Abstract
Acyclovir (ACV) and its derivatives have been highly effective for treating recurrent, lytic infections with Herpes Simplex Virus, type 1 (HSV-1), but searches for additional antiviral drugs are motivated by recent reports of resistance to ACV, particularly among immunocompromised patients. In addition, the relative neurotoxicity of ACV and its inability to prevent neurological sequelae among HSV-1 encephalitis survivors compel searches for new drugs to treat HSV-1 infections of the central nervous system (CNS). Primary drug screens for neurotropic viruses like HSV-1 typically utilize non-neuronal cell lines, but they may miss drugs that have neuron specific antiviral effects. Therefore, we compared the effects of a panel of conventional and novel anti-herpetic compounds in monkey epithelial (Vero) cells, human induced pluripotent stem cells (hiPSCs)-derived neural progenitor cells (NPCs) and hiPSC-derived neurons (N = 73 drugs). While the profiles of activity for the majority of the drugs were similar in all three tissues, Vero cells were less likely than NPCs to identify drugs with substantial inhibitory activity in hiPSC-derived neurons. We discuss the relative merits of each cell type for antiviral drug screens against neuronal infections with HSV-1.
HSV-1 causes lytic and productive infection in most human cells, but therapeutic options are limited and effective vaccines are currently unavailable (Kuo et al., 2014). Thus, HSV-1 causes lifelong recurrent infections in over 3.7 billion persons, world-wide (Looker et al., 2015). Derivatives of the acycloguanosines, such as Acyclovir (ACV) or its prodrug, Valacyclovir (VAL) can effectively suppress lytic infection through selective activation in virus-infected cells, followed by blockade of viral DNA replication. Yet, there is growing concern about HSV-1 resistance to these drugs (Frobert et al., 2014; Reardon and Spector, 1989; Stránská et al., 2005; Malvy et al., 2005), particularly in immune impaired patients. While the prevalence of resistance is approximately 0.5% in immunocompetent patients, it is over 30 fold higher in immunocompromised patients (Frobert et al., 2014). Resistance to ACV can develop from mutations in the viral thymidine kinase and/or DNA polymerase, with incidence rates estimated at 7.1% in immunocompromised persons (Reardon and Spector, 1989; Stránská et al., 2005; Malvy et al., 2005; Helldén et al., 2003; Chowdhury et al., 2014). Viral resistance is a common problem with known efficacious anti herpetic drugs, such as the helicase primase inhibitors (Piret and Boivin, 2014; Burrel et al., 2013; Hussin et al., 2013; Sukla et al., 2010). Foscarnet, the only approved second line antiviral drug to HSV-1, requires intravenous administration, and has considerable toxicity (Sukla et al., 2010; Mortality in patients; Markham and Faulds, 1994).
The rising prevalence of genital HSV-1 infections is raising concerns for fetal infections - particularly CNS infections that could affect neurodevelopment as a consequence of the high susceptibility of neuronal progenitor cells (NPCs) to HSV-1 (Chucair-Elliott et al., 2014). While ACV treatment improves survival from neonatal encephalitis, it does not improve the odds of normal neuronal development (Engman et al., 2008; Carter et al., 2003). A large proportion of adult encephalitis survivors also suffer permanent neurological sequelae. Evidently, ACV can cross the blood brain barrier, as neurotoxicity has been described in patients with renal failure (Chowdhury et al., 2016; Berry and Venkatesan, 2014). Thus, the limited efficacy of ACV against CNS infections suggests that lytic HSV-1 infection in the brain (and its successful treatment) are different from those in mucosal cells. For these reasons, even though most routine antiviral screening assays for HSV-1 utilize cell lines that support lytic infection, such as African green monkey (Vero) cells, antiviral drug screens in neuronal cells could be more informative. Further, in view of the human specific infection caused by HSV-1, assays using human neuronal cells would be preferable. Drug screening in human neuronal cells has been impractical in the past because it is difficult to obtain large numbers of primary human neural cells for culture (Immergluck et al., 1998; Richart et al., 2003) and neuron-like cells derived from cancer cell lines may not adequately mimic healthy neural tissues (Danaher et al., 1999; Su et al., 2000).
