klein, & Hajcak, 2011) and negative affect (Hajcak, McDonald, & Simons, 2004). Importantly, however, an increased ERN has not been as consistently observed in fear-based anxiety disorders, such as phobias (Hajcak, McDonald, & Simons, 2003; Moser, Hajcak, & Simons, 2005) and appears to be more related to disorders with increased anxious-misery/distress (Vaidyanathan, Nelson, & Patrick, 2011). Because PTSD symptoms are so heterogeneous and the disorder has been shown to overlap with both fear-based and anxious-misery/distress disorders (Zoellner, Pruitt, Farach, & Jun, 2014), it may not be as robustly related to the ERN as more classic distress disorders like OCD and GAD. The present findings underscore, though, that within PTSD there are groups of individuals who exhibit exaggerated defensive reactivity to errors/threats relative to other groups – namely, those with comorbid and without AUD. This implies that although the ERN may not relate to the pathophysiology of PTSD, it may nonetheless be an important marker of comorbidity status within trauma-exposed populations and shed light on of the mechanisms that may underlie PTSD+AUD comorbidity.
As hypothesized, current findings indicate that individuals with PTSD+AUD display heightened reactivity to threat, as reflected in the ERN following errors, relative to individuals with PTSD-AUD. There are several related processes that may contribute to this phenomenon. For example, it is possible that among those with PTSD, heightened defensive reactivity is a risk factor for onset of AUD and is therefore evident before onset of problematic drinking. As was previously discussed, alcohol consumption alleviates distress and has been shown to dampen threat reactivity (Moberg & Curtin, 2009) and reduce the ERN response to errors by dampening negative affect (Bartholow et al., 2012). Therefore, for individuals with PTSD that experience chronic, heightened defensive reactivity, alcohol consumption may be particularly negatively reinforcing, which could motivate this subset of individuals to continue drinking (Koob, 2013). In other words, those with comorbid PTSD+AUD may have had an existing affective vulnerability that predisposes them to developing a concurrent AUD.
If this is the case, screening and targeting PTSD patients with heightened defensive reactivity might help prevent the onset of AUD. It is also possible that within this group, heightened defensive reactivity does not emerge until after PTSD+AUD onset. Repeated alcohol use impacts important neural circuits, especially those that mediate stress and negative affective responding (Koob & Volkow, 2010). Relatedly, several studies have shown that chronic alcohol use exacerbates anxiety symptoms and can potentiate defensive responding (Cosci et al., 2007; Gorka et al., 2013). It is therefore possible that because of excessive drinking, individuals with PTSD+AUD exhibit exaggerated defensive reactivity to errors/threat. Although not a risk factor, this possibility nevertheless has important clinical implications as heightened defensive reactivity is likely to maintain drinking behaviors and contribute to the need and desire to maintain alcohol use. In order to disrupt the feed-forward cycle between negative affect and alcohol consumption directly targeting defensive reactivity within the context of PTSD+AUD treatment may prove to be beneficial. On a broader level, given that several studies have now demonstrated that comorbid AUD and anxiety disorders are associated with exaggerated reactivity to threat (i.e., Gorka et al., 2013; Schellekens et al., 2010), targeting this deficit in the context of any AUD+anxiety treatment may be beneficial as accumulating evidence suggests that aversive responding may be a transdiagnostic feature central to alcohol and anxiety/trauma-related comorbidity.
It is necessary to note that several prior studies have found that individuals with remitted AUD (not in the context of anxiety or trauma-related disorders) continue to display larger ERNs (Padilla et al., 2011; Schellekens et al., 2010); however the present study is the first to demonstrate that within the context of PTSD, individuals with current AUDs also exhibit this abnormality. Heightened defensive reactivity to errors may therefore represent a stable characteristic that is present within AUD populations regardless of disease state. There is also some evidence to suggest that it may be relatively specific to alcohol versus other drugs of abuse, as studies have shown that cocaine and opiate-dependent individuals (Marhe, van de Wetering, & Franken, 2013; Forman et al., 2004), and adolescent offspring of parents with illicit substance use disorders (Euser, Evans, Greaves-Lord, Huizink, & Franken, 2013), display a reduced ERN and/or error-related ACC activity. This is a potentially clinically important distinction between alcohol and other substances and additional studies are critically needed to explore the potential factors that contribute to these discrepant findings.
