Abstract
Purpose
Accurate glioma grading is crucial for treatment planning and predicting prognosis. We performed a quantitative volumetric analysis to assess the diagnostic accuracy of histogram analysis of diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) T1-weighted perfusion imaging in the preoperative evaluation of gliomas.
Methods
Sixty-three consecutive patients with pathologically-confirmed gliomas who underwent baseline DWI and DCE-MRI were enrolled. The patients were classified by histopathology according to tumor grade: 20 low-grade gliomas (grade II) and 43 high-grade gliomas (grades III and IV). Volumes-of-interest were calculated and transferred to DCE perfusion and ADC maps. Histogram analysis was performed to determine mean and maximum values for Vp and Ktrans, and mean and minimum values for ADC. Comparisons between high-grade and low-grade gliomas, and between grades II, III and IV, were performed. A Mann-Whitney U test at a significance level of corrected p≤0.01 was used to assess differences.
Results
All perfusion parameters could differentiate between high-grade and low-grade gliomas (p<0.001) and between grades II and IV, grades II and III and grades III and IV. Significant differences in minimum ADC were also found (p<0.01). Mean ADC only differed significantly between high and low grades and grades II and IV (p<0.01). There were no differences between grades II and III (p=0.1) and grades III and IV (p=0.71).
Conclusion
When derived from whole-tumor histogram analysis, DCE-MRI perfusion parameters performed better than ADC in non-invasively discriminating low- from high-grade gliomas.
INTRODUCTION
Gliomas account for nearly 30% of all brain and central nervous system tumors and 80% of all malignant brain tumors 1. Diffuse gliomas show a histologically continuous spectrum with varying grades of mitosis, necrosis, cellularity, and microvascular proliferation. The WHO has classified them as low-grade (grade II) or high-grade tumors (grades III and IV) based on their histopathologic characteristics. Low-grade gliomas are malignant, well-differentiated tumors. They are associated with a better prognosis than the high-grade gliomas 2. Accurate glioma grading is crucial for treatment planning and for determining the clinical outcome. Low-grade gliomas are usually subject to either strict follow-up or surgery 3. The treatment for high-grade gliomas is typically surgery, followed by concomitant radiation therapy and chemotherapy 3. Inaccurate grading represents a risk for the patient, since it could lead to an inappropriate therapy 3. Conventional histopathologic diagnosis has significant limitations: It is an invasive procedure that has inherent sampling error, especially for difficult-to-access tumors amenable only to stereotactic biopsy, and inability to evaluate residual tumor tissue after surgery 4.
MRI is a useful non-invasive imaging method for tissue characterization and glioma grading. Conventional MRI offers information on mass effect, edema, enhancement and necrosis that can be used to estimate tumor grade. However it has limitations for the grading of gliomas 5. In fact, prior studies have suggested that contrast enhancement alone is inadequate for tumor grading, since some low-grade gliomas may exhibit enhancement while some high-grade tumors may not 6–8. Enhancement may simply reflect disruption of the blood-brain barrier and increased vessel leakiness, rather than the neovascularity and angiogenesis that are the hallmarks of high-grade gliomas.
Advanced techniques such as perfusion and diffusion-weighted MRI can provide additional physiologic information unavailable from standard MRI 5, 9. Determination of perfusion and diffusion parameters is currently undertaken in the research and clinical settings using software that relies upon accurate ROI analysis. However, ROI-based analysis has a critical limitation, since only averaged values are evaluated. In addition, it may result in inaccuracies due to tumor heterogeneity and inter-observer variability in selecting ROIs 10.
Using the whole tumor as the volume-of-interest (VOI), we assessed the diagnostic accuracy of histogram analysis of the ADC and T1-weighted dynamic contrast-enhanced MRI perfusion parameters plasma volume (Vp) and permeability constant (Ktrans) in the preoperative evaluation of gliomas. The purpose of the study was to evaluate the sensitivity and specificity of Vp, Ktrans and ADC for distinguishing low- from high-grade tumors non-invasively. We hypothesized that T1-weighted DCE-MRI perfusion parameters would perform better than ADC in discriminating between glioma grades.
