Abstract
Phage peptide display technology has been used to identify IgE-binding mimotopes (mimics of natural epitopes) that mimic conformational epitopes. This approach is effective in the characterization of those epitopes that are important for eliciting IgE-mediated allergic responses by food allergens and those that are responsible for cross-reactivity among allergenic food proteins. Application of this technology will increase our understanding of the mechanisms whereby food allergens elicit allergic reactions, will facilitate the discovery of diagnostic reagents and may lead to mimotope-based immunotherapy.
Keywords: Food allergy, conformational epitope, IgE epitope, mimotopes, phage peptide display
Graphical Abstract
Phage peptide display is a valuable technology that can be used to characterize the interactions between protein and protein at the molecular level. Only recently, an increasing number of researchers have applied this technology to the study of food allergens. Successful application of phage peptide display technology in conjunction with computer-based algorithms can lead to the identification of IgE conformational epitopes of important food allergens. Overall, this approach offers a promising alternative to crystallography for identification of allergenic epitopes, which contribute to allergen-antibody complexes.
1. Introduction
Food allergy is a hypersensitivity reaction that affects approximately 4–10% of the population, and may be increasing in prevalence [1, 2].
The initiation of allergic reactions is due to allergen-induced cross-linking of allergen-specific IgE bound to high affinity receptors on mast cells and basophils, leading to cell degranulation and release of inflammatory mediators. Thus, binding of specific regions of an allergen to IgE that is part of IgE/FcεR1 complexes is a prerequisite for triggering allergic symptoms.
Binding sites recognized by IgE antibodies are called IgE epitopes. IgE epitopes are frequently categorized as either linear or conformational. Linear epitopes can be identified by analysis of IgE-binding to overlapping peptides derived from the primary sequence [3, 4]. Identification of conformational epitopes requires more elaborate methods. A variety of methods have been used to identify conformational epitopes including X-ray crystallography, nuclear magnetic resonance, hydrogen-deuterium exchange coupled to mass spectrometry, site-directed mutagenesis and shotgun mutagenesis [5, 6]. These approaches can, in varying degrees, provide high-resolution maps of antibody-antigen interactions and thus, a high-resolution structure of a mAb in complex with its target. However, these approaches are not always feasible because of the difficulty of obtaining sufficient quantities of correctly folded, properly processed allergens and can be laborious, time consuming and expensive. Phage display in combination with computational approaches is a cost-effective method to identify important conformational epitopes of clinically relevant allergens [7]. Strengths of this approach include the use of polyclonal antibodies from patients, the speed with which mimotopes can be identified, the relatively low amounts of allergen needed, the lack of need for crystals and, relative to some other techniques, technical simplicity. Drawbacks include the possibility of identifying off-target mimotopes, the need for sophisticated computer modeling to analyze the data and difficulties in validating the veracity of epitopes that are identified.
1. Phage display
Phage display, an advanced technology based on the expression of foreign peptides or proteins as fusions with coat proteins on the phage surface, was first described in 1985 by George P. Smith [8]. Phage display, due to its simplicity and efficacy, has proven to be a powerful versatile tool for studying specific interactions (protein with protein and protein with other molecules). Specific applications include, drug discovery [9–12], gene therapy, vaccine development [13–17], dissection of receptor interactions with agonists and antagonists [18, 19], epitope mapping [7, 20, 21] and identification of antagonists and inhibitors of enzymes [22–25].
Two types of phage are often employed for phage display. M13 filamentous phages are not only used for peptide display, but also for display of a variety of recombinant proteins. Most commonly, DNA encoding for a large library of random peptides is inserted into gene 3 or gene 8 of the filamentous phages. Filamentous phages infect Escherichia coli via the F pilus and fusion proteins are expressed on the surface of the bacteriophage. The infection caused by filamentous phage does not cause cell lysis, only “constant production”, albeit with slower bacterial growth [26, 27]. A similar approach can be taken with the lytic phage, T7, in which peptides or proteins are displayed as fusions with capsid proteins. In this approach, the lytic cycle results in the destruction of the infected bacteria cells and the mature virions can infect other cells [28, 29]. In each approach, the investigator must devise a process to screen the expressed peptides that will lead to identification of peptides that mimic the interaction to be studied.
2. Filamentous phage display
Filamentous phages have been most commonly used as a phage peptide display platform [27]. Phage peptide libraries used in allergen research usually consist of small peptides, 7 to 12 amino acids in length (Table 1). Even though B cell epitopes are reported to consist at least 8 amino acids, energy calculations imply that epitopes of 5–6 amino acids are the key contributors to the binding between an antibody and its epitope. Heptameric peptides can be used to select the epitopes with the highest affinity to the specific IgE antibodies, while longer peptides enhance the affinity of interaction and increase the ability to detect important conformational epitopes that may be of lower affinity [30–32].
Table 1.
Summary of studies using phage peptide display technology for identification of food allergen epitopes.
| Allergy | Allergen | Allergic sera (*)or antibodies | Libraries** | Biopanning (***) | References |
|---|---|---|---|---|---|
| Conformational IgE Epitopes | |||||
| Fish | Cy p 1 | Fish (16) | Cyc-10 on pIII | Solid surface (4) | 51 |
| Peach | Pru p 3 | Peach (23) | Ph.D. – 12 | Solid surface (3) | 41 |
| Peanut | Ara h 1 | Peanut (3) | Ph.D. – 7 | Solution (4) | 43 |
| Ara h 1 | Peanut (5) Peanut, rat IgG (5) |
Ph.D. – 7 | Solution (3) | 32 | |
| Peanut | Ara h 2 & 6 | Peanut (4) | Ph.D. – 12 | Solution (3) | 31 |
| Cross-reactive IgE epitopes in primary food allergies or pollen-associated food allergies | |||||
| Birch pollen | Bet v 1 | Birch pollen (5) | Ph.D. – 7 | Solution (2) | 35 |

|
|||||
| Peanut | Ara h 8 | ||||
| Soy | Gly m 4 | ||||
| Cherry | Pru av 1 | ||||
| Melon | Cuc m 2 | Melon (17) | Ph.D. – 12 | Solution (2) | 77 |

|
|||||
| Timothy grass | Phl p 12 | ||||
| Birch pollen | Bet v 2 | ||||
| Wheat | Tri a 14 | Baker’s asthma (8) | Ph.D. – 12 | Solution (2) | 40 |

|
|||||
| Peach | Pru p 3 | ||||
| The potential for mimotope-based vaccines in food allergy | |||||
| Celery-Mugwort-Birch-Spice Syndrome | Api g 5 | mAb BIP3 | Cyc-9 on pVIII | Solid surface (3) | 93 |
Number of individual sera or antibodies.
