Abstract
Background
Alphaviruses are arthropod borne RNA viruses of medical importance. Geographical expansion of mosquitoes of the Aedes genus in the past decades has been associated with major Alphavirus-associated outbreaks. Climate changes and intensification of air travels have favored vector expansion and virus dissemination in new territories leading to virus emergence not only in tropical areas but also in temperate regions. The detection of emergence is based upon surveillance networks with epidemiological and laboratory investigation.
Method
A specific, sensitive and rapid screening test for genus-specific Alphavirus is critically required. To address this issue, we developed a new molecular assay targeting nsP4 gene and using a TaqMan® real time RT-PCR method for the specific detection of all major Alphavirus genus members.
Results
This assay was tested for specificity using several Alphavirus species. We also tested successfully clinical sensitivity using patient’s samples collected during the Chikungunya outbreak of 2005–2006 in the Indian Ocean.
Conclusions
This new pan-Alphavirus molecular diagnostic tool offers great potential for exclusion diagnosis and emergence detection given its broad specificity restricted to Alphavirus genus.
Keywords: Alphavirus, Molecular diagnosis, Virus emergence
Background
The genus Alphavirus belongs to the family Togaviridae and comprises 29 virus species. Alphaviruses have a positive single stranded RNA. All of them are arthropod-borne except salmon pancreatic disease virus (SPDV) and southern elephant seal virus (SESV). Transmission involves intermediate host from avian or mammal origins for virus replication and mosquitoes as vectors for spillover infection in humans. Alphaviruses form icosahedral structures of 70 nm in diameter that contain a 5′ capped- and 3′ polyadenylated genome. Virus genome is ranging from 10 to 12 kb in length and comprises 2 open reading frames (ORF) separated by an intergenic sequence. 5’ORF encodes a polyprotein comprising 4 non-structural proteins (nsP) 1 to 4 and is involved in a replicative complex. The subgenomic RNA encodes for instance 3 structural proteins, E1, E2 and Capsid that interact to form virion envelope [1]. Alphaviruses form a small subset among all reported arboviruses. In the international arbovirus catalogue maintained by the Centers for Disease Control and Prevention (CDC – Atlanta), 537 viruses are actually reported; 9 of them are known to be Alphaviruses of medical relevance, Chikungunya virus (CHIKV), Ross river virus (RRV), O’nyong nyong virus (ONNV), Sindbis virus (SINV), Mayaro virus (MAYV), Barmah forest virus (BFV), Eastern equine encephalitis virus (EEEV), Western equine encephalitis virus (WEEV) and Venezuelan equine encephalitis virus (VEEV) [2].
Large outbreaks of CHIKV disease were documented in Gabon (1999), Indian Ocean (2006), India and Southern Asia (2006, 2007) [3–7]. ONNV was restricted to Africa until an imported case was described in a German traveler who stayed in Kenya [8]. MAYV was isolated from a human case in Trinidad (1954) then in several parts of South America and recently in French Guiana [9]. BFV is endemic in Western Australia as well as RRV. RRV was responsible for a large outbreak in Australia and in Western Pacific Ocean Islands; SINV related disease was widely distributed and associated to outbreaks in Africa, North Europe, Asia and Australia [10]. WEEV, EEEV and VEEV are zoonotic encephalitic alphaviruses distributed in North and South America and responsible for neurological symptoms in equines and humans [11].
Alphavirus epidemiology is intrinsically related to homophilic arthropod vectors distribution. Among these vectors, mosquitoes of Aedes and Culex genus are effective contributors of virus to human transmission. In the case of Aedes, 2 species Aedes aegypti and Aedes albopictus are involved. Aedes mosquitoes contribute to Alphavirus circulation inside the tropical and subtropical belt but Aedes albopictus is prone to geographical expansion to North countries from the late 1970s and spreading from East European countries to Mediterranean countries [12]. This is of great public health concern because of the risk of emergence that rises in novel temperate territories such as metropolitan France [13].
Human Alphavirus infections may be related to arthritogenic (Old World) alphaviruses or encephalitic (New World) alphaviruses. Classically, Old World alphaviruses like CHIKV cause an infection including a 4–7 days incubation period followed with the apparition of clinical signs including strong fever, headache, myalgia, arthralgia, maculopapular rash, edemas, abdominal pains and encephalitis in 5% of the [14]. New World alphaviruses like VEEV are responsible for central nervous system (CNS) disorders including severe encephalitis [15, 16]. Moreover, Alphavirus infections remain sometimes asymptomatic or can lead to chronic arthritic features over time [17].
Since 2006, imported CHIKV human cases were annually reported in France during the summer with limited autochthonous virus circulation in some cases. This was related to recreational activities and air travels over the world in CHIKV endemic regions [18–20]. This raised a concern about the ability to efficiently detect and report new emergence when it occurred in non-endemic geographical areas.
Alphavirus emergence is subjected to many determinants. CHIKV outbreak in Indian Ocean in 2005–2006 provided an example of adaptation of virus to vector. The adaptive CHIKV E1-A226V mutation was responsible for higher infectivity and virus transmission by Aedes albopictus [21–24]. The same observation was made with viruses from VEEV antigenic complex. Comparative viral replication studies showed Fort Morgan virus has lost its potential to infect mosquito cells because of two mutations occurring in nsP3 and nsP4, the latter impacting virus replication [25]. Virus adaptation resulted in the acquisition of a new host species through nucleotide changes and reflected the possibility for alphaviruses to jump species barriers and to promote new transmission cycles in case of emergence or resurgence.