MATERIALS AND METHODS
Subjects
This study was compliant with the Health Insurance Portability and Accountability Act. Informed consent was waived by the institutional review board. In a hospital database, records from the period of January 2012 through July 2014 were retrospectively reviewed to identify all patients meeting the following inclusion criteria: 1) pathologically-confirmed glioma diagnosis on the basis of the WHO classification for tumors of the central nervous system; 2) baseline diffusion-weighted MRI sequences with corresponding ADC maps and 3) baseline DCE-MRI perfusion scan with matching post-contrast axial T1-weighted images. A total of 63 consecutive patients (27 female and 36 male) with a mean age of 54 years were included in the study.
MRI Acquisition
MRI sequences were acquired with a 1.5 Tesla MRI scanner (GE Healthcare, Milwaukee, Wisconsin) and a standard 8-channel head coil. Gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, New Jersey) was injected via a venous catheter (18–21 gauge) at doses standardized by patient body weight (0.2 mL/kg body weight, maximum 20 mL) at 2–3 mL/sec. DCE-MRI of the brain was acquired as part of a standard clinical protocol with a 3D T1-weighted SPGR sequence (TR 4–5ms; TE 1–2ms; slice thickness, 3 mm; flip angle, 25°; field-of-view, 24 cm; matrix 256 × 128; temporal resolution (Δt) 5–6s. Ten-phase pre-injection time delay was applied along with 30-phase dynamic injection imaging. Matching contrast T1-weighted (TR/TE=600/8 ms; thickness=4.5 mm) and T2-weighted (TR/TE=4000/102 ms; thickness=4.5 mm) spin-echo images were obtained. Ten slices covering the lesion with slice thickness of 3mm were acquired.
Diffusion-weighted imaging (DWI) was performed in the transverse plane using a spin-echo, echo-planar imaging (EPI) sequence with the following parameters: TR/TE, 8000/104.2ms; diffusion gradient encoding in 3 orthogonal directions; b= 1000 s/mm2; FOV, 240 mm; matrix size, 128×128 pixels; slice thickness, 5 mm; section gap, 1 mm; and number of average=2. DWI scans were performed before DCE-MRI. The ADC values were calculated as follows: ADC= [ln(S/S0)]/b, where S is the SI of the region of interest (ROI) obtained through 3 orthogonally oriented DWIs or diffusion trace images, S0 is the SI of the ROI acquired through reference T2-weighted images, and b is the gradient b factor with a value of 1000 s/mm2. ADC maps were calculated on a pixel-by-pixel basis.
Data analysis
DCE-MRI perfusion raw data, ADC maps and T1-weighted images were transferred to an off-line workstation. Perfusion data were processed with FDA-approved commercial software (NordicICE; Nordic Neuro Lab, Bergen, Norway). Preprocessing for the perfusion data included noise adjustments and automatic selection of arterial input function (AIF). The AIF was obtained independently for every patient from the Middle Cerebral Artery. Linear assumption between change in signal intensity and gadolinium concentration was made to convert signal intensity curve to concentration-time curve. Curves showing an optimal relationship between AIF and concentration-time curve were carefully selected. We used the perfusion analysis method based on the 2-compartment pharmacokinetic model proposed by Tofts to calculate pharmacokinetic parameters, including blood plasma volume (Vp) and time-dependent leakage (Ktrans), and to display the results as parametric maps 11. A radiologist with 7 years of experience manually delineated a 3D ROI defining the margins of the enhancing tumor on each axial plane on T1 post-contrast images for enhancing tumors and on FLAIR for non enhancing tumors, creating a VOI. The radiologist was blinded to the results of the biopsies and other clinical data including age and gender. Special care was taken to exclude intralesional macrovessels in order not to contaminate the measurements. VOIs were transferred to parametric maps to obtain the pharmacokinetic parameters Vp and Ktrans.
Postprocessing of ADC maps was performed by using Functool software (AW 5.2, GE Healthcare, Milwaukee, Wisconsin), and ROIs were manually drawn on ADC maps throughout the entire tumor using post-contrast axial T1-weighted and FLAIR sequences. All parameters were normalized to a ratio of tumor / normal parenchyma by placing ROIs in normal white/gray matter of the contralateral hemisphere in a healthy-looking area of brain parenchyma.
