Abstract
Background
We assessed the influence of current cefepime minimal inhibitory concentration (MIC) breakpoints and the maximal cefepime dose on treatment outcomes in patients with bacteremia caused by cefepime-susceptible Pseudomonas aeruginosa.
Methods
Adult patients hospitalized between July 2010 and June 2014 with a positive blood culture for cefepime-susceptible P. aeruginosa and receipt of cefepime as the primary therapy throughout the course were reviewed. Cefepime Etest® MICs and clinical outcomes for P. aeruginosa bacteremia were reviewed to identify the MIC breakpoint influencing treatment outcomes.
Results
Of the 90 patients enrolled, 49 (54.4%) were male (mean age = 66.8 years). The mean Acute Physiology and Chronic Health Evaluation II score was 22.01. Sixty patients (66.7%) received a maximal cefepime dose, and the 30-day crude mortality rate was 36.7%. MIC90 of cefepime for P. aeruginosa was 8 mg/L. The cumulative survival rate at 30 days revealed that a lower cefepime MIC (<4 mg/L) for P. aeruginosa was associated with a higher survival rate than a higher MIC (≥4 mg/L) (72.6% vs. 23.5%, p < 0.0001). A cefepime MIC of ≥4 mg/L and age were independent risk factors for mortality, whereas the maximal cefepime dose was the independent protective factor. The use of a maximal cefepime dose did not improve the outcomes of patients with P. aeruginosa bacteremia at a MIC of ≥4 mg/L.
Conclusions
A cefepime MIC of 4 mg/L may predict an unfavorable outcome among patients with serious infections caused by P. aeruginosa, even the MICs still within the CLSI susceptibility breakpoint.
Keywords: Pseudomonas aeruginosa, Bacteremia, Cefepime, Minimal inhibitory concentrations, Maximal cefepime dose
Background
Pseudomonas aeruginosa is a leading cause of nosocomial infections [1, 2], which are often life threatening [3]. Recently, actual minimal inhibitory concentrations (MICs) of fluoroquinolones [4], extended-spectrum penicillins [5], and carbapenems [6] have predicted patient outcomes more accurately than did the categorical classification of MICs as susceptible, intermediate, and resistant. Cefepime is a fourth-generation cephalosporin with a broad-spectrum antibacterial activity; it has been widely used since its approval for clinical use in 1997 [7]. According to the Clinical and Laboratory Standards Institute (CLSI) criteria of 2016 [8], the susceptible range of cefepime MIC was ≤8 mg/L. However, the mortality rates of patients infected with gram-negative organisms treated with cefepime increased with increasing MICs [9]. Therefore, the primary aim of this study was to determine the predictive value of cefepime MICs on the therapeutic outcomes in patients with cefepime-susceptible P. aeruginosa bacteremia and to evaluate if the current cefepime breakpoints for P. aeruginosa require revision.
The present recommended cefepime dosage may be suboptimal for the treatment of infections caused by P. aeruginosa strains with a higher cefepime MIC value [10, 11], and therapy with a higher cefepime dose was associated with a lower mortality rate in patients with gram-negative bacilli (GNB) infections [12] and requirement of the intensive care [13, 14]. The secondary aim of this study was to evaluate whether the maximal cefepime dose could improve clinical outcomes in patients with cefepime-susceptible P. aeruginosa bacteremia.
Methods
Setting
This retrospective study was conducted at the Chang Gung Memorial Hospital (CGMH), Linkou, Northern Taiwan, a 3715-bed university-affiliated tertiary-care medical center with 308 intensive care unit (ICU) beds. All clinical specimens were processed using computer-assisted microbiology laboratory databases at a central microbiology laboratory. This study was approved by the Institutional Review Board of the CGMH (103-3354B).
Study design and patients
In this retrospective study, 586 patients admitted to CGMH from July 2010 to June 2014 with an unduplicated monomicrobial blood culture positive for cefepime-susceptible P. aeruginosa and a clinical syndrome suggestive of systemic infection were reviewed. The additional inclusion criteria are as follows: age ≥18 years, clear medical records, cefepime as the first-line therapy within 48 h of bacteremia onset and monotherapy against GNB throughout the treatment. Patients who met any of the following criteria were not eligible for the study: no receipt of cefepime therapy, receipt of cefepime <3 days, combination therapy with other antimicrobials against GNB including aminoglycosides, anti-pseudomonal β-lactams or anti-pseudomonal fluoroquinolones, and inadequate clinical information from the medical records. In this study, none of the patients had received cefepime more than 3 days initially, and then received other antibiotics instead. Finally, ninety patients were enrolled in this study (Fig. 1).
Fig. 1.