Given that human Alphavirus infections may result in a non-specific syndrome it is difficult to accurately diagnose these infections. Other arboviruses including Flavivirus, Bunyavirus, Rhabdovirus and Reovirus can lead to the apparition of the same symptoms in humans and this is also reported for infection by pathogenic leptospira and Plasmodium. Detection of virus emergence is sometimes complicated by the cocirculation of 2 distinct arboviruses circulating concomitantly in the same geographical area or by successive outbreaks involving different viruses of Alphavirus or Flavivirus genus. In French Polynesia, successive outbreaks of DENV, CHIKV, and ZIKA virus were reported in the past five years. Moreover, RRV circulation was serologically reported supporting autochthonous RRV circulation in a silent manner [26].
Differential diagnosis is often necessary to identify the infectious causative agent. Moreover, in case of a new emergence, an extensive panel of pathogen-specific detection tests can be difficult to set up. To overcome this constraint a genus-specific test is required to identify clinical samples positive for alphaviruses. In the acute phase of infection with high levels of viremia, RT-PCR is best suited for virus detection. In the last two decades, several nested or hemi-nested RT-PCR assays were designed to detect members of Alphavirus genus [27–30]. For this purpose, nsP1 and nsP4 gene were targeted to design an amplicon. In 2010, Grywna has tested a new primer design based upon nsP4 sequence displaying broader specificity for Alphavirus and good clinical sensitivity [31]. Unfortunately, the nested PCR approach used was time-consuming, subjected to risk of cross-contamination and requiring a gel electrophoresis step for amplicon visualization. We herein present a new real time RT-PCR assay based upon hydrolysis of a TaqMan® fluorescently labeled probe that allows rapid and specific detection of genomic Alphavirus nsP4 sequences with high sensitivity. Moreover, the format used for the assay design is compatible with routinely implemented molecular tests in clinical laboratories and will simplify diagnosis exclusion.
Methods
Ethics approval
Written informed consent was obtained from healthy subjects and patients undergoing arbovirus infection screening and attending Reunion Island University Hospital. The study was approved by the Human Ethics Committee of University of Bordeaux (‘Comité Consultatif de Protection de Personnes se prêtant à des Recherche Biomédicales’, Bordeaux France, ref. 2008-A00151–54).
Reference samples
Virus were obtained from several laboratories. SFV was from Pr Andres Merits in Estonia. ONNV, DENV species 1 to 4 and ZIKA virus were obtained from National Reference Laboratory for Arbovirus of Marseilles (France). RRV and SINV were purchased from the National Collection of Pathogenic Viruses (NCPV, UK). CHIKV strain clone #4.2 was isolated from a clinical sample in our laboratory during the CHIKV outbreak that occurred in Reunion Island in 2005 [32]. Other viruses used for validation studies were isolated from laboratory clinical samples. Rubella virus was detected using Priorix® (GlaxoSmithKline) a trivalent vaccine made of attenuated viral strains for measles virus, Myxovirus parotidis and Rubella.
Additionally we used RNA standards for quantification of WEEV, EEEV, VEEV, BFV and CHIKV. RNA standards were purchased from Eurogentec and designed with RNA nucleotides using specific viral sequences selected at the location corresponding to the expected amplicon generated by our RT-PCR assay. Accession number of selected sequences was AM258995 (CHIKV), NC001786 (BFV), GQ287640 (WEEV), KC344475 (VEEV), KP282670 (EEEV).
Nucleic acid isolation
Total nucleic acids were extracted from 200 μL aliquots of plasma human samples or supernatant from cell cultures using Nuclisens® reagents and EasyMAG® nucleic acid isolation platform according to Biomerieux’s recommendations. Final elution was done in 50 μL.
Design of primers and TaqMan® probes
Alphavirus genomic sequences were available from Virus Pathogen Database and Analysis Resource [33]. Sequence annotation was done using BioEdit Sequence Alignment Editor v7.1.3. Sequence alignment was performed with a Clustal tool provided as a plugin in BioEdit software [34]. Melting temperature and complementarity of primers were checked with Oligocalc calculator [35]. LNA™ probes were designed with LNA™ oligo tools from Exiqon.
To efficiently prime RT-PCR for pan-Alphavirus detection, we applied a degenerate primer design according to Li [36] and consisting of the following steps: 456 genomic downloaded sequences were used to generate consensus sequences specific to each of the 19 Alphavirus species mentioned in Table 1.
Table 1.