The measurements were binned and histogram analysis was performed to determine the mean and maximal values for Vp and Ktrans and the mean and minimum values for ADC.
Two experienced neuroradiologists visually assessed the radiological characteristics of the different groups on T1-weighted pre-contrast, post-contrast, T2-weighted, FLAIR sequences and parametric maps.
Statistical Analysis
A Mann-Whitney U test at a significance level of corrected p<0.01 was conducted to assess the differences between the DCE-MRI perfusion parameters (Vp and Ktrans) and ADC values for the different tumor grades. Significance was achieved after a Bonferroni adjustment. In addition, receiver operating characteristic (ROC) curve analysis was applied to assess the sensitivity and specificity of perfusion parameters and ADC values.
RESULTS
Patient Population
Our patient cohort was comprised of 63 individuals, 36 male (57 %) and 27 female (43%) with a mean age of 54.3 years. Twenty (31.5%) of the 63 patients, were in the low-grade glioma group (grade II); 10 (16.12%) of these patients had diffuse astrocytomas, 6 (9.6%) had oligodendrogliomas and 4 (6.45%) had oligoastrocytomas. Forty-three patients (68.5%) were in the high-grade glioma group (grades III and IV); 10 (16.12%) of these patients had anaplastic astrocytomas, and 33 (52.4%) of these patients had glioblastomas.
Conventional MRI and Perfusion Maps
In our study, low-grade gliomas (grade II) presented T1 hypointense or isointense signal and T2/FLAIR hyperintense signal compared to white matter. Little or no enhancement was conspicuous in the majority of low-grade tumors. Perfusion maps demonstrated modest or no elevation of Vp and Ktrans in these tumors (Figure 1A).
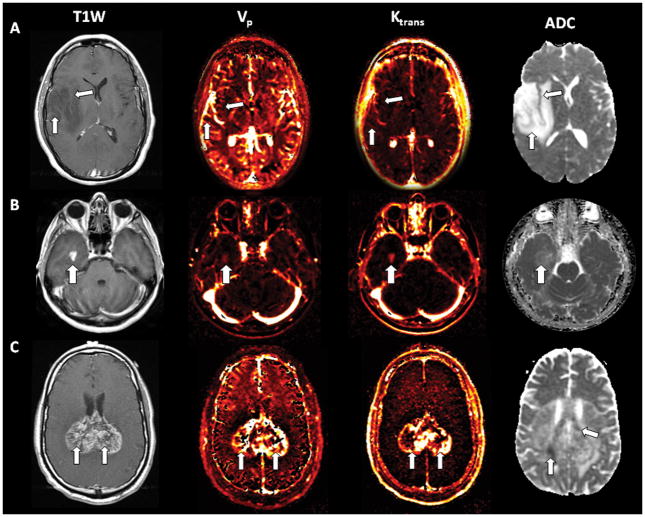
Fig 1.
A. T1-weighted post-Gd axial image of a right frontotemporal low-grade glioma demonstrating lack of enhancement (arrows). Corresponding perfusion maps Vp and Ktrans depicting no or little increased perfusion. ADC map of the same patient with manifest increased signal. B. T1-weighted post-Gd axial image of a right temporal anaplastic astrocytoma (grade III) with solid enhancement (arrow). The matching Vp and Ktrans perfusion maps illustrate increased perfusion. The corresponding ADC map of the same patient shows some areas of subtle decreased signal. C. T1-weighted post-Gd axial image of a biparietal glioblastoma (grade IV) crossing the splenium of the corpus callosum with marked heterogeneous enhancement (arrows). The matching Vp and Ktrans perfusion maps demonstrate increased perfusion . The corresponding ADC map of the same patient shows some areas of decreased signal (arrows) orienting towards high celularity.
Grade III gliomas (anaplastic astrocytomas) presented with T1 hypointense signal and T2/FLAIR hyperintense signal. Enhancement was variable, but most of these gliomas showed some degree of enhancement. Perfusion parameters were elevated in all cases(Figure 1B)..