The rapid advent of induced pluripotent stem cells (iPSCs)-based technologies has enabled virtually limitless numbers of human mitotic and postmitotic neural cells. Such cells possess many morphological and functional features of CNS neurons (D’Aiuto et al., 2014). Though iPSC-based cell platforms are increasingly used to screen for drugs against cardiac diseases, their utility for CNS infections has not been evaluated extensively. We therefore compared the anti-herpetic activity and toxicity of a panel of compounds in three different cell types; Vero cells, human iPSC-derived neural progenitor cells (NPCs) and neurons.
Human NPCs were generated from iPSCs as described (D’Aiuto et al., 2014). Neurons were generated by culturing NPCs in Neurobasal medium supplemented with 2% B27, BDNF 10 ng/ml, GDNF 20 ng/ml, CHIR99021 3 μM, forskolin 10 μM, dorsomorphin 1 μM, 50 U/ml penicillin G, and 50 mg/ml streptomycin (neurobasal BGCFC medium). After 4 days, CHIR99021, forskolin, and dorsomorphin were withdrawn and cells cultured for additional 25 days. Vero cells were cultured as previously described (McClain et al., 2015). Infection protocols used a modified KOS HSV-1 strain that expresses enhanced green fluorescent protein (EGFP) from the viral immediate early ICP0 promoter and monomeric red fluorescent protein (mRFP) from the promoter of the true late regulated Glycoprotein C (gC) (McClain et al., 2015). Infections of Vero cells and NPCs were approximately 1 pfu/cell for the multiplicity of infection (MOI), whilst neurons were infected with 0.3 pfu/cell. The virus was removed after 2 h. Drugs were added 2 h post infection (hpi), and Vero cells and NPCs were analyzed 24 h post infection (hpi), and neurons after 48 h. Flow cytometry was employed to analyze neuronal cultures because neuronal cultures produce multicellular aggregates that cannot be analyzed using high-content imaging system IXU software. The percentage of EGFP positive (EGFP+) cells in infected Vero cells and NPC cultures was analyzed using an ImageXpress Ultra (IXU) High Content imager. Each drugwas tested against each cell type and the proportion of inhibition in relation to untreated, infected cells was estimated.
The drugs screened in the three culture system represented different classes of molecules and their derivatives, including lysosomotropic agents and their derivatives, alkaloids derived from the Amaryllus plant, nostodiones, quinazolinones, and epigenetic inhibitors (Supplementary Fig. 1). We recently described the efficacy of lysosomotropic agents and derivatives of amaryllis derived alkaloids against lytic HSV-1 infections (McClain et al., 2015; McNulty et al., 2016). The former include 16F19, and 4F17; the latter include alkaloids R430, MP4B6, and MP4G10 referred to as C7, C5, and C3, respectively. A selected group of nostodiones were also tested, as nostodione A possesses proteasome inhibitory activity (Shim et al., 2008) and proteosome inhibitors exhibit antiviral activity, including effects against HSV-1 infection (La Frazia et al., 2006). We also tested a group of quinazolinones, because several derivatives have potential antiviral activity against influenza (Liu et al., 2015), HIV (Corbett et al., 2000), and TMV (Ma et al., 2014). Additionally, quinazolinone analogs can interfere with epigenetic regulation through inhibition of bromodomains (BET) (Gilham et al., 2016). It is well established that epigenetic regulation is involved in the reactivation of latent HSV-1; treatment with histone deacetylase inhibitors can promote reactivation of HSV-1 from quiescence in vitro and in vivo animal models (D’Aiuto et al., 2015) whereas inhibitors of histone demethylases can block reactivation (Messer et al., 2015). Thus, epigenetic inhibitors could potentially inhibit HSV-1 infections in CNS cells. Finally, ISO-SYN-CYB, was chosen because it is a phenylpropanoid dimer derived from the natural product eugenol, a potent antiviral agent (Benencia and Courrèges, 2000). All drugs were purchased from commercial vendors or synthesized locally (McNulty et al., in preparation).