Species-specific consensus sequence determined from 456 viral genomes
| Virus | Number of viral genomes used for consensus sequence | Virus | Number of viral genomes used for consensus sequence |
|---|---|---|---|
| CHIKV | 188 | BFV | 4 |
| ONNV | 5 | BEBV | 2 |
| WEEV | 12 | FMV | 3 |
| VEEV | 140 | HJV | 4 |
| EEEV | 14 | WHAV | 2 |
| SINV | 23 | MIDV | 1 |
| RRV | 14 | NDUV | 2 |
| SFV | 14 | SESV | 2 |
| MAYV | 3 | SPDV | 15 |
| GETV | 8 |
Clustal alignment of available genomic sequences was performed for each Alphavirus
A consensus sequence was determined for each viral species using Bioedit software
Consensus sequences were then aligned using CHIKV LR2006 sequence as a ruler guide
Alignment of consensus sequences was a prerequisite to select a nucleotide sequence with high similarity between viruses tested in our study. As mentioned by Grywna, nsP4 gene appeared to be highly conserved and thus acted as a good candidate for amplicon design [31]. Using Grywna’s primers as a starting point, we designed our own primers avoiding as much as possible degeneracy (See Table 2 and Fig. 1).
Table 2.
List of primers and probes
| Name | Fluorochrome | Sequence (5’➔ 3′) | Quencher | Nt | Tm (°C) | Degeneracy |
|---|---|---|---|---|---|---|
| F1 | GGTGCGATGATGAAGTCTGG | 20 | 60.5 | 0 | ||
| R1 | CTATGATATTGACTTCCATGTTCA | 24 | 58.3 | 0 | ||
| F2A | ATGATGAARTCIGGIATGTTYYT | 23 | 53.9–59.2 | 8 | ||
| F2B | ATGATGAARTCNGGNATGTT | 20 | 50.2–56.4 | 32 | ||
| R2A | ATYTTIACTTCCATGTTCATCCA | 23 | 55.5–57.6 | 2 | ||
| R3A | ATYTTIACTTCCATRTTCARCCA | 23 | 53.9–59.2 | 8 | ||
| R4A | ATYTTIACTTCCATGTTGACCCA | 23 | 57.6–59.2 | 2 | ||
| R2B | ATYTTNACTTCCATGTTCATCCA | 23 | 55.5–59.2 | 8 | ||
| R3B | ATYTTNACTTCCATRTTCARCCA | 23 | 53.9–60.9 | 32 | ||
| R4B | ATYTTNACTTCCATGTTGACCCA | 23 | 57.6–60.9 | 8 | ||
| P1 | ATTO425 | AT + GTT + GTC + GT + CN + CC | BHQ1/LNA™ | 14 | 40.8–43.7/66–69 | 4 |
| P2 | ATTO425 | AT + GTT + GTC + GT + CIC + CIAT | BHQ1/LNA™ | 17 | 47.5/67 | 0 |
Variable melting temperature was indicated for degenerate primers. Degenerate nucleotides were shown in bold. Y accounted for C/T, R for A/G, N for A or T or C or G. I was used as an alternative for N. ATTO425 labeled probes were quenched using Black Hole Quencher 1 (BHQ1). Locked Nucleic Acid nucleotides (LNA™) were prefixed with a “+” sign and the resulted increase in Tm was indicated following use of Exiqon™ tool for calculation
Fig. 1.
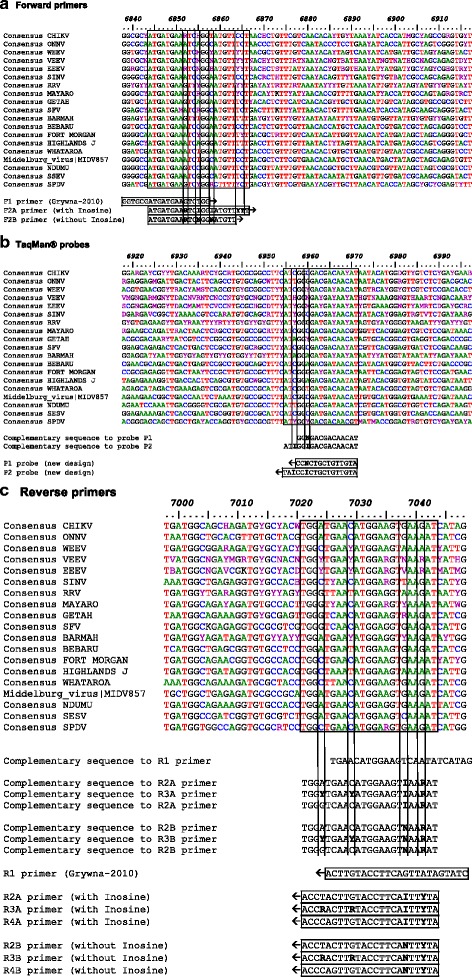
Design of primers and probes in Alphavirus nsP4 gene. Genome positions refer to CHIKV LR2006_OPY1|DQ443544 sequence. Arrows indicate the 5’➔3′ orientation. Location of forward primers (panel a), probes (panel b) and reverse primers (panel c) used for pan-alphavirus RT-PCR is indicated in boxes. Vertical bars delineate in primers wobble nucleotides that are considered for degeneracy. Use of inosine containing oligonucleotides was an alternative to decrease primers degeneracy and thus improve sensitivity of detection assay
Degeneracy of a primer is the total number of sequence combinations it contains. For this purpose, inosine was used as an alternative for N nucleotide into forward and reverse primers with ambiguous nucleotides. To take into account nucleotide variation inside the reverse primer, we designed a mix of 3 similar primers harboring different degenerated nucleotide at ambiguous positions. This allowed us to select for primers with 3′ extremity that will match with viral targeted sequences. Primers’ combinations with or without inosine were ultimately compared (See Table 3). These primers delineated a 200 nt long amplicon with a consensus sequence shared by all members of the Alphavirus genus. We designed within the amplified region two probes with a length ranging from 14 to 17 nucleotides and harboring LNA™ modifications to fit thermal requirement of a TaqMan® probe.