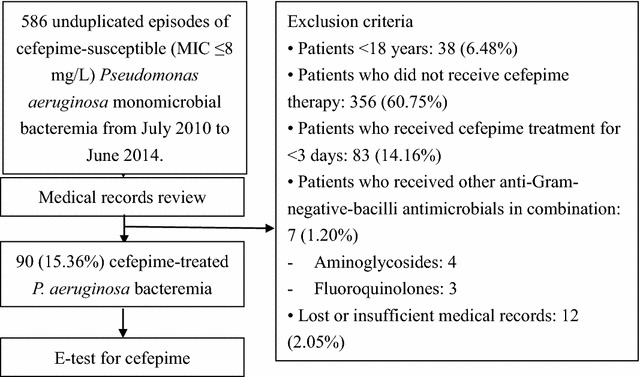
Flow chart of the exclusion of patients with Pseudomonas aeruginosa
Microbiology
Blood cultures were processed in the clinical microbiology laboratory by using an automated blood culture system (BACTEC 9240 system; Becton–Dickinson Diagnostic Instrument Systems, Sparks, MD, USA). Before June 2013, P. aeruginosa isolates were identified on the basis of the following properties: aerobic GNB on Gram staining with glucose nonfermentation, positive oxidase test, blue–green or yellow–green fluorescent pigment production, and growth at 42 °C [15]. After June 2013, bacterial species were identified through matrix-assisted laser desorption ionization-time of flight (MALDI-TOF). P. aeruginosa blood isolates are routinely preserved at our clinical microbiology laboratory in skimmed milk at −70 °C until further use. All the P. aeruginosa blood isolates investigated in this study were selected from these stocks and tested to determine the cefepime MICs using Etest® strips (bioMerieux, Lyon, France) according to the manufacturer instructions. An isolate of P. aeruginosa was defined as cefepime susceptible, intermediate, or resistant if its MIC was ≤8, 16, or ≥32 mg/L, respectively [8]. P. aeruginosa ATCC 27853 was the control.
Data collection and definition
Demographic data, such as age, sex, concomitant diseases, and clinical characteristics, of patients with P. aeruginosa bacteremia were retrieved by reviewing inpatient medical records. Concomitant diseases included severe renal impairment (defined as chronic kidney disease stage 4, 5 and needed renal replacement therapy), diabetes mellitus, cerebral vascular accident, liver cirrhosis, chronic pulmonary disease, and malignancy. Central venous catheter (CVC) placement, ventilator use, ICU stay, and the time interval between hospitalization and occurrence of P. aeruginosa bacteremia were recorded. Disease severity scores were calculated using the Acute Physiology and Chronic Health Evaluation II (APACHE II) score on the day P. aeruginosa bacteremia occurred. All the patients had collected the following parameters, age, comorbidities, systolic and mean arterial blood pressure (mmHg), heart rate, respiratory rate, body temperature, initial Glasgow Coma Scale score, arterial blood gas analysis: pH, arterial oxygen tension (PaO2), arterial carbon dioxide tension (pCO2), laboratory data (white blood cell count, hematocrit, sodium, potassium, and creatinine). However, the following values, if missing, were considered normal: PaO2, pH, and pCO2. Severe sepsis were defined as sepsis plus evidence of organ dysfunction included either one criteria as bellowed: (1) arterial hypoxemia (PaO2/fraction of inspiration O2; FiO2 <300), (2) acute oliguria (urine output <0.5 mL/kg per hour for at least 2 h despite adequate fluid resuscitation, (3) increase in creatinine >0.5 mg/dL, (4) coagulation abnormalities: international normalized ratio (INR) >1.5, activated partial thromboplastin time (aPTT) >60 s, platelets <100,000/μL, (5) hepatic dysfunction (elevated bilirubin), (6) paralytic ileus, and (7) decreased capillary refill or skin mottling. Septic shock was defined as sepsis with hypotension refractory to fluid resuscitation or hyperlactatemia. Neutropenia was defined as absolute neutrophil count of <0.5 × 109/L.
The sources of bacteremia determined from medical records, imaging studies, surgical findings, and microbiological evidences were categorized into lower respiratory and urinary tracts, skin and skin structure, central catheter-associated bloodstream infection (CABSI), and intra-abdominal infections. If no source was identified, the infection was categorized as primary bacteremia.
Treatment and outcomes
The dosage and dosing frequency of cefepime were reviewed from patient medical records. Cefepime was infused over 30 min. Creatinine clearance (CLCR) was calculated on the date of first dose of cefepime was given. CLCR was calculated using an adjusted Cockcroft–Gault equation that excluded patient weight [CLCR = (140 − age)/serum creatinine concentration]; the result was multiplied by 0.85 for female patients. The maximal cefepime dose adjusted by CLCR was defined as 2 g every 8 h, 2 g every 12 h, 2 g every 24 h, and 1 g every 24 h, while CLCR was ≥50, 30–49, 10–29, and <10 mL/min, respectively [10]. Patients receiving above CLCR-adjusted dosing regimens throughout the course of cefepime treatment were defined as using the maximal cefepime dose. Clinical outcomes were assessed using the 30-day crude mortality.