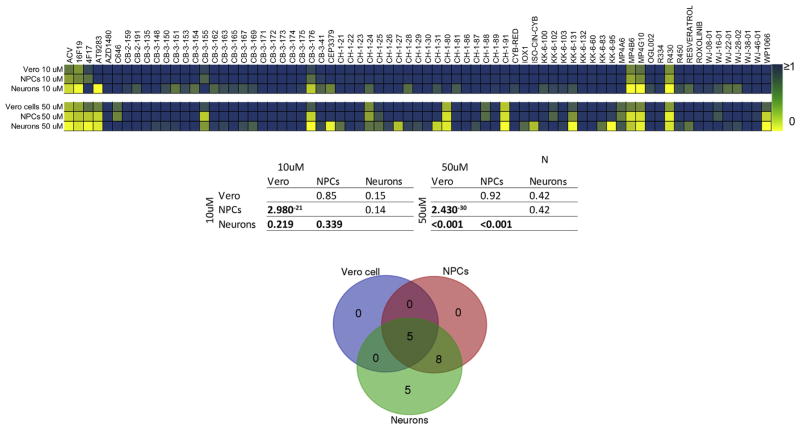
During initial drug screens, compounds are typically evaluated at a limited range of concentrations, and arbitrary cutoffs are used to identify potentially efficacious compounds that are assessed more intensively in a subsequent step. We thus tested each compound at two concentrations (10 μM and 50 μM, N = 73 drugs) and used a 50% inhibition as an arbitrary, predetermined cutoff value for go/no go on efficacy. At 10 μM, the majority of compounds inhibited HSV-1 infection by less than 50% in all three cell lines, when compared with untreated, infected cells (Fig. 1, Supplementary Fig. 2). Seven drugs reduced HSV-1 infection by 75–95% in neurons, but were less effective in NPCs and Vero cells. They included ACV; lysosomotropic agent 16F19; JAK2/3 inhibitor AT9283; quinazolinones CB-3-176; and alkaloids MP4B6, MP4G10, and R430 (Fig. 1, Supplementary Fig. 2). Three of the seven compounds that were highly active in neurons exhibited greater than 50% inhibition of infection in NPCs (ACV, MP4B6, and R430), but none of them inhibited infection to the same extent in infected Vero cells (Fig. 1, Supplementary Fig. 2). At 50 μM, eighteen compounds showed over 50% HSV-1 inhibition in neurons; of which only 13 had similar inhibitory activity in NPCs, but only 5 of 18 compounds were showed over 50% inhibition in Vero cells. Compounds CH1-27, CH-1-31, YSO-CYN-CYB, and CEP33779 exhibited inhibitory activity in neurons, but not in NPCs or Vero cells. Thus, a larger number of drugs were likely to show inhibitory activity in neurons than in NPCs, and the lowest number showed inhibitory activity in Vero cells.
Fig. 1. Summary of anti-HSV-1 drug activity in Vero cells, NPCs, and neurons at 10 μM and 50 μM.
Top panel: Heat map of drug activity. Cells were infected with an engineered HSV-1 construct expressing EGFP from an immediate early (IE) gene promoter and RFP from a late gene promoter. The drugs were added 2 h after the infections, and Vero cells and NPCs were analyzed 24 h post infection (hpi), and neurons after 48 h. The drug effect was calculated as the proportion of EGFP+ cells exposed to a specific drug by the proportion of EGFP+ cells in untreated infected cultures. Middle panel: We examined the correlation of drug effect between the three culture types within the two concentration conditions using a non-parametric Spearman’s rank correlation test. P-value is shown below the diagonal and the Spearman’s rank correlation coefficient is shown above the diagonal. Bottom panel: Ven diagram of the number of compounds that can inhibit HSV-1 infection by at least 50% at 50 μM.
A highly significant correlation was observed for drug effects in Vero cells and NPCs (Spearman’s correlation, rho = 0.85, p < 0.001). In contrast, the correlation between effects in Vero cells and neurons was much lower (rho = 0.15, p = 0.219). Correlations between NPCs and neurons were also relatively low (rho = 0.14, p < 0.339). Thus, the overall correlations between NPCs and Vero cells were greater than the correlations between neurons and NPCs.
