Abstract
Objective
To test the hypothesis that resuscitation with balanced fluids (lactated Ringer [LR]) is associated with improved outcomes compared with normal saline (NS) in pediatric sepsis.
Study design
We performed matched analyses using data from 12 529 patients <18 years of age with severe sepsis/septic shock at 382 US hospitals between 2000 and 2013 to compare outcomes with vs without LR as part of initial resuscitation. Patients receiving LR were matched 1:1 to patients receiving only NS (NS group), including separate matches for any (LR-any group) or exclusive (LR-only group) LR use. Outcomes included 30-day hospital mortality, acute kidney injury, new dialysis, and length of stay.
Results
The LR-any group was older, received larger crystalloid volumes, and was less likely to have malignancies than the NS group. After matching, mortality was not different between LR-any (7.2%) and NS (7.9%) groups (risk ratio 0.99, 95% CI 0.98, 1.01; P = .20). There were no differences in secondary outcomes except longer hospital length of stay in LR-any group (absolute difference 2.4, 95% CI 1.4, 5.0 days; P < .001). Although LR was preferentially used as adjunctive fluid with large-volume resuscitation or first-line fluid in patients with lower illness severity, outcomes were not different after matching stratified by volume and proportionate LR utilization, including for patients in the LR-only group.
Conclusions
Balanced fluid resuscitation with LR was not associated with improved outcomes compared with NS in pediatric sepsis. Although the current practice of NS resuscitation is justified, selective LR use necessitates a prospective trial to definitively determine comparative effectiveness among crystalloids.
Fluid resuscitation is the cornerstone of acute management for hypovolemia and shock, but there remains uncertainty as to the most appropriate fluid to restore blood volume and optimize organ perfusion.1–3 Isotonic crystalloid fluids are generally preferred, except in cases of hemorrhage, as they are inexpensive, easy to store, and available in a wide variety of settings.4,5 Sepsis guidelines for adults and pediatrics recommend initial crystalloid fluid resuscitation.6,7
Crystalloid fluids can be categorized as either nonbuffered/nonbalanced (eg, 0.9% normal saline [NS]) or balanced (eg, lactated Ringer [LR], Hartmann, Plasma-Lyte, Baxter, Deerfield, Illinois) solutions. Although balanced fluid have a more physiologic electrolyte composition and strong ion difference closer to plasma than NS, these fluids have not been preferentially used for sepsis resuscitation.4,5,8 However, large amounts of NS can induce a hyperchloremic metabolic acidosis and have been associated with adverse effects on kidney injury, coagulation, and death.9–12 Alternatively, balanced crystalloids have been associated with improved outcomes and decreased renal replacement therapy compared with NS in adult sepsis.11,13
In pediatric sepsis, there are limited data comparing clinical outcomes following LR vs NS resuscitation. Although Carcillo et al14 demonstrated the importance of early fluid resuscitation in pediatric septic shock, there was no differentiation between use of NS or LR. In a randomized trial of 4 fluid regimens in children with dengue fever, patients receiving LR were slower to recover from shock compared with NS, but the study was not powered for morbidity or mortality outcomes.15 The largest study of fluid resuscitation in children with severe infections restricted crystalloid fluids to NS.16 Consequently, guidelines for pediatric sepsis are unable to provide evidence-based recommendations to choose among available crystalloid solutions even despite emerging data questioning the relative safety of NS in adults.6 Because crystalloid fluids are so commonly used, even a small benefit attributable to type of fluid resuscitation could provide a substantial public health impact with only a minor shift in practice. We, therefore, sought to test the hypothesis that balanced fluid resuscitation is associated with improved outcomes in pediatric sepsis.
Methods
We conducted a matched retrospective cohort study of pediatric patients <18 years of age with severe sepsis or septic shock across 382 geographically diverse US hospitals between January 2000 and December 2013. Patients were identified from the Premier Healthcare Database, an administrative database established by the Premier healthcare alliance that contains itemized daily logs of all patient charges. The Premier Healthcare Database is the largest acute care database in the US with a complete census of all inpatients from more than 600 hospitals, of which approximately three-quarters are nonteaching hospitals. Pediatric data is contributed through a combination of community-based and specialty children’s hospitals. The study was considered exempt from human subjects research oversight by The Children’s Hospital of Philadelphia Institutional Review Board because only deidentified data were used.
