Abstract
Background
Intraoperative pain during Mohs micrographic surgery (MMS) has not been characterized. However, many patients report postoperative pain on the day of MMS.
Objective
We sought to determine if patients experience pain during their MMS visit.
Methods
In phase I of this study, patients were asked to report intraoperative pain level using the verbal numerical rating scale (0–10) at discharge. In phase II, pain levels were assessed before each Mohs layer and at discharge, to determine whether pain was experienced throughout the day.
Results
Pain was reported at some point during the MMS day for 32.8% of patients (n = 98). The mean pain number reported was 3.7 (range 1–8) out of 10. Pain was more commonly reported by patients who spent a longer time in the office, had 3 or more Mohs layers, and had a flap or graft repair. Patients most frequently reported pain with surgical sites of the periorbital area and nose.
Limitations
Time between Mohs layers was not measured. There was nonstandardized use of intraoperative local anesthesia volume and oral pain medications.
Conclusion
Some patients experience pain during MMS. However, the majority of patients report a low level of pain. Additional preventative measures could be considered in patients at higher risk.
Keywords: intraoperative pain, Mohs micrographic surgery, verbal numerical rating scale-11
Pain has been referred to as the fifth vital sign,1 and the assessment of pain is commonplace during inpatient hospitalization. However, standardized pain assessment is not universal in health care, including in the outpatient surgery setting.
Pain related to Mohs micrographic surgery (MMS) has been previously studied. However, existing studies focus on postoperative pain. These studies show that pain after MMS is prevalent, reported in over 50% of patients, and most frequently occurs on the day of surgery.2–4
Although data on postoperative pain after MMS exist, little is known about evaluation or management of intraoperative pain during the procedure. Because of the inherent wait times of MMS, pain may be relevant to some patients, influencing their experience and perception of the procedure. The objective of this study was to assess intraoperative pain during MMS. Our secondary objective was to identify variables associated with an increased likelihood of patient-reported pain during MMS.
METHODS
This quality improvement pilot project had 2 phases and was implemented in the practices of 3 Mohs surgeons (K. S. N., A. M. R., and E. H. L.) at a single cancer center. Data review was conducted under an institutional review board waiver. In the first phase, lasting 2 consecutive months, patients undergoing MMS were asked to verbally report their maximum pain number from any point during their MMS day on the verbal numerical rating scale (VNRS)-115 during standard discharge instructions. Questioning about pain was performed by the nurse or physician, who asked the standard question “did you have any pain during the day today?” If a patient responded that they had experienced pain during their MMS day, they were then asked to provide their pain number on the VNRS-11. A score of 0 indicates no pain and 10 indicates worst pain imaginable. On this scale, mild pain is considered to be in the range of 1 to 3; moderate, from 4 to 7; and severe, from 8 to 10.
Standard anesthesia used in our practice is buffered 1% lidocaine with epinephrine (1:200,000). Ice packs are frequently used after local anesthesia and in between Mohs layers. Oral analgesics are provided by patient request (most commonly acetaminophen 650 mg or acetaminophen and oxycodone) and are offered to patients reporting pain.
In the second phase, we implemented a quality improvement measure of routine pain assessment in the MMS unit. For 2 consecutive months, assessment of pain before each Mohs layer was initiated. During the second phase, oral analgesics were offered to patients who reported pain. Pain numbers were recorded on the MMS operative reports using the same methodology as for the participants in the earlier phase of the study. Patient charts were then retrospectively reviewed for age, sex, tumor type, preoperative and postoperative tumor size, location, number of Mohs stages, total time spent in the office, closure, analgesic medication given, and pain number as described above.
Descriptive statistics including relative frequencies, means, and SD were used to describe the study participants and aspects of the surgical procedure. Because many of the surgical characteristics have the potential of being highly correlated, pairwise correlations for the study variables were performed. Participant-reported pain was assessed as a dichotomous variable (no reported pain vs any reported pain). Cross-classifications of reported pain with participant and surgical characteristics along with χ2 statistics were calculated. The t tests were used to assess the differences in the distributions of age, preoperative size of the lesion, defect size, and the total elapsed time of the surgical procedure by participant-reported pain. Logistic regression was used to assess the association between participant-reported pain and the study surgical procedural variables. Robust SE were calculated to adjust variance estimates for multiple observations from a subset of participants. For these analyses, participant age, sex, and study phase were included in each of the models. All analyses were performed with software (Stata, v14.0, Stata Corp, College Station, TX).
RESULTS
A total of 299 skin cancers were included from 270 participants. Of these, 242 (89.6%) contributed 1 surgical site, 27 (10%) contributed 2, and 1 (0.4%) contributed 3. The average age of participants was 68.1 (SD 13.5) years and those who reported any pain during their surgery were younger than those who did not report pain (65.3 vs 69.4 years, respectively, P = .017). Participants reported pain during 98 (32.8%) of the procedures, with an average reported pain score of 3.7 of 10. A majority of the participants were female (n = 153, 56.7%), however, no difference in patient-reported pain was observed by sex (P = .91).
