Abstract
Transmissibility is a significant factor in parasite fitness. The rate and magnitude of parasite transmission affect prevalence and infection intensity in individual hosts and are influenced by environmental factors. In this context, the objectives of this study were: (i) to experimentally assess Opisthorchis viverrini miracidia survival and infectivity over time and across temperatures; and (ii) to combine these experimental results with environmental data to build a key component of a transmission model, identifying seasonal windows of transmission risk in hyper-endemic northeastern Thailand. Five replicates of 50 O. viverrini eggs were randomly distributed and maintained under four temperature conditions (25°C, 30°C, 35°C, 40°C). Microscopic observations were performed on all experimental units over a period of 3 months to record miracidia motility and mortality trends. Six infection trials were also conducted to assess infectivity of miracidia over time and across temperatures, using observations of egg hatching success and infection rates. Upon completion of experiments, data were integrated into a transmission model to create a Transmission Risk Index and to simulate seasonal transmission risk. Miracidia survival rate and motility decreased steadily with 50% mortality observed after 2 weeks. Hatching and infection success also decreased significantly after 3 weeks. Temperatures over 30°C were associated with increased mortality and decreased infectivity. When incorporating local environmental parameters into our model, we observed low transmission risk during the dry season and increasing transmission risk at the onset of the rainy season, culminating with the highest risk in September. We believe that our results provide the first estimates of O.viverrini miracidia survival and transmission potential under variable temperature conditions and suggest that high temperature treatment (> 40°C) of fecal waste could be an efficient control strategy.
Keywords: Transmission dynamics, Temperature-dependent, Host-parasite, Bithynia siamensis goniomphalos, Liver fluke, Thailand
Graphical abstract
1. Introduction
Transmissibility is a significant factor in parasite fitness, as it is a prerequisite for the completion of parasites’ life-cycles and hence their survival and reproductive success (Anderson and May, 1982; Day, 2001). The mode and magnitude of transmission also influence how a parasite affects its host population, including prevalence and individual host infection intensity, which often correlates with disease severity (Ewald, 1983). While understanding parasite life-cycles requires parasitological knowledge of the developmental stages of parasites in their hosts, a broader understanding of disease risk variability at the population level relies strongly on population biology, ecology and mathematical modeling (Crofton, 1971; Anderson and May, 1982). In natural settings, transmission dynamics vary in association with a wide range of environmental conditions, and infection incidence changes in response to fluctuations in encounter and compatibility filters between hosts and parasites (Combes, 2005; Thieltges et al., 2008). The environmental influence on transmission dynamics is particularly strong in the case of complex life-cycle endohelminths that require multiple intermediate hosts and exhibit one or two free-living parasitic stages whose likelihood of encountering a suitable host depends largely on abiotic factors including anthropogenic contaminants such as fertilizer residuals (e.g nitrite-nitrogen; Poulin, 1992; Lafferty, 1997; Pietrock and Marcogliese, 2003). Furthermore, biotic factors such as toxins produced by hosts, non-hosts, predators or decoy organisms may act simultaneously and in conjunction with abiotic factors to expose free-living endohelminth stages to a complex array of hazards on their way to the next host and thus further modulate transmission dynamics and infection patterns in a host population (Thieltges et al., 2008). Understanding the epidemiology of helminths of medical significance to humans therefore requires the adoption of a dynamic, population-focused, quantitative framework that assesses the influence of biotic and abiotic factors on hosts and infective stages in complex environmental settings. Quantitative modeling procedures coupled with robust statistical designs are essential components of such an endeavor (Basáñez et al., 2012).