Statistical analyses
All statistical analyses were performed using the Statistical Package for Social Sciences for Windows (version 18.0; SPSS Inc., Chicago, IL, USA). Categorical variables were compared using the χ2 or Fisher exact tests, as appropriate; continuous variables were compared using the Mann–Whitney U test. Variables with p < 0.1 in the univariate analysis were included in a multiple logistic regression model using the backward stepwise method for identifying the risk factors for the 30-day sepsis-related mortality. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. The survival curve was plotted by means of the Kaplan–Meier method, and the log rank test was used to compare univariate survival distribution. All tests were two-tailed, and p < 0.05 was considered significant.
Results
Patient enrollment and their clinical characteristics
A total of 586 unduplicated cefepime-susceptible P. aeruginosa blood isolates from 586 patients were identified. On the basis of our inclusion criteria, 496 patients were excluded because of age <18 years, no receipt of cefepime therapy or cefepime use <3 days, or receipt of combination therapy with other anti-GNB antimicrobials. Ninety patients with individual unduplicated P. aeruginosa blood isolates were enrolled (Fig. 1). Patient demographics and clinical characteristics are listed in Table 1. Of the 90 patients, 49 (54.4%) were male with a mean age of 66.8 years. The most common concomitant disease was solid organ malignancy (43.3%), followed by diabetes mellitus (32.2%), and chronic kidney disease stage IV and above (31.1%). Time between hospital admission and occurrence of P. aeruginosa bacteremia ranged from 0 to 252 days with a mean interval of 23.2 days.
Table 1.
Clinical characteristics of 90 patients with cefepime-susceptible Pseudomonas aeruginosa monomicrobial bacteremia receiving cefepime monotherapy
| Variables | Valuea |
|---|---|
| Demographic parameters | |
| Age, year | 66.8 (14.6) |
| Male gender | 49 (54.4) |
| Concomitant diseases | |
| Diabetes mellitus | 29 (32.2) |
| Severe renal impairment | 28 (31.1) |
| Liver cirrhosis | 10 (11.1) |
| Chronic pulmonary disease | 8 (8.9) |
| Cerebral vascular accident | 19 (21.1) |
| Solid organ malignancy | 39 (43.3) |
| Haematological malignancy | 16 (17.8) |
| Autoimmune disease | 5 (5.6) |
| Clinical conditions | |
| Time interval between admission and occurrence of bacteremia, day | 23.2 (36.3) |
| Central venous catheter use | 68 (75.6) |
| Patients’ severity | |
| APACHE II score | 22.07 (6.0) |
| Ventilator use | 23 (25.6) |
| Intensive care unit stay | 29 (32.2) |
| Severe sepsis or septic shock | 21 (23.3) |
| Neutropenia | 18 (20.0) |
| Source of bacteremia | |
| Primary bacteremia | 38 (42.2) |
| Lower respiratory tract | 27 (30) |
| Urinary tract | 7 (7.8) |
| Skin and skin structure | 2 (2.2) |
| Central catheter associated blood stream infection | 12 (13.3) |
| Intra-abdominal infection | 10 (11.1) |
| Treatment | |
| Use of maximum cefepime dose | 60 (66.7) |
| Treatment duration | 16.4 (7.031) |
| Remove catheter or operation | 8 (8.9) |
| Outcome | |
| 30-day crude mortality | 33 (36.7) |
APACHE II score Acute Physiology and Chronic Health Evaluation II score
aCategorical data: number (%) of patients; continuous data are expressed as mean (standard deviation)
Sixty-eight patients (75.6%) had received a CVC placement, 23 (25.6%) ever used a ventilator, 29 (32.2%) had ICU stay, 21 (23.3%) had severe sepsis or septic shock, and 18 (20%) had neutropenia. The mean APACHE II score was 22.01. Thirty-eight patients (38.8%) had primary bacteremia and the remaining 52 (61.2%) had identified sources of bacteremia. One case had vertebral osteomyelitis. The most common source of bacteremia was lower respiratory tract infection (27/52, 51.9%), followed by CABSI (12/52, 23.1%).
Treatment and outcomes
Sixty patients (66.7%) used the maximal cefepime dose. The treatment duration varied from 3 to 30 days with a mean duration of 16.4 days, and the 30-day crude mortality rate was 36.7%. Besides, none of the studied cases had reported the adverse effects including neurotoxicity during cefepime use.
MIC versus mortality
Figure 2 depicts the relationship between MICs and mortality rates. Cefepime MIC50 and MIC90 for P. aeruginosa were 1 and 8 mg/L, respectively. The lower MICs (0.5, 0.75, and 1 mg/L) were associated with the lower mortality rates (0, 15.8, and 36.4%, respectively). The mortality rate extended to 42.9 and 100% at the MICs of 4 and >4 mg/L, respectively.