Fig 1 presents the distribution of reported pain. Of those who reported pain during their procedure, 56% reported mild pain (scores 1–3), 39% reported moderate pain (scores >3–6), and only 5% reported severe pain (scores >6–10). Of patients who reported pain, 63 (64.3%) received an intervention for pain (eg, in-office oral pain medication, injection of bupivacaine 0.5% to the surgical site, or prescription for pain medication), with 57 receiving an intraoperative intervention. Characteristics of the surgical procedures and the distribution of reported pain by these characteristics are presented in Table I. Pain was found to be positively associated with the total number of surgical stages with patients being over 6 times more likely to report procedure-related pain when their surgery required 3 or more stages for complete clearance (odds ratio 6.1, 95% confidence interval 2.9–12.7, P < .001). The amount of elapsed time from the beginning of the first surgical layer until the patient leaves the office was also found to be positively associated with pain. Patients with procedures in the top quintile of elapsed time were 6.2 times more likely to report pain than those in the lowest quintile (odds ratio 6.2, 95% confidence interval 2.7–14.3, P < .001). Anatomic site was also associated with pain, with procedures on the periorbital area and nose more likely to be associated with pain, whereas trunk and extremity sites were less likely to be associated with pain.
Fig 1.
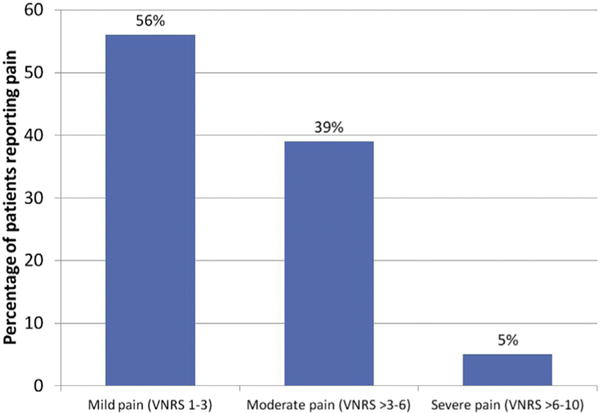
Percentage of patients reporting mild (verbal numerical rating scale [VNRS] 1–3), moderate (VNRS >3–6), and severe (VNRS >6–10) pain. The highest pain number reported at any point during the surgery day is represented.
Table I.
Logistic regression estimates of the association among pain, number of stages, final defect size, and closure type
| Pain
|
||||||
|---|---|---|---|---|---|---|
| Overall | No | Yes | P value | OR (95% CI) | P value | |
| Stages, N (%) | ||||||
| 1 | 112 (37.6) | 90 (45.0) | 22 (22.4) | <.001 | 1.0 (ref) | – |
| 2 | 131 (44.0) | 86 (43.0) | 45 (45.9) | 2.2 (1.2–4.0) | .011 | |
| ≥3 | 55 (18.4) | 24 (12.0) | 31 (31.6) | 6.1 (2.9–12.7) | <.001 | |
| Closure type, N (%) | ||||||
| Second intent | 60 (20.1) | 49 (24.4) | 11 (11.2) | <.001 | 1.0 | – |
| Flap | 51 (17.1) | 20 (10.0) | 31 (31.6) | 6.7 (2.7–16.5) | <.001 | |
| Plastics | 27 (9) | 12 (6.0) | 15 (15.3) | 5.8 (2.1–16.1) | .001 | |
| Graft | 9 (3) | 3 (1.5) | 6 (6.1) | 9.0 (1.9–42.1) | .005 | |
| Complex | 146 (48.8) | 114 (56.7) | 32 (32.7) | 1.3 (0.6–2.8) | .513 | |
| Other | 6 (2) | 3 (1.5) | 3 (3.1) | 4.6 (0.8–26.2) | .088 | |
| Diagnosis, N (%) | ||||||
| BCC | 190 (63.6) | 120 (63.2) | 70 (36.8) | .14 | ||
| SCC | 106 (35.5) | 79 (74.5) | 27 (25.5) | 0.7 (0.4–1.2) | .15 | |
| Other | 3 (1) | 2 (66.7) | 1 (33.3) | 0.6 (0.05–7.2) | .70 | |
| Preoperative size, cm | ||||||
| Longest dimension, mean (SD) | 0.85 (0.68) | 0.87 (0.73) | 0.81 (0.56) | .46 | 0.9 (0.6–1.3) | .46 |
| Defect size, cm | ||||||
| Longest dimension, mean (SD) | 1.51 (0.9) | 1.48 (1.0) | 1.54 (.075) | .69 | 1.3 (0.9–1.8) | .11 |
| Elapsed time, hours:min | ||||||
| Mean (SD) | 4:57 (1:54) | 4:34 (1:44) | 5:43 (1:58) | <.001 | 1.4 (1.2–1.6) | <.001 |
| Elapsed time quintiles, min, N (%) | ||||||
| 60–195 | 60 (20.4) | 47 (23.9) | 13 (13.4) | <.001 | 1.0 | – |
| 196–269 | 64 (21.8) | 47 (23.9) | 17 (17.5) | 1.32 (0.6–3.1) | .52 | |
| 270–326 | 53 (18) | 37 (18.8) | 16 (16.5) | 1.5 (0.7–3.6) | .33 | |
| 327–395 | 59 (20.1) | 43 (21.8) | 16 (16.5) | 1.4 (0.6–3.3) | .44 | |
| 396–599 | 58 (19.7) | 23 (11.7) | 35 (36.1) | 6.2 (2.7–14.3) | <.001 | |
| Location, N (%) | ||||||
| Cheek | 47 (15.8) | 34 (72.3) | 13 (27.7) | <.001 | 1.0 | – |