While there is literature on coupled laboratory-field quantitative approaches for the elucidation of Schistosoma spp. transmission dynamics and control (Liang et al., 2007), few such initiatives have been implemented with respect to liver flukes and other food-borne parasites (Lustigman et al., 2012). Opisthorchis viverrini is a food–borne parasite predominantly found in the Lower Mekong Region, endemic in Thailand, Lao PDR, Cambodia and Vietnam, where an estimated 10 million people harbor the infection (Sripa et al., 2010; Sithithaworn et al., 2012a). Adult Opisthorchis viverrini worms produce embryonated eggs that are released in the environment through the feces of their definitive (humans) or alternative (cats, dogs, rodents) hosts. Upon release into the environment, eggs will eventually be ingested by the next host, freshwater snails of the genus Bithynia, which includes three species and several potential cryptic sub-species (Petney et al., 2012; Sithithaworn et al., 2012b). Once in the snails, the miracidia hatch and penetrate snail tissue to develop into sporocysts and multiply asexually. The multiplied sporocysts then become rediae and subsequently cercariae that are gradually released into the environment, where they actively search for a fish host. Upon successful encounter with a fish, the cercariae will enter through its skin or gills, encyst in the flesh and develop into infective metacercariae (Wykoff et al., 1965; Kaewkes, 2003). Human infections occur by eating raw, fermented or undercooked cyprinid fish containing the infective metacercariae (Grundy-Warr et al., 2012). The adult worm inhabits the definitive or resevoir hosts’ bile ducts and, in cases of prolonged and heavy infection, provokes hepatobiliary damage of the bile duct wall, a condition generally accepted to be a risk factor associated with bile duct cancer known as cholangiocarcinoma (CCA) (Smout et al., 2011; Sripa et al., 2012, 2007; Sithithaworn et al., 2014).
Due to the distinct biological and epidemiological characteristics of parasitic stages of O. viverrini (i.e. miracidia, cercariae and metacercariae) and the resulting system’s complex transmission dynamics, it is practical and productive to develop experimental and mathematical procedures that explicitly break down the transmission system into its components, recognizing the agents (hosts and parasite stages) and the functional processes linking them. For example, the successful infection in snail hosts and subsequent cercarial release are contingent on at least seven steps: i) release of eggs from definitive hosts; ii) survival in the environment; iii) access to freshwater systems suitable for the snail and fish intermediate hosts; iv) ingestion by the snail host; v) hatching in the snail host; vi) multiplication and successful infection in the snail host; and vii) cercarial release; all of which are influenced by water parameters (Fig. 1). Although environmental parameters such as water salinity and temperature have been suggested to be important determinants of snail activity and abundance (see Petney et al., 2012 for a review) as well as infection success in snails (Prasopdee et al., 2015), variations in the survival of free living O. viverrini stages and their infectivity outside of their hosts has not been investigated in an explicitly quantitative framework (Lustigman et al., 2012; Sripa et al., 2015). The development of specific experiments that assess O. viverrini life stage survival and infectivity variation over time in different environmental settings, coupled with modeling procedures, appears critical to better identifying spatio-temporal windows of transmission risk and ultimately fostering more sustainable control strategies.
Fig. 1.
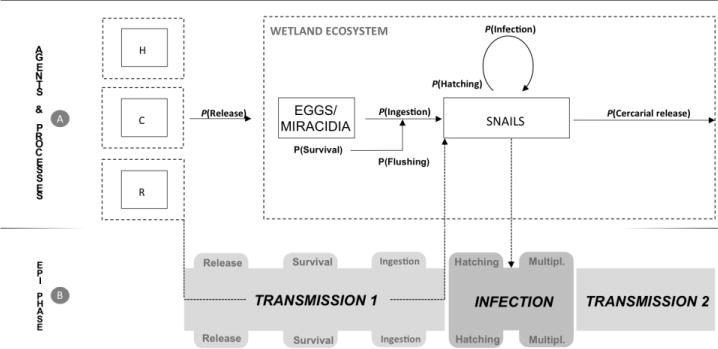
Opisthorchis viverrini transmission framework. (A) The identification of the agents (parasite stages, hosts and environmental features) as well as the relationship among them is critical to improving our understanding of (B) Opisthorchis viverrini transmission dynamics and likelihood of infection. We investigated the main processes that are likely contributing to modulation of transmission and infection during the first transmission episode involving O. viverrini eggs/miracidia and Bythinia snails. H, humans; C, cats and dogs; R, rodents and other alternative hosts.