Table 3.
List of primers and probe assayed in RT-PCR reactions
| RT-PCR MiX | Forward primer at final concentration of: | Reverse primers at final concentration of: | Probe at final concentration of: |
|---|---|---|---|
| #1 primers from Grywna (2010) | F1, 500 nM | R1, 500 nM | P1, 250 nM |
| #2 primers with deoxy- INOSINE | F2A, 500 nM | R2A, 250 nM +R3A, 250 nM +R4A, 250 nM |
P1, 250 nM |
| #3 primers without deoxy-INOSINE | F2B, 500 nM | R2B, 250 nM +R3B, 250 nM +R4B, 250 nM |
P1, 250 nM |
| #4 primers from Grywna (2010) | F1, 500 nM | R1, 500 nM | P2, 250 nM |
| #5 primers with deoxy- INOSINE | F2A, 500 nM | R2A, 250 nM +R3A, 250 nM +R4A, 250 nM |
P2, 250 nM |
| #6 primers without deoxy-INOSINE | F2B, 500 nM | R2B, 250 nM +R3B, 250 nM +R4B, 250 nM |
P2, 250 nM |
Each mix contains the forward primer, up to 3 reverse primers and a probe as mentioned. Combination of 3 reverse primers accounts for nucleotide variation by limiting the use of degenerated primers
Real time RT-PCR assay
Pan-Alphavirus assay was performed in a total volume of 10 μL including 2.5 μL of template, 2.5 μL of a 4X mix including selected primers and probe according to Table 3, 2.5 μL of molecular grade water and 2.5 μL of 4X ABI TaqMan® Fast Virus 1-Step Master Mix (Applied Biosystems, 850 Lincoln Centre Drive, Foster City CA94404, Cat #4444434). Oligonucleotides were purchased from Eurogentec®. TaqMan® hydrolysis probes were 5′ labeled using ATTO425 fluorochrome and 3′ quenched with BHQ1 (Black hole Quencher-1). Final concentration of primers and probes were respectively set at 500 μM and 250 μM. Cycling conditions were: 45 °C, 5 min, 98 °C, 20 s and 45 cycles comprising 2 steps, 98 °C, 3 s and 58 °C, 45 s with fluorescence reading using CYAN channel for detection of TaqMan® probe hydrolysis. RT-PCR cycling was set on a Roche LC480-II thermal cycler (Roche Applied Science, 68,298 Mannheim Germany, Cat #05015278001).
Linearity and PCR Efficiency of the pan-Alphavirus TaqMan® assay
RNA standards corresponding to designed amplicons were diluted at the concentration of 1.35 107 copies/μL. A 10-fold dilution series was prepared from RNA standards for WEEV, EEEV, VEEV, BFV and CHIKV. Ct values plotted against log10[concentration] were used for linear regression analysis. The coefficient of correlation was used to appreciate the linearity of the assay. The slope of the regression line was used to calculate the PCR efficiency: E = 100 × (10–1/slope – 1).
Specificity of pan-Alphavirus TaqMan® assay
Specificity of our TaqMan® assay was tested using 9 viruses which are known to belong to Alphavirus genus and 15 unrelated viruses from different lineages (Table 4).
Table 4.
Virus panel tested with the pan-Alphavirus RT-PCR assay
| Virus | Ct | Virus | Ct |
|---|---|---|---|
| SINV | 16.69 | ZIKA | nd |
| RRV | 13.22 | HIV1 | nd |
| BFV | 15.45 | HBV | nd |
| SFV | 16.85 | HCV | nd |
| ONN | 10.41 | EBV | nd |
| CHIKV | 20.96 | CMV | nd |
| WEEV | 18.20 | BKV | nd |
| EEEV | 19.08 | Flu B | nd |
| VEEV | 18.09 | Rubella, MeV, Myxovirus | nd |
| DENV-1 | nd | ||
| DENV-2 | nd | ||
| DENV-3 | nd | ||
| DENV-4 | nd |
Specificity of pan-Alphavirus assay was tested on a panel of 20 virus comprising 10 Alphaviruses and 15 unrelated viruses (nd, not detectable)
Sensitivity of pan-Alphavirus TaqMan® assay
Clinical sensitivity of our TaqMan® assay was tested by comparing of the Ct obtained from clinical samples positives for CHIKV. We compared the results obtained using a specific-CHIKV RT-PCR assay from Pastorino and our pan-Alphavirus assay [37]. Alternatively, we estimated the limit of detection (LOD) of our assay using a plasmid containing CHIKV amplicon or RNA standards for WEEV, EEEV, VEEV, BFV, and CHIKV.
Statistical analysis
Tests comparison was assayed for statistical significance using two-tailed Student test with α-risk set at 0.05 using GraphPad Prism v5.0 (GraphPad Software, La Jolla California USA, https://www.graphpad.com/).