Eligible patients were <18 years of age, diagnosed with severe sepsis or septic shock, received initial treatment at the Premier hospital, were not admitted to a neonatal intensive care unit (based on all patient refined-diagnosis related group codes), and were ordered to receive any combination of NS or LR fluid boluses during the first 3 days of hospital admission. To identify severe sepsis and septic shock, we used previously published combinations of International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes for either an invasive infection plus acute organ dysfunction (Tables I and II; available at www.jpeds.com) or the ICD-9-CM codes for severe sepsis (785.52) or septic shock (995.92).17,18 To increase the likelihood that initial fluid resuscitation was related to sepsis, we restricted inclusion to patients with blood cultures and broad-spectrum antibiotics (Table III; available at www.jpeds.com) ordered within the first 3 hospital days. We excluded patients with unknown hospital disposition at day 30.
Exposure to LR or NS was defined by type and amount of fluid recorded over the first three hospital days. Only LR or NS ordered as bolus therapy was considered. Because balanced fluids other than LR (eg, Plasma-Lyte) were rare (0.3%), we limited our analysis to LR and NS. Fluid volumes were billed as 250, 500, or 1000 mL units. Although some patients likely received only a portion of a unit because of weight-based fluid dosing in pediatrics, we considered the entire unit to have been administered. Patients were categorized as exposure to only NS (NS group) or to varying amounts LR and NS (LR-any group), similar to the methodology published by Raghunathan et al.13 We also performed a separate analysis of patients who received only NS vs only LR (LR-only group).
Demographics, month/site of admission, comorbid conditions, and intensive care therapies were obtained from the Premier Healthcare Database. Comorbid conditions were defined using pediatric complex chronic conditions.19 Therapies included use of the following on hospitals days 1, 2, or 3: noninvasive and invasive mechanical ventilation, vasoactive infusions, albumin, blood products, furosemide, corticosteroids, use of a central venous catheter, arterial line, or bladder catheter, and extracorporeal membrane oxygenation. Because doses of vasoactive infusions were not available, we summarized this variable as the total number of vasoactive infusions. Blood products were defined as any combination of red blood cells, platelets, fresh frozen plasma, or cryoprecipitate.
Outcomes
The primary outcome was all-cause 30-day hospital mortality in the NS vs LR-any groups. To increase the likelihood that death was related to the initial sepsis episode requiring fluid resuscitation, we censored the primary outcome at 30 days after admission. Secondary outcomes included uncensored hospital mortality, hospital mortality plus hospice, acute kidney injury (AKI) with and without dialysis, and pediatric intensive care unit (PICU) and hospital length of stay (LOS). AKI was defined by the ICD-9-CM code 584.x and AKI with dialysis was defined as an ICD-9-CM code for AKI (584.x) with either (a) a procedure charge for a dialysis catheter (38.95) with a charge for dialysis (39.95) or (b) charge codes for dialysis supplies.13 Patients with an ICD-9-CM code for end-stage renal disease already undergoing dialysis (ICD-9-CM 585.6) were excluded from the analysis of AKI with or without dialysis. All outcomes were also analyzed separately for patients in the LR-only group.
Statistical Analyses
Analyses were performed using R 2.13.1 (R Foundation) with mipmatch package20 and Stata v 12.1 (StataCorp, College Station, Texas). Data are presented as medians (IQR) or proportions. We used mixed integer programming 1:1 matching to minimize the within-pair Mahalanobis distance for key covariates that were both available within Premier and had a biologically plausible or previously demonstrated association, including demographics, comorbidities, and therapies, with risk of death. The Mahalanobis distance is the difference in covariate values for patients in the LR vs NS groups divided by the covariates’ SD.21 Unlike propensity scores that can produce stochastic balance, integer matching ensures a more predictable and precise balance on specific covariates.20 The specific patient-level covariates used for matching are listed in Tables IV and V (Table IV; available at www.jpeds.com). In addition, because LR use was likely to cluster by hospital, we also matched within site exactly except for hospitals that had ≤10 patients for which we allowed matching across sites. We also repeated the analysis excluding hospitals with ≤10 patients to ensure matching across low-volume hospitals did not impact our findings. Because prior studies have demonstrated differences in mortality for patients identified with specific severe sepsis/septic shock ICD-9-CM codes compared with codes for infection plus organ dysfunction,17,18 we used fine balance to match patients by sepsis identification strategy. Fine balance ensures matching on one variable without restricting matching on other variables.22 A similar approach matched patients by year and season.
Table V.