| Ear | 19 (6.4) | 13 (68.4) | 6 (31.6) | 1.2 (0.4–3.8) | .75 | |
| Forehead | 39 (13.1) | 26 (66.7) | 13 (33.3) | 1.3 (0.5–3.3) | .569 | |
| Nose | 60 (20.2) | 30 (50) | 30 (50) | 2.6 (1.2–5.9) | .021 | |
| Scalp | 22 (7.4) | 12 (54.6) | 10 (45.5) | 2.2 (0.8–6.3) | .148 | |
| Trunk/extremity | 73 (24.6) | 65 (89) | 8 (11) | 0.3 (0.1–0.9) | .022 | |
| Chin | 4 (1.4) | 3 (75) | 1 (25) | 0.9 (0.1–9.2) | .909 | |
| Lip | 16 (5.4) | 10 (62.5) | 6 (37.5) | 1.6 (0.5–5.2) | .461 | |
| Neck | 2 (0.7) | 2 (100) | 0 (0) | Omit | ||
| Periorbital | 15 (5.1) | 4 (26.7) | 11 (73.3) | 7.2 (1.9–26.7) | .003 | |
Each logistic model controlled for age, sex, and cohort (ie, phase) the participant was enrolled. BCC, Basal cell carcinoma; CI, confidence interval; OR, odds ratio; SCC, squamous cell carcinoma.
Elapsed procedure time and number of stages were found to be positively correlated (r = 0.6063) and were not included together in any analyses to mitigate any potential colinearity (Table II). The histopathologic diagnosis, overall preoperative size of the lesion, and resultant defect size were not found to be associated with patient-reported pain.
Table II.
Correlation matrix for study variables
| Stages | Elapsed time | Final defect size, cm | |
|---|---|---|---|
| Stages | 1 | ||
| Elapsed time | 0.6063 | 1 | |
| Final defect size, cm | 0.3397 | 0.3930 | 1 |
DISCUSSION
This study was undertaken to assess intraoperative pain during MMS. In this study, pain was experienced during 32.8% of procedures. Most patients experienced only minor pain, as expected, and declined intervention. Of patients, 5% experienced more severe pain, considered to be greater than 6 on the VNRS-11. In our population, pain was rarely reported by patients unless directly asked. On further questioning, a common response from patients was that they expected to be in pain because they were undergoing surgery, so did not think to report it. An increase in the percentage of patients reporting pain after instituting a protocol of regular pain assessment suggests that pain may be more prevalent during MMS than previously expected based on patient reporting.
Previous studies have shown multiple associations between postoperative pain and clinical characteristics. For example, Sniezek et al6 showed that the lip, followed by the nose, ear, and forehead, were the most painful sites. The scalp was the most painful area reported by Limthongkul et al.4 Similarly, our study showed that the anatomic sites of periorbital area and nose were significantly associated with pain. Firoz et al3 showed an increase in pain reporting for flap closures. Our population demonstrated an increase in pain associated with flaps and grafts. Similarly to other studies, we did not find an association between reported pain and defect size or gender.3,4 Although we observed that age may be associated with the reporting of pain, the difference was small and further studies are needed to determine if patient age influences the perception of pain during MMS.
Experiencing pain related to a surgical procedure can significantly alter a patient’s perception of their care and their satisfaction with treatment.7 Higher overall patient satisfaction after outpatient orthopedic procedures has been associated with low levels of postoperative pain and the perception that nurses and physicians showed concern for the patients’ pain levels.8 Even patients who do experience continued pain despite interventions have been shown to report increased satisfaction when their providers attempted to address the pain.9 Although patient satisfaction was not specifically assessed in our study, we can extrapolate that expressing concern and addressing pain, even when mild, may contribute to increased patient satisfaction.