Results
Pan-Alphavirus real time RT-PCR setup
Nineteen Alphaviruses were used to generate consensus sequences that were aligned prior to primer design in the nsP4 gene. 6 primer combinations were used to check for the effect of the presence of inosine in primers’ sequence. Using CHIKV RNA as a template, we failed to detect nsP4 amplicon with Grywna’s primers with mixes 1, 4 (Fig. 2). This might be due to a potential mismatch at the antepenultimate position in 3′ of the forward primer as evidenced on Fig. 1a. With highly degenerated primers (mixes 3, 6), amplification was weakly detectable (Fig. 2). In contrast, primers enriched with inosine (mixes 2, 5) gave better result as evidenced by earlier Cts and higher fluorescence signals (Fig. 2). From these experiments, we found that the two designed probes worked equally well.
Fig. 2.
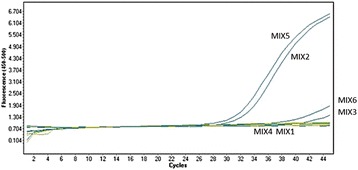
Comparison of 3 primers/probes combinations for pan-Alphavirus RT-PCR. Primers containing Inosine were assayed using MIX2 containing probe P1 or MIX5 containing probe P2. Primers without Inosine were tested using MIX3 containing probe P1 or MIX6 containing probe P2. Reference primers from Grywna [31] were used with probe P1 (MIX1) or probe P2 (MIX4)
We further investigated the performance of these two probes by calculating ∆Ct(Mix2-Mix5) obtained from 6 CHIKV RNA samples or 6 RRV RNA samples (data not shown). Averaged CHIKV ∆Ct was set at +0.27 and did not account for differences between probes. In contrast, in our pan-Alphavirus assay, averaged RRV ∆Ct at −1.80 clearly indicated a better performance with P1 probe, ie, the shorter LNA® probe. Therefore, we decided to select inosine-enriched primers in association with a short LNA® probe and use mix2 for the remaining validation studies of our assay.
Specificity of Pan-Alphavirus real time RT-PCR assay
The specificity for our assay was tested with positive controls for a large panel of viruses including 10 Alphavirus. We successfully detected genomic sequence of CHIKV, ONNV, RRV, SINV and SFV (Fig. 3). Unrelated viruses (DENV-1, DENV-2, DENV-3, DENV-4, ZIKA, HIV1, HBV, HCV, EBV, CMV, BKV, Influenza type B, Rubella, MeV, Myxovirus) were not detected with our pan-Alphavirus assay. Rubella virus is a Togavirus from Rubivirus genus. The genus Rubivirus is not closely related to the genus Alphavirus although they belong to the same family. Using our assay, we were not able to detect Rubella virus.
Fig. 3.
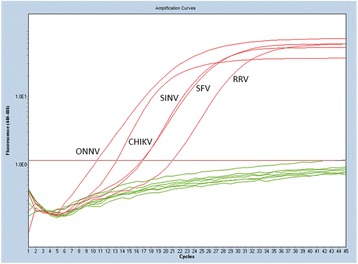
Specific detection of 5 Alphavirus species. Positive controls for CHIKV, ONNV, SINV, SFV or RRV were obtained from reference strains or were isolated from clinical samples in our laboratory. Negative controls included water and negative plasma controls. Fluorescence was read on LightCycler 480® (Roche) using Cyan filter (Excitation at 450 nM/Emission at 500 nM)
Sensitivity of Pan-Alphavirus real time RT-PCR assay
Clinical sensitivity was assessed based using a set of clinical samples positive for CHIKV that were dually tested using a CHIKV specific TaqMan® assay and our pan-Alphavirus assay. Cts obtained from both assays were similar and no significant difference between the two analyses was detected from data shown in Fig. 4.
Fig. 4.
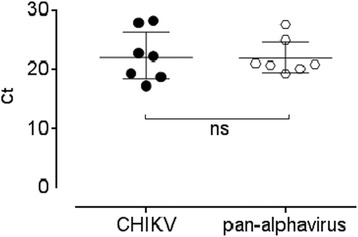
Clinical sensitivity of pan-Alphavirus assay versus CHIKV specific assay. (ns, no statistical difference observed under 5% risk α using a t-test)
The limit of detection of our assay to CHIKV was determined from serial dilutions of a titrated CHIKV RNA. We obtained a LOD at 40 copies per reaction. This was similar to the LOD at 27 copies per reaction reported by Pastorino in his CHIKV TaqMan® assay [37].
In addition we used quantified RNA standards with undergoing serial dilutions prior RT-PCR in order to qualify our assay. (See Table 5).
Table 5.
Linearity, PCR Efficiency and LOD of the pan-Alphavirus RT-PCR assay
| CHIKV | WEEV | EEEV | VEEV | BFV | |
|---|---|---|---|---|---|
| R2 | 0.994 | 0.998 | 0.995 | 0.993 | 0.991 |
| Slope | −3.39 | −3.81 | −3.77 | −3.55 | −3.67 |
| Intercept | 39.49 | 44.58 | 46.92 | 43.35 | 41.10 |
| Efficiency (%) | 97.24 | 82.99 | 84.18 | 91.35 | 87.12 |
| LOD (copies/run) | 26.6 | 59.5 | 42.6 | 111.2 | 87.5 |
Linearity of the assay was optimal for each specific RNA standard tested with R2 > 0.99. PCR efficiency was under expected range (75% to 110%) and the limit of detection was ranging from 26 to 111 copies/run depending on which RNA standard was used. This was also concordant with the different LODs ranging from 5 to 100 copies per reaction obtained with Grywna’s pan-Alphavirus nested RT-PCR assay [31].