Patient characteristics in matched cohort
| Variables* | LR-any group† N = 2117 |
NS group‡ N = 2117 |
P value§ |
|---|---|---|---|
| Age (y), median (IQR) | 8 (1–15) | 7 (1–14) | .01 |
| Male sex | 1119 (53) | 1149 (54) | .37 |
| Race/ethnicity | .31 | ||
| White | 983 (46) | 1021 (48) | |
| Black | 451 (21) | 466 (22) | |
| Hispanic | 249 (12) | 218 (10) | |
| Other | 434 (21) | 412 (19) | |
| Comorbid conditions¶ | |||
| Cardiovascular | 362 (17) | 312 (15) | .04 |
| Respiratory | 78 (4) | 51 (2) | .02 |
| Renal | 42 (2) | 29 (1) | .15 |
| Gastrointestinal | 62 (3) | 50 (2) | .29 |
| Malignancy | 187 (9) | 186 (9) | 1.0 |
| Hematologic/immunologic | 116 (5) | 97 (5) | .21 |
| Metabolic | 120 (6) | 105 (5) | .34 |
| Neuromuscular | 394 (19) | 377 (18) | .52 |
| Congenital disorders | 203 (10) | 164 (8) | .04 |
| PICU admission | 1729 (82) | 1664 (79) | .01 |
| Sepsis-related therapies** | |||
| Vasoactive infusion | 880 (42) | 824 (39) | .08 |
| Maximum number of concurrent vasoactives†† | 2 (1–3) | 2 (1–3) | .09 |
| Noninvasive mechanical ventilation | 181 (9) | 157 (7) | .19 |
| Invasive mechanical ventilation | 1389 (66) | 1347 (64) | .19 |
| Corticosteroids | 551 (26) | 488 (23) | .02 |
| Hydrocortisone | 230 (11) | 203 (10) | .19 |
| Methylprednisolone | 390 (18) | 347 (16) | .09 |
| Albumin 5% | 446 (21) | 409 (19) | .16 |
| Albumin 25% | 609 (29) | 552 (26) | .05 |
| Blood transfusion‡‡ | 995 (47) | 933 (44) | .06 |
| Furosemide | 947 (45) | 910 (43) | .25 |
| ECMO | 12 (<1) | 8 (<1) | .50 |
ECMO, extracorporeal membrane oxygenation.
Data presented as n (%), unless noted.
LR group included patients who received any amount of LR fluid resuscitation.
NS group included patients who received only NS fluid resuscitation.
Statistical comparison using Wilcoxon signed rank and McNemar test for matched pairs, as appropriate.
Comorbid conditions were categorized using ICD-9-CM codes defining pediatric complex chronic conditions.19
Includes therapies administered through hospital day 3.
Includes only patients who received at least 1 vasoactive infusion.
Includes administration of whole blood, packed red blood cells, platelets, plasma, and cryoprecipitate.
To assess match quality, we calculated standardized differences for each variable by dividing the mean difference between matched patients by the pooled SD before matching. We used the benchmark of <0.10, or less than one-tenth of a SD, as the maximum acceptable standardized difference.23,24
Matching was repeated within quartiles of total crystalloid volume, such that patients in the LR-any group were matched only to patients in the NS group who received a similar total volume of crystalloid fluid. We used sex-specific 50th percentile weight-for-age (Table VI; available at www.jpeds.com) to estimate age-related differences in volume administration. Next, matching was again repeated after stratifying patients by proportion of total fluid given as LR, including a separate match for the LR-only group. Stratifying by volume before matching ensured that matched pairs did not consist of patients who received substantially different total volumes of fluid. Finally, one last match was conducted using only those covariates unlikely to mediate the effect of fluid type on outcome, including age, sex, race/ethnicity, comorbidities, site, season, year, and sepsis identification strategy. For this final analysis, therapies that may have occurred following fluid resuscitation were not used in the match.
We tested for differences in outcomes using McNemar or Wilcoxon sign rank tests for matched pairs. To assess for potential confounding because of residual statistical differences in matching variables between groups, we used conditional logit regression to adjust for any covariates that differed between groups at P < .01.25 For 30-day mortality, we also performed a Kaplan-Meier analysis to determine if time-to-death differed between groups. For LOS, we used the Huber M estimate because of long tails and permutation distribution to test for statistical significance.22 Because LOS may be influenced by survival, we conducted 2 analyses to determine if LOS was sensitive to vital status. First, we set LOS to the sample maximum for all nonsurvivors, and, second, we set LOS to 30 days for all nonsurvivors.26 To account for the potential preference of LR utilization in surgical patients, the primary match and analyses were repeated after excluding all patients who underwent any surgery based on diagnosis related group codes. Statistical significance was defined as P < .05.
