Abstract
Here we address the effect of Akt signaling on endothelial progenitor cells (EPCs). Human peripheral blood mononuclear cells (PBMCs) were cultured on fibronectin-coated dishes in EPC differentiation medium. PBMCs differentiated in a series of three steps: proliferation for foci formation, tight attachment to the dishes in the early stages of differentiation, and maturation in the late stages. In Western blot analysis, Akt expression was attenuated in the early stages of differentiation and was gradually upregulated during EPC maturation. Forkhead box–containing protein, class O 3a (FOXO3a), an Akt downstream target, was down-regulated through phosphorylation in the late stages of EPC differentiation. Adenovirus-mediated overexpression of activated FOXO3a in PBMCs markedly increased the number of cell foci but reduced the number of DiI-acetyl LDL EPCs that appear at later time points. These data suggest that Akt/FOXO3a signaling is an important regulator of EPC maturation.
Keywords: Akt, FOXO3a, endothelial progenitor cells, mononuclear cells, differentiation
Introduction
The serine-threonine protein kinase Akt (also known as protein kinase B, or PKB) functions downstream from phosphatidylinositol 3-kinase (PI3K) as an important regulator of cell survival, growth, and glucose metabolism in many cell types (1). Previously, we reported the role of Akt in hematopoietic stem cells using an Akt-gene knockout mouse model (2). Bone marrow from Akt1-deficient mice exhibits a reduced side-population (SP) fraction due to attenuated translocation of Bcrp1 by Akt. PI3K/Akt signaling has also been implicated in the differentiation of a variety of cell types, including hematopoietic cells (3–5). However, the regulation and expression of Akt during differentiation are unclear.
Forkhead box–containing protein, O subfamily (FOXO) factors are downstream effectors of Akt that play a pivotal role in the regulation of cell-cycle progression and cell survival (6). FOXOs also respond to extracellular cues via changes in Akt signaling to control the differentiation and transformation of many cell types. Three FOXO factors (1, 3a, and 4) are substrates of the Akt protein kinase. They are inactivated through phosphorylation, which results in sustained nuclear exclusion (7, 8). FOXO factors are reported to be developmentally regulated during embryogenesis and myoblast differentiation (6). Although FOXO3a (FKHRL1) is reported to be expressed in hematopoietic progenitors (9, 10) and to affect hematopoiesis by controlling apoptosis signaling (11), FOXO3a’s effects on stem-progenitor cell differentiation remain unclear.
Recently, FOXO1 (FKHR)-deficient mice were reported to display abnormal angiogenesis, indicating that FOXO1 plays a critical role in normal vascular development in rodents (12) (13). However, little is known about the function of FOXO3a in endothelial progenitor cell (EPC) development. Therefore, to elucidate the intracellular signaling mechanisms that control EPCs during differentiation, we examined the detailed differentiation steps of EPCs, the expression of Akt and FOXO3a, and the effects of enforced FKHRL1 activation in EPCs. Here, we demonstrated that the Akt-FOXO3a signaling axis can control the differentiation of progenitor cells.
Methods
Endothelial Progenitor Assay
EPCs were cultured as described previously (14). Human peripheral blood monocytes (PBMCs) from healthy volunteers were isolated with Histopaque 1077 (Sigma, St. Louis, USA). PBMCs were cultured on fibronectin (Sigma)-coated 10-cm dishes or 6-well plates (BD Falcon, Franklin Lakes, USA) in EGM-2 medium (Clonetics, Walkersville, USA) containing 10% FBS, endothelial cell growth supplement, and antibiotics (Invitrogen, Carlsbad, USA) without corticosteroid. EPCs were defined 7 days after culture by staining with both fluorescein isothiocyanate (FITC)–labeled lectin (Sigma) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbo-cyanineperchlorate–labeledacetylatedlow-densitylipoprotein (LDL) [DiI-acLDL] (Biomedical Technologies, Stoughton, USA) in three different fields.