Fig. 2.
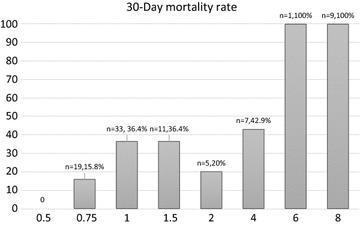
Cefepime minimal inhibitory concentrations versus rate of mortality. n numbers, presented as the blood isolate number and the following is mortality rate, MIC minimal inhibitory concentration (mg/L)
Risk factors for 30-day mortality of P. aeruginosa bacteremia
The cumulative survival rate at 30 days revealed that a lower cefepime MIC (<4 mg/L) for P. aeruginosa was associated with a significantly higher survival rate than a higher MIC (≥4 mg/L) (72.6% versus 23.5%, p < 0.0001) (Fig. 3).
Fig. 3.
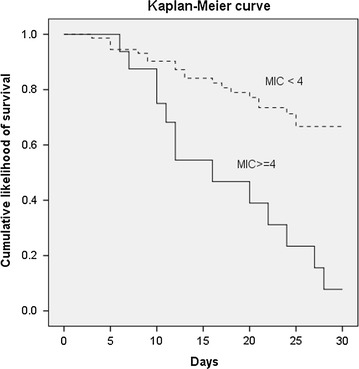
Kaplan-Meier survival curve in patients with cefepime-susceptible Pseudomonas aeruginosa bacteremia. Comparison of the cumulative survival between cefepime minimal inhibitory concentration (MIC) <4 and ≥4 mg/L (p < 0.0001)
The factors associated with the 30-day mortality in univariate analysis (Table 2) included older ages (71.5 ± 12.7 vs. 64.1 ± 15.1 years, p = 0.028), a longer time interval between the dates of admission and positive blood cultures (31.8 ± 34.6 vs. 18.3 ± 33.6 days, p = 0.02), a longer ICU stay (54.5% vs. 19.3%, p = 0.001), more episodes of severe sepsis or septic shock (36.4% vs. 15.8%, p = 0.038), more respiratory tract infections (48.5% vs. 19.3%, p = 0.004), a higher cefepime MIC (≥4 mg/L) (76.5% vs. 27.4%, p < 0.001), and fewer instances of maximal cefepime dose use (48.5% vs. 78.9%, p = 0.003). The mortality rate of patients with a cefepime MIC of ≥4 mg/L for P. aeruginosa was 76.5%, which was higher than those with a MIC of <4 mg/L (27.4%).
Table 2.
Univariate analyses of risk factors for 30-day crude mortality of cefepime-susceptible Pseudomonas aeruginosa bacteremia treated with cefepime
| Variables | Deceaseda | Surviveda | Univariate |
|---|---|---|---|
| n = 33 | n = 57 | p | |
| Demographic parameters | |||
| Age, year | 71.5 (12.7) | 64.1 (15.1) | 0.028 |
| Male gender | 21 (63.6) | 28 (49.1) | 0.183 |
| Concomitant diseases | |||
| Diabetes mellitus | 9 (27.3) | 20 (35.1) | 0.445 |
| Severe renal impairment | 13 (39.4) | 15 (26.3) | 0.197 |
| Liver cirrhosis | 4 (12.1) | 6 (10.5) | 1.000 |
| Chronic pulmonary disease | 4 (12.1) | 4 (7.0) | 0.458 |
| Cerebral vascular accident | 8 (24.2) | 11 (19.3) | 0.580 |
| Solid organ malignancy | 17 (51.5) | 22 (38.6) | 0.233 |
| Haematological malignancy | 4 (12.1) | 12 (21.1) | 0.394 |
| Autoimmune disease | 2 (6.1) | 3 (5.3) | 1.000 |
| Clinical conditions | |||
| Time interval between admission and occurrence of bacteremia, day | 31.8 (34.6) | 18.3 (33.6) | 0.019 |
| Central venous catheter use | 27 (81.8) | 41 (71.9) | 0.293 |
| Patients’ severity | |||
| APACHE II score | 23.9 (5.5) | 21.0 (6.1) | 0.091 |
| Intensive care unit stay | 18 (54.5) | 11 (19.3) | 0.001 |
| Severe sepsis or septic shock | 12 (36.4) | 9 (15.8) | 0.038 |
| Neutropenia | 7 (21.2) | 11 (19.3) | 0.827 |
| Source of bacteremia | |||
| Primary bacteremia | 11 (33.3) | 27 (47.4) | 0.194 |
| Lower respiratory tract | 16 (48.5) | 11 (19.3) | 0.004 |
| Urinary tract | 1 (3.0) | 6 (10.5) | 0.416 |
| Skin and skin structure | 1 (3.0) | 1 (1.8) | 1.000 |
| CABSI | 3 (9.1) | 9 (15.8) | 0.524 |
| Intra-abdominal site | 5 (15.2) | 5 (8.8) | 0.488 |
| Microbiology | |||
| MIC ≥4 mg/L | 13 (39.4) | 4 (7.0) | <0.001 |
| Treatment | |||
| Use of maximum cefepime dose | 15 (45.5) | 45 (78.9) | 0.001 |
| Treatment duration | 14.7 (7.2) | 17.5 (6.8) | 0.062 |
| Remove catheter or operation | 2 (6.1) | 6 (10.5) | 0.705 |
APACHE II score Acute Physiology and Chronic Health Evaluation II score, CABSI central catheter associated blood stream infection, MIC minimal inhibitory concentration
aCategorical data: number (%) of patients; continuous data are expressed as mean (standard deviation)
Those factors associated with the 30-day mortality in univariate analysis and APACHE II score were entered into multivariate analysis (Table 3), and the result showed that MIC ≥4 mg/L (adjusted OR 5.111; 95% CI 1.090–23.974; p = 0.039) and age (adjusted OR 1.065; 95% CI 1.011–1.122; p = 0.023) were the independent risk factors for the 30-day mortality. Maximal cefepime dose usage was an independent protecting factor (adjusted OR 0.271; 95% CI 0.08–0.889; p = 0.031).