In grade IV gliomas (GBM) enhancement was intense and heterogeneous and all cases demonstrated increased perfusion (Figure 1C).
Quantitative Perfusion Analysis
Values of all parameters are displayed in Table 1 and Figure 2. The measurements of mean Vp (VpMean) and maximum Vp (VpMax) in the low-grade and the high-grade groups differed significantly (p<0.001). When separated according to grades, VpMean and VpMax values differed significantly between grades II and III (p<0.01), grades III and IV (p<0.01) and grades II and IV (p<0.001). No differences were found among low-grade gliomas when comparing the different histologies (astrocytomas, oligodendrogliomas and oligoastrocytomas).
Table 1.
Quantitative Parameters According to Grade
| Low Grade | High Grade | |||
|---|---|---|---|---|
|
| ||||
| Grade II | Grade III | Grade IV | Grade III+IV | |
| KtransMean | 1.401 ± 0.596 | 2.765 ± 1.310 | 5.776 ± 3.906 | 5.076 ± 3.694 |
| KtransMax | 1.786 ± 0.920 | 3.391 ± 1.406 | 7.665 ± 6.399 | 6.671 ± 5.912 |
| VpMean | 1.294 ± 0.355 | 2.435 ± 1.128 | 6.497 ± 4.442 | 5.553 ± 4.280 |
| VpMax | 1.617 ± 0.492 | 2.792 ± 1.293 | 8.626 ± 5.273 | 7.269 ± 5.269 |
| ADCmean | 1.647 ± 0.427 | 1.354 ± 0.398 | 1.302 ± 0.327 | 1.315 ± 0.341 |
| ADCmin | 1.356 ± 0.386 | 1.008 ± 0.142 | 0.742 ± 0.249 | 0.804 ± 0.254 |
All values are normalized to a ratio of tumor / normal parenchyma.
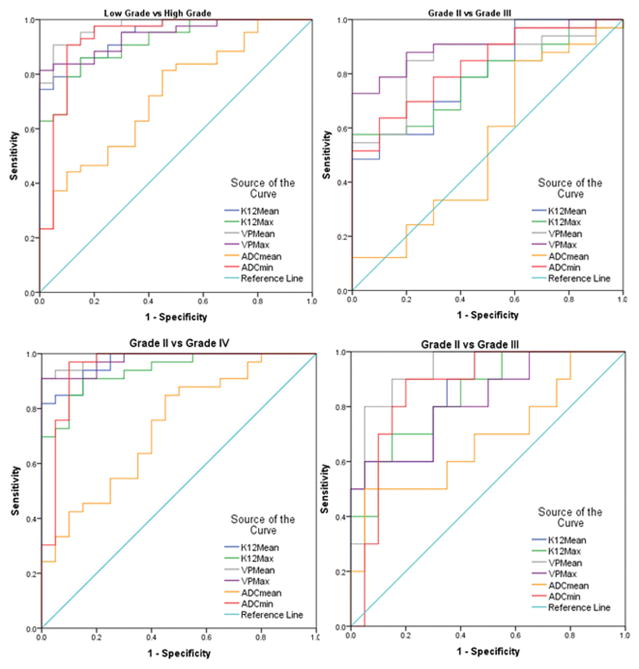
Fig 2.
Receiver operating characteristic (ROC) curves depicting the true positive rate (specificity) and the false positive rate (sensitivity) of DCE-MRI perfusion parameters Vp and Ktrans and ADC in classifying Low Grade vs High Grade gliomas, Grade II vs Grade III, Grade II vs Grade IV and Grade II vs grade III
Mean Ktrans (KtransMean) and maximum Ktrans (KtransMax) values were also lower in low-grade gliomas than in high-grade gliomas (p<0.001). Both parameters showed significant differences between grades II and III (p<0.01), grades III and IV (p<0.01) and grades II and IV (p<0.001).