Immunofluorescent Staining
Immunofluorescent staining was performed as described previously (2). Attached cells on fibronectin-coated dishes were washed and then fixed with ethanol and methanol solution (1:1 mixed) and permeabilized with 0.1% saponin prior to incubation with primary antibody. The following primary antibodies were used: anti–macrophage antigen-1 (Mac-1) and anti–von Willebrand factor (vWF) (BD Pharmingen, Franklin Lakes, USA), anti-flk-1 and anti–endothelial nitric-oxide synthase (eNOS) (Santa Cruz Biotechnology, Santa Cruz, USA) and anti-FOXO3a (Upstate Biotechnology, Waltham, USA). Cells were mounted using Vectashield→ mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, USA). Images were viewed and analyzed using automated image analysis software (OpenLab, Improvision, Lexington, USA).
Immunoblot Analysis
Immunoblot analysis was performed as described previously (15). The following primary antibodies were used: anti-Akt (Santa Cruz Biotechnology), anti-phospho-Akt (Cell Signaling Technology, Beverly, USA), anti-FOXO3a (rabbit polyclonal IgG, Upstate Biotechnology), anti-phospho-FOXO3a (Ser253) (rabbit polyclonal IgG, Upstate Biotechnology), anti-phospho-FOXO3a (Thr32) (rabbit polyclonal IgG, Upstate Biotechnology).
Adenovirus Gene Transfer
A green fluorescent protein (GFP)–containing adenoviral vector of FOXO3a-AAA triple mutant (TM-FOXO3a), which was not phosphorylatable because three phosphorylation sites (Thr32, Ser253, and Ser315) were replaced by alanine residues, was used as described previously (16). Transfection efficiency estimated by GFP was usually expressed in 50 to 70% of the cells.
Statistical Analysis
Data are shown as mean ± SEM All other data were evaluated with the two-tailed, unpaired Student’s t-test or compared by one-way analysis of variance.
Results and Discussion
Characterization of Human EPC Differentiation
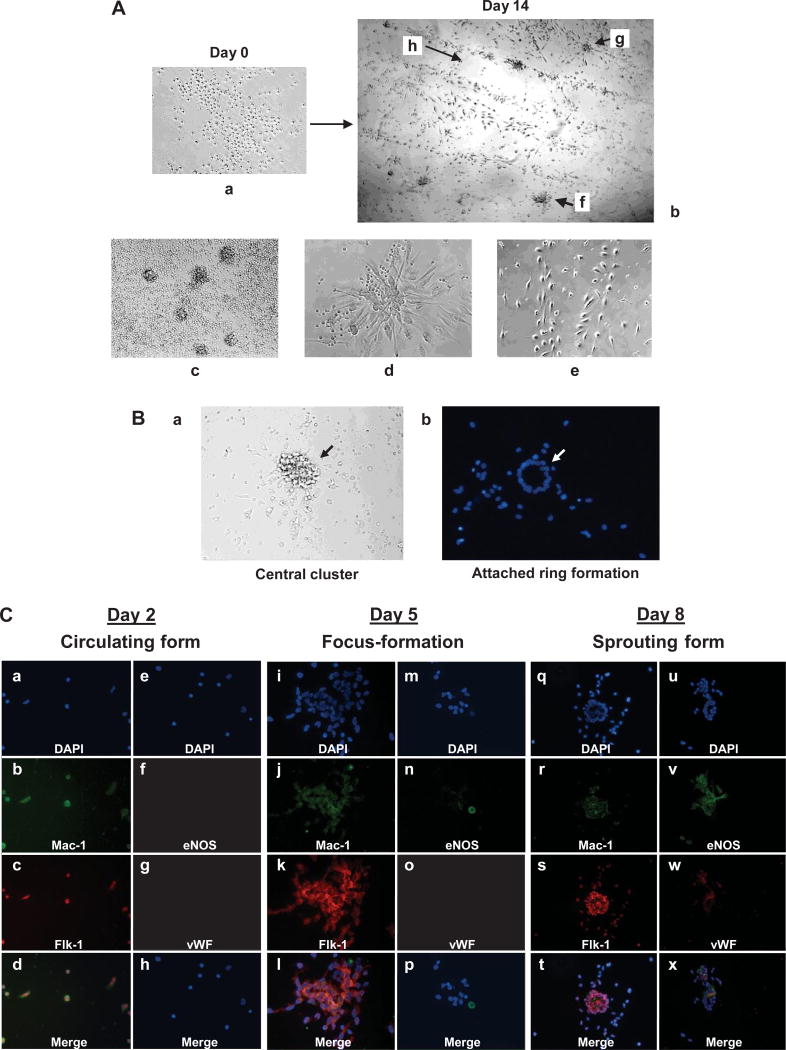
During differentiation, human PBMCs formed distinct colonies typically on day 4 of culture on fibronectin-coated dishes. These cells differentiated into endothelial-like cells in three steps: foci formation, sprouting from foci, and migration and maturation into cord-like structures (Fig. 1A). These steps typically occurred on days 4, 7, and 14, respectively, after culture on fibronectin. The steps were dependent on PBMC concentration, serum concentration, and whether or not corticosteroid was present in the serum (data not shown). Interestingly, these foci