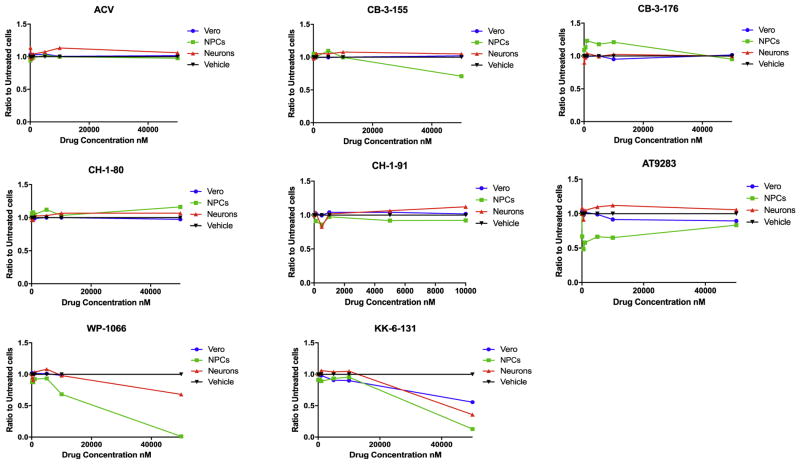
The apparently larger number of compounds that were more effective in neurons could reflect reduced levels of toxicity in these cells. Therefore, we estimated the toxicity of compounds that were more effective in both NPCs and neurons than in Vero cells, i.e., quinazolinones CB-3-176, CB-3-155, the nostodione KK-6-131, 4F17-derivatives CH-1-80 and CH-1-91, the epigenetic inhibitors AT9283, and WP1066. Cytotoxicity was assayed in all three cell types by flow cytometry using fixable viability dye (McClain et al., 2015), following exposure for 48 h at different concentrations (Fig. 2). No reduction in cell viability was observed in Vero cells, NPCs and neurons exposed to compounds CB-3-176, CH-1-80, and CH-1-91 at concentrations up to 50 μM (Fig. 2). Compound CB-3-155 produced a moderate cytotoxicity in NPCs at 50 μM (cell viability reduction: 30%) but not in Vero cells or neurons. The nostodione KK-6-131 showed significant cytotoxicity at 50 μM in Vero cells, NPCs and neuronal cultures (cell viability reduction: 44.5%, 86.32%, and 64.4%, respectively). The JAK inhibitor AT9283 showed significant cytotoxicity in NPCs starting from 100 nM (cell viability reduction: 33%), but not in Vero cells and neurons. A significant reduction in cell viability was observed in NPCs and neurons exposed to WP1066 at 10 μM and 50 μM, respectively (cell viability reduction: 31.8%, and 32.1%, respectively). Thus, the differential anti-HSV-1 drug activity in Vero cells, NPCs, and neurons cannot be explained merely by difference in drugs toxicity.
Fig. 2.
Cytotoxicity of selected drugs in Vero cells, NPCs, and neurons assessed by FC using fixable viability dye. The data represent an average of three replicates.
Taken together, our analyses highlight the need for carefully selecting the cell type used for an initial antiviral screen, particularly for viruses such as HSV-1 that can infect numerous tissues. Since initial HSV-1 infection occurs through mucosal tissues, epithelial or mucosal cell types are obvious choices for the primary drug screen. On the other hand, as the most profound damage from HSV-1 infections occurs in the CNS, it may be informative to consider neuronal tissues for the initial screen. The present studies indicate that while Vero cells are a reasonable choice, a number of drugs that have inhibitory properties in neurons would not be detected if screening was based on Vero cells alone. Using a relatively lax criterion of 50% inhibition, only 5 out of 18 compounds exhibiting significant antiviral activity in neurons were identified when using Vero cells at 50 μM. In particular, thirteen drugs that were highly efficacious in neurons showed modest or insignificant antiviral activity in Vero cells. On the other hand, 13 out of 18 compounds that were highly effective in neurons could be identified using our NPCs -based cellular platform. However, cell viability analysis showed cytotoxic effect of the epigenetic inhibitors WP1066 and AT9283 in NPCs and neurons at 50 μM. Furthermore, substantial cytotoxicity was observed in NPCs and neurons treated with CH-1-91 at 50 μM. The fact that antiviral activity of five compounds (CH-1-27, CH-1-31, iso-cyn-cyb, KK-6-95, and CEP33779) was restricted to neurons is particularly exciting, as it suggests that lead compound discovery tracks can be widened with such cell types.
Starting from the premise that an anti-HSV-1 drug should be efficacious not only in mature neurons but also during the different stages of neuronal differentiation, efficacy of a drug merely against neurons is not ideal. There is a need to identify drugs possessing robust inhibitory activity against HSV-1 in other stages of neuronal differentiation, such as NPCs, given the tropism of the virus toward NPCs (Braun et al., 2006), and the effect of NPC loss on cognitive dysfunction (Monje et al., 2002).