Minimum ADC (ADCmin) also proved to be useful for distinguishing between low-grade and high-grade gliomas (p<0.001) and differed significantly between grades II and III, grades III and IV (p<0.01), and grades II and IV (p<0.001). Mean ADC (ADCmean) values differed significantly between high- and low-grade gliomas and between grades II and IV (p<0.01). However, they did not differ significantly between grades II and III (p=0.1) or grades III and IV (p=0.73).
ROC Analysis
VpMean demonstrated the highest area under the curve (AUC) (0.974; see Fig. 3) when comparing high and low grade gliomas. VpMean also showed the highest AUC (0.93) for the comparison between grade II and grade III. VpMax had the highest AUC (0.906) in the evaluation of grade III and IV and also grade II and IV with an AUC (0.988). The cut-off values with corresponding sensitivity and specificity are displayed in Table 2.
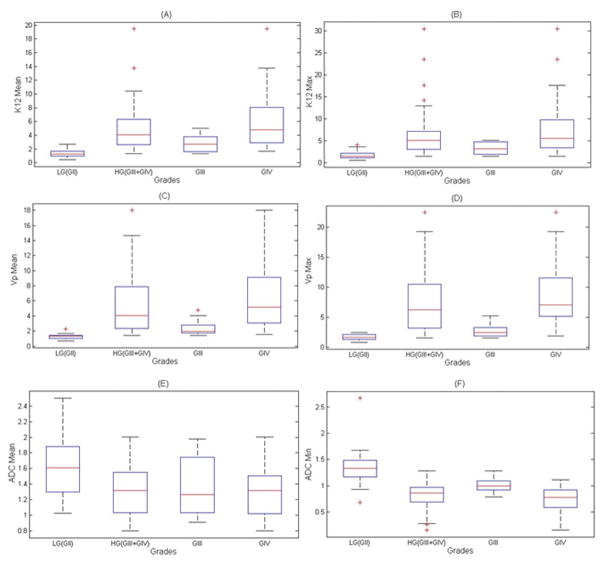
Fig 3.
A box plot illustrating the mean values and standard deviations for the DCE-MRI perfusion parameters Ktrans, Vp and ADC in Low Grade gliomas (Grade II), High Grade gliomas (Grade III + IV), Grade III, and Grade IV.
Table 2.
Cut-off Values with Sensitivity and Specificity
| Low vs High Grade | Grade II vs III | Grade III vs IV | Grade II vs IV | |
|---|---|---|---|---|
| KtransMean | 2.556; 79.1%; 95% | 1.608; 80%; 70% | 3.203; 69.7%; 70% | 1.928; 93.9%; 85% |
| Ktrans Max | 2.675; 86%; 85% | 1.915; 80%; 70% | 4.389; 66.7%; 70% | 2.675; 90.9%; 85% |
| Vp Mean | 1.726; 90.7%; 95% | 1.578; 90%; 85% | 2.864; 84.8%; 80% | 1.734; 93.9 %; 95% |
| Vp Max | 2.354; 83.7%; 95% | 1.778; 80%; 70% | 3.518; 87.9%; 80% | 2.793; 90.9%; 100% |
| ADC Min | 1.078; 90.7%; 90%. | 1.138; 90%; 80%. | 0.93; 78.8%; 70% | 1.075; 97%; 90% |
Cut-off value; Sensitivity; Specificity
DISCUSSION
This study was designed to assess and compare the use of histogram analysis of Vp and Ktrans (obtained from T1-weighted DCE-MRI) and ADC (obtained from diffusion-weighted MRI) to improve diagnostic accuracy in the differentiation of low- and high-grade gliomas in newly-diagnosed, untreated tumors. ADC and perfusion parameters are usually analyzed from multiple small region-of-interest (ROI) measurements. That method has been shown to be reasonably reproducible, though it remains a subjective, operator-dependent technique, inevitably associated with interobserver and intraobserver variability. In light of tissue heterogeneity, it has been suggested that the sampling error that occurs with the use of regions of interest contributes substantially to the unreliability of ADC maps for differentiating between tumor grades 2. Histogram analysis of imaging parameters is a more reproducible and objective method that allows inexperienced operators to obtain reliable data. In prior studies, histogram analysis has proven to be comparable to, if not superior to, current ROI-based methods for calculating the maximum value of relative cerebral blood volume (rCBV) (rCBVmax) 12. We hypothesized that by selecting the entire volume of the tumor, we could more accurately measure perfusion and diffusion within the tumor and use the histogram function to more objectively analyze the tumor results. Prior studies in the literature have found maximal perfusion values and minimum ADC values to be the most accurate for glioma grading 5. Thus we performed a histogram analysis of Vp, Ktrans and ADC, and calculated mean and maximum values for Vp and Ktrans, and mean and minimum values for ADC.