In this context, the objectives of this study were: (i) to experimentally assess O. viverrini miracidia survival and infectivity over time and at different temperatures; and (ii) to provide a first iteration of a process-specific model assessing transmission risk to snails using the present experimental data coupled with available geospatial and field parasitological survey data. Using these results, our goal is to identify seasonal transmission risk scenarios in northeastern Thailand, a hotspot of O. viverrini endemicity.
2. Materials and methods
2.1. Egg origin, collection and culture
One golden syrian hamster (Mesocricetus auratus) was experimentally infected with 50 O. viverrini metacercariae obtained from naturally infected cyprinid fish. Fecal pellets were collected 3 months after infection over a period of 48 h. Opisthorchis viverrini eggs were subsequently separated from the fecal material through a series of filtering and sedimentation steps. The raw fecal material was deposited in a 100 ml vial and rinsed with 45 ml of 0.85% sodium chloride solution. After soaking for 30 min, the fecal material was gently homogenized in normal saline solution by stirring with a wooden toothpick. Once a homogenous solution was obtained, it was filtered through 90 and 45 um sieves before being transferred into three 15 ml centrifuge tubes for the subsequent sedimentation and separation phases. The tubes were centrifuged for 3 min at 269 g, the supernatant discarded, and 5 ml of 0.85% sodium chloride solution were added to each tube. This solution was then homogenized and centrifuged at 120 g for 2 min. The supernatant was discarded and 5 ml of 0.85% sodium chloride solution were again added. An assessment of the number of eggs per volume was performed by counting the number of eggs per drop (three replicates) of purified fecal solution, which was deposited on a slide and observed under a microscope (200× magnification). The total number of drops was then counted and a final egg count per tube was estimated. This estimate was used to choose the number of replicates and the number of eggs per replicate for each experimental treatment.
2.2. Experimental design and data collection
2.2.1. Miracidia temperature-dependent survival and motility
In order to assess the influence of environmental temperature on O. viverrini miracidia survival and infectivity over time, we designed a factorial experiment in which eggs were randomly distributed and maintained under four temperature conditions (25°C, 30°C, 35°C and 40°C, more details below). The eggs were distributed and maintained in large 3.7 cm diameter × 1 cm deep (962 mm2) wells of tissue culture test-plate (SPL Life Science Co., Ltd.; South Korea), with one well corresponding to one replicate of approximately 300 eggs. We used five replicates (five wells) per temperature treatment for a total of approximately 1500 eggs per temperature regimen and 6000 eggs for the whole experiment. In each replicate/well we added 5 ml of distilled water. Under our experimental conditions, this volume was found to be optimal to allow microscopic observations. Before each observation by microscope, used water was replaced by new distilled water to promote aerobic conditions, minimize bacterial contamination and mimic natural water flow. To replace the solution while preserving as many eggs as possible, slow speed centrifugation 120 g, 1 min) was performed. The resulting supernatant was discarded and replaced by 5 ml of distilled water in each replicate.
In order to control temperature, the six-well plates containing the eggs were placed in four water bath incubators. The temperatures chosen were 25°C, 30°C, 35°C and 40°C. A similar temperature range was used in a recent study investigating temperature dependency of O. viverrini infection in snails (Prasopdee et al., 2015) and represents a relevant range of water temperatures in northeastern Thailand where O. viverrini is endemic. To assess the temperature difference between water in the incubator and temperature of the media inside the plate, controls were conducted prior to and during the experiment. An average non-significant difference of 0.3°C between water and media was found to be consistent across all temperature treatments and throughout the duration of the experiment.
Survival and motility of the miracidia were assessed every 2 days during the first month and every 10 days during the two subsequent months for a total of 22 observations. Observations were performed at a magnification of 400× using an inverted microscope (Nikon Eclipse TS100). Each observation consisted of randomly examining 50 eggs per replicate for egg shell and miracidia morphological features used as proxies to assess miracidia mortality. A miracidia was considered alive if it appeared “healthy,” with a marked U-shape, sharp, visible morphological features, a tightly closed operculum with no air bubble inside, and an ovoid egg with no noticeable deformities that could indicate a possible loss of biological integrity (Fig. 2A). Additionally, each egg was observed continuously for 45 s to record miracidia motility which, coupled with the mortality assessment described above, was used as a proxy to further assess functional viability.