Discussion
The aim of our study was to implement a new molecular method for Alphavirus detection. A couple of RT-PCR methods were already available but were difficult to use in clinical laboratories because of their endpoint PCR format involving amplicon detection. Gel electrophoresis, amplicon sequencing even mass spectrophotometry were used for PCR product visualization. All these methods involved the need to work with open tubes thus raising the risk of cross-over contamination. Our real time RT PCR assay addressed and solved this issue by generating and detecting amplicon at the same time in closed tubes.
Our approach took benefits from careful alignments of available genomic sequences prior to virus-specific consensus sequence generation. In silico analysis was crucial to select for virus consensus sequences, allowing accurate determination of ambiguous nucleotide positions in primers and probes. We especially took into account primers’ nucleotide variation in the 3′ extremity. Primers’ 3′ ends are important for primer extension as unfortunate mismatch in the 5 last 3′ nucleotides usually compromise efficient priming of the PCR reaction. To avoid mismatches between primers and template, 3 strategies were developed: we used degenerated primers; we chose to blast our reverse primer into 3 distinct primers harboring different ambiguous nucleotide combination and to mix them; we used inosine enriched primers instead A or T or C or G containing primers. The latter approach allowed us to select for primers with limited degeneracy [38]. We observed better results with low degenerated primers due to the presence of inosine. This approach may be useful for other pan-PCR system setups.
We also checked for the performance of two Taqman® probe designs incorporating LNA modified nucleotides; the former consisted of a short 14 nucleotide stretch with ambiguous position, the latter was longer with inosine enrichment. The two probes performed equally well but in some cases a better result was obtained with the shorter probe despite of its residual degeneracy. Classically shorter LNA® probes behave better and our design was not affected by a low level of degeneracy.
Our test was validated using clinical samples for CHIKV, ONNV, SINV, SFV and RRV. We also used synthetic RNA standards to detect WEEV, EEEV, VEEV and BFV sequences. Alignments of genomic sequences indicated a potential for the detection of other Alphaviruses using this test. These Alphaviruses include members of the SFV antigenic complex, Getah virus and Bebaru virus; members of the VEEV complex, Fort Morgan virus, Highlands J virus; Ndumu virus and Middelburg virus that are related to 2 distinct antigenic complexes and responsible for human illness [39]. Interestingly, Getah virus infects horses, pigs and goats and Highland J virus is found in horses and poultry [40, 41]. There are some precedents where viruses infecting horses can cross species’ barrier and infect humans. Hendra virus, a member of Henipavirus genus is not an arbovirus. It is hosted in frugivorous bats causing a zoonosis affecting horses and incidentally humans. Due to increased exposure to natural host’s reservoir, human could contract infection with different and multiple Alphaviruses that usually target animals. Risk assessment leading to cross species’ barrier is hard to establish but can result in new virus emergence. The potential of our test to detect unsuspected Alphavirus in human related disease may be of great clinical interest.
Conclusion
TaqMan® assays perfectly suit constraints for virus emergence detection at early stage of disease. In the acute phase of the disease, Alphavirus infections are known to cause high viremia in humans and should be easily detectable by RT-PCR. Serological assays could be used in complement at a later stage of the disease [42]. Our test will provide a convenient tool to early incriminate or exclude Alphavirus in disease etiology.
Acknowledgements
We thank Pr Andres Merits (University of Tartu, Estonia) and Dr. Isabelle Leparc-Goffart (National Reference Centre on Arboviruses, Marseille, France).
Funding
This study was supported by a grant from the National Reference Laboratory for Arbovirus of Marseille (France).
Availability of data and materials
The datasets generated or analyzed during the current study are available from the corresponding author.
