Abstract
In a sample of children with traumatic brain injury, this magnetic resonance imaging (MRI) based investigation examined whether presence of a focal lesion uniquely influenced cortical thickness in any brain region. Specifically, the study explored the relation of cortical thickness to injury severity as measured by Glasgow Coma Scale (GCS) score and length of stay (LOS), along with presence of encephalomalacia, focal white matter lesions (WMLs) or presence of hemosiderin deposition as a marker of shear injury. For comparison, a group of children without head injury but with orthopedic injury (OI) of similar age and sex were also examined. Both TBI and OI children had normally reduced cortical thickness with age, assumed to reflect neuronal pruning. However, the reductions observed within the TBI sample were similar to those in the OI group, suggesting that in this sample TBI, per se, did not uniquely alter cortical thickness in any brain region at the group level. Injury severity in terms of GCS or longer LOS was associated with greater reductions in frontal and occipitoparietal cortical thickness. However, presence of focal lesions were not related to unique changes in cortical thickness despite having a prominent distribution of lesions within frontotemporal regions among children with TBI. Because focal lesions were highly heterogeneous, their association with cortical thickness and development appeared to be idiosyncratic, and not associated with group level effects.
Keywords: Pediatric Traumatic Brain Injury, Cortical Thickness, Brain Development, Lesion Analysis
Traumatic brain injury (TBI) is typically classified by severity of injury and whether neuroimaging studies identify focal lesions either acutely or during the chronic phase1-4. Interestingly, focal lesions may occur at any injury severity level although the size and number of focal lesions typically increase with injury severity5. Since the brain is in its most dynamic phase of development from infancy through early adulthood 6, there has long been speculation that age at the time TBI occurs may moderate brain development post-injury, although only few studies have addressed this topic 7-10.
One of the well-established dynamic changes with brain maturation in typically developing children is a reduction in cortical thickness11-13. Assumed to be the consequence of neuronal pruning, reduction in gray matter as measureable by various magnetic resonance imaging (MRI) techniques has been reported by numerous studies12, 14-16. In a child's brain that otherwise was healthy until sustaining a TBI, how focal lesions from the injury might influence cortical thickness and development is not understood. In adult cases of stroke or disorders like multiple sclerosis, subcortical lesions impact regional cortical thickness depending on the area and location of the lesion or infarct17-21. In adult TBI reduced cortical thickness has been observed and may be related to injury severity22, 23. Whether focal lesions systematically alter cortical development in pediatric TBI has not been previously examined.
The Social Outcomes in Brain Injured Kids (SOBIK) project is a multicenter investigation that has examined neuroimaging, neurocognitive and social behavioral outcome during the chronic phase (> 12 months) of pediatric TBI as compared to group-matched children with orthopedic injury (OI) but meeting no criteria for having sustained a TBI see 5, 24, 25-27. For the children with TBI, the SOBIK inclusion criteria required head injured participants with a Glasgow Coma Scale (GCS) of 13 or higher also to have positive day-of-injury (DOI) neuroimaging findings, typically based on computed tomography (CT). Those with moderate-to severe TBI were included regardless of neuroimaging findings as long as GCS was ≤ 12. Accordingly, injury severity in the SOBIK study ranged from complicated mild TBI (cm-TBI) to moderate (GCS 9-12) and severe (GCS ≤ 8)see 5.
On MRI studies performed during the chronic phase (> 6 months post-injury) of TBI, three commonly identified abnormalities are areas with encephalomalacia, white matter lesions (WMLs) or regions of hemosiderin deposition 1, 2. WMLs are typically defined by abnormally bright or “hyperintense” signal within the white matter, best viewed within a T2- weighted fluid- attenuated inversion recovery (FLAIR) sequence, and referred to as a white matter hyperintense (WMH) signal abnormality. When observed after TBI, large WMH signal abnormalities reflect major disruptions in white matter integrity28-30, but the role that smaller, punctate WMHs play as a pathological consequence of brain injury is less well understood31. A by-product of hemorrhage is hemosiderin, because of retained ferritin within degraded blood either intraparenchymally or on the surface of the brain from prior contusion, hemosiderin has a “hypointense” or dark signal, best viewed in the gradient recalled or susceptibility weighted sequences 32. Regions of focal encephalomalacia often arise from where prior cortical contusions have occurred, including those associated with depressed skull fractures that mechanically distort brain parenchyma. Encephalomalacia reflects focal degeneration with loss of brain parenchyma combined with localized increased cerebrospinal fluid (CSF) that backfills where tissue loss has occurred 1, 2. Such regions are often best defined within T1- and T2-weighted images where the mixture of parenchymal reduction combined with localized CSF increase can be visualized.
Surface contusions are more likely to occur in frontal and temporal lobes, especially the polar areas, orbitofrontal regions and the ventral and mesial aspects of the temporal lobe, because of impact of brain tissue against the bony ridges within the base of the skull 1, 2. WMLs in TBI are thought to reflect more specific trauma to white matter likely a result of deformation forces that either shear axons or degrade axon integrity as a result of focal damage and/or secondary (Wallerian) degeneration. Hemosiderin deposition in the brain of the child who experiences a TBI is also thought to be a reflection of shear injury wherein vascular integrity has been compromised associated with blood extravasation33. While regions of hemosiderin deposition often occur within the white matter they may occur anywhere in the brain, especially where contusions have occurred. Punctate areas of hemosiderin deposition may or may not overlap with WMHs. Each of these lesion types has unique neuroimaging features that readily permit differentiation using different MRI sequences and classification parameters 34. Likewise, focal lesions from TBI occur from different underlying neuropathological and biomechanical origins as a result of the trauma. While focal lesions may co-occur they often do not overlap5. How such lesions influence cortical thickness or volume in pediatric TBI is unknown and is the focus of the current investigation. Following TBI focal areas of cortical contusion would likely alter local cortical development but whether this affects adjacent or contralateral regions of cortex in a consistent manner at the group level is unknown.