Fig. 2.
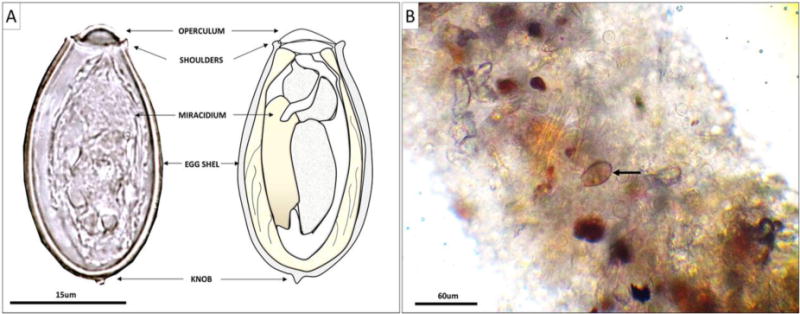
Morphology of Opisthorchis viverrini eggs. (A) Photographic and diagrammatic representations of O. viverrini egg morphological characteristics used to assess viability and (B) microscopic observation of snail feces to assess egg ingestion and hatching rates. The picture shows a well/clean open shell, suggesting successful hatching of the miracidia (black arrow) resulting from mechanical and biochemical stimuli. Alternatively, degraded eggs frequently still harbor their operculum and present air bubbles, suggesting the loss of osmotic balance and death of the miracidia.
2.2.2. Snail infections
Bithynia siamensis goniomphalos adult snails were collected from public ponds in the Muang district, Khon Kaen Province, Thailand, and maintained in the laboratory in 50 L plastic mesocosms containing dechlorinated tap water and provided with fresh boiled lettuce every 3 days. Prior the start of the experiment, the snails were examined for trematode infection by cercarial shedding (Suwannatrai et al., 2011) weekly for 8 weeks. Snails that were free from trematode infection were then used in the experiment. In order to assess miracidia infectivity over time and at different temperatures we conducted infection trials every week for the first month (four infection trials) and once each month during the two subsequent months for a total of six infection trials. Five snails (8 – 10.0 mm shell length, all males) per temperature treatment (a total of 20 snails per trial) were used during each infection trial. Five snails were placed in individual wells of a 6-well cell culture plate (one plate with five snails per temperature treatment) with 5 ml of dechlorinated tap water and exposed to a sub-sample of 50 viable O. viverrini eggs (Chanawong and Waikagul, 1965) taken from the incubation plates. Snails were not given food for 48 h prior to exposure and their feeding activity was activated by exposure to 8 W lights for 8 h during daytime for the duration of the infection trial. Since we were not investigating the effect of temperature on snail susceptibility but were more interested in investigating the carryover effect of temperature on miracidia infectivity, we chose to run the infection trial at a “neutral” temperature of 34°C known to be optimal for infection to occur (Prasopdee et al., 2015). By doing so, observed differences in infectivity, if any, would result from the physiological effect of temperature on miracidia infectivity and not due to the snail’s ability to prevent infection through temperature-dependent metabolic mechanisms. Each infection trial ran for 7 days to ensure that hatching could take place and that miracidia had time to establish infection and multiply in the snail, making subsequent detection of infection more successful. Snail feces were collected every 24 h and fixed in 10% formalin. Each snail fecal sample was carefully screened under a microscope (400× magnification) to assess hatching and ingestion rates (Khampoosa et al., 2012; Prasopdee et al., 2015). Hatching rate was estimated as the ratio of hatched versus non-hatched eggs found in snail feces per sample while the ingestion rate was estimated as the ratio of the total number of eggs (hatched or not hatched) present in the feces compared with the initial 50 deposited in the well (Fig. 2). At the end of the 7-day trials, individual snails were rinsed to eliminate potential remnants of infection and stored, without their shells, at −20°C prior to DNA extraction.