Potential limitations of our study include the small sample size. Pain is also difficult to quantify as it is very subjective; however, our intent was to measure perceived pain. The influence of individual factors such as anxiety may play a role in pain perception, and can be a confounder when predicting which patients will experience pain. Chen et al10 showed that, specifically in an MMS population, patients with high anxiety toward pain experienced greater postoperative pain. We did not systematically assess patient anxiety, which may have contributed to greater pain perception in some patients. The volume of local anesthesia delivered is also not standardized as multiple practitioners perform injections. Possibly, the volume of anesthesia is a contributing factor and could be evaluated in a separate study. Bupivacaine is not used as part of standard anesthesia in our practice; however, 1 patient with a large forehead site (>3 cm postoperative size) received bupivacaine with initial injection of anesthesia and did not report pain; therefore, bupivacaine was a potential confounder in this 1 case. Although total time was measured, time between MMS layers was not measured or correlated with pain. Further, the administration of intraoperative oral pain medication was not standardized. Therefore, the subsequent pain numbers reported after receiving oral pain medications may have been underestimated.
Conclusions
Based on this study, instituting a policy of routine pain assessment in the MMS day better identifies patients who are in pain. Identifying this group of patients allows the surgical team to address pain earlier and offer additional treatment such as ice packs, oral analgesics, and use of longer-acting local anesthetic agents for patients throughout the surgical day. The VNRS-11 is a quick and simple tool for assessment of MMS pain and was easily integrated into our practice. In addition, many patients expressed gratitude for the attention paid to their comfort during their surgery day. Simply asking patients about whether they are experiencing pain may allow interventions to control pain and may improve the quality of the patient care experience. Based on these findings, standard questioning on intraoperative pain during MMS has become our practice’s standard of care.
CAPSULE SUMMARY.
The incidence of postoperative pain after Mohs micrographic surgery is estimated at 50%. However, to our knowledge, pain during Mohs micrographic surgery has not been characterized.
Almost 30% of patients experience intraoperative pain during Mohs micrographic surgery, identified by standard patient questioning.
Three or more Mohs layers, flap or graft repair, and surgical sites in the periorbital and nose areas were associated with intraoperative pain.
Acknowledgments
The authors would like to acknowledge the Mohs team at Memorial Sloan-Kettering Cancer Center, including the nurses.
Funding sources: None.
Footnotes
Conflicts of interest: None declared.
Presented in the Tromovitch Award Abstract Session at the American College of Mohs Surgery Annual Meeting, San Antonio, TX, April 30, 2015.
References
- 1.Sayers M, Marando R, Fisher S, Aquila A, Morrison B, Dailey T. No need for pain. J Healthc Qual. 2000;22:10–15. doi: 10.1111/j.1945-1474.2000.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 2.Harris K, Curtis J, Larsen B, et al. Opioid pain medication use after dermatologic surgery: a prospective observational study of 212 dermatologic surgery patients. JAMA Dermatol. 2013;149:317–321. doi: 10.1001/jamadermatol.2013.1871. [DOI] [PubMed] [Google Scholar]
- 3.Firoz BF, Goldberg LH, Arnon O, Mamelak AJ. An analysis of pain and analgesia after Mohs micrographic surgery. J Am Acad Dermatol. 2010;63:79–86. doi: 10.1016/j.jaad.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 4.Limthongkul B, Samie F, Humphreys TR. Assessment of postoperative pain after Mohs micrographic surgery. Dermatol Surg. 2013;39:857–863. doi: 10.1111/dsu.12166. [DOI] [PubMed] [Google Scholar]
- 5.Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analog scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41:1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007–1013. doi: 10.1111/j.1524-4725.2011.02022.x. [DOI] [PubMed] [Google Scholar]
- 7.Glass JS, Hardy CL, Meeks NM, Carroll BT. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543–560. doi: 10.1016/j.jaad.2015.04.050. [DOI] [PubMed] [Google Scholar]
- 8.Jamison RN, Ross MJ, Hoopman P, et al. Assessment of postoperative pain management: patient satisfaction and perceived helpfulness. Clin J Pain. 1997;13:229–236. doi: 10.1097/00002508-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Sherwood G, Adams-McNeill J, Starck PL, Nieto B, Thompson CJ. Qualitative assessment of hospitalized patients’ satisfaction with pain management. Res Nurs Health. 2000;23:486–495. doi: 10.1002/1098-240X(200012)23:6<486::AID-NUR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Chen AF, Landy DC, Kumetz E, Smith G, Weiss E, Saleeby ER. Prediction of postoperative pain after Mohs micrographic surgery with 2 validated pain anxiety scales. Dermatol Surg. 2015;41:40–47. doi: 10.1097/DSS.0000000000000224. [DOI] [PubMed] [Google Scholar]