Injury severity was examined in two ways, as assessed by GCS and length of stay LOS, see 35 for using LOS as a marker of overall injury severity, 36. LOS is an accepted proxy for injury severity reflecting the assumption that more severe injuries result in longer LOS37. Longer LOS has been associated with greater levels of overall brain atrophy in previous studies36. However, LOS is also influenced by non-CNS injuries along with other healthcare and treatment issues during acute and rehabilitative in-patient care 38; it therefore is not as direct a measure of TBI severity as GCS. The current study examined the relation of cortical thickness to injury severity (GCS and LOS) and type of injury (TBI, OI or combined), as well as association with the presence of focal lesions. Cortical thickness was derived from analyses using the ‘Query, Design, Estimate, Contrast’ (QDEC) function from FreeSurfer (freesurfer.net/fswiki/FreeSurferWiki). In previous voxel-based analyses of MRI findings in this cohort, because of the heterogeneity of lesion location, the only consistent gray matter differences between TBI and OI were in the basal forebrain region5, 39. Because lesion heterogeneity is substantial and each traumatic injury is unique to the individual, we hypothesized that at a group level of analysis uniform changes in cortical thickness would not occur in relation to focal lesions.
Methods
Participants
From 2006 through 2010, a total of 143 participants were recruited into a multi-site (Toronto, Canada, Columbus, US, and Cleveland, US) study of social outcomes in children with TBI, of whom 124 underwent MRI scanning at a minimum of 12 months post injury. Study details have been previously published5. The study was conducted in accordance with established ethical guidelines and received institutional ethics approval from all three data collection sites (The Hospital for Sick Children and the University of Toronto; Rainbow Babies & Children's Hospital and Case Western Reserve University; The Research Institute at Nationwide Children's Hospital and The Ohio State University) and the neuroimaging analysis site (Brigham Young University); written informed consent and/or assent was obtained from all participants. The TBI group was composed of 82 children (n= 72 scanned) who experienced head trauma resulting in hospitalization and who had a recorded DOI post-resuscitation GCS score of 12 or less, or a score of 13-15 with positive CT imaging for brain insult or skull fracture. For the 61 children with OI (n = 52 scanned), some form of orthopedic injury had occurred but not in association with head trauma; if GCS was recorded for whatever reason it had to be scored as a 15. In addition to GCS as an index of severity, LOS was also obtained for each child as the total number of days hospitalized starting with the emergency department (ED) visit.
Injury Severity
Two measures were used to examine injury severity, the GCS score and LOS. GCS is the most common metric for examining injury severity in neuroimaging studies 40. In the current study, the initial post-resuscitation GCS score was used. LOS was defined as the number of in-patient days until the day of discharge.
MRI
MRI was performed during the chronic phase of injury (i.e., an average of 2.7 years post trauma). Magnetic field strength was 1.5 Tesla for all studies with specifics for image sequences reported in Bigler et al. 5 . Briefly, the Toronto and Columbus sites used GE Signa Excite scanners and the Cleveland site used a Siemens Symphony scanner. All sites acquired the following sequences on each participant: thin slice (1.2 mm thick, no gap), volume acquisition T1-weighted Ultrafast 3D Gradient Echo commonly referred to as MPRAGE or FSPGR (depending on scanner manufacturer) for FreeSurfer analyses; a dual-echo proton density (PD)/T2 weighted sequence (thickness: 3mm); FLAIR (thickness: 3mm); and GRE (thickness: 3mm).
The FreeSurfer analyses used version 5.3 (http://surfer.nmr.mgh.harvard.edu/). Images were processed through the automated pipeline, which corrects for motion, normalizes voxel intensity throughout, skull strips the image, registers each subject to a standard template, performs a pial surface correction, and segments cortical and subcortical regions. To insure image fidelity sufficient for the purpose of examining cortical thickness, following automated processing, manual inspection of each subject was performed for accuracy and any visually identifiable errors corrected through the FreeSurfer toolbox “tkmedit.” Following post-processing, each subject was re-processed through the automated pipeline to account for manual intervention.
A QDEC (Query, Design, Estimate, and Contrast) group analysis was then performed separately for OI and TBI participants. For all analyses, each subject was first smoothed with a 15mm FWHM (full-width, half-maximum) Gaussian kernel followed by the use of a general linear model (GLM) for analysis of each hemisphere independently. Cortical thickness was assessed in relation to injury severity in terms of GCS and LOS, as well as contrasting groups defined in terms of white matter pathology on the FLAIR, hemosiderin deposition on the GRE, or regional focal encephalomalacia as originally identified by a clinical neuroradiologist. Thickness plots, when significant after correction, were shown in each hemisphere while accounting for any differences resulting from sex or age. A Monte-Carlo simulation was performed to correct for multiple comparisons and family-wise error (FWE, p < 0.05). Only regions that survived FWE correction were shown on an ‘inflated’ cortical surface map that accounts for cortical volume not only at the crest of a gyrus, but deep within the sulcus.
White Matter Lesions, Hemosiderin Deposition and Encephalomalacia Identification
Preprocessing
All preprocessing of the original DICOM images was implemented using the Advanced Normalization Tools 41 toolset.
ROI Creation
To create uniform images that represent lesion data for the entire dataset across the different lesion types, separate lesion mapping protocols for region of interest (ROI) creation were generated using automated processes that included some operator editing, especially for certain lesion margins with indistinct boundaries or for small punctate WMHs or hemosiderin deposits. Each ROI created was also compared to a radiological report to confirm lesion identification.
White Matter Lesion ROI
All WMHs were automatically traced using the Lesion Segmentation Toolbox (version 1.2.3; 2013; http://www.applied-statistics.de/lst.html) for SPM8 see 42 following the recommended protocol 43, with the binary lesion map threshold set to .3. As a part of this protocol, all of the ROIs that were created were placed in MNI space 44. Once all of the WML ROI's were established and reviewed for accuracy, they were summed together using the “fslmaths” tool from the FMRIB Software Library (version 5.0.7; 2014: http://fsl.fmrib.ox.ac.uk/fsldownloads/fsldownloadmain.html) to form one glass brain output representative of all WMLs for TBI subjects.