2.2.3. DNA extraction and infection screening
Snail tissue was crushed using pestles and then incubated in CTAB buffer (2% w/v CTAB, 1.4 M NaCl, 0.2% v/v -mercaptoethanol, 20 mM EDTA, 100 mM Tris–HCl, pH 8.0, 0.2 mg/ml of proteinase K; Winnepenninckx et al., 1993) at 55°C for 6 h. Snail homogenate proteins were precipitated with phenol/chloroform, centrifuged at 12,000 g for 10 min at 4°C. DNA was precipitated with isopropanol, then washed once with 70% ethanol and once with absolute ethanol. The DNA pellet was air-dried, re-dissolved with TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) and diluted to 10 ng.
To verify infection, we used the specific primers OV-6F (5_-CTG AAT CTC TCG TTT GTTCA-3_) and OV-6R (5_-GTT CCA GGT GAG TCT CTC TA-3; Wongratanacheewin et al., 2001). The primers were designed based on the pOV-A6-specific DNA probe sequence which gave a 330 bp product corresponding to a highly repeated sequence in the genome of O. viverrini exhibiting no significant homology with related parasites such as Clonorchis sinensis, Paragonimus siamensis and minute flukes (Wongratanacheewin et al., 2001). Conventional PCR was performed using a DNA Thermal cycler (GeneAmp®PCRSystem 9700, Applied Biosystems, Foster City, CA, USA) to confirm infection and estimate infection rate. The reaction was carried out with a total volume of 10 μl containing 0.04 μl of TaKaRa Ex Taq 250 U, 1 μl of dNTP mixture, 1 μl of 10× Ex Taq buffer, 3 μl of DNA sample, 3 μl of distilled water, and 5 pmol of each primer. The PCR procedure was performed with cycling conditions as follows: initial denaturation at 94°C for 5 min followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min, followed by a final extension at 72°C for 7 min. PCR products were analyzed by 1.5% agarose gel electrophoresis in TBE buffer.
2.3. Statistical analyses
We computed generalized mixed models (logistic regressions) with binomial error distribution and a logit link function to analyze the effects of the explanatory variables “temperature,” “time” (fixed factors) and “replicate” (random factor) on binary response variables (mortality, motility, ingestion, hatching, and infection). To select the most appropriate explanatory model (i.e. what factors and their interactions should be included), we used the R package “gmulti”, which automatically generates all possible models under certain constraints with the specified response and explanatory variables, and finds the best models based on the Akaike Information Criterion (AIC). For assessment of factor level (e.g. temperature category) influence on response variables (e.g., mortality), the estimates of the coefficients and the intercepts of the selected logistic regression model were calculated via maximum likelihood estimation and differences among factor levels (e.g., among temperature or time levels) were assessed using z-scores (Wald tests). Odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated from the model coefficients. All statistical analyses were performed using “R” version 3.0.2 (R Core Team, 2013). Statistically significant results were determined to be those where the observed P-value was less than 0.05.
2.4. Modeling Transmission Risk Index
Using the Nova multipurpose modeling software (Salter, 2013), output data were generated using input from the aforementioned experiments to create an integrated Transmission Risk Index (TRI), defined as the likelihood of infection success in the snails and showing change over time and temperature. The rationale was to develop a baseline model capable of forecasting transmission risk under various temperature and time conditions. The model that produces the TRI is an algebraic model that treats each of the four processes studied (mortality, motility, ingestion, hatching) as a strictly discrete step in a linear sequence of events. Because mortality and motility arguably represent a similar stage in the egg-to-cercaria transformation, some iterations of the model exclude motility, as it is a more selective measure than mortality for whether the egg is viable when it is ingested by a snail. In the algebraic model, each process is treated as a Bernoulli trial, so the TRI is the product of the probability of four (or three) trials, representing the likelihood that a given egg successfully hatches within a snail at a certain temperature and at a certain time point in the lifespan of the egg.