Abbreviations
- BEBV
Bebaru virus
- BFV
Barmah forest virus
- CHIKV
Chikungunya virus
- EEEV
Eastern equine encephalitis virus
- FMV
Fort Morgan virus
- GETV
Getah virus
- HJV
Highlands J virus
- MAYV
Mayaro virus
- MeV
Measles virus
- MIDV
Middelburg virus
- NDUV
Ndumu virus
- ONNV
O’nyong nyong virus
- RRV
Ross river virus
- SESV
Southern elephant seal virus
- SFV
Semliki forest virus
- SINV
Sindbis virus
- SPDV
Salmon pancreas disease virus
- VEEV
Venezuelan equine encephalitis virus
- WEEV
Western equine encephalitis virus
- WHAV
Whataroa virus
Authors’ contributions
CG designed primers and LNA® probes and optimized PCR setup and performed TaqMan® assays. GLPY was involved in sample management, CG, BR, PG and MCBJ analyzed and interpreted data. CG and PG wrote the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from healthy subjects or patients undergoing virus screening and attending Reunion Island University Hospital. Our validation study involved healthy controls, patients with confirmed CHIKV and patients with viral infection not related to Alphavirus. The study was approved by the Human Ethics Committee of University of Bordeaux (‘Comité Consultatif de Protection de Personnes se prêtant à des Recherche Biomédicales’, Bordeaux France, ref. 2008-A00151–54).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weaver SC, Winegar R, Manger ID, Forrester NL. Alphaviruses: population genetics and determinants of emergence. Antivir Res. 2012;94(3):242–257. doi: 10.1016/j.antiviral.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33(4):330–342. doi: 10.1016/S0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 3.Dash AP, Bhatia R, Sunyoto T, Mourya DT. Emerging and re-emerging arboviral diseases in Southeast Asia. J vector borne diseases. 2013;50(2):77–84. [PubMed] [Google Scholar]
- 4.Lima-Camara TN. Emerging arboviruses and public health challenges in Brazil. Rev Saude Publica. 2016;50:1–7. [DOI] [PMC free article] [PubMed]
- 5.Vasconcelos PF, Calisher CH. Emergence of human arboviral diseases in the Americas, 2000-2016. Vector Borne Zoonotic Dis. 2016;16(5):295–301. doi: 10.1089/vbz.2016.1952. [DOI] [PubMed] [Google Scholar]
- 6.Jaffar-Bandjee MC, Ramful D, Gauzere BA, Hoarau JJ, Krejbich-Trotot P, Robin S, Ribera A, Selambarom J, Gasque P. Emergence and clinical insights into the pathology of Chikungunya virus infection. Expert Rev Anti-Infect Ther. 2010;8(9):987–996. doi: 10.1586/eri.10.92. [DOI] [PubMed] [Google Scholar]
- 7.Sissoko D, Malvy D, Giry C, Delmas G, Paquet C, Gabrie P, Pettinelli F, Sanquer MA, Pierre V. Outbreak of Chikungunya fever in Mayotte, Comoros archipelago, 2005-2006. Trans R Soc Trop Med Hyg. 2008;102(8):780–786. doi: 10.1016/j.trstmh.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Tappe D, Kapaun A, Emmerich P, Campos Rde M, Cadar D, Gunther S, Schmidt-Chanasit J. O’nyong-nyong virus infection imported to Europe from Kenya by a traveler. Emerg Infect Dis. 2014;20(10):1766–1767. doi: 10.3201/eid2010.140823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llagonne-Barets M, Icard V, Leparc-Goffart I, Prat C, Perpoint T, Andre P, Ramiere C. A case of Mayaro virus infection imported from French Guiana. J Clin Virol. 2016;77:66–68. doi: 10.1016/j.jcv.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Lwande OW, Obanda V, Bucht G, Mosomtai G, Otieno V, Ahlm C, Evander M. Global emergence of Alphaviruses that cause arthritis in humans. Infect Ecol Epidemiol. 2015;5:29853. doi: 10.3402/iee.v5.29853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zacks MA, Paessler S. Encephalitic alphaviruses. Vet Microbiol. 2010;140(3–4):281–286. doi: 10.1016/j.vetmic.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7(1):76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roche B, Leger L, L'Ambert G, Lacour G, Foussadier R, Besnard G, Barre-Cardi H, Simard F, Fontenille D. The spread of Aedes albopictus in Metropolitan France: contribution of environmental drivers and human activities and predictions for a near future. PLoS One. 2015;10(5):e0125600. doi: 10.1371/journal.pone.0125600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakshmi V, Neeraja M, Subbalaxmi MV, Parida MM, Dash PK, Santhosh SR, Rao PV. Clinical features and molecular diagnosis of Chikungunya fever from South India. Clin Infect Dis. 2008;46(9):1436–1442. doi: 10.1086/529444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salimi H, Cain MD, Klein RS. Encephalitic arboviruses: emergence, clinical presentation, and neuropathogenesis. Neurotherapeutics. 2016;13(3):514–534. doi: 10.1007/s13311-016-0443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paredes A, Weaver S, Watowich S, Chiu W. Structural biology of old world and new world alphaviruses. Arch Virol Suppl. 2005;19:179–185. doi: 10.1007/3-211-29981-5_14. [DOI] [PubMed] [Google Scholar]
- 17.Suhrbier A, Jaffar-Bandjee MC, Gasque P. Arthritogenic alphaviruses--an overview. Nat Rev Rheumatol. 2012;8(7):420–429. doi: 10.1038/nrrheum.2012.64. [DOI] [PubMed] [Google Scholar]
- 18.Krastinova E, Quatresous I, Tarantola A. Imported cases of chikungunya in metropolitan France: update to June 2006. Euro surveillance. 2006;11(8):E060824.1. doi: 10.2807/esw.11.34.03030-en. [DOI] [PubMed] [Google Scholar]
- 19.Paty MC, Six C, Charlet F, Heuze G, Cochet A, Wiegandt A, Chappert JL, Dejour-Salamanca D, Guinard A, Soler P, et al. Large number of imported chikungunya cases in mainland France, 2014: a challenge for surveillance and response. Euro surveillance. 2014;19(28):20856. doi: 10.2807/1560-7917.ES2014.19.28.20856. [DOI] [PubMed] [Google Scholar]