Table 3.
Multivariate analyses of risk factors for 30-day crude mortality of cefepime-susceptible Pseudomonas aeruginosa bacteremia treated with cefepime
| Variables | p | OR | 95% CI |
|---|---|---|---|
| MIC ≥4 mg/L | 0.039 | 5.111 | 1.090–23.974 |
| Use of maximum cefepime dose | 0.031 | 0.271 | 0.082–0.889 |
| Age, year | 0.023 | 1.065 | 1.011–1.122 |
| Lower respiratory tract infections | 0.056 | 4.008 | 0.967–16.621 |
| APACHE II score | 0.824 | 0.986 | 0.869–1.119 |
| Intensive care unit staya | 0.146 | 2.945 | 0.687–12.619 |
| Severe sepsis or septic shocka | 0.210 | 2.609 | 0.582–11.706 |
| Time interval between admission and occurrence of bacteremia | 0.861 | 1.002 | 0.983–1.021 |
| Treatment duration | 0.063 | 0.923 | 0.848–1.004 |
All variables with p < 0.1 in univariate analysis were included in a multivariate regression model using the backward stepwise method
OR odds ratio, CI confidence interval, MIC minimal inhibitory concentration
aThe factors of intensive care unit stay and severe sepsis or septic shock had strongly correlation (correlation coefficient 0.9), however, single factor with either intensive care unit stay or severe sepsis and septic shock were still remained insignificantly in the multivariate analyses model (factor with intensive care unit only: adjusted OR 3.127, 95% CI 0.746–13.111, p = 0.119; factor with severe sepsis or septic shock only: adjusted OR 2.813, 95% CI 0.647–12.226, p = 0.168)
Relationship between MIC and the maximal dose of cefepime
Seventy-three patients had P. aeruginosa blood isolates with a cefepime MIC of <4 mg/L. Among them, compared with the survived, the deceased had fewer patients receiving a maximal dose of cefepime (50.0% vs. 81.1%, p = 0.008), more solid organ malignancy (65% vs 35.8%, p = 0.025) and a shorter treatment duration (14.0 ± 7.0 vs. 17. 8 ± 6.6 days, p = 0.048). In the multivariate analysis, use of the maximal cefepime dose was the only protecting factor for mortality (adjusted OR 0.244; 95% CI 0.077–0.771; p = 0.016). This protection was not found among patients with a MIC of ≥4 mg/L for P. aeruginosa. When the MIC was ≥4 mg/L, the mortality rate of patients using the maximal cefepime dose was 75% (6 of 8 patients), which is similar to those using a lower dose of cefepime (7 of 9 patients, 77.8%, p = 1.000). For those patients receiving a maximal dose of cefepime (n = 60), patients with a cefepime MIC of ≥4 mg/L for P. aeruginosa had a higher 30-day crude mortality rate than those with a MIC of <4 mg/L (33.3% vs. 4.4%, p = 0.008).