The probabilities of success for each trial are based on the experimental data and interpolated between time points of observation. The output of this algebraic model is subsequently used to estimate seasonal transmission risk in an endemic transmission area, incorporating regional environmental and epidemiological parameters. The seasonal TRI algebraic model uses a framework of risk normalized to the highest value by month for each step to estimate seasonal risk. The four steps are: i) egg abundance in the environment; ii) precipitation to mobilize the eggs to susceptible snail habitat; iii) snail abundance in the environment; and iv) appropriate temperature for successful infection to occur based on the experimentally-based TRI. Model assumptions and descriptions are provided in the Supplementary Data S1, Supplementary Fig. S1 and Supplementary Table S1.
3. Results
3.1. Experimental observations
3.1.1. Miracidia mortality and motility over time and across temperatures
For miracidia mortality, the statistical model that best fit mortality data was composed of independent temperature and time factors plus the modifying term representing their interaction (Table 1). According to the selected model, miracidia survival decreased when temperature increased (P <0.001) and over time (P <0.001). However, a significant interaction term between temperature and time (P <0.001) suggests that the degradation patterns observed at each temperature did not vary in the same way over time (Fig. 3A, Table 2).
Table 1.
Statistical model selection scores. For each response variable (mortality, motility, hatching, ingestion, infectivity) an analysis of deviance was computed to select the model that best fitted the data. The factors assessed were temperature, time and replicate, and their interactions. The deviance and Akaike Information Criterion (AIC) scores for the best models selected are given. The lowest deviance and AIC scores characterize the model that best fit the data.
| Response variable | Best Explanatory Model | df | Deviance | AIC |
|---|---|---|---|---|
| Mortality rate | Temp. + Time + Temp. × Time | 176 | 66.499 | 1359.60 |
| Motility rate | Temp. + Time + Replicate | 195 | 154.430 | 348.86 |
| Ingestion rate | Temperature + Time | 111 | 424.940 | 698.08 |
| Hatching rate | Temperature + Time + Replicate | 83 | 98.354 | 209.25 |
| Infection rate | Temperature + Time | 47 | 167.016 | 101.84 |
df, degrees of freedom.
Fig. 3.
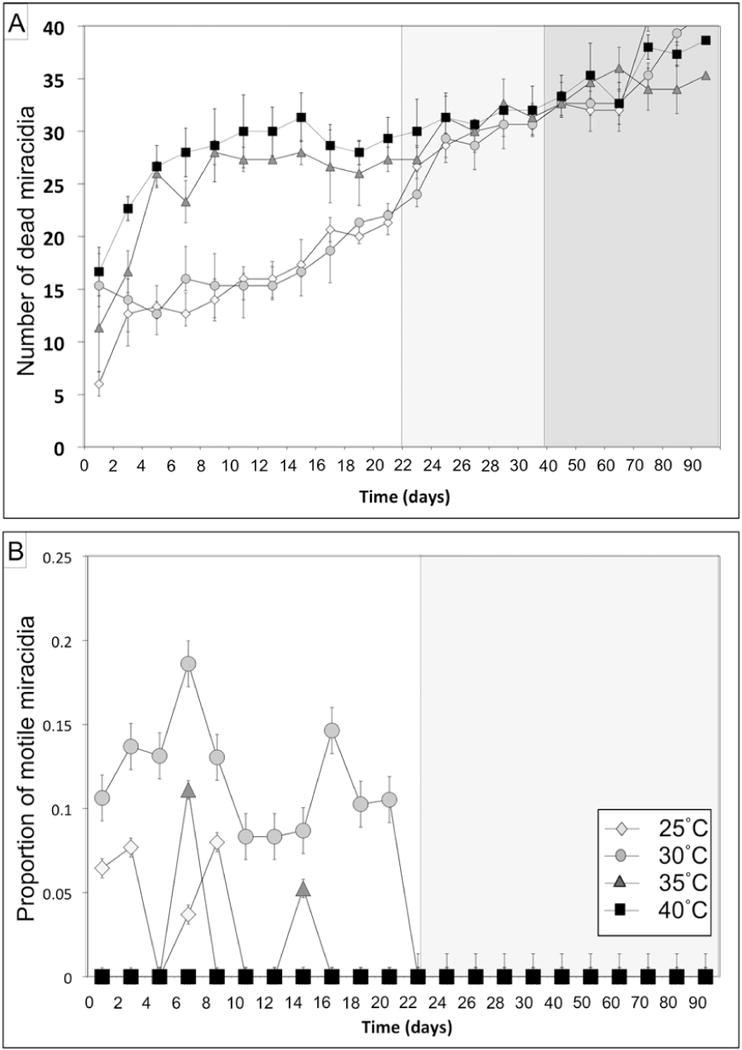
Graphical representations of Opisthorchis viverrini miracidia mortality and motility. (A) Number of deceased miracidia and (B) proportion of motile miracidia over time and across temperatures. Different background shades represent temporal thresholds beyond which significant differences were observed.