Numbers refer to the number of amino acids in the peptides.
Number of rounds of panning.
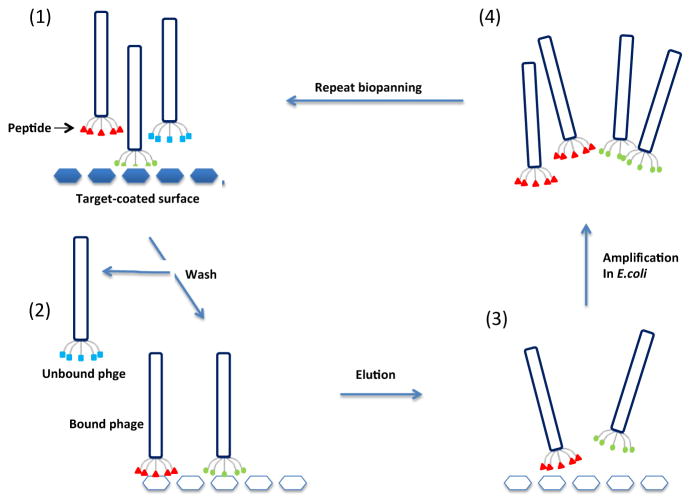
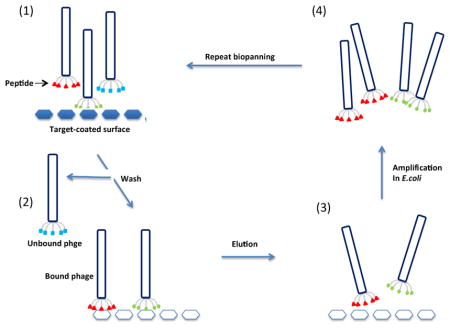
The most straightforward strategy for screening a phage library is the surface biopanning procedure, which consists of three main steps (Figure 1): (i) coating a plastic surface with the target, (ii) adding the phage library onto the immobilized target followed by removal of unbound phages by washing and (iii) eluting the bound phages usually with an acidic solution or by competition with the original antigen. Eluted phages are titrated and amplified in host bacteria and subjected to a second round of selection. Ideally, three rounds of selection are necessary to isolate target-specific phages [33, 34]. Because direct coating may cause some binding sites to be inaccessible due to steric blocking or target denaturation, an alternative method is phage panning in solution: the target is incubated with the phage in solution followed by affinity capture of the phage-target complexes onto an affinity matrix (usually beads) specific for the target protein [35]. For example, if the target protein has a polyhistidine affinity tag, the target-phage complexes can be captured on chelated nickel beads. Methods can be further optimized by using the avidin-biotin system which will increase sensitivity and enhance detection of relevant mimotopes [31]. A negative selection step can be introduced in which the amplified phage is pre-incubated with the beads in the absence of target to further minimize the retention off-target phages [36, 37]. Following the last selection round, the bound phages are eluted and individual clones from the final eluate are isolated and characterized for target specificity in a phage ELISA. The primary structure of the identified phages is then determined by sequencing the corresponding insert.
Fig. 1.
Schematic representation of biopanning. (1) Target proteins are immobilized on a solid surface and incubated with the phage peptide display library, (2) Unbound phage is washed away, (3) Bound phage is eluted through acidic solution or affinity eluted with specific ligands and (4) Eluted phage is amplified and used for repeated cycles of selection.
Validation of these peptides as true mimotopes is a challenge. The most direct way to address this is with inhibition assays characterizing the mimotope’s ability to inhibit the binding of antibody to the allergen. This is most successful with monoclonal and not polyclonal antibodies. Inhibition assays using polyclonal IgE have not been successful because of the polyclonal nature of the interaction (clearly one peptide cannot be enough). Unfortunately, patient-derived monoclonal IgE antibodies are not available to fully validate mimotopes. For additional validation, one can test the binding of peptides expressed on phage to specific IgE isolated from allergic sera as well as by their ability to elicit blocking IgG following immunization[37].
Phage libraries can be screened with monoclonal or polyclonal antibodies to identify multiple mimics of antigenic epitopes. In this application, the selected phage peptides mimic the native antibody-binding epitopes of the antigen by virtue of their physico-chemical properties [7], making this an ideal method for determination of individual epitope recognition patterns [38, 39]. Screening phage-displayed peptides with polyclonal IgE from allergic patients in combination with computer-based mapping of the peptide mimics onto the surface of the three-dimensional structure of the allergen is a promising novel tool to investigate IgE epitope specificity in individual patients [32, 40, 41]. Phage peptide display technology has been used to investigate allergen-antibody interactions for both respiratory and food allergens [7, 38, 42–44]. A summary of published reports in which phage peptide display technology has been applied for the identification of food allergen epitopes and of those food allergen epitopes that contribute to cross-reactivity among allergens is shown in Table 1.
3. Identification of conformational IgE epitopes
Extensive research has been performed to identify sequential epitopes on food allergens [45–47]. In contrast, few conformational epitopes have been identified, a situation resulting from the technical challenges associated with their characterization. Recently, increasing evidence indicates that conformational IgE epitopes may be particularly important in food allergen-induced allergic reactivity [48–50]. The identification of conformational IgE epitopes has also enhanced our understanding of the structural requirements for allergen-induced crosslinking of IgE/FcεR1 complexes that is necessary to activate basophils and mast cells. Specifically, the phage display technique has been used to define peptides (mimotopes) that mimic conformational IgE epitopes of food allergens. Alignment and mapping of identified mimotopes onto the allergen structure surface provide important information about epitope localization and the three-dimensional structure of the antibody binding sites [7].
Parvalbumin, a Ca2+/Mg+-binding protein, is a major fish allergen. Untersmayr et al. [51] screened a cysteine-flanked peptide phage library with Cyp c 1-specific IgE purified from fish-allergic patients and identified five mimotopes that mimic conformational epitopes. All showed similar binding patterns with both parvalbumin specific IgE and IgG in an ELISA, indicating that these mimotopes bind to both isotypes with similar specificity. Epitope mapping identified three matching regions. One of the matching regions was located in the EF domain, indicating that the Ca 2+ binding loop of the EF domain is an immunogenic area. It was previously reported that Ca 2+ depletion reduced the IgE binding capacity of parvalbumin because of dramatic conformational changes in the Ca 2+ binding site [52]. Thus, it is likely that these conformational changes in the Ca 2+ binding loop of the EF domain result in inaccessibility of critical residues for the binding of antibody.