Although the current study addressed a significant gap in the existing literature, there were several limitations that should be noted. First, many of the PTSD participants in the current sample were taking psychiatric medications and although the PTSD+AUD and PTSD-AUD groups were matched on prevalence of medications, and medication status was included as a covariate in all analyses, it is unclear to what extent this may have impacted the current results. Second, the majority of the participants in the current study were male, and all were veterans, making the generalizability of the findings to female and civilian PTSD populations unclear. Third, the sample size was relatively small, which may have limited our statistical power and ability to detect other important group differences. Lastly, the study was cross-sectional and thus, we cannot infer whether heightened reactivity to errors among PTSD+AUD individuals occurred prior or subsequent to the comorbidity. Future studies are therefore needed to clarify the present findings and elucidate the temporal relations between responding to errors and onset of PTSD+AUD.
With these limitations in mind, there are several important implications of these findings. The current study indicates that individuals with PTSD+AUD display a greater neural response to threat/errors than individuals with PTSD-AUD. It is possible that this affective abnormality predisposes individuals to comorbidity and/or maintains the retractable nature of PTSD+AUD. Defensive reactivity may therefore be a valuable target for prevention and intervention within this subgroup, and perhaps all comorbid AUD and anxiety or trauma/stress related disorders. Given that accumulating evidence implicates defensive reactivity as a key factor underlying anxiety and AUD pathology, future research is needed to continue to explore how individual differences in threat responding and underlying neurobiological mechanisms may contribute to the common co-occurrence of these disorders.
Acknowledgments
This material is based on work supported by Veterans Affairs Merit Review Program Awards (to KLP) from Clinical Sciences Research and Development, Office of Research and Development of the U.S. Department of Veterans Affairs.
References
- Babor TF, de la Fuente JR, Saunders J, Grant M. AUDIT: The Alcohol Use Disorders Identification Test. Guidelines for use in primary health care. Geneva: World Health Organization; 1992. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. http://dx.doi.org/10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Henry EA, Lust SA, Saults JS, Wood PK. Alcohol effects on performance monitoring and adjustment: affect modulation and impairment of evaluative cognitive control. Journal of Abnormal Psychology. 2012;121(1):173–186. doi: 10.1037/a0023664. http://dx.doi.org/10.1037/a0023664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory manual. San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. http://dx.doi.org/10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. http://dx.doi.org/10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forenia CA. Psychometric properties of the PTSD checklist (PCL) Behavior Research and Therapy. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. http://dx.doi.org/10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biological Psychiatry. 2005;57(8):832–840. doi: 10.1016/j.biopsych.2004.12.025. http://dx.doi.org/10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Cosci F, Schruers KR, Abrams K, Griez EJ. Alcohol use disorders and panic disorder: a review of the evidence of a direct relationship. The Journal of Clinical Psychiatry. 2007;68(6):1–478. doi: 10.4088/jcp.v68n0608. http://dx.doi.org/10.4088/JCP.v68n0608. [DOI] [PubMed] [Google Scholar]
- Debell F, Fear NT, Head M, Batt-Rawden S, Greenberg N, Wessely S, Goodwin L. A systematic review of the comorbidity between PTSD and alcohol misuse. Social Psychiatry and Psychiatric Epidemiology. 2014;49(9):1401–1425. doi: 10.1007/s00127-014-0855-7. http://dx.doi.org/10.1007/s00127-014-0855-7. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. http://dx.doi.org/10.1111/j.1467-9280.1994.tb00630.x. [Google Scholar]
- Easdon C, Izenberg A, Armilio ML, Yu H, Alain C. Alcohol consumption impairs stimulus-and error-related processing during a Go/No-Go Task. Cognitive Brain Research. 2005;25(3):873–883. doi: 10.1016/j.cogbrainres.2005.09.009. http://dx.doi.org/10.1016/j.cogbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Endrass T, Klawohn J, Gruetzmann R, Ischebeck M, Kathmann N. Response- related negativities following correct and incorrect responses: Evidence from a temporospatial principal component analysis. Psychophysiology. 2012;49(6):733–743. doi: 10.1111/j.1469-8986.2012.01365.x. http://dx.doi.org/10.1111/j.1469-8986.2012.01365.x. [DOI] [PubMed] [Google Scholar]