separated into two parts after gentle washing: an easily detached central region and a tightly attached ring formation at the periphery (Fig. 1B). The removed central region of foci could proliferate and form sprouting spindle-like cells again if re-cultured on fibronectin-coated dishes, whereas spindle-like cells sprouting from foci might have less proliferative ability than cells in the central region (data not shown). This suggests that, in such a center region, cells remain in a less-differentiated state and increase in number, then tightly attach to fibronectin and differentiate into matured cells. We also examined the characteristics of foci formation with immunofluorescent staining as shown in Fig. 1C. In the early differentiation step, cells in the foci expressed both flk-1 and Mac-1, which represent cell markers for endothelial progenitor and monocytic lineages, respectively. However, these cells did not express detectable eNOS or vWF. After 8 days, cells expressed flk-1, eNOS, and vWF in sprouting cells at the periphery of foci, but not Mac-1, indicating that these cells are more differentiated endothelial progenitors. These results support our expected characterization of the foci from Fig. 1A and B, and indicate that PBMCs differentiate in a series of three steps: proliferation, attachment, and maturation.
Fig. 1.
Characterization of human endothelial progenitor cell differentiation. A: Isolated peripheral blood mononuclear cells (PBMCs) (a) can differentiate into endothelial-like cells (b) in vascular endothelial growth factor–containing medium on fibronectin-coated dishes in about 2 weeks. Various steps (c–e) of differentiation were observed. f: Foci formation; g: migration (sprouting from foci); h: cord-like structure. B: Foci showing loose central cluster (a) and tightly attached peripheral ring formation of cells (b). DAPI staining of foci after gentle washing. Representative images are shown. C: Expression of cell surface markers in early EPC differentiation steps by immunofluorescent staining of EPCs with antibodies against Mac-1, flk-1, eNOS, and vWF with DAPI counterstaining. Human PBMCs were isolated from healthy volunteers and cultured on fibronectin-coated 2-well slide chambers. Images were viewed with a confocal microscope. a–h: Attached cells on Day 2; i–p: focus formation on Day 5; q–x: foci with sprouting cells on Day 8 of culture of PBMCs. d: Merged a–c; h: merged e–g; l: merged i–k; p: merged m–o; t: merged q–s; x: merged u–w.