Our comparative drug screening identified new compounds with anti-HSV-1 properties in neuronal lineages, such as the quinazolinones, using a IPSC-based cellular platform that would not have been discovered using Vero cells. The modification of such molecular scaffolds can lead to additional compounds with increased efficacy, lower toxicity, and activity in a broader range of cell types.
Some limitations should be noted. Only two concentrations of each drug were tested; additional concentrations would enable more precise IC50 estimates. MOIs can also influence the antiviral activity in different cells. The potency of a compound could be related to the MOI such that a compound effect will appear to be more potent when cells are infected at a lower MOI and could be one of the explanations for the different efficacy observed in the neuronal cells. Indeed, the five compounds that were effective in all three cell lines show lower potencies in Vero and NPC cells than neurons. We infected neurons were at a lower infective dose (MOI = 0.3), versus NPCs and Vero cells (MOI = 1), due to their higher susceptibility to HSV-1 infection and as noted in our earlier studies (McClain et al., 2015). At MOI = 1, the optimal condition for infecting Vero cells and NPCs, the viral infectionwas highly toxic for the neurons. We considered using the lower MOI (0.3) for the Vero cells and NPCs to enable uniformity in the present study, but at this MOI, infection rates were unacceptably slow.
Our study suggests several new avenues for research. The present studies focused on antiviral candidate drugs that act intracellularly, primarily on the lytic phase. Our approach could complement other novel antiviral strategies, such as the search for drugs that inhibit viral entry to host cells (Antoine et al., 2013). Ongoing advances in iPSC techniques could also enable high-throughput cell-based drug screening campaigns. Human neural progenitor cells can also be utilized against other neurotropic viruses.
In summary, our study highlights the utility of hiPSC-based neural cellular platforms to identify new anti-HSV-1 molecules.
Supplementary Material
Acknowledgments
This project was funded from NIH grants 5R01MH063480-12 and 5R01MH045817; Scioto Foundation grant07R-1712; and Pittsburgh Center for Kidney Research Kidney Imaging Core NIH P30 DK079307.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.antiviral.2017.03.016.
References
- Antoine TE, Park PJ, Shukla D. Glycoprotein targeted therapeutics: a new era of anti-herpes simplex virus-1 therapeutics. Rev Med Virol. 2013;23:194–208. doi: 10.1002/rmv.1740. http://dx.doi.org/10.1002/rmv.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benencia F, Courrèges MC. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother Res. 2000;14:495–500. doi: 10.1002/1099-1573(200011)14:7<495::aid-ptr650>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Berry L, Venkatesan P. Aciclovir-induced neurotoxicity: utility of CSF and serum CMMG levels in diagnosis. J Clin Virol. 2014;61:608–610. doi: 10.1016/j.jcv.2014.09.001. http://dx.doi.org/10.1016/j.jcv.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Braun E, et al. Neurotropism of herpes simplex virus type 1 in brain organ cultures. J Gen Virol. 2006;87:2827–2837. doi: 10.1099/vir.0.81850-0. http://dx.doi.org/10.1099/vir.0.81850-0. [DOI] [PubMed] [Google Scholar]
- Burrel S, et al. Surveillance of herpes simplex virus resistance to antivirals: a 4-year survey. Antivir Res. 2013;100:365–372. doi: 10.1016/j.antiviral.2013.09.012. http://dx.doi.org/10.1016/j.antiviral.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Carter JA, Neville BG, Newton CR. Neuro-cognitive impairment following acquired central nervous system infections in childhood: a systematic review. Brain Res Brain Res Rev. 2003;43:57–69. doi: 10.1016/s0165-0173(03)00192-9. [DOI] [PubMed] [Google Scholar]
- Chowdhury MA, et al. Acyclovir-induced neurotoxicity: a case report and review of literature. Am J Ther. 2014 doi: 10.1097/MJT.0000000000000093. http://dx.doi.org/10.1097/MJT.0000000000000093. [DOI] [PubMed]