- Euser AS, Evans BE, Greaves-Lord K, Huizink AC, Franken IH. Diminished error-related brain activity as a promising endophenotype for substance-use disorders: evidence from high-risk offspring. Addiction Biology. 2013;18(6):970–984. doi: 10.1111/adb.12002. http://dx.doi.org/10.1111/adb.12002. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. http://dx.doi.org/10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, … Jovanovic T. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychological Medicine. 2012;42(03):533–543. doi: 10.1017/S0033291711001565. http://dx.doi.org/10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version – Non-patient Edition (SCID- I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, … Lorensen E. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biological Psychiatry. 2004;55(5):531–537. doi: 10.1016/j.biopsych.2003.09.011. http://dx.doi.org/10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A Neural System for Error Detection and Compensation. Psychological Science. 1993;4(6):385–390. http://doi.org/10.1111/j.1467-9280.1993.tb00586.x. [Google Scholar]
- Goldstein H. Multilevel statistical models. Vol. 922. John Wiley & Sons; 2011. [Google Scholar]
- Gorka SM, Nelson BD, Shankman SA. Startle response to unpredictable threat in comorbid panic disorder and alcohol dependence. Drug and Alcohol Dependence. 2013;132(1):216–222. doi: 10.1016/j.drugalcdep.2013.02.003. http://doi.org/10.1016/j.drugalcdep.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry. 2008;165:898– 904. doi: 10.1176/appi.ajp.2007.07101581. http://doi.org/10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry. 2009;66(1):47–53. doi: 10.1016/j.biopsych.2008.12.028. http://doi.org/10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Foti D. Errors are aversive defensive motivation and the error-related negativity. Psychological Science. 2008;19(2):103–108. doi: 10.1111/j.1467-9280.2008.02053.x. http://doi.org/10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Franklin M, Foa E, Simons R. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. American Journal of Psychiatry. 2008;165(1):116–123. doi: 10.1176/appi.ajp.2007.07010143. http://dx.doi.org/10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biological Psychology. 2003;64(1):77–90. doi: 10.1016/s0301-0511(03)00103-0. http://dx.doi.org/10.1016/S0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons R. Error-related psychophysiology and negative affect. Brain and Cognition. 2004;56(2):189–197. doi: 10.1016/j.bandc.2003.11.001. http://dx.doi.org/10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42(2):151–160. doi: 10.1111/j.1469-8986.2005.00270.x. http://dx.doi.org/10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hedges LV. Estimation of effect size from a series of independent experiments. Psychological Bulletin. 1982;92(2):490–499. [Google Scholar]
- Henderson HA, Ono KE, McMahon CM, Schwartz CB, Usher LV, Mundy PC. The costs and benefits of self-monitoring for higher functioning children and adolescents with autism. Journal of Autism and Developmental Disorders. 2015;45(2):548–559. doi: 10.1007/s10803-013-1968-7. http://doi.org/10.1007/s10803-013-1968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S, Falkenstein M. Independent component analysis of erroneous and correct responses suggests online response control. Human Brain Mapping. 2010;31(9):1305–1315. doi: 10.1002/hbm.20937. http://dx.doi.org/10.1002/hbm.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Jackson SI, Unrod M. Generalized expectancies for negative mood regulation and problem drinking among college students. Journal of Studies on Alcohol and Drugs. 2000;61(2):332–340. doi: 10.15288/jsa.2000.61.332. http://dx.doi.org/10.15288/jsa.2000.61.332. [DOI] [PubMed] [Google Scholar]
- Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, Mora CA. Clinical evaluation of a measure to assess combat exposure. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1989;1:53–55. http://dx.doi.org/10.1037/1040-3590.1.1.53. [Google Scholar]