Discussion
According to our review of relevant literature, our study is the first one to provide clinical data demonstrating that treatment of cefepime-susceptible P. aeruginosa bacteremia with a maximal dose of cefepime improved the outcomes of patients with a lower cefepime MIC for P. aeruginosa. Besides, the current CLSI criteria for cefepime susceptibility did not predict clinical outcomes appropriately in this study. The 30-day crude mortality rate was 36.7% and the mortality rate was higher at the group of patients with a MIC of ≥4 mg/L for P. aeruginosa than those with a MIC of <4 mg/L (76.5% vs. 27.4%). Cefepime MIC ≥4 mg/L influenced patient outcomes independently, whereas using a maximal dose of cefepime in patients with various degrees of renal function was the only independent protecting factor for mortality. In addition, using the maximal dose of cefepime significantly decreased the mortality rate at patients with a MIC of <4 mg/L for P. aeruginosa. However, the protective effect vanished at a MIC of ≥4 mg/L. Our results revealed that using the maximal cefepime dose could improve patient outcomes at a lower MIC level. In this study, the antibiotic susceptibility testing was performed using Etest, not broth microdilution (BMD) methods, which is the CLSI criteria based on. However, Etest results generally have correlated well with MICs generated by BMD method [16]. Thus, the current CLSI criteria for cefepime susceptibility breakpoint of ≤8 mg/L may be reevaluated for severe P. aeruginosa infections.
In optimal situations, antibiotic susceptibility breakpoints are determined by integrating various microbiologic, pharmacokinetic/pharmacodynamic (PK/PD), and clinical data. However, after antibiotics were released commercially, new mechanisms of antibiotic resistance developed and probably affected the efficacy of antibiotics. Falagas et al. [17] described that high MICs of GNB, particularly in Salmonella enterica and P. aeruginosa infections, within the currently accepted “susceptible” range were associated with worse outcomes. Several studies have revealed that high piperacillin MICs are associated with increasing mortality rates and microbiological treatment failure. This led to lowering of the CLSI recommendation of the breakpoint of piperacillin against P. aeruginosa from ≤64 to ≤16 mg/L [5, 18, 19]. Worse outcomes related to high MICs were also found on carbapenem use for patients with either bloodstream [6] or lower respiratory tract infections [20]. Patients with levofloxacin-treated gram-negative bloodstream infections, who have elevated levofloxacin MICs but are nevertheless categorized as susceptible, had worse outcomes than those infected with gram-negative organisms, which had lower MICs [4]. Cefepime was inferior to carbapenems in treating patients with bacteremia caused by cefepime-susceptible extended-spectrum ß-lactamase producing strains. The mortality rate increased significantly because cefepime MICs increased (p = 0.004) [21]. Compared with a cefepime MIC of ≤4 mg/L for P. aeruginosa, patients with a MIC of 8 mg/L had a significantly higher mortality rate (66.7% versus 20.8%, p = 0.01) regardless of the cefepime dosage [9].
Studies have demonstrated that free or nonprotein-bound drug concentration over the MIC of the organism (fT > MIC) is the ideal predictor for bactericidal and microbiologic response for β-lactams. A larger fT > MIC (50–70%) is required for the maximal activity against gram-negative organisms [22]. However, several studies have now assessed the PK/PD profile of cefepime and support a change in cefepime dose or breakpoints for susceptibility. Crandon et al. [10] revealed that at the CLSI MIC breakpoint of cefepime susceptibility for P. aeruginosa (≤8 mg/L), a dose of only 2 g every 8 h has a ≥82% likelihood of achieving at least 60% fT > MIC in patients with normal renal function. At this MIC (≤8 mg/L), the dose of 1 or 2 g every 12 h for immunocompetent patients with severe P. aeruginosa infections has a target attainment rate of only 47.7 or 65.8%, respectively. Another PK/PD study of cefepime revealed that when C 67% >MIC was used as the pharmacodynamic target, a dose of 2 g every 12 h had a more than 80% likelihood of achieving the optimal target with an MIC of up to 4 mg/L, whereas a dose of 2 g every 24 h can probably achieve a target attainment rate of up to 80% only when the MICs were ≤2 mg/L [11]. The aforementioned studies explain the failure of achieving pharmacodynamics and the possible microbiological failure in cefepime-treated P. aeruginosa infections with a high cefepime MIC. In addition, they revealed the influence of different cefepime dosages on pharmacodynamics.
Alves et al. [12] demonstrated that treatment with cefepime at a dose of 2 g every 8 h over a 30-min infusion was associated with significantly lower hospital mortality rates in patients with GNB bloodstream infection compared with the usual dosage regimens, such as 1 or 2 g every 12 h and 1 g every 8 h. Moreover, they included 113 patients with Escherichia coli (62, 54.9%) and P. aeruginosa (19, 16.8%) infections. The median MIC of all GNB was 0.0625 mg/L, and most (78.8%) MICs were ≤0.25 mg/L; MIC90 was 2 mg/L. High-dose cefepime therapy was associated with lower mortality rates in patients with GNB infection, including GNB with a low cefepime MIC.
Our study has the limitations for being a retrospective design with the treatment decisions dependent on the physicians’ judgments and the hospital antimicrobial stewardship program [23]. However, some study results suggested E test provides equal or more clear and accurate results in clinical set-up [24, 25]. Furthermore, the mechanisms for increasing cefepime MICs in P. aeruginosa isolates remain unclear. Additional investigations concerning the resistance are thus necessary.