Peach is frequently involved in allergic reactions in the adult population from the Mediterranean area [41]. In order to define peptides that mimicking conformational epitopes on Pru p 3, a major peach allergen, and to analyze the location of such epitopes on its molecular surface, Pacios et al. screened a random peptide phage display with Pru p 3 – specific IgE and performed three-dimensional modeling analysis. Pru p 3-specific IgE was purified from two pools of sera grouped by symptoms, OAS (Oral Allergy Syndrome) and SYS (Systemic Symptoms). A total of 21 individual phage colonies were identified that were confirmed by ELISA with individual patient sera from each pool. Peptide alignments revealed two consensus sequences, one for the OAS pool and the other for the SYS pool. The two consensus sequences mapped to the same surface area of Pru p 3, corresponding to the helix 2-loop-helix 3 region and to part of the non-structured C-terminal coil. These two relevant conformational IgE-binding regions of Pru p 3 may be useful for design of hypoallergenic variants of the allergen.
The peanut protein, Ara h 1, is an important allergen that is recognized by serum IgE from more that 80% of peanut allergic patients [53, 54]. In its native form Ara h 1 is a 63.5 kDa protein that forms stable homotrimers maintained by hydrophobic interactions between amino acids at the monomer-monomer contact points [55, 56]. Following digestion, Ara h 1 retains both its ability to sensitize and to elicit an allergic reaction, but not if subjected to chromatography. This suggests that, following digestion, small peptide fragments aggregate to form larger complexes [32, 57].
A phage peptide display library was screened with IgE from peanut allergic patients as well as IgE from Brown Norway rats immunized with either purified intact Ara h 1 or gastro-duodenal digested Ara h 1 products [32]. The bound phages were eluted with either intact or digested Ara h 1. Sequence analysis of the identified mimotopes with patient serum IgE revealed five epitope motifs that showed patterns of a minimum of five amino acids. All five motifs were identified on both intact and digested Ara h 1. However, such motifs were not identified by IgG from the immunized rats which showed a much more heterogeneous pattern. Despite the finding that the occurrence of motifs recognized by immunoglobulins was different between peanut allergic patients (IgE) and Ara h 1 immunized rats (IgG), the epitope mapping profile of IgE mimotopes from humans and IgG mimotopes from rats were very similar in terms of the immunoglobulin-binding fingerprints, amino acid distribution and the localization and clustering of epitopes on the Ara h 1 molecule. This indicates that humans and rats direct their antibody-response against the same areas of the allergen. Similarly, the IgE epitope profiles of intact and digested Ara h 1 were very similar when elution was made with intact or digested Ara h 1, respectively. These results suggest that the digested Ara h 1 is kept in a conformation resembling the native structure of Ara h 1, probably by non-covalently interactions, such as hydrophobic interactions.
Increased allergen-specific IgG4 concentrations are associated with development of tolerance to food allergens. This has been demonstrated in the natural development of tolerance to several food allergens and in desensitization by allergen-specific immunotherapy [58–62]. Recently, several studies compared the specific IgE and IgG4 antibody-epitope recognition pattern in food allergic patients [45, 58, 60, 63] and suggested that similarities between IgE and IgG4 binding epitopes could be critical for the development of tolerance. However, these studies focused only on IgE and IgG4 linear epitopes. Bogh et al. [43] used the phage peptide display technique to study Ara h 1 specific IgE and IgG4 epitope recognition patterns, taking conformational epitopes into account. Phage library screening with IgE from three peanut allergic sera identified 146 Ara h 1 mimotopes. All mimotopes represented Ara h 1 conformational epitopes as none of the mimotope sequences corresponded to a full linear stretch of the Ara h 1 primary sequence and all mimotopes could be mapped to the surface of the Ara h 1 molecule by a computer-based algorithm. Three consensus sequences were recognized by IgE for all three patients. However, the IgG4 epitope pattern was more heterogeneous than the IgE pattern and no consensus sequence was detected. Furthermore, IgG4 epitopes did not coincide with IgE epitopes and were thought to have lower affinity than the IgE epitopes. Each patient had a distinct IgE and IgG4 epitope recognition profile, although some important IgE epitopes were common to all patients. These results demonstrate that the phage peptide display technique can be successful in distinguishing between the epitope patterns of IgE and IgG4, giving detailed information on fine specificity and relative affinity.
Ara h 2 and Ara h 6 are the two most potent peanut allergens [64–67]. Recently, Chen at al screened a phage peptide display library with IgE from four peanut allergic sera [31]. In order to increase the ability to detect important conformational epitopes, the authors optimized the method by using avidin-biotin system to enhance signal intensity. A total of 41 mimotopes that mimic conformational epitopes were identified that specifically bound to Ara h 2/Ara h 6-specific IgE. A computer-based algorithm [7, 68] showed that all mimotopes mapped to surface-exposed patches on the 3D model structures of Ara h 2 and Ara h 6. In the case of Ara h2, the highest scoring patches were located in the vicinity of a patch centered on Y63 and in the case of Ara h 6, the four top scoring patches centered on M80, T68, C73 and H29. Three of these patches, those centered on M80, T68, and C73, share several overlapping residues. Further structural alignment between Ara h 2 and Ara h 6 showed that the patch centered on H29 in Ara h 6 partially overlaps with the high scoring patch centered on Y63 in Ara h 2. These mimotopes were also recognized by IgG from rabbits immunized with either Ara h 2 or Ara h 6. Furthermore, the binding intensity of the mimotopes to rabbit anti-Ara h 2 and Ara h 6 specific IgG was highly correlated, which was consistent with the findings from the structural analysis. The mimotopes were further tested for their binding to IgE from 29 peanut-allergic sera. Eight of the 41 mimotopes were recognized by more than 90% of the tested peanut-allergic sera, suggesting that these 8 mimotopes mimic immunodominant epitopes. Each serum had distinct IgE recognition patterns but the patterns were not correlated to the concentration of peanut specific IgE or the clinical histories, indicating a broad variation in IgE conformational epitopes among patients suffering from peanut allergy.
4. Identification of cross-reactive IgE epitopes in primary food allergies and in pollen-associated food allergies
Cross-reactivity among foods or between food allergens and aeroallergens is a common clinical issue in patients with food allergy. Mimotope peptides are useful for studying potential cross-reactivity between homologous allergens.