- Kline A, Weiner MD, Ciccone DS, Interian A, Hill LS, Losonczy M. Increased risk of alcohol dependency in a cohort of National Guard troops with PTSD: a longitudinal study. Journal of Psychiatric Research. 2014;50:18–25. doi: 10.1016/j.jpsychires.2013.11.007. http://dx.doi.org/10.1016/j.jpsychires.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. http://dx.doi.org/10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacological Review. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. http://dx.doi.org/10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, … Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. American Journal of Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. http://dx.doi.org/10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, … Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biological Psychiatry. 1999;45(7):817–826. doi: 10.1016/s0006-3223(98)00246-7. http://dx.doi.org/10.1016/S0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Post D, Kennedy AE, Rabinak CA, Phan KL. Electrocortical processing of social signals of threat in combat-related post-traumatic stress disorder. Biological Psychology. 2013;94(2):441–449. doi: 10.1016/j.biopsycho.2013.08.009. http://dx.doi.org/10.1016/j.biopsycho.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. Journal of Affective Disorders. 1988;14:61–68. doi: 10.1016/0165-0327(88)90072-9. http://dx.doi.org/10.1016/0165-0327(88)90072-9. [DOI] [PubMed] [Google Scholar]
- Marhe R, van de Wetering BJ, Franken IH. Error-related brain activity predicts cocaine use after treatment at 3-month follow-up. Biological Psychiatry. 2013;73(8):782–788. doi: 10.1016/j.biopsych.2012.12.016. http://dx.doi.org/10.1016/j.biopsych.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error- related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year- olds. Developmental Cognitive Neuroscience. 2011;2(1):152–161. doi: 10.1016/j.dcn.2011.09.005. http://dx.doi.org/10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Gratton G, Yee CM. Generalized implementation of an eye movement correction procedure. Psychophysiology. 1988;25:241–243. http://doi.org/doi:10.1111/j.1469-8986.1988.tb00999.x. [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable versus predictable threat. Journal of Abnormal Psychology. 2009;118(2):335–347. doi: 10.1037/a0015636. http://psycnet.apa.org/doi/10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Simons RF. The effects of fear on performance monitoring and attentional allocation. Psychophysiology. 2005;42:261–268. doi: 10.1111/j.1469-8986.2005.00290.x. http://doi.org/10.1111/j.1469-8986.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38(5):752–760. http://doi.org/10.1111/1469-8986.3850752. [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review. 2008;28(8):1343–1354. doi: 10.1016/j.cpr.2008.07.003. http://doi.org/10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46(5):957–961. doi: 10.1111/j.1469-8986.2009.00848.x. http://doi.org/10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Ouimette PC, Ahrens C, Moos RH, Finney JW. Posttraumatic stress disorder in substance abuse patients: Relationship to 1-year posttreatment outcomes. Psychology of Addictive Behaviors. 1997;11(1):34–47. http://psycnet.apa.org/doi/10.1037/0893-164X.11.1.34. [Google Scholar]
- Padilla ML, Colrain IM, Sullivan EV, Mayer BZ, Turlington SR, Hoffman LR, … Pfefferbaum A. Electrophysiological evidence of enhanced performance monitoring in recently abstinent alcoholic men. Psychopharmacology. 2011;213(1):81–91. doi: 10.1007/s00213-010-2018-1. http://doi.org/10.1007/s00213-010-2018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Holman A, Angstadt M, Kennedy AE, Hajcak G, Phan KL. Neural response to errors in combat-exposed returning veterans with and without post- traumatic stress disorder: a preliminary event-related potential study. Psychiatry Research. 2013;213(1):71–78. doi: 10.1016/j.pscychresns.2013.01.002. http://doi.org/10.1016/j.pscychresns.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GP. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298(5601):2209–2211. doi: 10.1126/science.1076929. http://doi.org/10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as an endophenotype for obsessive-compulsive disorder: Evidence from unaffected firstdegree relatives. American Journal of Psychiatry. 2011;168:317. doi: 10.1176/appi.ajp.2010.10030416. http://doi.org/10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Meyer A, Hajcak G. The ERN is the ERN is the ERN? Convergent validity of error-related brain activity across different tasks. Biological Psychology. 2013;93(3):377–385. doi: 10.1016/j.biopsycho.2013.04.007. http://doi.org/10.1016/j.biopsycho.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Robinson J, Sareen J, Cox BJ, Bolton JM. Role of self-medication in the development of comorbid anxiety and substance use disorders: a longitudinal investigation. Archives of General Psychiatry. 2011;68(8):800–807. doi: 10.1001/archgenpsychiatry.2011.75. http://doi.org/10.1001/archgenpsychiatry.2011.75. [DOI] [PubMed] [Google Scholar]