Results
An initial cohort of 12 529 patients met all inclusion/exclusion criteria, including 10 379 ordered for only NS fluid (NS group) and 2150 patients ordered for at least 1 LR fluid bolus (LR-any group) (Figure 1; available at www.jpeds.com). Only 459 patients received exclusive LR resuscitation (LR-only group). Use of LR decreased slightly between 2000 and 2005 (31% to 16%) but remained between 14% and 16% through 2013. The median (IQR) volume of total fluid resuscitation was 1000 mL (500–2000 mL) and by estimated weight-for-age was 24 mL/kg (13–45 mL/kg). Before matching, the LR-any group was older (median 8 vs 5 years, P < .001), received larger crystalloid volumes (median 2000 vs 1000 mL [40 vs 22 mL/kg], P < .001), was less likely to have malignancies, and more likely to receive intensive care therapies (Table IV). Unadjusted 30-day hospital mortality was not different between the LR-any (7.4%) and NS (6.9%) groups (P = .33). However, unadjusted 30-daymortality varied significantly for patients who received only LR (5.0%), a combination of LR and NS (8.1%), and only NS (6.9%) fluid (P = .04).
A total of 2117 patients who received any LR were matched 1:1 with patients that received only NS on the variables listed in Table V, as well as site, season, and sepsis identification strategy. Patient characteristics, comorbidities, and nonfluid therapies were similar after matching (Table V). For several covariates, P values remained <.05 reflecting the large sample, but standardized differences were all <.10 in the matched cohort (Figure 2; available at www.jpeds.com). The majority of patients included in the matched analysis were identified with severe sepsis using ICD-9-CM codes for infection plus organ dysfunction (80.4%) vs specific severe sepsis/septic shock codes (19.6%).
LR-Any Matched Analysis
In the matched cohort of 4234 patients, 30-day hospital mortality was 7.2% in the LR-any group and 7.9% in the NS group (risk ratio 0.99, 95% CI 0.98, 1.01; P = .20). There were no significant differences in overall hospital mortality, hospital mortality plus hospice, or AKI with and without dialysis (Table VII). There remained no differences in these outcomes after further adjusting for imperfectly balanced covariates in multivariable analyses, after repeating the match using only those covariates unlikely to mediate the effect of fluid type on outcome, or after excluding surgical patients (data not shown). Hospital LOS was longer for the LR-any group compared with the NS group (absolute difference 2.4, 95% CI 1.4, 5.0 days; P < .001). Analyses setting LOS to the sample maximum or to 30 days for all nonsurvivors did not impact these findings (Table VIII; available at www.jpeds.com), nor did excluding 234 matched pairs from hospitals with ≤10 patients (data not shown). The Kaplan-Meier analysis demonstrated no difference in time-to-death between matched groups (log-rank P = .11) (Figure 3; available at www.jpeds.com).
Table VII.
Outcomes in matched cohorts for LR-any and LR-only groups
| Outcomes* | LR-any group† (n = 2117) | NS group‡ (n = 2117) | Risk ratio/difference 95% CI) | P value§ |
|
| ||||
| Mortality, 30-d | 153 (7.2) | 168 (7.9) | 0.99 (0.9, 1.09) | .20 |
| Mortality, hospital | 194 (9.2) | 199 (9.4) | 1.0 (0.98, 1.02) | .41 |
| Mortality, including hospice | 200 (9.4) | 211 (10.0) | 0.99 (0.98, 1.01) | .29 |
| AKI | 334 (15.8) | 337 (15.9) | 1.0 (0.97, 1.02) | .41 |
| New dialysis | 27 (1.3) | 33 (1.6) | 1.0 (0.99, 1.00) | .21 |
| PICU LOS¶, median (IQR) | 7.8 (1.0, 13.0) | 7.3 (1.0, 12.0) | 0.5 (0.2, 0.8) | .01 |
| Hospital LOS¶, median (IQR) | 15.5 (6.0, 22.0) | 13.1 (4.0, 20.0) | 2.4 (1.4, 5.0) | <.001 |
|
| ||||
| Outcomes* | LR-only group** (n = 459) | NS group‡ (n = 459) | Risk ratio/difference (95% CI) | P value§ |
|
| ||||
| Mortality, 30-d | 23 (5.0) | 21 (4.6) | 1.01 (0.98, 1.03) | .69 |
| Mortality, hospital | 29 (6.3) | 28 (6.1) | 1.0 (0.97, 1.03) | .62 |
| Mortality, including hospice | 30 (6.5) | 29 (6.3) | 1.0 (0.97, 1.03) | .62 |
| AKI | 45 (9.8) | 49 (10.7) | 0.99 (0.95, 1.03) | .32 |
| New dialysis | 5 (1.1) | 5 (1.1) | 1.0 (0.99, 1.01) | .50 |
| PICU LOS¶, median (IQR) | 5.8 (1.0, 10.0) | 5.5 (1.0, 9.0) | 0.33 (−0.1, 0.7) | .27 |
| Hospital LOS¶, median (IQR) | 11.9 (5.0, 18.0) | 10.5 (4.0, 14.0) | 1.35 (0.5, 2.2) | .01 |
Data presented as median (IQR).