Perfusion parameters are potentially useful in the characterization of gliomas, since they tend to correlate with the level of neovascularization and consequently the degree of malignancy 4, 5, 13, 14. Considering that the degree of malignancy of a tumor can be represented by the most malignant area, it is reasonable to expect perfusion parameters to have potential for categorizing tumor malignancy 15. Prior studies have successfully characterized high- and low-grade gliomas using perfusion parameters 5, 16. Some of these studies were performed using Dynamic Susceptibility Contrast (DSC) MRI. However, this technique has some limitations: It is difficult to derive absolute quantitative measurements from DSC MRI, since there is no linear relationship between contrast medium concentration and signal changes 17. DSC MRI is susceptible to artifacts from large vessels or bones 18. Also, contrast medium leakage through the blood brain barrier is not accounted for 17, 19. DCE-MRI, on the other hand, looks at the brain vessels in a different way that permits quantitative assessment of the blood-brain barrier and vascular permeability using compartment modeling 18. Estimations of tumor blood volume and permeability obtained with dynamic contrast-enhanced imaging techniques (MRI or Perfusion CT) have been found to correlate with tumor grade, prognosis, and treatment response. 13, 20. These imaging parameters have been shown to be markers of tumor vascular attenuation and angiogenesis in gliomas 21.
Using DSC perfusion, Hilario et al. demonstrated significant differences (p<0.001) in CBV values between high- and low-grade gliomas, between grades II and IV, and also between grades III and IV. They could not show significant differences, however, between grade II and grade III gliomas 5. Jung et al performed a histogram analysis of the DCE MRI variables Vp, Ktrans and Ve from a slightly smaller sample of patients (n=28), which demonstrated that Ktrans was the most significant pharmacokinetic parameter for glioma grading 16. We postulated that high-grade gliomas would present significantly higher vascularity and permeability than would low-grade gliomas. In our study, all perfusion parameters proved to be good biomarkers for distinguishing between high-grade and low-grade gliomas (p<0.001). In contrast to Jung et al. 16, we found that Vp mean was the best discriminator of glioma grade in most comparisons (grade II vs. grade III, grade II vs. grade IV, and high grade vs. low grade). VpMax was the best parameter, however, to differentiate between grades III and IV, with a cutoff value of 3.52 demonstrating high sensitivity (88%) and specificity (80%). The reason for this difference in results may be due to the fact that our data set included a larger number of patients (63 patients versus 28 patients in Jung et al study). Further analysis with larger cohorts to test both parameters Ktrans and Vp would be useful.
DWI has been found to be a helpful noninvasive method for evaluating the cellularity of tumors. ADC maps illustrate the reduction of mobility of water molecules due to high cellularity, with a decrease in ADC values suggesting higher-grade tumor 5, 13. In our study, ADCmean demonstrated statistically significant differences only between low-grade and high-grade gliomas, whereas ADCmin proved to be a useful biomarker to distinguish between all grades, with high sensitivity and specificity for all comparisons. The reason for this results may be that we analyzed the entire volume of the tumor, including cystic and necrotic areas. Cystic and necrotic areas could influence ADC measurements, since they are frequently present in high-grade gliomas and therefore increase the ADC values 2. Necrotic areas display low intensity in diffusion-weighted MR images and high intensity in ADC maps, since necrosis is associated with low cellularity. Some studies have demonstrated a high correlation between the degree of tumor necrosis at pathology and the ADC values of ROIs 2. Lee et al. considered that necrosis could be possibly a confusing factor for the grading of gliomas based on ADC maps since mean ADC values of the entire tumor including cystic or necrotic areas are higher than those in active tumor lesions 2. In their study they demonstrated that the exclusion of cystic or necrotic portions from the ROI improved the differentiation between low- and high-grade gliomas 2. The results obtained from our histogram analysis of mean ADC values are in agreement with previously published findings. Our histogram analysis of the ADC minimum values, however, showed highly significant differences between all grades of gliomas.