Table 2.
Results of the logistic regressions. The main effects of time and temperature, as well as their interaction, on mortality, motility, hatching, ingestion and infection are presented.
| Response variable | Factors | df | X2 | P value |
|---|---|---|---|---|
| Temperature | 3 | 1446.560 | <0.0001 | |
| Mortality rate | Time | 21 | 310.130 | <0.0001 |
| Temperature × Time | 21 | 66.500 | <0.0001 | |
| Temperature | 3 | 486.990 | <0.0001 | |
| Motility rate | Time | 21 | 331.150 | <0.0001 |
| Replicate | 2 | 261.950 | <0.0001 | |
| Temperature | 3 | 13.600 | 0.003* | |
| Ingestion rate | Time | 5 | 20.540 | 0.0001 |
| Temperature | 3 | 130.360 | 0.02341 | |
| Hatching | Time | 5 | 94.570 | <0.0001 |
| Replicate | 4 | 87.770 | 0.14660 | |
| Temperature | 3 | 157.590 | 0.02412 | |
| Infection rate | Time | 5 | 28.334 | <0.0001 |
df, degrees of freedom
More specifically, although miracidia mortality increased gradually until the end of the experiment (from 22% to 82.5%), three distinct periods of time can be contrasted. Miracidia observed during the intermediate (from day 22 to day 40) and late (from day 41 to day 90) periods of the experiment had 3.07 and 6.37 times the odds of mortality compared with miracidia observed early in the experiment (both P <0.001, Table 3). Mortality also slightly increased from the intermediate to late observation periods (ORcrude=1.74, P <0.001, 95% CI: 1.56, 1.93). Notably, the observed increase in mortality over time was strongly contingent upon the temperature treatment. During the early phase of the experiment (until day 22), miracidia exposed to warmer conditions (35°C and 40°C between which mortality was similar Df = 1, χ2 = 0.0609, P = 0.805) had 2.28 times the odds of dying compared with miracidia held under cooler conditions (25°C and 30°C between which mortality was similar, degrees of freedom (Df) = 1, χ2 = 2.29, P = 0.13; Table 3). Subsequently, the mortality rate of miracidia under warmer conditions plateaued while the mortality rate of miracidia under cooler conditions kept increasing steadily (Fig. 3A). As a consequence, the initial difference in mortality between the two temperature groups almost disappeared after day 22 (P = 0.048) and then trended in reverse as the experiment entered its third phase after 40 days (Fig. 3A).
Table 3.