Encephalomalacia ROI
Encephalomalacia ROIs were generated through an automated pipeline created for this project. In an attempt to keep operator interface to a minimum, a simple algorithm was applied to all of the images to automatically designate lesions. To begin, all of the control brains were smoothed and then used to create a template brain that represented the average thresholds for the entire dataset. Next, the standard deviation of the control population was used to create an upper and lower bound representation of what could be defined as normal variation. Greater focus was on the lower boundary because it defines which voxels represent atrophied tissue (represented as thresholds below the normal variation). Using this method, all of the voxels that were below the lower-bound threshold, were marked as possible lesions in each TBI subject. The output lesion masks from this method were fairly good representations of atrophied tissues as compared to the template. However, hand edits were made to remove any “noisy” voxels that were mislabeled. Once this was done, 20 participants were identified with each lesion map compared to the original T1 images to improve accuracy. Each ROI was corrected to better represent each participant's traumatic parenchymal changes and then saved accordingly.
After all the ROI's were collected for these 20 participants with identifiable encephalomalacia, “fslmaths” was used to add all of them together into one overlay. The resulting image was an ROI that not only encoded lesion location, but also a frequency color map that reflected how many participants had overlapping features of encephalomalacia within a particular location.
Hemosiderin ROI
To create an ROI that represented identifiable hemosiderin deposits in the dataset, findings of signal hypointensity compared the participants’ GRE sequence with a template brain. For each finding, the hemosiderin ROI was traced onto the template to ensure that all of the participants ROI's would be in the same space. Once all of the tracings were made and compared to the radiological report, all of the ROI's were aggregated into a single image accomplished by using “fslmaths” to combine all of the images together.
Image Generation
Once the different type of lesion ROI's were completed, they were loaded into Mango version 3.2.6; 45 for viewing. Each ROI was individually placed into the program with a corresponding template mask. Using the “build surface” feature, a three-dimensional representation of each lesion type then was created. The colors and contrasts of each image were adjusted to facilitate interpretation. Screenshots were then taken of each view to generate the final images. The resulting images represent the lesion distribution for the SOBIK children with TBI.
Results
Injury Severity and Cortical Thickness
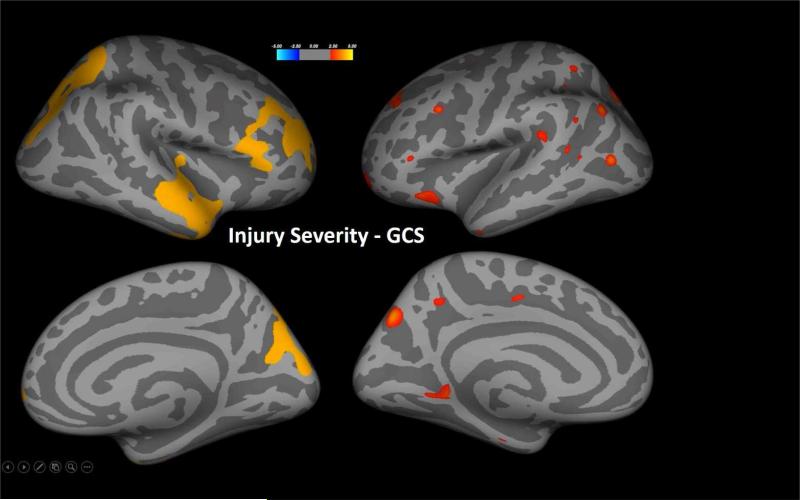
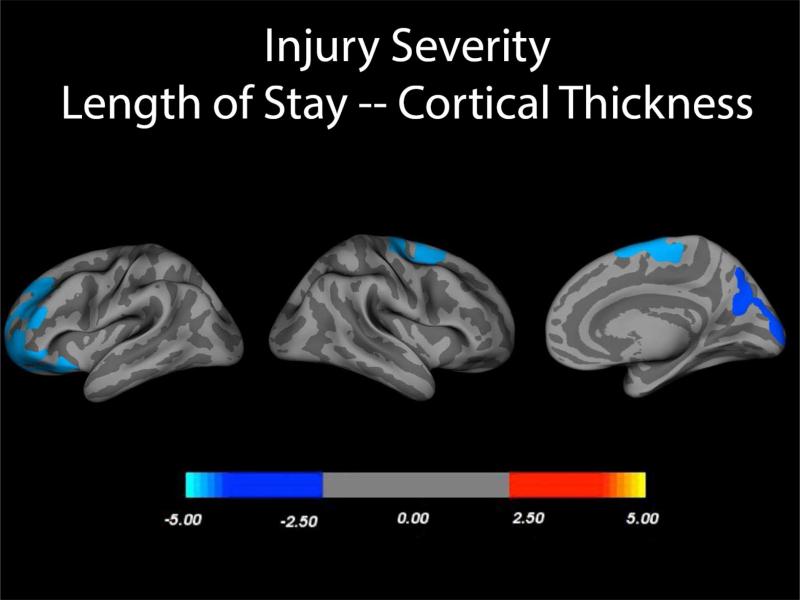
As shown in Figures 1 and 2, injury severity as measured by GCS and LOS related to cortical thickness but interestingly, with different distributions of cortical thinning. For both analyses the contrasts were made just within the TBI participants and either the GCS or LOS variable. In children with TBI, GCS was related to cortical thickness after controlling for age and sex wherein the right frontal and temporal polar regions along with an occipitoparietal distribution (see Figure 1) exhibited reduced thickness with lower GCS. In contrast, GCS-related findings in the left hemisphere were less robust and more scattered. Longer LOS, a proxy for more severe injury was associated with reduced thickness in the anterior left frontal, posterior right frontal and posterior right occipitoparietal cortices as shown in Figure 2. This association remained even after controlling for age and sex. Although some OI children had significant orthopedic and other injuries that resulted in an extended LOS, LOS was not related to cortical thickness in this group.
Figure 1.
In child participants with TBI, statistical p-maps derived from FreeSurfer QDEC analyses demonstrated several regions with significant reduction in cortical thickness related to GCS, after Monte Carlo correction for familywise error. Lower GCS reflective of more severe injury was associated with reduced cortical thickness, most notable in the right frontal and temporal polar regions along with regions in an occipitoparietal distribution.