- Chowdhury MA, et al. Acyclovir-induced neurotoxicity: a case report and review of literature. Am J Ther. 2016;23:e941–943. doi: 10.1097/MJT.0000000000000093. http://dx.doi.org/10.1097/MJT.0000000000000093. [DOI] [PubMed] [Google Scholar]
- Chucair-Elliott AJ, et al. Microglia-induced IL-6 protects against neuronal loss following HSV-1 infection of neural progenitor cells. Glia. 2014;62:1418–1434. doi: 10.1002/glia.22689. http://dx.doi.org/10.1002/glia.22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett JW, et al. Inhibition of clinically relevant mutant variants of HIV-1 by quinazolinone non-nucleoside reverse transcriptase inhibitors. J Med Chem. 2000;43:2019–2030. doi: 10.1021/jm990580e. [DOI] [PubMed] [Google Scholar]
- D’Aiuto L, et al. Large-scale generation of human iPSC-derived neural stem cells/early neural progenitor cells and their neuronal differentiation. Organogenesis. 2014;10:365–377. doi: 10.1080/15476278.2015.1011921. http://dx.doi.org/10.1080/15476278.2015.1011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aiuto L, et al. Persistent infection by HSV-1 is associated with changes in functional architecture of iPSC-derived neurons and brain activation patterns underlying working memory performance. Schizophr Bull. 2015;41:123–132. doi: 10.1093/schbul/sbu032. http://dx.doi.org/10.1093/schbul/sbu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaher RJ, Jacob RJ, Miller CS. Establishment of a quiescent herpes simplex virus type 1 infection in neurally-differentiated PC12 cells. J Neurovirol. 1999;5:258–267. doi: 10.3109/13550289909015812. [DOI] [PubMed] [Google Scholar]
- Engman ML, et al. Neuropsychologic outcomes in children with neonatal herpes encephalitis. Pediatr Neurol. 2008;38:398–405. doi: 10.1016/j.pediatrneurol.2008.02.005. http://dx.doi.org/10.1016/j.pediatrneurol.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Frobert E, et al. Resistance of herpes simplex viruses to acyclovir: an update from a ten-year survey in France. Antivir Res. 2014;111:36–41. doi: 10.1016/j.antiviral.2014.08.013. http://dx.doi.org/10.1016/j.antiviral.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Gilham D, et al. Corrigendum to “RVX-208, a BET-inhibitor for treating atherosclerotic cardiovascular disease, raises ApoA-I/HDL and represses pathways that contribute to cardiovascular disease” [Atherosclerosis 247 (2016) 48–57] Atherosclerosis. 2016;253:345. doi: 10.1016/j.atherosclerosis.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Helldén A, et al. High serum concentrations of the acyclovir main metabolite 9-carboxymethoxymethylguanine in renal failure patients with acyclovirrelated neuropsychiatric side effects: an observational study. Nephrol Dial Transpl. 2003;18:1135–1141. doi: 10.1093/ndt/gfg119. [DOI] [PubMed] [Google Scholar]
- Hussin A, Md Nor NS, Ibrahim N. Phenotypic and genotypic characterization of induced acyclovir-resistant clinical isolates of herpes simplex virus type 1. Antivir Res. 2013;100:306–313. doi: 10.1016/j.antiviral.2013.09.008. http://dx.doi.org/10.1016/j.antiviral.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Immergluck LC, Domowicz MS, Schwartz NB, Herold BC. Viral and cellular requirements for entry of herpes simplex virus type 1 into primary neuronal cells. J Gen Virol. 1998;79(Pt 3):549–559. doi: 10.1099/0022-1317-79-3-549. http://dx.doi.org/10.1099/0022-1317-79-3-549. [DOI] [PubMed] [Google Scholar]
- Kuo T, Wang C, Badakhshan T, Chilukuri S, BenMohamed L. The challenges and opportunities for the development of a T-cell epitope-based herpes simplex vaccine. Vaccine. 2014;32:6733–6745. doi: 10.1016/j.vaccine.2014.10.002. http://dx.doi.org/10.1016/j.vaccine.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Frazia S, Amici C, Santoro MG. Antiviral activity of proteasome inhibitors in herpes simplex virus-1 infection: role of nuclear factor-kappaB. Antivir Ther. 2006;11:995–1004. [PubMed] [Google Scholar]