- 20.Roiz D, Bousses P, Simard F, Paupy C, Fontenille D. Autochthonous Chikungunya transmission and extreme climate events in Southern France. PLoS Negl Trop Dis. 2015;9(6):e0003854. doi: 10.1371/journal.pntd.0003854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, Lavenir R, Pardigon N, Reynes JM, Pettinelli F, et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3(7):e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coffey LL, Forrester N, Tsetsarkin K, Vasilakis N, Weaver SC. Factors shaping the adaptive landscape for arboviruses: implications for the emergence of disease. Future Microbiol. 2013;8(2):155–176. doi: 10.2217/fmb.12.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang G, Gao X, Gould EA. Factors responsible for the emergence of arboviruses; strategies, challenges and limitations for their control. Emerg Microbes Infect. 2015;4(3):e18. doi: 10.1038/emi.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould EA, Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg. 2009;103(2):109–121. doi: 10.1016/j.trstmh.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allison AB, Stallknecht DE, Holmes EC. Evolutionary genetics and vector adaptation of recombinant viruses of the western equine encephalitis antigenic complex provides new insights into alphavirus diversity and host switching. Virology. 2015;474:154–162. doi: 10.1016/j.virol.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aubry M, Finke J, Teissier A, Roche C, Broult J, Paulous S, Despres P, Cao-Lormeau VM, Musso D. Silent circulation of ross river virus in French Polynesia. Int J Infect Dis. 2015;37:19–24. doi: 10.1016/j.ijid.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Bronzoni RV, Moreli ML, Cruz AC, Figueiredo LT. Multiplex nested PCR for Brazilian Alphavirus diagnosis. Trans R Soc Trop Med Hyg. 2004;98(8):456–461. doi: 10.1016/j.trstmh.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Eshoo MW, Whitehouse CA, Zoll ST, Massire C, Pennella TT, Blyn LB, Sampath R, Hall TA, Ecker JA, Desai A, et al. Direct broad-range detection of alphaviruses in mosquito extracts. Virology. 2007;368(2):286–295. doi: 10.1016/j.virol.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer M, Proebster B, Kinney RM, Kaaden OR. Genus-specific detection of alphaviruses by a semi-nested reverse transcription-polymerase chain reaction. Am J Trop Med Hyg. 1997;57(6):709–718. doi: 10.4269/ajtmh.1997.57.709. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Seco MP, Rosario D, Quiroz E, Guzman G, Tenorio A. A generic nested-RT-PCR followed by sequencing for detection and identification of members of the alphavirus genus. J Virol Methods. 2001;95(1–2):153–161. doi: 10.1016/S0166-0934(01)00306-8. [DOI] [PubMed] [Google Scholar]
- 31.Grywna K, Kupfer B, Panning M, Drexler JF, Emmerich P, Drosten C, Kummerer BM. Detection of all species of the genus Alphavirus by reverse transcription-PCR with diagnostic sensitivity. J Clin Microbiol. 2010;48(9):3386–3387. doi: 10.1128/JCM.00317-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, Denizot M, Guichard E, Ribera A, Henni T, et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184(10):5914–5927. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 33.Pickett BE, Sadat EL, Zhang Y, Noronha JM, Squires RB, Hunt V, Liu M, Kumar S, Zaremba S, Gu Z, et al. ViPR: an open bioinformatics database and analysis resource for virology research. Nucleic Acids Res. 2012;40(Database issue):D593–D598. doi: 10.1093/nar/gkr859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:4. [Google Scholar]
- 35.Kibbe WA. OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res. 2007;35(Web Server issue):W43–W46. doi: 10.1093/nar/gkm234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li K, Shrivastava S, Stockwell TB. Degenerate primer design for highly variable genomes. Methods Mol Biol. 2015;1275:103–115. doi: 10.1007/978-1-4939-2365-6_7. [DOI] [PubMed] [Google Scholar]
- 37.Pastorino B, Bessaud M, Grandadam M, Murri S, Tolou HJ, Peyrefitte CN. Development of a TaqMan RT-PCR assay without RNA extraction step for the detection and quantification of African Chikungunya viruses. J Virol Methods. 2005;124(1–2):65–71. doi: 10.1016/j.jviromet.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Dov E, Kushmaro A. Inosine at different primer positions to study structure and diversity of prokaryotic populations. Curr Issues Mol Biol. 2015;17:53–56. [PubMed] [Google Scholar]
- 39.Forrester NL, Palacios G, Tesh RB, Savji N, Guzman H, Sherman M, Weaver SC, Lipkin WI. Genome-scale phylogeny of the alphavirus genus suggests a marine origin. J Virol. 2012;86(5):2729–2738. doi: 10.1128/JVI.05591-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karabatsos N, Lewis AL, Calisher CH, Hunt AR, Roehrig JT. Identification of Highlands J virus from a Florida horse. Am J Trop Med Hyg. 1988;39(6):603–606. doi: 10.4269/ajtmh.1988.39.603. [DOI] [PubMed] [Google Scholar]
- 41.Nemoto M, Bannai H, Tsujimura K, Yamanaka T, Kondo T. Genomic, pathogenic, and antigenic comparisons of Getah virus strains isolated in 1978 and 2014 in Japan. Arch Virol. 2016;161(6):1691–1695. doi: 10.1007/s00705-016-2840-9. [DOI] [PubMed] [Google Scholar]
- 42.Panning M, Grywna K, van Esbroeck M, Emmerich P, Drosten C. Chikungunya fever in travelers returning to Europe from the Indian Ocean region, 2006. Emerg Infect Dis. 2008;14(3):416–422. doi: 10.3201/eid1403.070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the current study are available from the corresponding author.