Figure 2.
In child participants with TBI, statistical p-maps derived from FreeSurfer QDEC analyses demonstrated several regions with significant reduction in cortical thickness, after Monte Carlo correction for family wise error, related to length of stay (LOS), co-varying for sex and age. Similar analyses run with OI participants yielded no significant differences in cortical thickness related to LOS.
Cortical Thickness, Age and Injury
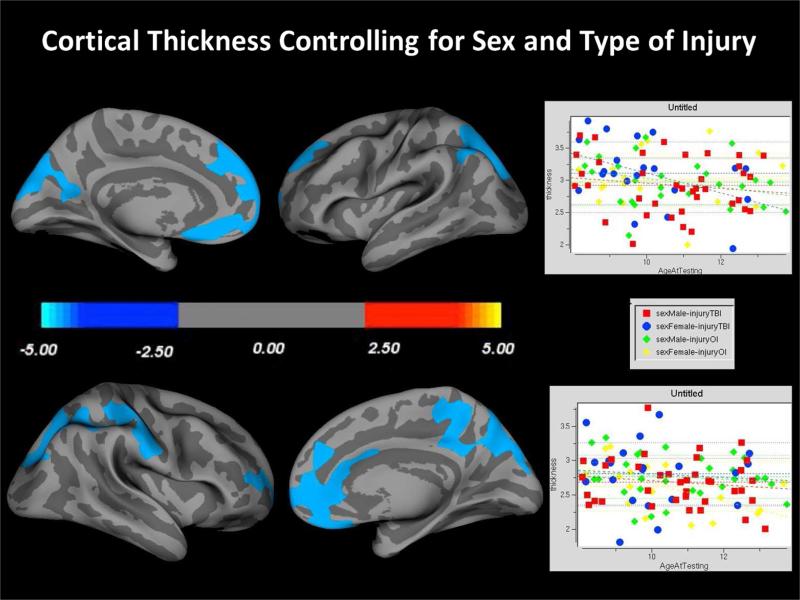
Both the OI and TBI groups exhibited reduced cortical thickness with age. Interestingly, the distributions of age-related changes were similar in the two groups and so the cortical thickness plots presented in Figure 3 combined all SOBIK children into a single group but retained the individual thickness values by age, sex and whether TBI or OI. As visualized, age was associated with decreasing cortical thickness regardless of whether a TBI or OI participant.
Figure 3.
Typical regions of age mediated reductions with increasing age are plotted examining all participants in an age X cortical thickness interaction analysis. At a group level, there were no significant findings suggesting a unique contribution of TBI or OI to altered trajectories of cortical thickness by age.
Distributions of Focal Lesions
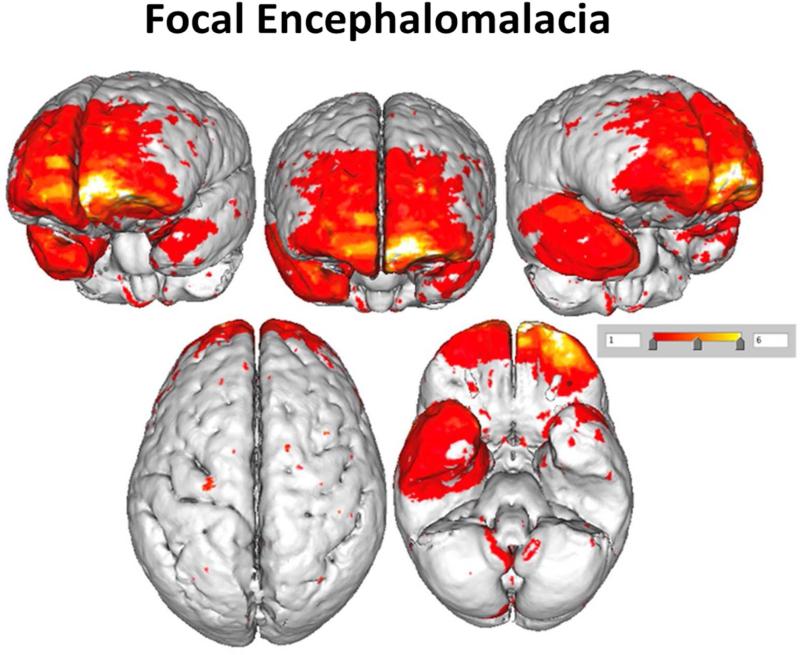
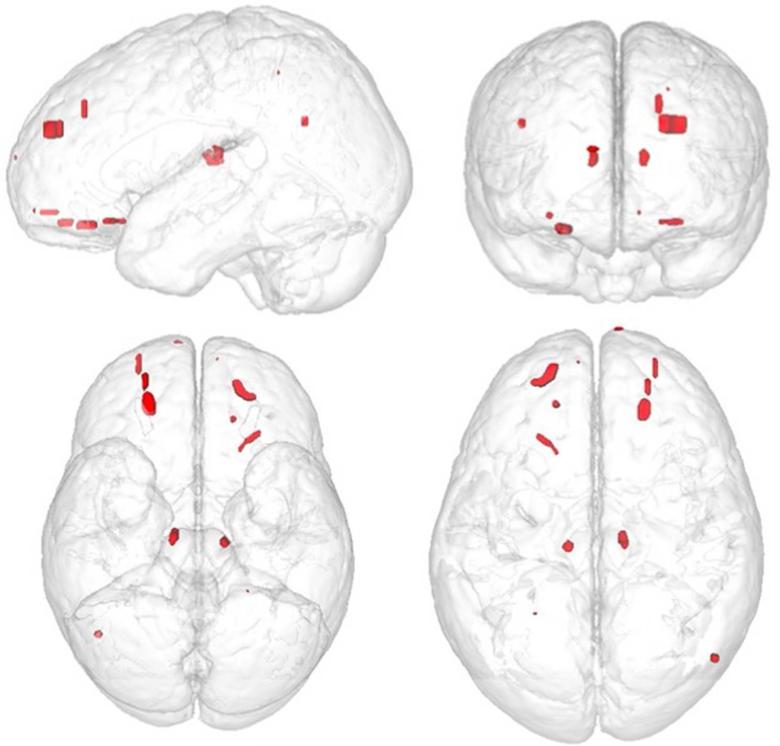
Figure 4 depicts the surface areas where overlapping encephalomalacia occurred. The most distinct regions where encephalomalacia occurred were primarily within frontotemporal regions but as can be seen from Figure 4 there was very little overlap in encephalomalacic lesions except within the frontal polar region, especially on the left. Despite the presence of focal pathology when the participants with just focal encephalomalacia were compared to the TBI participants without focal pathology or to the OI group, no regional differences in cortical thickness as a function of encephalomalacia survived statistical correction for multiple comparisons.