Akt and FKHRL1 Expression during EPC Differentiation
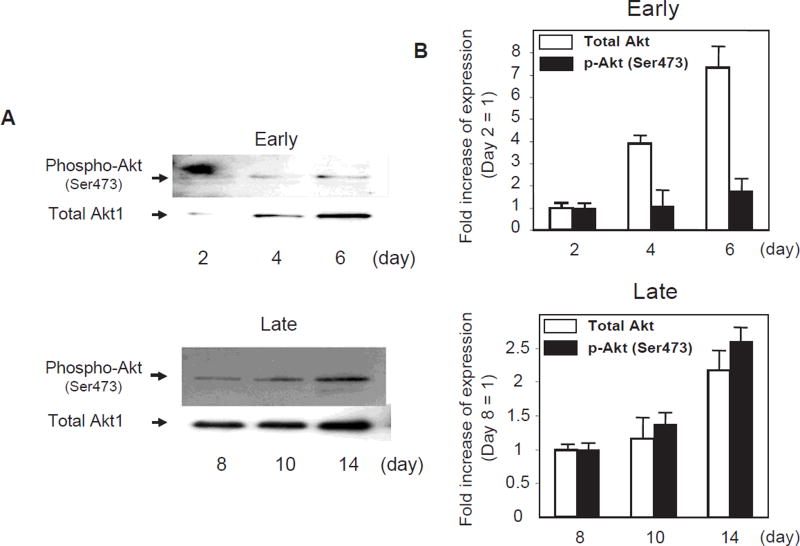
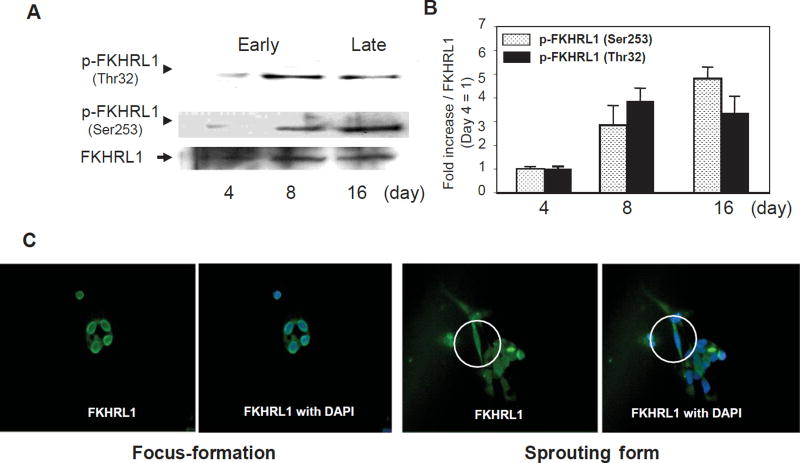
Next, we examined signal transduction in attached PBMCs by immunoblot assays. As shown in Fig. 2, Akt1 protein expression and phosphorylation at Ser473 were low at early stages of differentiation but gradually increased with time in culture. Consistent with these observations, FOXO3a was under-phosphorylated at the early stages of differentiation, but phosphorylation at Thr32 and Ser253 increased with time in culture (Fig. 3A, B). Urbich et al. observed the higher expression of FOXO4 in EPCs, whereas the expression of FOXO1 and FOXO3a is very low or absent in EPCs (17). However, the lower expression levels of FOXO1 and FOXO3a in EPC were observed within 1 week of culture from PBMNCs. Our results also showed a lower level of “phosphorylated” FOXO3a expression in EPC within 1 week of culture. However, FOXO3a phosphorylation gradually increased (i.e., the “inactive form” increased) during EPC maturation. Immunofluorescent staining also showed that FOXO3a was translocated into the cytosol at the time the foci sprouted out; however, FOXO3a was located in the nucleus in the early foci without sprouting cells (Fig. 3C). Although we observed the increases in the expression of Akt and FOXO3a phosphorylation separately, whether each phosphorylation occurs sequentially or separately could not be determined in this experiment. This may be a limitation of this study.
Fig. 2.
