Abstract
Background
An upward trending incidence in cervical adenocarcinoma (ADC) has been reported in many countries. Because non-squamous histology has been associated with increased risk of ovarian metastases (OM), bilateral oophorectomy is commonly performed for ADC without due consideration for ovarian preservation, degrading the quality of life for young premenopausal patients.
Methods
Subjects were patients with International Federation of Gynecology and Obstetrics (FIGO) stage I–IIB cervical ADC who underwent radical hysterectomy, including pelvic lymphadenectomy and bilateral salpingo-oophorectomy at our institution between Oct. 2006 and Sept. 2014. Clinicopathologic variables were studied by univariate and multivariate analyses.
Results
Of the 312 patients enrolled in the study, 14 patients (4.5%) developed OM. Multivariate analysis revealed that uterine corpus involvement (odds ratio [OR] 5.178, p = 0.019), parametrial involvement (OR 14.125, p = 0.005) and vaginal infiltration (OR 4.167, p = 0.047) were independently associated with metastasis. OM had no effect on either relapse-free survival (95% confidence interval [CI]: 0.077–4.095, p = 0.57) or overall survival (95% CI: 0.893–9.820, p = 0.076).
Conclusion
Cervical ADC is associated with an increased risk of OM. Ovarian preservation surgery in cervical ADC may be safe for young patients at an early FIGO stage without deep stromal, endometrial or perineural invasion, and particularly without uterine corpus invasion, parametrial involvement and infiltration into the vagina.
Keywords: Adenocarcinoma, Ovarian metastasis, Ovarian preservation
Background
Invasive cervical cancer (ICC) ranks as the third most common malignancy and is the fourth leading cause of female cancer deaths worldwide [1]. Patients with FIGO early stage disease generally undergo radical hysterectomy and pelvic lymphadenectomy. However, bilateral oophorectomy is not part of standard surgical management of ICC.
Currently, an upward trend in the incidence of adenocarcinoma (ADC) has been reported in many countries, particularly among women under the age of 40 [2–5]. Since Shimada et al. and Ronnett et al. have reported that approximately 5% of women with cervical ADC are at an increased risk of ovarian metastases (OM), which occurs in about half of ADC cases post-hysterectomy [6, 7], oophorectomy is commonly performed in ADC to preclude OM.
Ovarian preservation, which is beneficial to the physiologic and psychosexual well-being of premenopausal women affected by cervical cancer, remains a challenge in clinical practice. Some believe that non-squamous histology should be a deterrent to ovarian transposition [8]. Others advocate lymphovascular space invasion (LVSI), deep stromal invasion (DSI) and uterine corpus involvement as contraindications [8, 9]. The aim of the present retrospective study was to identify the clinicopathological factors associated with OM in ADC.
Methods
Patients
Study participants were patients diagnosed with FIGO stage I–IIB invasive ADC of the uterine cervix and treated by radical hysterectomy, bilateral salpingo-oophorectomy and pelvic lymphadenectomy at the Zhejiang Cancer Hospital, Zhejiang Province, China between Oct. 2006 and Sep. 2014. Clinical data were extracted from the institution’s electronic databases after informed consent was obtained from all patients. The Medical Ethics Committee of Zhejiang Cancer Hospital approved the study.
The following clinical and histological parameters were evaluated in relation to OM: age at surgery, FIGO stage, bulky tumor size (>4 cm), differentiation, morphology, DSI (≥2/3), LVSI, parametrial involvement, uterine corpus involvement, endometrial invasion, fallopian tube invasion, vaginal infiltration, perineural invasion (PNI) and para-aortic/pelvic lymph node status.
Statistical analysis
Statistical analyses were performed with the SPSS 16.0 software package (IBM, Armonk, NY, USA). P-values <0.05 were considered statistically significant. Summary statistics are presented as frequencies and percentages. The Pearson χ2 test was used to assess the association between clinicopathologic parameters and the presence of OM. Multivariate analysis was used to detect independent risk factors for OM and results are presented as odds ratios (OR).
Results
Patient characteristics
A total of 312 ADC patients were enrolled into the study, including 9 patients (2.9%) with FIGO stage IA, 217 patients (69.6%) with stage IB, 74 patients (23.7%) with stage IIA and 12 patients (3.8%) with stage IIB. The median age of patients was 46 years (range: 19–73 years).