LR-any group included patients who received any amount of LR fluid resuscitation.
NS group included patients who received only NS fluid resuscitation.
Statistical comparison using McNemar test for matched pairs or Wilcoxon sign rank test, as appropriate.
LOS is reported in days.
LR-only group included patients who only received LR fluid resuscitation.
Patients with specific ICD-9-CM codes for severe sepsis/septic shock had higher 30-day hospital mortality than patients identified by infection plus organ dysfunction codes (15.0% vs 5.8%, P < .001). However, there were no differences in outcomes between the matched LR-any and NS groups stratified by sepsis identification strategy except for longer PICU and hospital LOS for the LR group identified by combination codes (Table IX; available at www.jpeds.com).
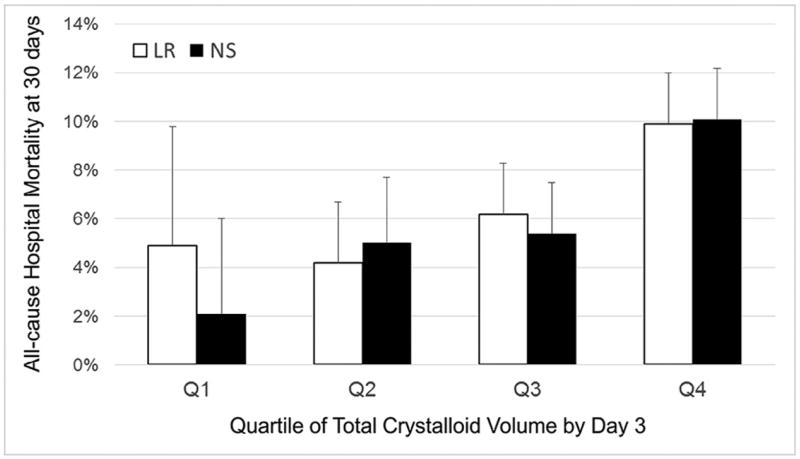
Volume-Stratified Analysis
Although mortality rose with increasing weight-adjusted crystalloid fluid volume, 30-day hospital mortality did not differ between the LR-any and NS groups after matching within volume quartiles (Figure 4). However, only 142 patients received LR in the first quartile of total crystalloid fluid volume, and there was an increase in LR utilization with successive quartiles of total volume administration (Q1: 5.0%; Q2: 11.5%; Q3: 20.6%; Q4: 30.2%), supporting the preferential use of LR in patients who required larger total fluid volumes. In addition, PICU admission and the use of nonfluid intensive therapies were more common in each successive quartile (Table X; available at www.jpeds.com) making it difficult to disentangle proportionate LR use, volume of fluid resuscitation, and illness severity. Finally, matching performed least effectively in the fourth quartile of patients who received the largest total fluid volumes and with the most severe illness severity (Figure 5; available at www.jpeds.com).
Figure 4.
Hospital mortality for LR-any and NS groups matched within quartile of total crystalloid fluid volume. The x-axis categorizes patients based on quartile of total fluid crystalloid volume received after correcting by estimated weight for age (median total volume in Q1 = 8 mL/kg, Q2 = 17 mL/kg, Q3 = 32 mL/kg, and Q4 = 68 mL/kg). The y-axis shows the adjusted 30-day mortality rate. Patients in the LR-any group were matched within volume quartile to patients who received only NS. There were no significant differences in mortality between the LR-any and NS groups within any of the total volume quartiles.
Dose-Response Analysis
To account for variability in the proportionate use of LR, patients were separately matched after stratifying by proportion of total crystalloid volume ordered as LR. Patients with an increasing proportionate LR use received fewer intensive therapies and had a lower rate of adverse outcomes (Tables XI and XII; available at www.jpeds.com).However, there were no differences in 30-day hospital mortality or AKI (Figure 6; available at www.jpeds.com) or dialysis (data not shown) between the LR groups stratified by proportionate LR utilization and the NS group.