Expression of Akt during endothelial progenitor cell differentiation. A: Immunoblot analysis of EPCs with antibodies against total Akt1 and phosphorylated Akt. Akt-immunoblot assay was performed as described in Methods, separately with samples at early differentiation (days 2, 4, and 6) and late differentiation (days 8, 10, and 14). B: Comparison of the expression of total Akt1 and phosphorylated Akt in early and late differentiation stages by densitometric analysis. n = 4.
Fig. 3.
Expression of FOXO3a during endothelial progenitor cell differentiation. A: Immunoblot analysis of EPCs with antibodies against total FOXO3a and phosphorylated FOXO3a. FOXO3a-immunoblot assay was performed as described in Methods. B: Comparison of the expression of phosphorylated FOXO3a (Thr32 and Ser253) by densitometric analysis. n = 3. C: Immunofluorescence of EPC with antibodies against total FOXO3a. Circles in figures indicate sprouting cells from foci.
Recently, Kim et al. reported that the PI3K/Akt pathway contributes to the maintenance of self-renewal in human embryonic stem cells after basic fibroblast growth factor (bFGF) stimulation (18). Collectively, these data indicate that Akt signaling not only maintains stem cells in a self-renewal state, but also regulates progenitor cell maturation. Glycogen synthase kinase 3β (GSK3β), another Akt target, is also reported to be involved in the determination of stem cell self-renewal or differentiation through its ability to negatively regulate the Wnt pathway (19, 20). During EPC differentiation, we observed that GSK3β was also markedly phosphorylated (data not shown). Thus, multiple downstream effectors of Akt may cooperate to control EPC differentiation and phenotype.
Enforced Expression of FOXO3a Increases Foci Number but Not Endothelial-Like Cells in Human EPC Assay
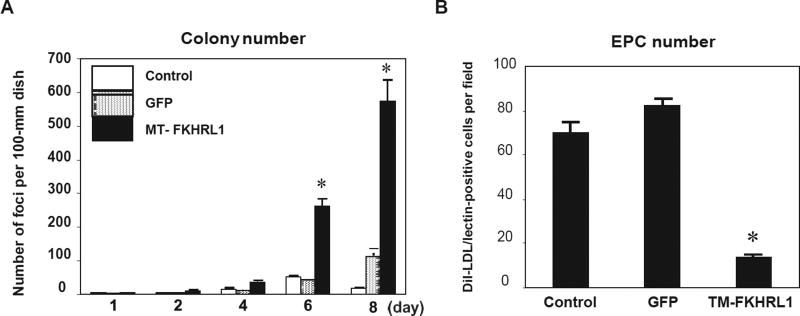
Finally, we performed virus-mediated overexpression gene studies in PBMCs to test the functional significance of FOXO phosphorylation during EPC differentiation. Transfection efficiency with adenovirus was approximately 50–70% of total cells on each dish, based on an analysis of GFP-positive cells (data not shown). In cultured human PBMCs, adenovirus-mediated transduction of FOXO3a triple mutant, in which the three Akt phosphorylation sites are replaced by alanine residues, significantly increased the number of foci formed in these cultures (Fig. 4A). This increase in foci formation was accompanied by a reduction in cells stained with DiI-acLDL (Fig. 4B), suggesting that enforced expression of a non-phosphorylatable form of FOXO3a markedly increased foci formation but not spindle-like EPCs. This, in turn, indicated a block in EPC differentiation. To further examine the reduction in the number of EPCs, we found that formed foci failed to attach to the fibronectin-coated dishes and to differentiate into spindle-like cells. These results indicate that the FOXO3a signal is necessary to form foci but should be inactivated in the late stage of differentiation.
Fig. 4.