OM were diagnosed in 14 patients (4.5%). A summary of patients with OM is shown in Table 1. OM occurred in five of the 217 patients (2.3%) in stage IB, eight of the 74 patients (10.8%) in stage IIA and one of the 12 patients (8.3%) in stage IIB. A significantly higher incidence of OM was observed in stage II ADC (p = 0.002, OR 9.899). The mean age of these 14 patients was 46 (range 32–68) years. Potential risk factors for OM are listed in Table 2. Patients with OM were frequently observed by univariate analysis with FIGO stage I or II (p = 0.002), DSI (p = 0.002), uterine corpus involvement (p < 0.001), endometrial invasion (p < 0.001), parametrial involvement (p < 0.001), PNI (p = 0.011), fallopian tube invasion (p < 0.001) or vaginal infiltration (p < 0.001). However, outcomes for patients with OM did not correlate with age at surgery, bulky tumor size, differentiation, morphology, LVSI or lymph node metastasis.
Table 1.
Summary of patients with ovarian metastasis
| Case | Age | FIGO stage | Grade | DSI | Morphology | UCI | Endometrial invasion | Fallopian tube invasion | Perineural invasion | LVSI | Infiltration to vagina | Lymph node metastasis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Para-aortic | Pelvic | ||||||||||||
| 1 | 42 | IIA | G3 | + | Endophytic | + | − | − | + | + | − | + | + |
| 2 | 43 | IIA | G2 | + | Exophytic | − | − | − | + | − | + | − | − |
| 3 | 62 | IIA | G2 | + | Endophytic | + | − | − | + | − | + | − | + |
| 4 | 49 | IB | G3 | + | Exophytic | + | − | + | − | + | + | − | − |
| 5 | 41 | IIA | G2 | + | Exophytic | − | − | − | − | + | + | − | + |
| 6 | 51 | IIB | G2 | + | Exophytic | − | − | − | − | − | − | − | − |
| 7 | 36 | IIA | G3 | − | Exophytic | + | + | − | − | − | − | − | − |
| 8 | 55 | IIA | G2 | Exophytic | − | − | − | − | − | + | − | + | |
| 9 | 48 | IIA | G2 | Endophytic | − | − | − | + | + | + | − | − | |
| 10 | 36 | IB | G3 | − | Exophytic | + | − | − | − | − | − | − | − |
| 11 | 43 | IB | G2 | − | Exophytic | + | − | − | − | − | + | − | − |
| 12 | 68 | IB | G3 | − | Endophytic | + | + | + | − | + | + | − | − |
| 13 | 43 | IIA | G2 | + | Endophytic | + | + | + | + | + | − | − | − |
| 14 | 32 | IB | G3 | + | Endophytic | + | − | + | − | + | − | − | − |
DSI deep stromal invasion
UCI uterine corpus involvement
LVSI lymphovascular space invasion
Table 2.
Univariate and multivariate analyses of risk factors for ovarian metastasis in patients with cervical adenocarcinoma
| Parameter | N (%) | Univariate analyses | Multivariate analyses | |||
|---|---|---|---|---|---|---|
| Total no. of patients enrolled | 312 | Ovarian metastasis | Odds ratio | p-value | ||
| Yes | No | p-value | ||||
| Age at surgery | ||||||
| < 60 | 282 (90.4) | 12 | 270 | 0.544 | ||
| ≥ 60 | 30 (9.6) | 2 | 28 | |||
| FIGO stage | ||||||
| I | 226 (72.4) | 5 | 221 | 0.002* | 2.445 | 0.243 |
| II | 86 (27.6) | 9 | 77 | |||
| Tumor diameter (cm) | ||||||
| < 4 | 221 (70.8) | 7 | 214 | 0.079 | ||
| ≥ 4 | 91 (29.2) | 7 | 84 | |||
| Differentiation | ||||||
| G1 + G2 | 191 (61.2) | 8 | 182 | 0.756 | ||
| G3 | 121 (38.8) | 6 | 115 | |||
| Tumour morphology | ||||||
| Exophytic | 109 (34.9) | 8 | 101 | 0.075 | ||
| Endophytic | 203 (65.1) | 6 | 197 | |||
| Deep stromal invasion | ||||||
| Superficial (<2/3) | 210 (67.3) | 4 | 206 | 0.002* | 1.307 | 0.743 |
| Deep (≥2/3) | 102 (32.7) | 10 | 92 | |||
| Parametrial invasion | ||||||
| Negative | 296 (94.9) | 8 | 288 | 5.80E-11* | 14.125 | 0.005* |
| Positive | 16 (5.1) | 6 | 10 | |||