- Liu S, et al. 2-Pyridinyl-4(3H)-quinazolinone: a scaffold for anti-influenza A virus compounds. Chem Biol Drug Des. 2015;86:1221–1225. doi: 10.1111/cbdd.12589. http://dx.doi.org/10.1111/cbdd.12589. [DOI] [PubMed] [Google Scholar]
- Looker KJ, et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One. 2015;10:e0140765. doi: 10.1371/journal.pone.0140765. http://dx.doi.org/10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, et al. Synthesis and antiviral bioactivity of novel 3-((2-((1E,4E)-3-oxo-5-arylpenta-1,4-dien-1-yl)phenoxy)methyl)-4(3H)-quinazolinone derivatives. J Agric Food Chem. 2014;62:8928–8934. doi: 10.1021/jf502162y. http://dx.doi.org/10.1021/jf502162y. [DOI] [PubMed] [Google Scholar]
- Malvy D, et al. A retrospective, case-control study of acyclovir resistance in herpes simplex virus. Clin Infect Dis. 2005;41:320–326. doi: 10.1086/431585. http://dx.doi.org/10.1086/431585. [DOI] [PubMed] [Google Scholar]
- Markham A, Faulds D. Ganciclovir. An update of its therapeutic use in cytomegalovirus infection. Drugs. 1994;48:455–484. doi: 10.2165/00003495-199448030-00009. [DOI] [PubMed] [Google Scholar]
- McClain L, et al. Broad-spectrum non-nucleoside inhibitors of human herpesviruses. Antivir Res. 2015;121:16–23. doi: 10.1016/j.antiviral.2015.06.005. http://dx.doi.org/10.1016/j.antiviral.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty J, et al. iPSC neuronal assay identifies amaryllidaceae pharmacophore with multiple effects against herpesvirus infections. ACS Med Chem Lett. 2016;7:46–50. doi: 10.1021/acsmedchemlett.5b00318. http://dx.doi.org/10.1021/acsmedchemlett.5b00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer HG, Jacobs D, Dhummakupt A, Bloom DC. Inhibition of H3K27me3-specific histone demethylases JMJD3 and UTX blocks reactivation of herpes simplex virus 1 in trigeminal ganglion neurons. J Virol. 2015;89:3417–3420. doi: 10.1128/JVI.03052-14. http://dx.doi.org/10.1128/JVI.03052-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. http://dx.doi.org/10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Mortality in patients with the acquired immunodeficiency syndrome treated with either foscarnet or ganciclovir for cytomegalovirus retinitis. Studies of Ocular Complications of AIDS Research Group, in collaboration with the AIDS Clinical Trials Group. N Engl J Med. 1992;326:213–220. doi: 10.1056/NEJM199201233260401. [DOI] [PubMed] [Google Scholar]
- Piret J, Boivin G. Antiviral drug resistance in herpesviruses other than cytomegalovirus. Rev Med Virol. 2014;24:186–218. doi: 10.1002/rmv.1787. http://dx.doi.org/10.1002/rmv.1787. [DOI] [PubMed] [Google Scholar]
- Reardon JE, Spector T. Herpes simplex virus type 1 DNA polymerase. Mechanism of inhibition by acyclovir triphosphate. J Biol Chem. 1989;264:7405–7411. [PubMed] [Google Scholar]
- Richart SM, et al. Entry of herpes simplex virus type 1 into primary sensory neurons in vitro is mediated by Nectin-1/HveC. J Virol. 2003;77:3307–3311. doi: 10.1128/JVI.77.5.3307-3311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim SH, Chlipala G, Orjala J. Isolation and structure determination of a proteasome inhibitory metabolite from a culture of Scytonema hofmanni. J Microbiol Biotechnol. 2008;18:1655–1658. [PMC free article] [PubMed] [Google Scholar]
- Stránská R, et al. Survey of acyclovir-resistant herpes simplex virus in The Netherlands: prevalence and characterization. J Clin Virol. 2005;32:7–18. doi: 10.1016/j.jcv.2004.04.002. http://dx.doi.org/10.1016/j.jcv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Su YH, et al. The HSV 1 genome in quiescently infected NGF differentiated PC12 cells can not be stimulated by HSV superinfection. J Neurovirol. 2000;6:341–349. doi: 10.3109/13550280009030760. [DOI] [PubMed] [Google Scholar]
- Sukla S, et al. Mismatch primer-based PCR reveals that helicase-primase inhibitor resistance mutations pre-exist in herpes simplex virus type 1 clinical isolates and are not induced during incubation with the inhibitor. J Antimicrob Chemother. 2010;65:1347–1352. doi: 10.1093/jac/dkq026. http://dx.doi.org/10.1093/jac/dkq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.