The role of FOXO3a during endothelial progenitor cell differentiation, using enforced expression of activated FOXO3a. Transfection efficiency for adenovirus into human EPCs was estimated by counting GFP-expressing cells. A: Quantification of foci with enforced expression of TM-FOXO3a vs. controls. Means ± SEM, n = 3. *p < 0.01 vs. control. B: Quantification of EPCs with enforced expression of TM-FOXO3a vs. controls. Means ± SEM, n = 4. *p < 0.05 vs. control.
Timely Akt-Controlled Activation and Inactivation of FOXO3a May Be Required in Human EPC Differentiation
It is well known that in “differentiated” cells, FOXO3a activation enhances apoptosis involving Fas/FasL signaling. It was reported that the downregulation of FOXO3a (active form) enhanced neovascularization in vivo (21). Indeed, we also observed that “overexpression” of the active form of FOXO3a (MT-FOXO3a) in “mature” endothelial cells and well-differentiated EPCs enhanced apoptosis and inhibited endothelial cell function (data not shown). These papers mainly explained the effects of FOXO3a on “differentiated” cells. However, FOXO3a is also a pivotal differentiation factor in undifferentiated cells as described by Bakker et al. (9). A recent report also demonstrates that mice lacking the myocyte nuclear factor (MNF), one of the forkhead family members, die in the first few weeks of life with a severe myopathy that is similar to Duchenne’s muscular dystrophy in humans (22). Therefore, MNF acts to regulate genes that coordinate the proliferation and differentiation of myogenic stem cells after muscle injury. Another forkhead protein is known to have important regulatory functions during development in the control of cell proliferation and differentiation, as well as in tissue morphogenesis (23–25). These reports support our findings that indicate the regulation of stem-progenitor cell differentiation by FOXO3a. Activation of FOXO3a may be required for the initiation of cell differentiation, but not for maturation. Further examination will be needed to confirm the contributions of timely activation and inactivation of FOXO3a to EPC differentiation.
EPCs are now being targeted as therapeutic agents for ischemic organs and as a pathophysiological target of diseases, such as cancer progression (26) and retinopathy (27). It has been shown that the number of EPC foci is inversely associated with cardiovascular risk factors (28), suggesting that these foci are clusters of a progenitor cell population that have a critical role in angiogenesis. Here, we demonstrated that overexpression of MT-FOXO3a (“active form”) increased nondifferentiated EPC foci, resulting in reduced EPC number. Thus, the elevated level of the “active” form of FOXO3a inhibited EPC maturation and angiogenesis. Moreover, we observed the tendency of atherosclerosis-related factors to affect the number of EPC foci. For example, treatment with oxidative low-density lipoprotein (ox-LDL) in PBMCs probably increased the number of cell foci. However, these foci were easily detached, resulting in the reduction in the number of EPCs (our observation results). This observation is similar to that by enforced expression of activated FOXO3a. Therefore, the Akt-FOXO3a signaling axis may be involved not only in regulating angiogenesis but also in influencing the induction of atherosclerosis.
In conclusion, we have shown that the Akt-FOXO3a signaling axis can control the differentiation of endothelial progenitor cells. In the early stage of differentiation, FOXO3a signals may be required for cell proliferation and initiation, whereas Akt signals could be needed to mature the initiated cells by inactivated FOXO3a through phosphorylation. Therefore, the proper regulation of Akt-FOXO3a signaling plays a pivotal role in stem-progenitor cell differentiation.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to M.H. and M.M.); from the Suzuken Memorial Foundation (to M.M.); and by the NIH (no. HL081587; to K.W.).