LR-Only Matched Analysis
In the separate matched cohort of 918 patients receiving either exclusive LR or NS fluid, 30-day hospital mortality was 5% in the LR-only group and 4.6% in the NS group (risk ratio 1.01, 95% CI 0.98, 1.03; P = .69). There were no significant differences in secondary outcomes, except a longer hospital LOS in the LR-only group (Table VII).
Discussion
In this large matched cohort study of pediatric severe sepsis and septic shock, balanced fluid resuscitation with LR was not associated with improved mortality, AKI, or dialysis, even when matched by fluid volume and proportionate LR utilization. However, LR was preferentially used either as first-line fluid in patients with lower illness severity or as an adjunctive fluid in patients who received large amounts of fluid resuscitation, and the matching algorithm was least effective in the most severely ill patients who received the largest total fluid volumes. Consequently, the results of our study are best interpreted as establishing the need for and the equipoise to conduct a prospective randomized trial to definitely address the comparative effectiveness of balanced fluids and NS in pediatric sepsis.
Prior data suggest that the supraphysiologic chloride content of NS may be detrimental to renal function and acid-base balance.1–3,9–11 Infusion of chloride-rich fluids reduced renal blood flow in dogs and healthy human volunteers to a greater extent than more balanced fluids.27,28 NS also induced abdominal discomfort, drowsiness, and impaired cognition, compared with LR and other balanced fluids, in a human study.29 In a sequential period study of critically ill adults, use of chloride-restrictive fluids reduced the odds of AKI and dialysis by almost 50%.11 Infusion of large volumes of chloride-rich solutions can also cause a hyperchloremic metabolic acidosis, which has been shown to be proinflammatory.30
Our work builds on previous clinical studies that have overwhelmingly focused on adult populations. Most studies have demonstrated either no difference31–34 or a benefit of at least some proportion of resuscitation fluids given as balanced solutions.11–13 For example, Raghunathan et a13 found that receipt of at least some balanced fluids during initial resuscitation was associated with lower hospital mortality in adults with vasopressor-dependent septic shock. This contrast with our results may be attributable to age-related biological differences, such as a lower rate of baseline subclinical cardiac and renal disease in children, or methodological differences, such as limiting the adult study to vasopressor-dependent shock. Unfortunately, insufficient sample size precluded limiting our pediatric analysis to vasopressor-dependent shock. Moreover, even though Premier offers a geographically diverse sample, the relatively low median fluid volume resuscitation and mortality <8%suggests an overall moderate illness severity that may not reflect more severe pediatric sepsis cases that tend to concentrate at specialty children’s hospitals. It is also possible that potential detrimental effects of LR, including microvascular thromboses (because of calcium activating the clotting cascade)9 or cerebral edema (because of mild fluid hypotonicity),35 may be more problematic in children. For example, in Vietnamese children with Dengue shock, patients randomized to resuscitation with LR had a longer time to recovery than patients resuscitated with NS.15 As with other critical therapies, benefits seen in adult populations may not translate to children.
Prior studies suggest that potential adverse effects associated with NS are dose-related such that LR may only be beneficial for patients requiring large-volume fluid resuscitation.1–3 In our study, mortality increased with larger fluid volumes and decreased with a greater proportion of fluid given as LR, but there were no differences between LR and NS groups after matching within volume quartiles, by proportionate LR utilization, or in the separate matched analysis of LR-only patients. However, the preference for LR as first-line fluid in patients with low illness severity or as an adjunctive fluid in patients who received a large amount of total fluid could have masked a true benefit of LR.