- Schellekens AF, De Bruijn ER, Van Lankveld CA, Hulstijn W, Buitelaar JK, De Jong CA, Verkes RJ. Alcohol dependence and anxiety increase error- related brain activity. Addiction. 2010;105(11):1928–1934. doi: 10.1111/j.1360-0443.2010.03065.x. http://doi.org/10.1111/j.1360-0443.2010.03065.x. [DOI] [PubMed] [Google Scholar]
- Schroder KE, Perrine MW. Covariations of emotional states and alcohol consumption: evidence from 2 years of daily data collection. Social Science & Medicine. 2007;65(12):2588–2602. doi: 10.1016/j.socscimed.2007.07.011. http://doi.org/10.1016/j.socscimed.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, … Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122(2):322–338. doi: 10.1037/a0030747. http://psycnet.apa.org/doi/10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. http://doi.org/Retrieved from https://www.psychiatrist.com/default2.asp. [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, … Alpert NM. Regional cerebral blood flow in the amygdala and medial prefrontalcortex during traumatic imagery in male and female vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. http://doi.org/10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addictive Behaviors. 1982;7(4):363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Stewart SH. Trauma memory and alochol abuse: Drinking to forget? In: Read D, Lindsay DS, editors. Recollections of trauma: Scientific evidence and clinical practice. New York: Plenum; 1997. pp. 461–467. [Google Scholar]
- Swick D, Honzel N, Turken U. Intact error monitoring in combat Veterans with post-traumatic stress disorder. Psychiatry Research: Neuroimaging. 2015;234(2):227–238. doi: 10.1016/j.pscychresns.2015.09.016. http://doi.org/10.1016/j.pscychresns.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Nelson LD, Patrick CJ. Clarifying domains of internalizing psychopathology using neurophysiology. Psychological Medicine. 2012;42(03):447–459. doi: 10.1017/S0033291711001528. http://dx.doi.org/10.1017/S0033291711001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior. 2002;77(4–5):477–482. doi: 10.1016/s0031-9384(02)00930-7. http://dx.doi.org/10.1016/S0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, Bonnet M. Is the ‘error negativity’ specific to errors? Biological Psychology. 2000;51(2):109–128. doi: 10.1016/s0301-0511(99)00032-0. http://dx.doi.org/10.1016/S0301-0511(99)00032-0. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Dieterich R, Riesel A. Error-related brain activity in the age of RDoC: a review of the literature. International Journal of Psychophysiology. 2015;98(2):276–299. doi: 10.1016/j.ijpsycho.2015.02.029. http://dx.doi.org/10.1016/j.ijpsycho.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Longer term test–retest reliability of error-related brain activity. Psychophysiology. 2011;48(10):1420–1425. doi: 10.1111/j.1469-8986.2011.01206.x. http://dx.doi.org/10.1111/j.1469-8986.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Olvet D, Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biological Psychology. 2010;85:472–480. doi: 10.1016/j.biopsycho.2010.09.011. http://dx.doi.org/10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Riesel A, Hajcak G. Integrating multiple perspectives on error-related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motivation and Emotion. 2012;36(1):84–100. http://dx.doi.org/10.1007/s11031-011-9269-y. [Google Scholar]
- Xiao Z, Wang J, Zhang M, Li H, Tang Y, Wang Y, … Fromson JA. Error- related negativity abnormalities in generalized anxiety disorder and obsessive–compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(1):265–272. doi: 10.1016/j.pnpbp.2010.11.022. http://dx.doi.org/10.1016/j.pnpbp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Yeater EA, Austin JL, Green MJ, Smith JE. Coping mediates the relationship between posttraumatic stress disorder (PTSD) symptoms and alcohol use in homeless, ethnically diverse women: A preliminary study. Psychological trauma: Theory, Research, Practice, and Policy. 2010;2(4):307–310. http://psycnet.apa.org/doi/10.1037/a0021779. [Google Scholar]
- Zoellner LA, Pruitt LD, Farach FJ, Jun JJ. Understanding heterogeneity in PTSD: fear, dysphoria, and distress. Depression and Anxiety. 2014;31(2):97–106. doi: 10.1002/da.22133. http://dx.doi.org/10.1002/da.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]