Conclusions
In summary, our data showed that patients treated with cefepime for cefepime-susceptible P. aeruginosa bloodstream infections had a worse outcome while the isolates had a higher MIC value that was still within the susceptible category. Use of a higher cefepime dose in cases with a MIC of <4 mg/L for P. aeruginosa improved patient outcomes. Mortality rate increased in patients with a higher cefepime MIC (≥4 mg/L) for P. aeruginosa even with a maximal cefepime dose. Thus, when using cefepime to treat serious P. aeruginosa infections, the current CLSI cefepime MIC of 8 mg/L as the susceptibility breakpoint may not predict the clinical outcome well.
Authors’ contributions
TYS made substantial contributions to concept and design, acquisition of data, analysis, interpretation of data and drafted the manuscript. JJY participated in the design of the study, drafted of article and critical revision. CCY and CTH participated in the design of the study and drafted of article. JHC made substantial contributions to acquisition of data, analysis, and interpretation of data. MHL is the corresponding author and made substantial contributions to concept and design, acquisition of data, analysis, interpretation of data and responsible to all the training sessions. All authors read and approved the final manuscript.
Acknowledgements
We wish to thank Department of Laboratory Medicine, Chang Gung Memorial Hospital at Linkou for laboratory support and data analysis.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Our data are available on request because we do not have ethics approval to upload the dataset online. However, the anonymized minimal dataset will be available upon request to all interested researchers, by contacting the corresponding author.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the CGMH (103-3354B).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- aPTT
activated partial thromboplastin time
- BMD
broth microdilution
- CABSI
central catheter-associated bloodstream infection
- CGMH
Chang Gung Memorial Hospital
- CIs
confidence intervals
- CLCR
creatinine clearance
- CLSI
Clinical and Laboratory Standards Institute
- CVC
central venous catheter
- GNB
gram-negative bacilli
- FiO2
fraction of inspiration O2
- ICU
intensive care unit
- INR
international normalized ratio
- MALDI-TOF
matrix-assisted laser desorption ionization-time of flight
- MICs
minimal inhibitory concentrations
- ORs
odds ratios
- PaO2
arterial oxygen tension
- pCO2
arterial carbon dioxide tension
- PK/PD
pharmacokinetic/pharmacodynamic
Contributor Information
Ting-Yi Su, Email: midorisu1983@gmail.com.
Jung-Jr Ye, Email: loyalwise@gmail.com.
Chien-Chang Yang, Email: bears112@gmail.com.
Ching-Tai Huang, Email: chingtaihuang@gmail.com.
Ju-Hsin Chia, Email: juhsin@cgmh.org.tw.
Ming-Hsun Lee, Phone: +886 3 328 1200, Email: drharrylee@gmail.com.
References
- 1.Eliopoulos GM, Cosgrove SE, Carmeli Y. The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis. 2003;36:1433–1437. doi: 10.1086/375081. [DOI] [PubMed] [Google Scholar]
- 2.Gaynes RP, Edwards JR. The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin Infect Dis. 2008;47:927–930. doi: 10.1086/591698. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch EB, Cottreau JM, Chang K-T, Caeiro J-P, Johnson ML, Tam VH. A model to predict mortality following Pseudomonas aeruginosa bacteremia. Diagn Microbiol Infect Dis. 2012;72:97–102. doi: 10.1016/j.diagmicrobio.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 4.DeFife R, Scheetz MH, Feinglass JM, Postelnick MJ, Scarsi KK. Effect of differences in MIC values on clinical outcomes in patients with bloodstream infections caused by gram-negative organisms treated with levofloxacin. Antimicrob Agents Chemother. 2009;53:1074–1079. doi: 10.1128/AAC.00580-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tam VH, Gamez EA, Weston JS, Gerard LN, LaRocco MT, Caeiro JP, et al. Outcomes of bacteremia due to Pseudomonas aeruginosa with reduced susceptibility to piperacillin–tazobactam: implications on the appropriateness of the resistance breakpoint. Clin Infect Dis. 2008;46:862–867. doi: 10.1086/528712. [DOI] [PubMed] [Google Scholar]
- 6.Esterly JS, Wagner J, McLaughlin MM, Postelnick MJ, Qi C, Scheetz MH. Evaluation of clinical outcomes in patients with bloodstream infections due to gram-negative bacteria according to carbapenem MIC stratification. Antimicrob Agents Chemother. 2012;56:4885–4890. doi: 10.1128/AAC.06365-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endimiani A, Perez F, Bonomo RA. Cefepime: a reappraisal in an era of increasing antimicrobial resistance. Expert Rev Anti Infect Ther. 2008;6:805–824. doi: 10.1586/14787210.6.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement M100-S26 2016 USA.