There are several limitations. First, claims-based data may lead to misclassification bias if ordered and administered therapies are discrepant, although this is unlikely to produce differential bias between groups. Also, we were not able to account for prehospital fluid administration, partial administration of fluid units (which is common in pediatrics because of weight-based fluid administration), or account for deviations in weight from published growth curves. Even though this may have over-or underestimated fluid administration, these are unlikely to have been sources of differential bias between LR and NS. However, the younger age of the NS-only group (before and after matching) could have introduced slightly more error in estimated total fluid volumes than in the LR-group because younger patients are more likely to receive partial administration of a fluid bag and rounding to median weight represents a larger proportional change relative to true weight. Second, using ICD-9-CM codes for infection plus organ dysfunction to identify pediatric sepsis is controversial.17,18,36 Notably, patients with more specific ICD-9-CM sepsis codes had a trend toward decreased mortality and less dialysis in the LR group. Moreover, because identification and timing of surgical interventions is limited using administrative codes, it was difficult to fully account for a possible surgical preference to use LR. Third, differences in demographics, comorbidities, and intensive therapies indicate nonrandom selective use of LR over NS. Although statistical matching was able to remove much of these baseline differences, we cannot rule out residual confounding within unmeasured covariates. In addition, in the absence of physiologic and laboratory data, we used intensive care therapies for illness severity but could not determine if these therapies occurred after—or as a result of—differential use of LR vs NS. Because excluding these variables from the match posed risk for increased confounding, we chose a conservative approach similar to Raghunathan et al13 fluid study in adult sepsis despite the possibility that matching on these covariates could have masked outcome differences. However, secondary analyses using patients matched only on covariates unlikely to mediate the effect of fluid type on outcome also showed no differential effect of LR vs NS. Finally, because LR was the predominant balanced fluid used, our data may not be generalizable to other balanced fluids.
In this large matched observational study, use of LR (alone or in combination with NS) was not associated with improved outcomes compared with exclusive NS resuscitation in pediatric septic shock. These findings support the current practice of using NS as the first choice for crystalloid fluid resuscitation in pediatric sepsis. However, given the limitations of matching within a retrospective observational study to fully account for the nonrandom selective use of LR, our findings also emphasize the need for a large-scale prospective randomized trial to definitely determine the comparative effectiveness of balanced fluids and NS in pediatric sepsis.
Supplementary Material
Acknowledgments
Supported by the Department of Anesthesiology and Critical Care, Division of Emergency Medicine, and Center for Pediatric Clinical Effectiveness at The Children’s Hospital of Philadelphia. S.W. receives support from National Institute of General Medical Sciences (K23GM110496). F.B. receives support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23HD082368).
Glossary
- AKI
Acute kidney injury
- ICD-9-CM
International Classification of Diseases, Ninth Edition, Clinical Modification
- LOS
Length of stay
- LR
Lactated Ringer
- NS
Normal saline
- PICU
Pediatric intensive care unit
Footnotes
The authors declare no conflicts of interest.
References
- 1.Karakala N, Raghunathan K, Shaw AD. Intravenous fluids in sepsis: what to use and what to avoid. Curr Opin Crit Care. 2013;19:537–43. doi: 10.1097/MCC.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 2.Santi M, Lava SA, Camozzi P, Giannini O, Milani GP, Simonetti GD, et al. The great fluid debate: saline or so-called “balanced” salt solutions? Ital J Pediatr. 2015;41:47. doi: 10.1186/s13052-015-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369:1243–51. doi: 10.1056/NEJMra1208627. [DOI] [PubMed] [Google Scholar]
- 4.Boulain T, Boisrame-Helms J, Ehrmann S, Lascarrou JB, Bougle A, Chiche A, et al. Volume expansion in the first 4 days of shock: a prospective multicentre study in 19 French intensive care units. Intensive Care Med. 2015;41:248–56. doi: 10.1007/s00134-014-3576-1. [DOI] [PubMed] [Google Scholar]
- 5.Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, et al. Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intensive Care Med. 2015;41:1529–37. doi: 10.1007/s00134-015-3850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–88. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 8.Ventura AM, Shieh HH, Bousso A, Goes PF, de Cassia FOFI, de Souza DC, et al. Double-blind prospective randomized controlled trial of dopamine versus epinephrine as first-line vasoactive drugs in pediatric septic shock. Crit Care Med. 2015;43:2292–302. doi: 10.1097/CCM.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 9.Kiraly LN, Differding JA, Enomoto TM, Sawai RS, Muller PJ, Diggs B, et al. Resuscitation with normal saline (NS) vs. lactated ringers (LR) modulates hypercoagulability and leads to increased blood loss in an uncontrolled hemorrhagic shock swine model. J Trauma. 2006;61:57–64. doi: 10.1097/01.ta.0000220373.29743.69. discussion-5. [DOI] [PubMed] [Google Scholar]