Birch pollen-allergic patients often have birch pollen-associated food allergy which is mediated by cross-reactive IgE that reacts with Bet v 1-homologous allergens from foods [69]. Bet v 1-homologous food allergens are found in fresh fruits, vegetables, tree nuts, and legumes including peanut [69, 70]. Mittag et al. investigated cross-reactive IgE epitopes and epitope recognition patterns in individual patients [35]. A phage peptide-display library was screened with polyclonal IgE from five patients with birch pollen allergy in combination with several food allergies. Competitive ELISA confirmed the cross-reactivity between Bet v 1 (birch pollen), Ara h 8 (peanut), Gly m 4 (soy) and Pru av 1 (cherry) in the patient sera. The IgE bound phages specific for Bet v 1 or the homologous food proteins (Gly m 4, Ara h 8, and Pru av 1) were eluted competitively with the corresponding allergen. Thirty seven mimotopes with strong binding to specific IgE were identified and three potential IgE binding areas were identified on all four cross-reactive food allergens. One area was recognized by IgE from all patients, whereas, the other two areas were recognized by only 3 of the 5 patients. The results were consistent with the findings from cross-competitive ELISA and demonstrated that phage peptide screening in combination with computer-based mapping of the mimotopes on the surface of the three dimensional structure of the allergen is a promising tool to investigate IgE epitope specificity and to predict cross-reactivity in individual patients.
Melon allergy, an important food allergy in Spain [71, 72], is frequently associated with the sensitization to several pollens (e.g. birch, Parieteria, and grass) [73–75]. This cross-reactivity is due to homology among plant profilins. Cuc m 2, a melon profilin, is a major melon allergen [76]. By screening a phage peptide library with IgE from melon allergic patients, Tordesillas et al. identified and sequenced 12 individual Cuc m 2 specific mimotopes [77]. The mimotopes were mapped onto the 3D structure of the Cuc m 2 model and a consensus sequence S2W3A5Y6D9H10T111P112G113Q114N116M117R121L122 was identified. This sequence was identical to homologous residues in Phl p 12 (timothy grass) and Bet v 2 (birch) but not to the homologous sequence in human profilin. The identified mimotopes most likely identify surface regions in Cuc m 2 that are involved in cross-reactions among food and pollen profilins and appear to explain the cross-reactivity observed in patients.
Peach Pru p 3, a major food allergen discussed above, is a lipid transfer protein [78, 79]. The homologous protein in wheat, Tri a 14, is thought to be important in occupational baker’s asthma. Although Tri a 14 and peach Pru p 3 share 45% sequence identity, competitive ELISA results showed highly variable cross-reactivity between the two allergens among patients with baker’s asthma, indicating different sensitization patterns to these allergens [80]. Tordesillas et al. used three approaches to characterize the IgE-binding epitopes of Tri a 14 and Pru p3: i) identifying linear IgE epitopes of Tri a 14 and Pru p 3 by IgE immunodetection of synthetic decapeptides with IgE from patient with baker’s asthma, ii) identifying Tri a 14 and Pru p 3 specific conformational epitopes by screening phage peptide display library with the same IgE, and iii) analysis of the surface electrostatic potential of both allergens [40]. Four linear epitopes were identified by IgE immunodetection, two of which were found to be shared by both allergens. However, one of the remaining epitopes was found only in Tri a 14 and the other, only in Pru p 3. By phage peptide library screening, a mimotope that mimics an important conformational epitope on both allergens was identified. Both Tri a 14 and Pru p 3 share the conformational regions involved in IgE-binding, but with different electrostatic features [40]. Thus, differences in the two linear epitopes and in the electrostatic potentials of the conformational epitope may explain the different sensitization patterns to the two allergens.
6. The potential for mimotope-based vaccines in food allergy
Filamentous phage are highly immunogenic and are known to induce humoral and cellular immune responses directed to their coat proteins resulting in a strong activation response [81–83]. Filamentous phage carriers have also been shown to elicit a more focused anti-peptide response compared to traditional carrier proteins [84]. Although still in early stages of development, phage-based vaccines have been used to induce protection against infectious diseases and cancers in preclinical studies [14, 85–89] and also have been tested in phase I/II human immunization studies [90].
Allergy to plant-derived foods is often associated with sensitization to pollen allergens. In the celery-mugwort-birch-spice syndrome, IgE cross-reactivity is associated with several classes of allergens [91, 92]. The celery allergen, Api g 5, a glycoprotein carrying fucosylated and xylosylated complex N-glycans, has been recognized as an important allergen. Lukschal et al. screened a phage peptide library with BIP3, a monoclonal antibody originally raised against birch pollen high molecular weight allergens, which also reacts to Api g 5 [93]. After phage screening and evaluation by an inhibitory ELISA, three mimotopes were identified, which specifically inhibited the binding of patient serum IgE to birch pollen allergens. These mimotopes were then used for immunization of BALB/c mice. The mimotopes were highly immunogenic and induced a strong IgG response against Api g 5 and birch pollen high molecular weight allergens, indicating that Api g 5 and its cross-reactive homologues are the major targets of BIP3. In order to determine whether the mimotopes are mimics of carbohydrate motifs of the epitopes, the authors used non-glycosylated grass pollen allergen, Phl p 5, and a model glycoprotein representing multiple carbohydrate species as controls. The results showed weak binding of the specific IgG to carbohydrates, suggesting that the BIP3-specific mimotopes predominantly mimic protein epitopes. These results demonstrate that the specific IgG was directed towards allergens relevant for human IgE binding in the celery-birch-mugwort-spice syndrome.
7. Conclusion
Phage peptide display is a valuable technology that can be used to characterize the interactions between proteins at the molecular level. It has been used to investigate protein interactions for drug discovery including anticancer peptides and proteins, receptor agonists and enzyme inhibitors. Only recently, an increasing number of researchers have applied this technology to the study of food allergens. Successful application of phage peptide display technology in conjunction with computer-based algorithms can lead to the identification of IgE conformational epitopes of important food allergens. However, IgE mimotopes, when analyzed only through computational surface mapping, do not provide reliable evidence of true epitopes and should be further validated. Overall, this approach offers a promising alternative to crystallography for identification of allergenic epitopes that contribute to allergen-antibody complexes. When the interaction between serum IgE and specific allergens has been fully characterized, it will be possible to develop novel therapeutic agents based on the development of high-affinity competing antibodies and/or peptides.
Acknowledgments
This review was supported by R21-AI112792 (XC) and R01-AI099029 (SCD), both from the National Institute of Allergy and Infectious Diseases and by divisional funds. Also, this work was supported by Colorado CTSI Grant Number UL1 RR025780 to XC.