Figure 4.
This illustration plots where focal encephalomalacic changes occurred in TBI participants using a prototype brain image. No focal areas of encephalomalacia were identified in the OI controls. Note that the ‘heat map’ shows relatively few regions of overlap. Red depicts solitary areas of focal encephalomalacia that do not overlap with other participants, and overlap is depicted where the color is orange to white.
Distribution of White Matter Lesions (WMLs)
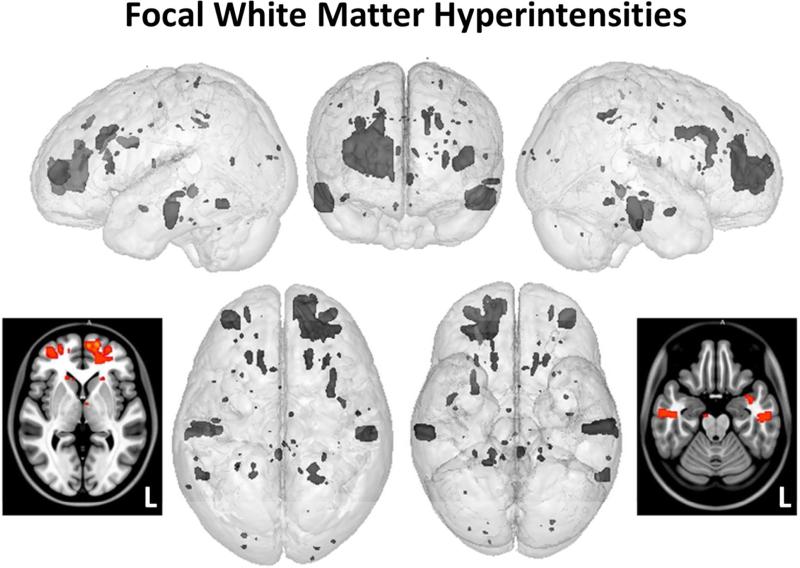
The distribution of FLAIR-identified WMLs also followed a frontotemporal pattern as shown in Figure 5. Despite the frontotemporal pattern of focal white matter pathology, when participants with only focal WMLs were compared to the TBI participants without such pathology or the OI group, no regional differences in cortical thickness as a function of WMLs survived statistical correction for multiple comparisons.
Figure 5.
Also using the same rendered prototype brain model as in Figure 3, but now spatially localizing where T2-FLAIR hyperintense signal abnormalities were identified, the loci of these areas were plotted. The axial smoothed renderings provide a ‘heat map’ (same scale as in Figure 3) identifying where overlap occurred, which was sparse and only in the left frontal lobe. Note – some of the hyperintense T2-FLAIR signal abnormalities occurred in predominantly subcortical gray matter structures, a less frequent location than within the white matter but nonetheless, known areas where hyperintense signal abnormalities may occur see 57, 58.
Distribution of Hemosiderin Deposition
The distribution of hemosiderin deposition likewise was predominantly frontal as shown in Figure 6. However, when the participants with only focal hemosiderin deposition were compared to TBI participants without focal pathology or the OI group, no regional differences in cortical thickness in relation to the presence of hemosiderin deposition survived statistical correction for multiple comparisons.
Figure 6.
Also using the same rendered prototype brain model as in Figure 3, but now spatially localizing where GRE hypointense signal abnormalities were identified, the loci of these areas were plotted. None overlapped and accordingly, only red is depicted.
Combined Focal Lesion Effects on Cortical Thickness
When all subjects with focal lesion, regardless of type, were pooled together and cortical thickness examined, as a function of presence or absence of focal lesions, no group differences survived statistical correction for multiple comparisons.
DISCUSSION
Injury severity based on GCS and LOS was associated with regional reductions in cortical thickness although the patterns differed for these two measures of severity. As previously stated GCS is likely a more direct measure of actual CNS injury where LOS captures not just the brain injury but systemic injuries and other potential secondary injuries (i.e. metabolic, respiratory care, etc.). Accordingly, it is not surprising that these two measures of injury severity differed in their relation to cortical thickness. Interestingly, the presence of any kind of focal lesion did not specifically relate to any unique distribution of reduced cortical thickness. However, as a group, those with any focal findings had longer LOS, so LOS and focal pathology were both associated with injury severity. Indeed, as shown in Figure 4, there was greater left frontal encephalomalacia and so the LOS findings may reflect the interaction between severity and LOS. Sample size was insufficient to explore these relations any further but they are likely to be complex as focal injuries are known to be related to greater neuroinflammation and secondary brain injury30. Both OI and TBI children displayed the expected age-related reductions in thickness, and this finding was not unique to the TBI group. In this sense, within the SOBIK sample merely experiencing a TBI at the group level of analysis did not distinctively alter or accelerate any apparent systematic age-dependent reductions in cortical thickness.
For consistent changes in cortical thickness to be manifested within a particular region, as a function of the brain injury there would need to be consistency in the underlying pathology. Figures 4-5 show just the opposite, with little or no consistency in the distribution of lesions across participants. As shown in Figure 4, although most of the frontal lobe is colorized, only a few areas within the frontal polar region overlap (orange to white colorization). The few overlapping regions of direct parenchymal damage, combined with the heterogeneity of mechanism along with individual differences across children diminishes the likelihood of any uniformity in the parenchymal injury from trauma. To fully examine outcomes related to focal injury, a much larger sample size would be needed, so that pathology could be categorized by location and also to better control for injury severity as well as age and age at the time of injury.