| Lymph-vascular space invasion | ||||||
| Negative | 197 (63.1) | 7 | 190 | 0.297 | ||
| Positive | 115 (36.9) | 7 | 108 | |||
| Pelvic node status | ||||||
| Negative | 227 (72.8) | 10 | 217 | 0.909 | ||
| Positive | 85 (27.2) | 4 | 81 | |||
| Fallopian tube invasion | ||||||
| Negative | 302 (96.8) | 10 | 292 | 3.51E-8* | 1.837 | 0.338 |
| Positive | 10 (3.2) | 4 | 6 | |||
| Para-aortic node status | ||||||
| Negative | 305 (97.8) | 13 | 292 | 0.205 | ||
| Positive | 7 (2.2) | 1 | 6 | |||
| Infiltration to vagina | ||||||
| Yes | 58 (18.6) | 8 | 50 | 1.48E-4* | 4.167 | 0.047* |
| No | 254 (81.4) | 6 | 248 | |||
| Endometrial invasion | ||||||
| Negative | 300 (96.2) | 11 | 289 | 4.65E-4* | 3.156 | 0.213 |
| Positive | 12 (3.8) | 3 | 9 | |||
| Uterine corpus invasion | ||||||
| Negative | 252 (80.8) | 5 | 247 | 1.20E-5* | 5.178 | 0.019* |
| Positive | 60 (19.2) | 9 | 51 | |||
| Perineural invasion | ||||||
| Negative | 271 (86.9) | 9 | 262 | 0.011* | 0.273 | 0.174 |
| Positive | 41 (13.1) | 5 | 36 | |||
FIGO The International Federation of Gynecology and Obstetrics
* p < 0.05
Furthermore, multivariate analysis identified uterine corpus involvement (OR 5.178, p = 0.019), parametrial involvement (OR 14.125, p = 0.005) and vaginal infiltration (OR 4.167, p = 0.047) to be independently associated with OM. Meanwhile, OM had no effect on either relapse-free survival (95% confidence interval [CI]: 0.077–4.095, p = 0.57) or overall survival (95% CI: 0.893–9.820, p = 0.076) (Fig. 1).
Fig. 1.
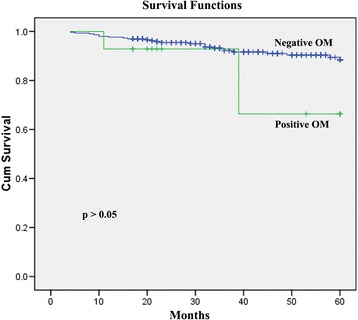
Overall survival of 14 patients positive for ovarian metastases (OM) and 298 patients negative for OM. OM had no effect on overall survival (95% CI: 0.893–9.820, p = 0.076)
Discussion
ICC is still a leading cause of cancer-related deaths in women worldwide. For decades, an upward trending incidence of ADC has been reported in many countries, accounting for approximately one-quarter of all ICC cases [10]. ADC is distinguished from squamous cell carcinoma (SCC) by virtue of different human papillomavirus types, patterns of spread, prognosis and recurrence [11, 12]. Furthermore, published data has revealed that the incidence of OM in the presence of non-squamous histology is increased compared to SCC [8]; hence, bilateral oophorectomy is frequently recommended in cases of ADC.
Approximately 50% of ICC patients are premenopausal and under 45 years old [13]. Ovarian preservation has proven invaluable for improving the quality of life of these young women. According to the literature, ovarian metastatic incidence occurs more frequently among patients with ADC than in those with SCC, ranging between 1.7% and 18.9% and from 0.4% to 1.3%, respectively [6, 8, 14, 15]. Moreover, Ronnett et al. reported that in about half of ADC cases, metastases occurred post-hysterectomy [7]. Therefore, the benefits of preserving hormonal function may be offset by a potentially higher risk of recurrence in ADC. Conversely, Gubbala et al. concluded that ovarian transposition resulted in significant preservation of ovarian function with negligible risk of metastases to the transposed ovaries despite the common incidence of ovarian cysts [16]. Lyu et al. found that ovarian preservation provided oncological safety for young women with stage I cervical adenocarcinoma [17]. In the present study, the incidence of OM in ADC was 4.5% (14/312), and a significantly higher incidence of OM was observed in stage II compared to stage I cancers.