References
- 1.Patel DA, Holdford DA, Edwards E, Carroll NV. Estimating the economic burden of food-induced allergic reactions and anaphylaxis in the United States. J Allergy Clin Immunol. 2011;128:110–115. e115. doi: 10.1016/j.jaci.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Greenhawt M. Food allergy quality of life and living with food allergy. Curr Opin Allergy Clin Immunol. 2016;16:284–290. doi: 10.1097/ACI.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 3.Shreffler WG, Beyer K, Chu TH, Burks AW, Sampson HA. Microarray immunoassay: association of clinical history, in vitro IgE function, and heterogeneity of allergenic peanut epitopes. J Allergy Clin Immunol. 2004;113:776–782. doi: 10.1016/j.jaci.2003.12.588. [DOI] [PubMed] [Google Scholar]
- 4.Flinterman AE, Knol EF, Lencer DA, Bardina L, et al. Peanut epitopes for IgE and IgG4 in peanut-sensitized children in relation to severity of peanut allergy. J Allergy Clin Immunol. 2008;121:737–743. e710. doi: 10.1016/j.jaci.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Abbott WM, Damschroder MM, Lowe DC. Current approaches to fine mapping of antigen-antibody interactions. Immunology. 2014;142:526–535. doi: 10.1111/imm.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson E, Doranz BJ. A high-throughput shotgun mutagenesis approach to mapping B-cell antibody epitopes. Immunology. 2014;143:13–20. doi: 10.1111/imm.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiwari R, Negi SS, Braun B, Braun W, et al. Validation of a phage display and computational algorithm by mapping a conformational epitope of Bla g 2. Int Arch Allergy Immunol. 2012;157:323–330. doi: 10.1159/000330108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 9.Su JL, Lai KP, Chen CA, Yang CY, et al. A novel peptide specifically binding to interleukin-6 receptor (gp80) inhibits angiogenesis and tumor growth. Cancer Res. 2005;65:4827–4835. doi: 10.1158/0008-5472.CAN-05-0188. [DOI] [PubMed] [Google Scholar]
- 10.Tipps ME, Lawshe JE, Ellington AD, Mihic SJ. Identification of novel specific allosteric modulators of the glycine receptor using phage display. J Biol Chem. 2010;285:22840–22845. doi: 10.1074/jbc.M110.130815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schooltink H, Rose-John S. Designing cytokine variants by phage-display. Comb Chem High Throughput Screen. 2005;8:173–179. doi: 10.2174/1386207053258550. [DOI] [PubMed] [Google Scholar]
- 12.Loi M, Di Paolo D, Soster M, Brignole C, et al. Novel phage display-derived neuroblastoma-targeting peptides potentiate the effect of drug nanocarriers in preclinical settings. J Control Release. 2013;170:233–241. doi: 10.1016/j.jconrel.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Berardinis P, Haigwood NL. New recombinant vaccines based on the use of prokaryotic antigen-display systems. Expert Rev Vaccines. 2004;3:673–679. doi: 10.1586/14760584.3.6.673. [DOI] [PubMed] [Google Scholar]
- 14.Sathaliyawala T, Rao M, Maclean DM, Birx DL, et al. Assembly of human immunodeficiency virus (HIV) antigens on bacteriophage T4: a novel in vitro approach to construct multicomponent HIV vaccines. J Virol. 2006;80:7688–7698. doi: 10.1128/JVI.00235-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Scala G, Quinto I, Liu W, et al. Protection of rhesus macaques against disease progression from pathogenic SHIV-89.6PD by vaccination with phage-displayed HIV-1 epitopes. Nat Med. 2001;7:1225–1231. doi: 10.1038/nm1101-1225. [DOI] [PubMed] [Google Scholar]
- 16.Gu Y, Wei J, Yang J, Huang J, et al. Protective immunity against Trichinella spiralis infection induced by a multi-epitope vaccine in a murine model. PLoS One. 2013;8:e77238. doi: 10.1371/journal.pone.0077238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asadi-Ghalehni M, Ghaemmaghami M, Klimka A, Javanmardi M, et al. Cancer immunotherapy by a recombinant phage vaccine displaying EGFR mimotope: an in vivo study. Immunopharmacol Immunotoxicol. 2015;37:274–279. doi: 10.3109/08923973.2015.1027917. [DOI] [PubMed] [Google Scholar]
- 18.Lee SC, Ibdah R, Van Valkenburgh C, Rowold E, et al. Phage display mutagenesis of the chimeric dual cytokine receptor agonist myelopoietin. Leukemia. 2001;15:1277–1285. doi: 10.1038/sj.leu.2402163. [DOI] [PubMed] [Google Scholar]
- 19.Peng L, Oganesyan V, Wu H, Dall’Acqua WF, Damschroder MM. Molecular basis for antagonistic activity of anifrolumab, an anti-interferon-alpha receptor 1 antibody. MAbs. 2015;7:428–439. doi: 10.1080/19420862.2015.1007810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojas G, Tundidor Y, Infante YC. High throughput functional epitope mapping: revisiting phage display platform to scan target antigen surface. MAbs. 2014;6:1368–1376. doi: 10.4161/mabs.36144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Infante YC, Pupo A, Rojas G. A combinatorial mutagenesis approach for functional epitope mapping on phage-displayed target antigen: application to antibodies against epidermal growth factor. MAbs. 2014;6:637–648. doi: 10.4161/mabs.28395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diamond SL. Methods for mapping protease specificity. Curr Opin Chem Biol. 2007;11:46–51. doi: 10.1016/j.cbpa.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Hawinkels LJ, van Rossenberg SM, de Jonge-Muller ES, Molenaar TJ, et al. Efficient degradation-aided selection of protease inhibitors by phage display. Biochem Biophys Res Commun. 2007;364:549–555. doi: 10.1016/j.bbrc.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Delespaul W, Peeters Y, Herdewijn P, Robben J. A novel helper phage for HaloTag-mediated co-display of enzyme and substrate on phage. Biochem Biophys Res Commun. 2015;460:245–249. doi: 10.1016/j.bbrc.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Lee YC, Hsiao NW, Tseng TS, Chen WC, et al. Phage display-mediated discovery of novel tyrosinase-targeting tetrapeptide inhibitors reveals the significance of N-terminal preference of cysteine residues and their functional sulfur atom. Mol Pharmacol. 2015;87:218–230. doi: 10.1124/mol.114.094185. [DOI] [PubMed] [Google Scholar]