- 10.Zhou F, Peng ZY, Bishop JV, Cove ME, Singbartl K, Kellum JA. Effects of fluid resuscitation with 0.9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis. Crit Care Med. 2014;42:e270–8. doi: 10.1097/CCM.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–72. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 12.Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–9. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 13.Raghunathan K, Shaw A, Nathanson B, Sturmer T, Brookhart A, Stefan MS, et al. Association between the choice of IV crystalloid and inhospital mortality among critically ill adults with sepsis. Crit Care Med. 2014;42:1585–91. doi: 10.1097/CCM.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 14.Carcillo JA, Davis AL, Zaritsky A. Role of early fluid resuscitation in pediatric septic shock. JAMA. 1991;266:1242–5. [PubMed] [Google Scholar]
- 15.Ngo NT, Cao XT, Kneen R, Wills B, Nguyen VM, Nguyen TQ, et al. Acute management of dengue shock syndrome: a randomized double-blind comparison of 4 intravenous fluid regimens in the first hour. Clin Infect Dis. 2001;32:204–13. doi: 10.1086/318479. [DOI] [PubMed] [Google Scholar]
- 16.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–95. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 17.Balamuth F, Weiss SL, Neuman MI, Scott H, Brady PW, Paul R, et al. Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med. 2014;15:798–805. doi: 10.1097/PCC.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss SL, Parker B, Bullock ME, Swartz S, Price C, Wainwright MS, et al. Defining pediatric sepsis by different criteria: discrepancies in populations and implications for clinical practice. Pediatr Crit Care Med. 2012;13:e219–26. doi: 10.1097/PCC.0b013e31823c98da. [DOI] [PubMed] [Google Scholar]
- 19.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107:E99. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 20.Zubizarreta JR. Using mixed integer programming for matching in an observational study of kidney failure after surgery. J Am Stat Assoc. 2012;107:1360–71. [Google Scholar]
- 21.Rubin DB. Bias reduction using Mahalanobis-metric matching. Biometrics. 1980;36:293–8. [Google Scholar]
- 22.Rosenbaum PR. Sensitivity analysis for m-estimates, tests, and confidence intervals in matched observational studies. Biometrics. 2007;63:456–64. doi: 10.1111/j.1541-0420.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 23.Neuman MD, Rosenbaum PR, Ludwig JM, Zubizarreta JR, Silber JH. Anesthesia technique, mortality, and length of stay after hip fracture surgery. JAMA. 2014;311:2508–17. doi: 10.1001/jama.2014.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silber JH, Rosenbaum PR, Trudeau ME, Even-Shoshan O, Chen W, Zhang X, et al. Multivariate matching and bias reduction in the surgical outcomes study. Med Care. 2001;39:1048–64. doi: 10.1097/00005650-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:i969. doi: 10.1136/bmj.i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin W, Halpern SD, Prasad Kerlin M, Small DS. A “placement of death” approach for studies of treatment effects on ICU length of stay. Stat Methods Med Res. 2014 Aug 1; doi: 10.1177/0962280214545121. pii: 0962280214545121. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–35. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88:999–1003. doi: 10.1097/00000539-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Kellum JA, Song M, Li J. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264.7 cells. Am J Physiol Regul Integr Comp Physiol. 2004;286:R686–92. doi: 10.1152/ajpregu.00564.2003. [DOI] [PubMed] [Google Scholar]
- 31.Annane D, Siami S, Jaber S, Martin C, Elatrous S, Declere AD, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310:1809–17. doi: 10.1001/jama.2013.280502. [DOI] [PubMed] [Google Scholar]
- 32.Rochwerg B, Alhazzani W, Gibson A, Ribic CM, Sindi A, Heels-Ansdell D, et al. Fluid type and the use of renal replacement therapy in sepsis: a systematic review and network meta-analysis. Intensive Care Med. 2015;41:1561–71. doi: 10.1007/s00134-015-3794-1. [DOI] [PubMed] [Google Scholar]
- 33.Rochwerg B, Alhazzani W, Sindi A, Heels-Ansdell D, Thabane L, Fox-Robichaud A, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med. 2014;161:347–55. doi: 10.7326/M14-0178. [DOI] [PubMed] [Google Scholar]
- 34.Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314:1701–10. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 35.Ayus JC, Achinger SG, Arieff A. Brain cell volume regulation in hyponatremia: role of sex, age, vasopressin, and hypoxia. Am J Physiol Renal Physiol. 2008;295:F619–24. doi: 10.1152/ajprenal.00502.2007. [DOI] [PubMed] [Google Scholar]
- 36.Balamuth F, Weiss SL, Hall M, Neuman MI, Scott H, Brady PW, et al. Identifying pediatric severe sepsis and septic shock: accuracy of diagnosis codes. J Pediatr. 2015;167:1295–300. doi: 10.1016/j.jpeds.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.