Our study had several potential limitations. First, some of the tumors included in our study had ill-defined margins that made it more difficult to distinguish tumor from healthy parenchyma and thus to properly delineate the tumor contour. Although this problem could lead to errors in reproducibility, the VOI included the entirety of the tumor and the histogram analysis could display the full range of values in order to obtain reliable voxel values. Second, pathologic misdiagnosis due to sampling error, although unlikely, was possible due to heterogeneity of specimens in which various cell populations may coexist; such heterogeneity is typical in high-grade tumors. In the low-grade group, we included a heterogeneous group of tumors with different histologies, which presented with different perfusion patterns. This may have affected the overall results, since oligodendrogliomas have been reported to have elevated perfusion parameters values 22. However, in our study, possibly due to the rather small number of oligodendroglial tumors in the sample, these tumors did not show significantly increased perfusion parameters, so they could not have skewed the results in the low-grade group. In addition, the sample sizes for the low-grade and high-grade glioma groups were somewhat uneven, with a larger number of patients in the high-grade group. However, this circumstance could not be avoided, because high-grade gliomas are more common than low-grade gliomas among the general population, and we recruited consecutive patients.
CONCLUSION
The results of our study demonstrate that quantitative histogram analysis of T1-weighted perfusion parameters Vp and Ktrans from DCE-MRI, as well as ADC from diffusion-weighted MRI, can be useful for the non-invasive assessment of glioma grade. Though all measurements in our study were significant predictors of glioma grade, Vp mean stood out as the best predictor. Combining T1-weighted perfusion parameters Vp and Ktrans with ADC could potentially improve diagnostic accuracy and have an impact on clinical outcome.
Acknowledgments
grant :P30 CA004748
Abbreviations
- WHO
World Health Organization
- VOI
volume-of-interest
- FDA
Food and Drug Administration
- AIF
arterial input function
- ROC
receiver operating characteristic
- Vp
plasma volume
- Ktrans
permeability constant
- AUC
Area Under the Curve
Contributor Information
Julio Arevalo Perez, Memorial Sloan-Kettering Cancer Center, Radiology.
Kyung Peck, MSKCC, Radiology.
Robert Young, Memorial Sloan-Kettering Cancer Center, Radiology.
Andrei Holodny, Memorial Sloan-Kettering Cancer Center, Radiology.
Sasan Karimi, Memorial Sloan-Kettering Cancer Center, Radiology.
John Lyo, Memorial Sloan-Kettering Cancer Center, Radiology.
References
- 1.Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer genetics. 2012;205:613–21. doi: 10.1016/j.cancergen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Choi SH, Kim JH, Sohn CH, Lee S, Jeong J. Glioma grading using apparent diffusion coefficient map: application of histogram analysis based on automatic segmentation. NMR Biomed. 2014;27:1046–52. doi: 10.1002/nbm.3153. [DOI] [PubMed] [Google Scholar]
- 3.Caulo M, Panara V, Tortora D, et al. Data-driven Grading of Brain Gliomas: A Multiparametric MR Imaging Study. Radiology. 2014;272:494–503. doi: 10.1148/radiol.14132040. [DOI] [PubMed] [Google Scholar]
- 4.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24:1989–98. [PMC free article] [PubMed] [Google Scholar]
- 5.Hilario A, Ramos A, Perez-Nunez A, et al. The added value of apparent diffusion coefficient to cerebral blood volume in the preoperative grading of diffuse gliomas. AJNR Am J Neuroradiol. 2012;33:701–7. doi: 10.3174/ajnr.A2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Law M, Yang S, Babb JS, et al. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol. 2004;25:746–55. [PMC free article] [PubMed] [Google Scholar]