- 26.Smith GP, Petrenko VA. Phage Display. Chem Rev. 1997;97:391–410. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- 27.Huang JX, Bishop-Hurley SL, Cooper MA. Development of anti-infectives using phage display: biological agents against bacteria, viruses, and parasites. Antimicrob Agents Chemother. 2012;56:4569–4582. doi: 10.1128/AAC.00567-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg AH, Griffin K, Washington MT, Patel SS, Studier FW. Selection, identification and genetic analysis of random mutants in the cloned primase/helicase gene of bacteriophage T7. J Biol Chem. 1996;271:26819–26824. [PubMed] [Google Scholar]
- 29.Condron BG, Atkins JF, Gesteland RF. Frameshifting in gene 10 of bacteriophage T7. J Bacteriol. 1991;173:6998–7003. doi: 10.1128/jb.173.21.6998-7003.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bannon GA, Ogawa T. Evaluation of available IgE-binding epitope data and its utility in bioinformatics. Mol Nutr Food Res. 2006;50:638–644. doi: 10.1002/mnfr.200500276. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Negi SS, Liao S, Gao V, et al. Conformational IgE epitopes of peanut allergens Ara h 2 and Ara h 6. Clin Exp Allergy. 2016;46:1120–1128. doi: 10.1111/cea.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bogh KL, Nielsen H, Madsen CB, Mills EN, et al. IgE epitopes of intact and digested Ara h 1: a comparative study in humans and rats. Mol Immunol. 2012;51:337–346. doi: 10.1016/j.molimm.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda MN. Screening of peptide-displaying phage libraries to identify short peptides mimicking carbohydrates. Methods Enzymol. 2006;416:51–60. doi: 10.1016/S0076-6879(06)16004-8. [DOI] [PubMed] [Google Scholar]
- 34.Taki T, Ishikawa D, Hamasaki H, Handa S. Preparation of peptides which mimic glycosphingolipids by using phage peptide library and their modulation on beta-galactosidase activity. FEBS Lett. 1997;418:219–223. doi: 10.1016/s0014-5793(97)01386-0. [DOI] [PubMed] [Google Scholar]
- 35.Mittag D, Batori V, Neudecker P, Wiche R, et al. A novel approach for investigation of specific and cross-reactive IgE epitopes on Bet v 1 and homologous food allergens in individual patients. Mol Immunol. 2006;43:268–278. doi: 10.1016/j.molimm.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Jensen-Jarolim E, Wiedermann U, Ganglberger E, Zurcher A, et al. Allergen mimotopes in food enhance type I allergic reactions in mice. FASEB J. 1999;13:1586–1592. doi: 10.1096/fasebj.13.12.1586. [DOI] [PubMed] [Google Scholar]
- 37.Ganglberger E, Grunberger K, Sponer B, Radauer C, et al. Allergen mimotopes for 3-dimensional epitope search and induction of antibodies inhibiting human IgE. FASEB J. 2000;14:2177–2184. doi: 10.1096/fj.99-1000com. [DOI] [PubMed] [Google Scholar]
- 38.Hantusch B, Krieger S, Untersmayr E, Scholl I, et al. Mapping of conformational IgE epitopes on Phl p 5a by using mimotopes from a phage display library. J Allergy Clin Immunol. 2004;114:1294–1300. doi: 10.1016/j.jaci.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Cao MJ, Alcocer M, Liu QM, et al. Mapping and characterization of antigenic epitopes of arginine kinase of Scylla paramamosain. Mol Immunol. 2015;65:310–320. doi: 10.1016/j.molimm.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Tordesillas L, Pacios LF, Palacin A, Quirce S, et al. Molecular basis of allergen cross-reactivity: non-specific lipid transfer proteins from wheat flour and peach fruit as models. Mol Immunol. 2009;47:534–540. doi: 10.1016/j.molimm.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 41.Pacios LF, Tordesillas L, Cuesta-Herranz J, Compes E, et al. Mimotope mapping as a complementary strategy to define allergen IgE-epitopes: peach Pru p 3 allergen as a model. Mol Immunol. 2008;45:2269–2276. doi: 10.1016/j.molimm.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 42.Szalai K, Fuhrmann J, Pavkov T, Scheidl M, et al. Mimotopes identify conformational B-cell epitopes on the two major house dust mite allergens Der p 1 and Der p 2. Mol Immunol. 2008;45:1308–1317. doi: 10.1016/j.molimm.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Bogh KL, Nielsen H, Eiwegger T, Madsen CB, et al. IgE versus IgG4 epitopes of the peanut allergen Ara h 1 in patients with severe allergy. Mol Immunol. 2014;58:169–176. doi: 10.1016/j.molimm.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Davies JM, O’Hehir RE, Suphioglu C. Use of phage display technology to investigate allergen-antibody interactions. J Allergy Clin Immunol. 2000;105:1085–1092. doi: 10.1067/mai.2000.107040. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Botas J, de la Hoz B. IgE and IgG4 Epitope Mapping of Food Allergens with a Peptide Microarray Immunoassay. Methods Mol Biol. 2016;1352:235–249. doi: 10.1007/978-1-4939-3037-1_18. [DOI] [PubMed] [Google Scholar]