While GCS was predictive of systematic change in cortical thickness, the differences were not uniformly distributed across the two hemispheres. GCS was based on the post-resuscitation score but several children within the moderate or severe TBI categories did not have visibly identifiable neuroimaging abnormalities despite having a GCS ≤ 12. In these children the initially low GCS was likely associated with an upper brainstem injury resulting in loss or alteration of consciousness, but no focal or otherwise diffuse injury notable on neuroimaging studies. Some of these children recovered quickly and had short LOS. In contrast, more severely injured children would likely remain longer in the hospital regardless of GCS and in this instance, for the SOBIK TBI group, LOS and GCS controlling for age and sex resulted in different distributions of reduced cortical thickness with both showing reductions in occipitoparietal regions. There may also be a host of other variables that could relate to cortical thickness including pre-injury factors, neuropsychiatric sequelae and medication treatment and/or medical interventions that the SOBIK investigation was not designed to assess 46, 47. Future studies will need to more thoroughly explore pre-injury and potential treatment variables as they relate to cortical thickness in pediatric TBI.
Although the occipital region is far removed from where the majority of focal lesions in the frontal region occurred, one potential explanation is that the greater frontal pathology from trauma disrupts the long-coursing parietofrontal and occipitofrontal tracts. As demonstrated in Figures 4-6 the lesion patterns are diverse with minimal overlap, but the majority of children with focal lesions, regardless of type, had a frontal distribution. While the lesions across numerous frontal lobe sites, the projection of occipitofrontal and parietofrontal fasciculi could be commonly affected48. Since the final common white matter pathway of the inferior fronto- occipital fasciculus (IFOF) courses along the inferior frontal lobe, regardless of where a focal inferior frontal lesion may occur, it would potentially affect the IFOF. Similarly, coursing in parallel with but superior to the IFOF, the superior longitudinal fasciculus (SLF) connects frontal and parietal regions, and is vulnerable to the effects of TBI 49. Likewise, biomechanical, post-mortem neuropathological studies and advanced neuroimaging techniques like diffusion tensor imaging have demonstrated the likelihood of injury to these major white matter tracts even when focal lesions are not present50-54. Disrupting the IFOF and SLF with disparate lesions in the frontal lobe may be responsible for the occipital and parietal cortical thinning. Another factor associated with more posterior changes in cortical thickness in the absence of focal lesions in occipitoparietal regions may be the distribution of biomechanical deformation that comes with coup–contrecoup injuries to brain parenchyma during trauma 55.
The next step in these analyses will be to explore how thickness relates to outcome7, 10, 56. The current investigation demonstrates that there is not a simple relationship between presence of focal lesions and cortical thickness. Certainly, focal lesions adversely influence brain development at some level, but given their idiosyncratic and highly heterogeneous distribution -- albeit mostly within frontal and temporal areas -- uniform changes at the group level in terms of cortical thickness were not found to be associated with focal lesions. How best to characterize the neuropathological and neurobehavioral influences of these lesions is the challenge. It may be that in modelling injury factors from TBI related to neurocognitive and neurobehavioral sequelae, more complex models that include added information about both cortical and subcortical pathologies and their network connectivity will better delineate relations between injury and outcome.
Acknowledgment
Supported in part by grants from the National Institute of Child Health and Human Development (Grant Nos. 5R01HD048946 and 3R01HD048946-05S1). Dr. Bigler co-directs the Neuropsychological Assessment and Research Laboratory at Brigham Young University which provides forensic consultation. Manuscript assistance of Kaylee Sowards is greatly appreciated.
Contributor Information
Erin D. Bigler, Department of Psychology and the Neuroscience Center, Brigham Young University, Provo, UT; Department of Psychiatry, University of Utah, Salt Lake City.
Brandon A. Zielinski, Departments of Pediatrics and Neurology, University of Utah, Salt Lake City
Naomi Goodrich-Hunsaker, Department of Psychology, Brigham Young University, Provo, Utah
Garrett M. Black, Department of Psychology, Brigham Young University, Provo, Utah
Trevor Huff, Department of Psychology, Brigham Young University, Provo, Utah.
Zachary Christiansen, Department of Psychology, Brigham Young University, Provo, Utah.
Dawn-Marie Wood, Department of Psychology, Brigham Young University, Provo, Utah
Tracy J. Abildskov, Department of Psychology, Brigham Young University, Provo, Utah
Maureen Dennis, Program in Neuroscience & Mental Health, The Hospital for Sick Children, Toronto Department of Surgery and Department of Psychology, University of Toronto, Toronto.
H. Gerry Taylor, Department of Pediatrics, Case Western Reserve University and Rainbow Babies & Children's Hospital, University Hospitals Case Medical Center, Cleveland, Ohio
Kenneth Rubin, Department of Psychology, University of Maryland, College Park, Maryland
Kathryn Vannatta, Department of Pediatrics, The Ohio State University, Center for Behavioral Health, Columbus Children's Research Institute, Columbus, Ohio
Cynthia A. Gerhardt, Department of Pediatrics, The Ohio State University, Center for Behavioral Health, Columbus Children's Research Institute, Columbus, Ohio
Terry Stancin, Department of Pediatrics, Case Western Reserve University, Rainbow Babies & Children's Hospital, University Hospitals Case Medical Center Department of Psychiatry, MetroHealth Medical Center, Cleveland, Ohio.
Keith Owen Yeates, Department of Pediatrics, The Ohio State University Center for Biobehavioral Health, The Research Institute at Nationwide Children's Hospital.