References
- 1.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 2.Mogi M, Yang J, Lambert JF, et al. Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J Biol Chem. 2003;278:39068–39075. doi: 10.1074/jbc.M306362200. [DOI] [PubMed] [Google Scholar]
- 3.Fujio Y, Guo K, Mano T, Mitsuuchi Y, Testa JR, Walsh K. Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol Cell Biol. 1999;19:5073–5082. doi: 10.1128/mcb.19.7.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rommel C, Clarke BA, Zimmermann S, et al. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Li X, Eswarakumar VP, Seger R, Lonai P. Fibroblast growth factor (FGF) signaling through PI 3-kinase and Akt/PKB is required for embryoid body differentiation. Oncogene. 2000;19:3750–3756. doi: 10.1038/sj.onc.1203726. [DOI] [PubMed] [Google Scholar]
- 6.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 7.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 8.Birkenkamp KU, Coffer PJ. Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem Soc Trans. 2003;31:292–297. doi: 10.1042/bst0310292. [DOI] [PubMed] [Google Scholar]
- 9.Bakker WJ, Blazquez-Domingo M, Kolbus A, et al. FoxO3a regulates erythroid differentiation and induces BTG1, an activator of protein arginine methyl transferase 1. J Cell Biol. 2004;164:175–184. doi: 10.1083/jcb.200307056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engstruöm M, Karlsson R, Jönsson JI. Inactivation of the forkhead transcription factor FoxO3 is essential for PKB-mediated survival of hematopoietic progenitor cells by kit ligand. Exp Hematol. 2003;31:316–323. doi: 10.1016/s0301-472x(03)00002-x. [DOI] [PubMed] [Google Scholar]
- 11.Kashii Y, Uchida M, Kirito K, et al. A member of Forkhead family transcription factor, FKHRL1, is one of the downstream molecules of phosphatidylinositol 3-kinase–Akt activation pathway in erythropoietin signal transduction. Blood. 2000;96:941–949. [PubMed] [Google Scholar]
- 12.Furuyama T, Kitayama K, Shimoda Y, et al. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 13.Hosaka T, Biggs WH, 3rd, Tieu D, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 16.Skurk C, Maatz H, Kim HS, et al. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J Biol Chem. 2004;279:1513–1525. doi: 10.1074/jbc.M304736200. [DOI] [PubMed] [Google Scholar]
- 17.Urbich C, Knau A, Fichtlscherer S, et al. FOXO-dependent expression of the proapoptotic protein Bim: pivotal role for apoptosis signaling in endothelial progenitor cells. FASEB J. 2005;19:974–976. doi: 10.1096/fj.04-2727fje. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Cheon SH, Yoo SJ, et al. Contribution of the PI3K/Akt/PKB signal pathway to maintenance of self-renewal in human embryonic stem cells. FEBS Lett. 2005;579:534–540. doi: 10.1016/j.febslet.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 20.Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase–dependent signaling. J Biol Chem. 2004;279:48063–48070. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 21.Potente M, Urbich C, Sasaki K, et al. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garry DJ, Meeson A, Elterman J, et al. Myogenic stem cell function is impaired in mice lacking the forkhead/winged helix protein MNF. Proc Natl Acad Sci U S A. 2000;97:5416–5421. doi: 10.1073/pnas.100501197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winnier GE, Hargett L, Hogan BL. The winged helix transcription factor MFH1 is required for proliferation and patterning of paraxial mesoderm in the mouse embryo. Genes Dev. 1997;11:926–940. doi: 10.1101/gad.11.7.926. [DOI] [PubMed] [Google Scholar]
- 24.Xuan S, Baptista CA, Balas G, Tao W, Soares VC, Lai E. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron. 1995;14:1141–1152. doi: 10.1016/0896-6273(95)90262-7. [DOI] [PubMed] [Google Scholar]
- 25.Freyaldenhoven BS, Freyaldenhoven MP, Iacovoni JS, Vogt PK. Aberrant cell growth induced by avian winged helix proteins. Cancer Res. 1997;57:123–129. [PubMed] [Google Scholar]
- 26.Rumpold H, Wolf D, Koeck R, Gunsilius E. Endothelial progenitor cells: a source for therapeutic vasculogenesis? J Cell Mol Med. 2004;8:509–518. doi: 10.1111/j.1582-4934.2004.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant MB, May WS, Caballero S, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 28.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]