- 46.Shreffler WG, Lencer DA, Bardina L, Sampson HA. IgE and IgG4 epitope mapping by microarray immunoassay reveals the diversity of immune response to the peanut allergen, Ara h 2. J Allergy Clin Immunol. 2005;116:893–899. doi: 10.1016/j.jaci.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 47.Otsu K, Guo R, Dreskin SC. Epitope analysis of Ara h 2 and Ara h 6: characteristic patterns of IgE-binding fingerprints among individuals with similar clinical histories. Clin Exp Allergy. 2015;45:471–484. doi: 10.1111/cea.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albrecht M, Kuhne Y, Ballmer-Weber BK, Becker WM, et al. Relevance of IgE binding to short peptides for the allergenic activity of food allergens. J Allergy Clin Immunol. 2009;124:328–336. 336e321–326. doi: 10.1016/j.jaci.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 49.Wood RASS, Burks AW, Grishin A, Henning AK, Lindblad R, Stablein D, Sampson HA. A phase 1 study of heat/phenol-killed, E. coli-encapsulated, recombinant modified peanut proteins Ara h 1 Ara h 2 and Ara h 3 (EMP-123) for the treatment of peanut allergy. Allergy. 2013;68:803–808. doi: 10.1111/all.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernard H, Guillon B, Drumare MF, Paty E, et al. Allergenicity of peanut component Ara h 2: Contribution of conformational versus linear hydroxyproline-containing epitopes. J Allergy Clin Immunol. 2015;135:1267–1274. e1261–1268. doi: 10.1016/j.jaci.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 51.Untersmayr E, Szalai K, Riemer AB, Hemmer W, et al. Mimotopes identify conformational epitopes on parvalbumin, the major fish allergen. Mol Immunol. 2006;43:1454–1461. doi: 10.1016/j.molimm.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 52.Bousquet J, Lockey R, Malling HJ, Alvarez-Cuesta E, et al. Allergen immunotherapy: therapeutic vaccines for allergic diseases. World Health Organization. American academy of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1998;81:401–405. doi: 10.1016/s1081-1206(10)63136-5. [DOI] [PubMed] [Google Scholar]
- 53.Burks AW, Shin D, Cockrell G, Stanley JS, et al. Mapping and mutational analysis of the IgE-binding epitopes on Ara h 1, a legume vicilin protein and a major allergen in peanut hypersensitivity. Eur J Biochem. 1997;245:334–339. doi: 10.1111/j.1432-1033.1997.t01-1-00334.x. [DOI] [PubMed] [Google Scholar]
- 54.Burks AW, Williams LW, Helm RM, Connaughton C, et al. Identification of a major peanut allergen, Ara h I, in patients with atopic dermatitis and positive peanut challenges. J Allergy Clin Immunol. 1991;88:172–179. doi: 10.1016/0091-6749(91)90325-i. [DOI] [PubMed] [Google Scholar]
- 55.Maleki SJ, Chung SY, Champagne ET, Raufman JP. The effects of roasting on the allergenic properties of peanut proteins. J Allergy Clin Immunol. 2000;106:763–768. doi: 10.1067/mai.2000.109620. [DOI] [PubMed] [Google Scholar]
- 56.Shin DS, Compadre CM, Maleki SJ, Kopper RA, et al. Biochemical and structural analysis of the IgE binding sites on ara h1, an abundant and highly allergenic peanut protein. J Biol Chem. 1998;273:13753–13759. doi: 10.1074/jbc.273.22.13753. [DOI] [PubMed] [Google Scholar]
- 57.Bogh KL, Kroghsbo S, Dahl L, Rigby NM, et al. Digested Ara h 1 has sensitizing capacity in Brown Norway rats. Clin Exp Allergy. 2009;39:1611–1621. doi: 10.1111/j.1365-2222.2009.03333.x. [DOI] [PubMed] [Google Scholar]
- 58.Savilahti EM, Rantanen V, Lin JS, Karinen S, et al. Early recovery from cow’s milk allergy is associated with decreasing IgE and increasing IgG4 binding to cow’s milk epitopes. J Allergy Clin Immunol. 2010;125:1315–1321. e1319. doi: 10.1016/j.jaci.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomicic S, Norrman G, Falth-Magnusson K, Jenmalm MC, et al. High levels of IgG4 antibodies to foods during infancy are associated with tolerance to corresponding foods later in life. Pediatr Allergy Immunol. 2009;20:35–41. doi: 10.1111/j.1399-3038.2008.00738.x. [DOI] [PubMed] [Google Scholar]
- 60.Vickery BP, Lin J, Kulis M, Fu Z, et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J Allergy Clin Immunol. 2013;131:128–134. e121–123. doi: 10.1016/j.jaci.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandez-Rivas M, Garrido Fernandez S, Nadal JA, Diaz de Durana MD, et al. Randomized double-blind, placebo-controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy. 2009;64:876–883. doi: 10.1111/j.1398-9995.2008.01921.x. [DOI] [PubMed] [Google Scholar]
- 62.Burks AW, Jones SM, Wood RA, Fleischer DM, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez-Botas J, Cerecedo I, Zamora J, Vlaicu C, et al. Mapping of the IgE and IgG4 sequential epitopes of ovomucoid with a peptide microarray immunoassay. Int Arch Allergy Immunol. 2013;161:11–20. doi: 10.1159/000343040. [DOI] [PubMed] [Google Scholar]
- 64.Koppelman SJ, de Jong GA, Laaper-Ertmann M, Peeters KA, et al. Purification and immunoglobulin E-binding properties of peanut allergen Ara h 6: evidence for cross-reactivity with Ara h 2. Clin Exp Allergy. 2005;35:490–497. doi: 10.1111/j.1365-2222.2005.02204.x. [DOI] [PubMed] [Google Scholar]
- 65.Kulis M, Chen X, Lew J, Wang Q, et al. The 2S albumin allergens of Arachis hypogaea, Ara h 2 and Ara h 6, are the major elicitors of anaphylaxis and can effectively desensitize peanut-allergic mice. Clin Exp Allergy. 2012;42:326–336. doi: 10.1111/j.1365-2222.2011.03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Porterfield HS, Murray KS, Schlichting DG, Chen X, et al. Effector activity of peanut allergens: a critical role for Ara h 2, Ara h 6, and their variants. Clin Exp Allergy. 2009;39:1099–1108. doi: 10.1111/j.1365-2222.2009.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen X, Zhuang Y, Wang Q, Moutsoglou D, et al. Analysis of the effector activity of Ara h 2 and Ara h 6 by selective depletion from a crude peanut extract. J Immunol Methods. 2011;372:65–70. doi: 10.1016/j.jim.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Negi SS, Braun W. Automated detection of conformational epitopes using phage display Peptide sequences. Bioinform Biol Insights. 2009;3:71–81. doi: 10.4137/bbi.s2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vieths S, Scheurer S, Ballmer-Weber B. Current understanding of cross-reactivity of food allergens and pollen. Ann N Y Acad Sci. 2002;964:47–68. doi: 10.1111/j.1749-6632.2002.tb04132.x. [DOI] [PubMed] [Google Scholar]