References
- 1.Gean AD. Brain Injury: Applications from War and Terrorism. Wolters Kluwer; 2014. [Google Scholar]
- 2.Gean AD. Neuroimaging of Traumatic Brain Injury. Raven Press; New York: 1994. [DOI] [PubMed] [Google Scholar]
- 3.Levine B, Kovacevic N, Nica EI, et al. Quantified MRI and cognition in TBI with diffuse and focal damage. NeuroImage Clinical. 2013:2534–41. doi: 10.1016/j.nicl.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimi A, Fink KR. Thieme Medical Pub. 2015 [Google Scholar]
- 5.Bigler ED, Abildskov TJ, Petrie J, et al. Heterogeneity of brain lesions in pediatric traumatic brain injury. Neuropsychology. 2013;27(4):438–51. doi: 10.1037/a0032837. [DOI] [PubMed] [Google Scholar]
- 6.Staudt M. Brain plasticity following early life brain injury: insights from neuroimaging. Semin Perinatol. 2010;34(1):87–92. doi: 10.1053/j.semperi.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Wilde EA, Merkley TL, Bigler ED, et al. Longitudinal changes in cortical thickness in children after traumatic brain injury and their relation to behavioral regulation and emotional control. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2012;30(3):267–76. doi: 10.1016/j.ijdevneu.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewing-Cobbs L, Barnes MA, Fletcher JM. Early brain injury in children: development and reorganization of cognitive function. Developmental neuropsychology. 2003;24(2-3):669–704. doi: 10.1080/87565641.2003.9651915. [DOI] [PubMed] [Google Scholar]
- 9.Ewing-Cobbs L, Prasad MR, Swank P, et al. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. NeuroImage. 2008;42(4):1305–15. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levan A, Baxter L, Kirwan CB, et al. Right frontal pole cortical thickness and social competence in children with chronic traumatic brain injury: cognitive proficiency as a mediator. The Journal of head trauma rehabilitation. 2015;30(2):E24–31. doi: 10.1097/HTR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 11.Jernigan TL, Brown TT, Bartsch H, Dale AM. Toward an integrative science of the developing human mind and brain: Focus on the developing cortex. Dev Cogn Neurosci. 2015 doi: 10.1016/j.dcn.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zielinski BA, Prigge MB, Nielsen JA, et al. Longitudinal changes in cortical thickness in autism and typical development. Brain : a journal of neurology. 2014;137(Pt 6):1799–812. doi: 10.1093/brain/awu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–9. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 14.Vijayakumar N, Whittle S, Yucel M, et al. Thinning of the lateral prefrontal cortex during adolescence predicts emotion regulation in females. Soc Cogn Affect Neurosci. 2014;9(11):1845–54. doi: 10.1093/scan/nst183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014;87120-6 doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Squeglia LM, Jacobus J, Sorg SF, et al. Early adolescent cortical thinning is related to better neuropsychological performance. J Int Neuropsychol Soc. 2013;19(9):962–70. doi: 10.1017/S1355617713000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charil A, Dagher A, Lerch JP, et al. Focal cortical atrophy in multiple sclerosis: relation to lesion load and disability. NeuroImage. 2007;34(2):509–17. doi: 10.1016/j.neuroimage.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Righart R, Duering M, Gonik M, et al. Impact of regional cortical and subcortical changes on processing speed in cerebral small vessel disease. NeuroImage Clinical. 2013;2854-61 doi: 10.1016/j.nicl.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuladhar AM, Reid AT, Shumskaya E, et al. Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke; a journal of cerebral circulation. 2015;46(2):425–32. doi: 10.1161/STROKEAHA.114.007146. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Hodge J, Wei XC, Kirton A. Reduced ipsilesional cortical volumes in fetal periventricular venous infarction. Stroke; a journal of cerebral circulation. 2012;43(5):1404–7. doi: 10.1161/STROKEAHA.111.645077. [DOI] [PubMed] [Google Scholar]
- 21.Bava S, Archibald SL, Trauner DA. Brain structure in prenatal stroke: quantitative magnetic resonance imaging (MRI) analysis. Journal of child neurology. 2007;22(7):841–7. doi: 10.1177/0883073807304700. [DOI] [PubMed] [Google Scholar]
- 22.Michael AP, Stout J, Roskos PT, et al. Evaluation of Cortical Thickness after Traumatic Brain Injury in Military Veterans. J Neurotrauma. 2015;32(22):1751–8. doi: 10.1089/neu.2015.3918. [DOI] [PubMed] [Google Scholar]
- 23.Govindarajan KA, Narayana PA, Hasan KM, et al. Cortical Thickness in Mild Traumatic Brain Injury. J Neurotrauma. 2016 doi: 10.1089/neu.2015.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bigler ED, Yeates KO, Dennis M, et al. Neuroimaging and social behavior in children after traumatic brain injury: findings from the Social Outcomes of Brain Injury in Kids (SOBIK) study. NeuroRehabilitation. 2013;32(4):707–20. doi: 10.3233/NRE-130896. [DOI] [PubMed] [Google Scholar]
- 25.Yeates KO, Gerhardt CA, Bigler ED, et al. Peer relationships of children with traumatic brain injury. Journal of the International Neuropsychological Society : JINS. 2013;19(5):518–27. doi: 10.1017/S1355617712001531. [DOI] [PubMed] [Google Scholar]
- 26.Dennis M, Simic N, Bigler ED, et al. Cognitive, affective, and conative theory of mind (ToM) in children with traumatic brain injury. Developmental cognitive neuroscience. 2013;525-39 doi: 10.1016/j.dcn.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennis M, Simic N, Gerry Taylor H, et al. Theory of mind in children with traumatic brain injury. Journal of the International Neuropsychological Society : JINS. 2012;18(5):908–16. doi: 10.1017/S1355617712000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding K, Marquez de la Plata C, Wang JY, et al. Cerebral atrophy after traumatic white matter injury: correlation with acute neuroimaging and outcome. J Neurotrauma. 2008;25(12):1433–4. doi: 10.1089/neu.2008.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riedy G, Senseney JS, Liu W, et al. Findings from Structural MR Imaging in Military Traumatic Brain Injury. Radiology. 2015;150438 doi: 10.1148/radiol.2015150438. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM. White matter involvement after TBI: Clues to axon and myelin repair capacity. Exp Neurol. 