The selection of premenopausal patients who would benefit from ovarian preservation to improve the quality of life is challenging. A thorough understanding of the risk factors involved would be of great value for following a patient with ovarian preservation postoperatively [17]. The majority of cases we have examined have been diagnosed through primary radical surgery with bilateral oophorectomy. However, in the past two decades, increasing attention has been paid to OM and contributing factors. Age, FIGO stage, histology, lymph node metastases, DSI, LVSI, bulky tumor size, parametrial invasion and corpus uteri invasion have been determined to be independent risk factors for OM in ADC [8, 18–21]. In our study, we identified FIGO stage, DSI, uterine corpus invasion, endometrial invasion, parametrial involvement, PNI, fallopian tube invasion and vaginal infiltration as significantly related to OM in ADC. In our multivariate analysis, uterine corpus invasion, parametrial involvement and vaginal infiltration were independent risk or protective factors for OM in patients with ADC. The occurrence of OM had no significant relationship to either relapse-free survival or overall survival.
The routes by which ICC spreads to the ovary remain unclear. Wu et al. proposed that lymphatic spread and transtubal implantation may be possible pathways of metastases from the cervix to the ovaries [18]. Tabata et al. reported that OM may take place via hematogenous spread of cervical carcinoma [22]. Further research efforts will be required to establish the pathways involved.
Conclusions
In conclusion, we found that cervical ADC is associated with a relatively higher risk of OM. Based on our data, we suggest that ovarian preservation may be safely performed in young patients with early FIGO stage cervical ADC without deep stromal invasion, endometrial invasion or perineural invasion, and particularly in the absence of uterine corpus invasion, parametrial involvement and infiltration to the vagina.
Acknowledgements
Not applicable.
Funding
The Zhejiang Province Nature Foundation (grant No. LQ16H160016).
Availability of data and materials
Please contact the corresponding author for data requests.
Abbreviations
- ADC
Adenocarcinoma
- DSI
Deep stromal invasion
- ICC
Invasive cervical cancer
- LVSI
Lymphovascular space invasion
- OM
Ovarian metastases
- PNI
Perineural invasion
- SCC
Squamous cell carcinoma
Authors’ contributions
PZ and HL participated in the study design, and critically appraised and revised the manuscript. JZ conducted the literature search, extracted the data, drafted the manuscript and participated in data analysis. YC performed data analysis and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Local Institutional Ethics Committee approved the study, and informed consent for publication was obtained from all patients.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiansong Zhou, Email: zjzhoujiansong@163.com.
Yuanyuan Chen, Email: chenyuan061001@163.com.
Ping Zhang, Email: zhangping@zjcc.org.cn.
Hanmei Lou, Email: louhm@zjcc.org.cn.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the United States--a 24-year population-based study. Gynecol Oncol. 2000;78:97–105. doi: 10.1006/gyno.2000.5826. [DOI] [PubMed] [Google Scholar]
- 3.Vizcaino AP, Moreno V, Bosch FX, Muñoz N, Barros-Dios XM, Borras J, et al. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int J Cancer. 2000;86:429–435. doi: 10.1002/(SICI)1097-0215(20000501)86:3<429::AID-IJC20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.Visioli CB, Zappa M, Ciatto S, Iossa A, Crocetti E. Increasing trends of cervical adenocarcinoma incidence in Central Italy despite Extensive Screening Programme, 1985–2000. Cancer Detect Prev. 2004;28:461–464. doi: 10.1016/j.cdp.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Bray F, Carstensen B, Møller H, Zappa M, Zakelj MP, Lawrence G, et al. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomark Prev. 2005;4:2191–2199. doi: 10.1158/1055-9965.EPI-05-0231. [DOI] [PubMed] [Google Scholar]
- 6.Shimada M, Kigawa J, Nishimura R, Yamaguchi S, Kuzuya K, Nakanishi T, et al. Ovarian metastasis in carcinoma of the uterine cervix. Gynecol Oncol. 2006;101:234–237. doi: 10.1016/j.ygyno.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Ronnett BM, Yemelyanova AV, Vang R, Gilks CB, Miller D, Gravitt PE, et al. Endocervical adenocarcinomas with ovarian metastases: analysis of 29 cases with emphasis on minimally invasive cervical tumours and the ability of the metastases to simulate primary ovarian neoplasms. Am J Surg Pathol. 2008;32:1835–1853. doi: 10.1097/PAS.0b013e3181758831. [DOI] [PubMed] [Google Scholar]