- 70.Mittag D, Akkerdaas J, Ballmer-Weber BK, Vogel L, et al. Ara h 8, a Bet v 1-homologous allergen from peanut, is a major allergen in patients with combined birch pollen and peanut allergy. J Allergy Clin Immunol. 2004;114:1410–1417. doi: 10.1016/j.jaci.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 71.Cuesta-Herranz J, Lazaro M, Figueredo E, Igea JM, et al. Allergy to plant-derived fresh foods in a birch- and ragweed-free area. Clin Exp Allergy. 2000;30:1411–1416. doi: 10.1046/j.1365-2222.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez-Perez R, Crespo JF, Rodriguez J, Salcedo G. Profilin is a relevant melon allergen susceptible to pepsin digestion in patients with oral allergy syndrome. J Allergy Clin Immunol. 2003;111:634–639. doi: 10.1067/mai.2003.74. [DOI] [PubMed] [Google Scholar]
- 73.Barber D, de la Torre F, Feo F, Florido F, et al. Understanding patient sensitization profiles in complex pollen areas: a molecular epidemiological study. Allergy. 2008;63:1550–1558. doi: 10.1111/j.1398-9995.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- 74.Cuesta-Herranz J, Lazaro M, Martinez A, Figueredo E, et al. Pollen allergy in peach-allergic patients: sensitization and cross-reactivity to taxonomically unrelated pollens. J Allergy Clin Immunol. 1999;104:688–694. doi: 10.1016/s0091-6749(99)70343-x. [DOI] [PubMed] [Google Scholar]
- 75.Pastor C, Cuesta-Herranz J, Cases B, Perez-Gordo M, et al. Identification of major allergens in watermelon. Int Arch Allergy Immunol. 2009;149:291–298. doi: 10.1159/000205574. [DOI] [PubMed] [Google Scholar]
- 76.Lopez-Torrejon G, Crespo JF, Sanchez-Monge R, Sanchez-Jimenez M, et al. Allergenic reactivity of the melon profilin Cuc m 2 and its identification as major allergen. Clin Exp Allergy. 2005;35:1065–1072. doi: 10.1111/j.1365-2222.2005.02303.x. [DOI] [PubMed] [Google Scholar]
- 77.Tordesillas L, Pacios LF, Palacin A, Cuesta-Herranz J, et al. Characterization of IgE epitopes of Cuc m 2, the major melon allergen, and their role in cross-reactivity with pollen profilins. Clin Exp Allergy. 2010;40:174–181. doi: 10.1111/j.1365-2222.2009.03401.x. [DOI] [PubMed] [Google Scholar]
- 78.Zuidmeer L, van Ree R. Lipid transfer protein allergy: primary food allergy or pollen/food syndrome in some cases. Curr Opin Allergy Clin Immunol. 2007;7:269–273. doi: 10.1097/ACI.0b013e32814a5401. [DOI] [PubMed] [Google Scholar]
- 79.Fernandez-Rivas M, Gonzalez-Mancebo E, Rodriguez-Perez R, Benito C, et al. Clinically relevant peach allergy is related to peach lipid transfer protein, Pru p 3, in the Spanish population. J Allergy Clin Immunol. 2003;112:789–795. doi: 10.1016/S0091. [DOI] [PubMed] [Google Scholar]
- 80.Palacin A, Varela J, Quirce S, del Pozo V, et al. Recombinant lipid transfer protein Tri a 14: a novel heat and proteolytic resistant tool for the diagnosis of baker’s asthma. Clin Exp Allergy. 2009;39:1267–1276. doi: 10.1111/j.1365-2222.2009.03280.x. [DOI] [PubMed] [Google Scholar]
- 81.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 82.Sartorius R, D’Apice L, Trovato M, Cuccaro F, et al. Antigen delivery by filamentous bacteriophage fd displaying an anti-DEC-205 single-chain variable fragment confers adjuvanticity by triggering a TLR9-mediated immune response. EMBO Mol Med. 2015;7:973–988. doi: 10.15252/emmm.201404525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hashiguchi S, Yamaguchi Y, Takeuchi O, Akira S, Sugimura K. Immunological basis of M13 phage vaccine: Regulation under MyD88 and TLR9 signaling. Biochem Biophys Res Commun. 2010;402:19–22. doi: 10.1016/j.bbrc.2010.09.094. [DOI] [PubMed] [Google Scholar]
- 84.van Houten NE, Zwick MB, Menendez A, Scott JK. Filamentous phage as an immunogenic carrier to elicit focused antibody responses against a synthetic peptide. Vaccine. 2006;24:4188–4200. doi: 10.1016/j.vaccine.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Costa LE, Goulart LR, Pereira NC, Lima MI, et al. Mimotope-Based Vaccines of Leishmania infantum Antigens and Their Protective Efficacy against Visceral Leishmaniasis. PLoS One. 2014;9:e110014. doi: 10.1371/journal.pone.0110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manoutcharian K, Diaz-Orea A, Gevorkian G, Fragoso G, et al. Recombinant bacteriophage-based multiepitope vaccine against Taenia solium pig cysticercosis. Vet Immunol Immunopathol. 2004;99:11–24. doi: 10.1016/j.vetimm.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 87.Hardy B, Raiter A. A mimotope peptide-based anti-cancer vaccine selected by BAT monoclonal antibody. Vaccine. 2005;23:4283–4291. doi: 10.1016/j.vaccine.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 88.Wagner S, Hafner C, Allwardt D, Jasinska J, et al. Vaccination with a human high molecular weight melanoma-associated antigen mimotope induces a humoral response inhibiting melanoma cell growth in vitro. J Immunol. 2005;174:976–982. doi: 10.4049/jimmunol.174.2.976. [DOI] [PubMed] [Google Scholar]
- 89.Zhang D, Chen Y, Fan D. MG7 mimotope-based DNA vaccination for gastric cancer. Expert Rev Vaccines. 2006;5:223–231. doi: 10.1586/14760584.5.2.223. [DOI] [PubMed] [Google Scholar]
- 90.Roehnisch T, Then C, Nagel W, Blumenthal C, et al. Phage idiotype vaccination: first phase I/II clinical trial in patients with multiple myeloma. J Transl Med. 2014;12:119. doi: 10.1186/1479-5876-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dewitt AM, Andersson K, Peltre G, Lidholm J. Cloning, expression and immunological characterization of full-length timothy grass pollen allergen Phl p 4, a berberine bridge enzyme-like protein with homology to celery allergen Api g 5. Clin Exp Allergy. 2006;36:77–86. doi: 10.1111/j.1365-2222.2006.02399.x. [DOI] [PubMed] [Google Scholar]
- 92.Borghesan F, Mistrello G, Amato S, Giuffrida MG, et al. Mugwort-fennel-allergy-syndrome associated with sensitization to an allergen homologous to Api g 5. Eur Ann Allergy Clin Immunol. 2013;45:130–137. [PubMed] [Google Scholar]
- 93.Lukschal A, Wallmann J, Bublin M, Hofstetter G, et al. Mimotopes for Api g 5, a Relevant Cross-reactive Allergen, in the Celery-Mugwort-Birch-Spice Syndrome. Allergy Asthma Immunol Res. 2016;8:124–131. doi: 10.4168/aair.2016.8.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]