2016;275(Pt):3328–33. doi: 10.1016/j.expneurol.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Kanekar S, Devgun P. A pattern approach to focal white matter hyperintensities on magnetic resonance imaging. Radiol Clin North Am. 2014;52(2):241–61. doi: 10.1016/j.rcl.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Soderlund K, Senseney JS, et al. Imaging Cerebral Microhemorrhages in Military Service Members with Chronic Traumatic Brain Injury. Radiology. 2015;150160 doi: 10.1148/radiol.2015150160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beauchamp MH, Beare R, Ditchfield M, et al. Susceptibility weighted imaging and its relationship to outcome after pediatric traumatic brain injury. Cortex. 2013;49(2):591–8.. doi: 10.1016/j.cortex.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Bigler ED. Structural Image Analysis of the Brain in Neuropsychology Using Magnetic Resonance Imaging (MRI) Techniques. Neuropsychol Rev. 2015;25(3):224–49. doi: 10.1007/s11065-015-9290-0. [DOI] [PubMed] [Google Scholar]
- 35.Dijkers M, Brandstater M, Horn S, et al. Inpatient rehabilitation for traumatic brain injury: the influence of age on treatments and outcomes. NeuroRehabilitation. 2013;32(2):233–52. doi: 10.3233/NRE-130841. [DOI] [PubMed] [Google Scholar]
- 36.Bigler ED, Ryser DK, Gandhi P, et al. Day-of-injury computerized tomography, rehabilitation status, and development of cerebral atrophy in persons with traumatic brain injury. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2006;85(10):793–80. doi: 10.1097/01.phm.0000237873.26250.e1. [DOI] [PubMed] [Google Scholar]
- 37.Lazaridis C, Yang M, DeSantis SM, et al. Predictors of intensive care unit length of stay and intracranial pressure in severe traumatic brain injury. J Crit Care. 2015;30(6):1258–6. doi: 10.1016/j.jcrc.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cowen TD, Meythaler JM, DeVivo MJ, et al. Influence of early variables in traumatic brain injury on functional independence measure scores and rehabilitation length of stay and charges. Arch Phys Med Rehabil. 1995;76(9):797–80. doi: 10.1016/s0003-9993(95)80542-7. [DOI] [PubMed] [Google Scholar]
- 39.Bigler ED, Jantz PB, Farrer TJ, et al. Day of injury CT and late MRI findings: Cognitive outcome in a paediatric sample with complicated mild traumatic brain injury. Brain Inj. 2015;29(9):1062–7. doi: 10.3109/02699052.2015.1011234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saatman KE, Duhaime AC, Bullock R, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25(7):719–38. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avants BB, Tustison NJ, Johnson HJ. Advanced Normalization Tools Computer Software. 2013 http://stnava.github.io/ANTs/
- 42.Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59(4):3774–83. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt P, Mühlau M, Christian G and Wink L. A Lesion Segmentation Tool For SPM manual for version 1.2.3. 2013 http://www.applied-statistics.de/LST_manual.pdf.
- 44.Carmack PS, Spence J, Gunst RF, et al. Improved agreement between Talairach and MNI coordinate spaces in deep brain regions. Neuroimage. 2004;22(1):367–71. doi: 10.1016/j.neuroimage.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 45.Lancaster JL, Martinez MJ. Mango Computer Software. 2014 http://rii.uthscsa.edu/mango/
- 46.Abe C, Ekman CJ, Sellgren C, et al. Cortical thickness, volume and surface area in patients with bipolar disorder types I and II. J Psychiatry Neurosci. 2015;41(1):150093. doi: 10.1503/jpn.150093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lisdahl KM, Tamm L, Epstein JN, et al. The impact of ADHD persistence, recent cannabis use, and age of regular cannabis use onset on subcortical volume and cortical thickness in young adults. Drug Alcohol Depend. 2016;161135-46 doi: 10.1016/j.drugalcdep.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex; a journal devoted to the study of the nervous system and behavior. 2008;44(8):1105–3. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Farbota KD, Bendlin BB, Alexander AL, et al. Longitudinal diffusion tensor imaging and neuropsychological correlates in traumatic brain injury patients. Front Hum Neurosci. 2012;6160 doi: 10.3389/fnhum.2012.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu L, Li J, Feng DF, et al. Detection of white matter lesions in the acute stage of diffuse axonal injury predicts long-term cognitive impairments: a clinical diffusion tensor imaging study. J Trauma Acute Care Surg. 2013;74(1):242–7. doi: 10.1097/TA.0b013e3182684fe8. [DOI] [PubMed] [Google Scholar]
- 51.Koerte IK, Lin AP, Willems A, et al. A review of neuroimaging findings in repetitive brain trauma. Brain Pathol. 2015;25(3):318–49. doi: 10.1111/bpa.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adams JH, Doyle D, Ford I, et al. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15(1):49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 53.Dennis EL, Jin Y, Villalon-Reina JE, et al. White matter disruption in moderate/severe pediatric traumatic brain injury: advanced tract-based analyses. Neuroimage Clin. 2015;7493-505 doi: 10.1016/j.nicl.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kraft RH, McKee PJ, Dagro AM, Grafton ST. Combining the finite element method with structural connectome-based analysis for modeling neurotrauma: connectome neurotrauma mechanics. PLoS Comput Biol. 2012;8(8):e1002619. doi: 10.1371/journal.pcbi.1002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bayly PV, Clayton EH, Genin GM. Quantitative imaging methods for the development and validation of brain biomechanics models. Annu Rev Biomed Eng. 2012;14369-96 doi: 10.1146/annurev-bioeng-071811-150032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merkley TL, Bigler ED, Wilde EA, et al. Diffuse changes in cortical thickness in pediatric moderate-to-severe traumatic brain injury. Journal of neurotrauma. 2008;25(11):1343–5. doi: 10.1089/neu.2008.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duhaime AC, Gean AD, Haacke EM, et al. Common data elements in radiologic imaging of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1661–6. doi: 10.1016/j.apmr.2010.07.238. [DOI] [PubMed] [Google Scholar]
- 58.Haacke EM, Duhaime AC, Gean AD, et al. Common data elements in radiologic imaging of traumatic brain injury. J Magn Reson Imaging. 2010;32(3):516–43. doi: 10.1002/jmri.22259. [DOI] [PubMed] [Google Scholar]