- 7.Ginsberg LE, Fuller GN, Hashmi M, Leeds NE, Schomer DF. The significance of lack of MR contrast enhancement of supratentorial brain tumors in adults: histopathological evaluation of a series. Surg Neurol. 1998;49:436–40. doi: 10.1016/s0090-3019(97)00360-1. [DOI] [PubMed] [Google Scholar]
- 8.Knopp EA, Cha S, Johnson G, et al. Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology. 1999;211:791–8. doi: 10.1148/radiology.211.3.r99jn46791. [DOI] [PubMed] [Google Scholar]
- 9.Hirai T, Murakami R, Nakamura H, et al. Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol. 2008;29:1505–10. doi: 10.3174/ajnr.A1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Law M, Young R, Babb J, Pollack E, Johnson G. Histogram analysis versus region of interest analysis of dynamic susceptibility contrast perfusion MR imaging data in the grading of cerebral gliomas. AJNR Am J Neuroradiol. 2007;28:761–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–32. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Young R, Babb J, Law M, Pollack E, Johnson G. Comparison of region-of-interest analysis with three different histogram analysis methods in the determination of perfusion metrics in patients with brain gliomas. J Magn Reson Imaging. 2007;26:1053–63. doi: 10.1002/jmri.21064. [DOI] [PubMed] [Google Scholar]
- 13.Aronen HJ, Gazit IE, Louis DN, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology. 1994;191:41–51. doi: 10.1148/radiology.191.1.8134596. [DOI] [PubMed] [Google Scholar]
- 14.Rollin N, Guyotat J, Streichenberger N, Honnorat J, Tran Minh VA, Cotton F. Clinical relevance of diffusion and perfusion magnetic resonance imaging in assessing intra-axial brain tumors. Neuroradiology. 2006;48:150–9. doi: 10.1007/s00234-005-0030-7. [DOI] [PubMed] [Google Scholar]
- 15.Wetzel SG, Cha S, Johnson G, et al. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology. 2002;224:797–803. doi: 10.1148/radiol.2243011014. [DOI] [PubMed] [Google Scholar]
- 16.Jung SC, Yeom JA, Kim JH, et al. Glioma: Application of histogram analysis of pharmacokinetic parameters from T1-weighted dynamic contrast-enhanced MR imaging to tumor grading. AJNR Am J Neuroradiol. 2014;35:1103–10. doi: 10.3174/ajnr.A3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin KE, Ahn KJ, Choi HS, et al. DCE and DSC MR perfusion imaging in the differentiation of recurrent tumour from treatment-related changes in patients with glioma. Clin Radiol. 2014;69:e264–72. doi: 10.1016/j.crad.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Essig M, Shiroishi MS, Nguyen TB, et al. Perfusion MRI: the five most frequently asked technical questions. AJR Am J Roentgenol. 2013;200:24–34. doi: 10.2214/AJR.12.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fatterpekar GM, Galheigo D, Narayana A, Johnson G, Knopp E. Treatment-Related Change Versus Tumor Recurrence in High-Grade Gliomas: A Diagnostic Conundrum—Use of Dynamic Susceptibility Contrast-Enhanced (DSC) Perfusion MRI. American Journal of Roentgenology. 2012;198:19–26. doi: 10.2214/AJR.11.7417. [DOI] [PubMed] [Google Scholar]
- 20.Law M, Oh S, Babb JS, et al. Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging--prediction of patient clinical response. Radiology. 2006;238:658–67. doi: 10.1148/radiol.2382042180. [DOI] [PubMed] [Google Scholar]
- 21.Jain R, Poisson L, Narang J, et al. Correlation of perfusion parameters with genes related to angiogenesis regulation in glioblastoma: a feasibility study. AJNR Am J Neuroradiol. 2012;33:1343–8. doi: 10.3174/ajnr.A2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito T, Yamasaki F, Kajiwara Y, et al. Role of perfusion-weighted imaging at 3T in the histopathological differentiation between astrocytic and oligodendroglial tumors. Eur J Radiol. 2012;81:1863–9. doi: 10.1016/j.ejrad.2011.04.009. [DOI] [PubMed] [Google Scholar]