- 9.Bhat SV, Peleg AY, Lodise TP, Shutt KA, Capitano B, Potoski BA, et al. Failure of current cefepime breakpoints to predict clinical outcomes of bacteremia caused by gram-negative organisms. Antimicrob Agents Chemother. 2007;51:4390–4395. doi: 10.1128/AAC.01487-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crandon JL, Bulik CC, Kuti JL, Nicolau DP. Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54:1111–1116. doi: 10.1128/AAC.01183-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tam VH, McKinnon PS, Akins RL, Drusano GL, Rybak MJ. Pharmacokinetics and pharmacodynamics of cefepime in patients with various degrees of renal function. Antimicrob Agents Chemother. 2003;47:1853–1861. doi: 10.1128/AAC.47.6.1853-1861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alves MD, Ribeiro VB, Tessari JP, Mattiello F, De Bacco G, Luz DI, et al. Effect of cefepime dose on mortality of patients with gram-negative bacterial bloodstream infections: a prospective cohort study. J Antimicrob Chemother. 2014;69:1681–1687. doi: 10.1093/jac/dku001. [DOI] [PubMed] [Google Scholar]
- 13.Nicasio AM, Eagye KJ, Nicolau DP, Shore E, Palter M, Pepe J, et al. Pharmacodynamic-based clinical pathway for empiric antibiotic choice in patients with ventilator-associated pneumonia. J Crit Care. 2010;25:69–77. doi: 10.1016/j.jcrc.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Roos JF, Bulitta J, Lipman J, Kirkpatrick CMJ. Pharmacokinetic–pharmacodynamic rationale for cefepime dosing regimens in intensive care units. J Antimicrob Chemother. 2006;58:987–993. doi: 10.1093/jac/dkl349. [DOI] [PubMed] [Google Scholar]
- 15.Gaby WL, Hadley C. Practical laboratory test for the identification of Pseudomonas aeruginosa. J Bacteriol. 1957;74:356–358. doi: 10.1128/jb.74.3.356-358.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jorgensen JH, Ferraro MJ. antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis. 2009;49:1749–1755. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- 17.Falagas ME, Tansarli GS, Rafailidis PI, Kapaskelis A, Vardakas KZ. Impact of antibiotic MIC on infection outcome in patients with susceptible gram-negative bacteria: a systematic review and meta-analysis. Antimicrob Agents Chemother. 2012;56:4214–4222. doi: 10.1128/AAC.00663-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamma PD, Turnbull AE, Milstone AM, Hsu AJ, Carroll KC, Cosgrove SE. Does the piperacillin minimum inhibitory concentration for Pseudomonas aeruginosa influence clinical outcomes of children with pseudomonal bacteremia? Clin Infect Dis. 2012;55:799–806. doi: 10.1093/cid/cis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamagishi Y, Terada M, Ohki E, Miura Y, Umemura T, Mikamo H. Investigation of the clinical breakpoints of piperacillin–tazobactam against infections caused by Pseudomonas aeruginosa. J Infect Chemother. 2012;18:127–129. doi: 10.1007/s10156-011-0285-3. [DOI] [PubMed] [Google Scholar]
- 20.Drusano GL, Lode H, Edwards JR. Meropenem: clinical response in relation to in vitro susceptibility. Clin Microbiol Infect. 2000;6:185–194. doi: 10.1046/j.1469-0691.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee NY, Lee CC, Huang WH, Tsui KC, Hsueh PR, Ko WC. Cefepime therapy for monomicrobial bacteremia caused by cefepime-susceptible extended-spectrum beta-lactamase-producing Enterobacteriaceae: MIC matters. Clin Infect Dis. 2013;56:488–495. doi: 10.1093/cid/cis916. [DOI] [PubMed] [Google Scholar]
- 22.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 23.Wang HY, Chiu CH, Huang CT, Cheng CW, Lin YJ, Hsu YJ, et al. Blood culture-guided de-escalation of empirical antimicrobial regimen for critical patients in an online antimicrobial stewardship programme. Int J Antimicrob Agents. 2014;44:520–527. doi: 10.1016/j.ijantimicag.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Rajkumari N, Mathur P, Bhardwaj N, Gupta G, Dahiya R, Behera B, et al. Resistance pattern of mupirocin in methicillin-resistant Staphylococcus aureus in trauma patients and comparison between disc diffusion and E-test for better detection of resistance in low resource countries. J Lab Physicians. 2014;6:91–95. doi: 10.4103/0974-2727.141505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogbolu DO, Terry-Alli OA, Daini OA, Olabiyi FA, Igharo EA. Comparison of E-test with other conventional susceptibility testing methods for ciprofloxacin and gentamicin against gram negative enteric bacilli. Afr J Med Med Sci. 2012;41:135–140. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our data are available on request because we do not have ethics approval to upload the dataset online. However, the anonymized minimal dataset will be available upon request to all interested researchers, by contacting the corresponding author.