- 8.Landoni F, Zanagnolo V, Lovato-Diaz L, Maneo A, Rossi R, Gadducci A, et al. Ovarian metastases in early-stage cervical cancer (IA2-IIA): a multicenter retrospective study of 1965 patients (a Cooperative Task Force study) Int J Gynecol Cancer. 2007;17:623–628. doi: 10.1111/j.1525-1438.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 9.Morice P, Haie-Meder C, Pautier P, Lhomme C, Castaigne D. Ovarian metastasis on transposed ovary in patients treated for squamous cell carcinoma of the uterine cervix: report of two cases and surgical implications. Gynecol Oncol. 2001;83:605–607. doi: 10.1006/gyno.2001.6447. [DOI] [PubMed] [Google Scholar]
- 10.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(Suppl. 3):S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara H, Yokota H, Monk B, Treilleux I, Devouassoux-Shisheboran M, Davis A, et al. Gynecologic Cancer InterGroup (GCIG) Consensus Review for Cervical Adenocarcinoma. Int J Gynecol Cancer. 2014;24(9 Suppl 3):S96–101. doi: 10.1097/IGC.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 12.Irie T, Kigawa J, Minagawa Y, Itamochi H, Sato S, Akeshima R, et al. Prognosis and clinicopathological characteristics of IB-IIB adenocarcinoma of the uterine cervix in patients who have had radical hysterectomy. Eur J Surg Oncol. 2000;26:464–467. doi: 10.1053/ejso.1999.0923. [DOI] [PubMed] [Google Scholar]
- 13.Winarto H, Febia E, Purwoto G, Nuranna L. The Need for Laparoscopic Ovarian Transposition in Young Patients with Cervical Cancer Undergoing Radiotherapy. Int J Reprod Med. 2013;2013:173568. doi: 10.1155/2013/173568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MJ, Chung HH, Kim JW, Park NH, Song YS, Kang SB. Uterine corpus involvement as well as histologic type is an independent predictor of ovarian metastasis in uterine cervical cancer. J Gynecol Oncol. 2008;19:181–184. doi: 10.3802/jgo.2008.19.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natsume N, Aoki Y, Kase H, Kashima K, Sugaya S, Tanaka K. Ovarian Metastasis in Stage IB and II Cervical Adenocarcinoma. Gynecol Oncol. 1999;74:255–258. doi: 10.1006/gyno.1999.5442. [DOI] [PubMed] [Google Scholar]
- 16.Gubbala K, Laios A, Gallos I, Pathiraja P, Haldar K, Ind T. Outcomes of ovarian transposition in gynaecological cancers; a systematic review and meta-analysis. J Ovarian Res. 2014;7:69. doi: 10.1186/1757-2215-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyu J, Sun T, Tan X. Ovarian Preservation in Young Patients With Stage I Cervical Adenocarcinoma. Int J Gynecol Cancer. 2014;24:1513–1520. doi: 10.1097/IGC.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 18.Wu HS, Yen MS, Lai CR, Ng HT. Ovarian metastasis from cervical carcinoma. Int J Gynaecol Obstet. 1997;57:173–178. doi: 10.1016/S0020-7292(97)02848-8. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto R, Okamoto K, Yukiharu T, Kaneuchi M, Negishi H, Sakuragi N, et al. A study of risk factors for ovarian metastases in stage Ib–IIIb cervical carcinoma and analysis of ovarian function after a transposition. Gynecol Oncol. 2001;82:312–316. doi: 10.1006/gyno.2001.6277. [DOI] [PubMed] [Google Scholar]
- 20.Sakuragi N, Takeda N, Hareyama H, Fujimoto T, Todo Y, Okamoto K, et al. A multivariate analysis of blood vessel and lymph vessel invasion as predictors of ovarian and lymph node metastases in patients with cervical carcinoma. Cancer. 2000;88:2578–2583. doi: 10.1002/1097-0142(20000601)88:11<2578::AID-CNCR21>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 21.Hu T, Wu L, Xing H, Yang R, Li X, Huang K, et al. Development of Criteria for Ovarian Preservation in Cervical Cancer Patients Treated with Radical Surgery With or Without Neoadjuvant Chemotherapy: A Multicenter Retrospective Study and Meta-analysis. Ann Surg Oncol. 2013;20:881–890. doi: 10.1245/s10434-012-2632-8. [DOI] [PubMed] [Google Scholar]
- 22.Tabata M, Ichinoe K, Sakuragi N, Shiina Y, Yamaguchi T, Mabuchi Y. Incidence of ovarian metastasis in patients with cancer of the uterine cervix. Gynecol Oncol. 1987;28:255–261. doi: 10.1016/0090-8258(87)90170-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the corresponding author for data requests.