Response variables, factors, levels and adjusted Odds Ratios (OR).
| Response variable | Factors and Levels | OR | 95% CI |
|---|---|---|---|
| MORTALITY | Temperature | ||
| Warmer | 1.48 | 1.38 – 1.58 | |
| Time | |||
| Intermediate | 2.15 | 1.98 2.34 | |
| Late | 3.75 | 3.42 – 4.13 | |
| Temperature × Time | |||
| Cooler and Intermediate | 3.07 | 2.73, 3.46 | |
| Cooler and Late | 6.37 | 5.56, 7.33 | |
| Warmer and Intermediate | 1.55 | 1.38 – 1.75 | |
| Warmer and Late | 2.28 | 2.01 – 2.61 | |
| MOTILITY | Temperature | ||
| T2 | 1.97 | 1.36 – 2.22 | |
| T3 | 1.08 | 0.81 – 1.51 | |
| T4 | 1.10 | 0.81 – 1.55 | |
| Time | |||
| Intermediate | 0.69 | 0.43 – 1.13 | |
| Late | 0.63 | 0.37 – 1.08 | |
| INGESTION | Temperature | ||
| T2 | 0.82 | 0.59 – 1.11 | |
| T3 | 0.64 | 0.47 – 0.87 | |
| T4 | 0.89 | 0.65 – 1.23 | |
| Time | |||
| 2 | 0.95 | 0.64 – 1.41 | |
| 3 | 0.69 | 0.46 – 1.05 | |
| 4 | 0.53 | 0.35 – 0.80 | |
| 5 | 0.59 | 0.39 – 0.88 | |
| 6 | 0.96 | 0.67 – 1.40 | |
| HATCHING | Temperature | ||
| T2 | 0.54. | 0.27 – 1.09 | |
| T3 | 0.89 | 0.45 – 1.80 | |
| T4 | 0.45 | 0.21 – 0.96 | |
| Time | |||
| 2 | 0.61 | 0.25 – 1.5 | |
| 3 | 0.54 | 0.22 – 1.31 | |
| 4 | 0.26 | 0.11 – 0.63 | |
| 5 | 0.16 | 0.07 – 0.39 | |
| 6 | 0.17 | 0.07 – 0.41 | |
| INFECTION | Temperature | ||
| T2 | 1.62 | 0.62 – 4.34 | |
| T3 | 0.60 | 0.22 – 1.62 | |
| T4 | 0.19 | 0.06 – 0.55 | |
| Time | |||
| 2 | 0.71 | 0.13 – 3.62 | |
| 3 | 0.06 | 0.01 – 0.21 | |
| 4 | 0.02 | 0.004 – 0.087 | |
| 5 | 0.01 | 0.002 – 0.059 | |
| 6 | 0.002 | 0.0002 – 0.014 |
T1 is not shown in the table as it is the reference category for this variable. T2, T3, T4, temperature treatments 25°C, 30°C, 35°C and 40°C, respectively.
Time 1 is not shown in the table as it is the reference category for this variable. Times 2, 3, 4 each correspond to an infection trial performed at regular time intervals (see Section 2.2.2).
NS, not significant; CI, confidence interval.
For miracidia motility, the model that best fit motility data was composed of independent temperature and time fixed factors as well as a replicate random factor (Table 1). Miracidia motility was strongly influenced by both temperature (P <0.0001) and time (P <0.0001, Table 2) as motility decreased over time with marked differences between temperature regimes. These trends, however, were particularly apparent for certain replicates (P <0.001), reflecting a significant effect of the replicate factor that may reveal an experimental artifact. The incorporation of the replicate random factor in the model distributes replicate-associated variance over the whole analysis and controls for its potential confounding effect (Fig. 3B, Table 2).
Miracidia motility was observed from the beginning of the experiment (3 days after the eggs had been released) until day 22 under all temperature regimes except 40°C. Using the same time categories as for mortality, miracidia had 0.69 and 063 times the odds of being motile during the intermediate and late phase of the experiment compared with the early phase (both P <0.001; Table 3). Miracidia incubated at 30°C were more consistently motile and had 1.97 times the odd of moving when observed compared with miracidia held under other temperature conditions (P < 0.001, Fig. 3B, Table 3). After day 22, no motile miracidia were observed.
3.1.2. Egg hatching, ingestion and infection rate
The model that best fit the data for egg ingestion rate per snail was composed of time and temperature (Table 1). Ingestion rate tended to decrease over time but low ingestion rates were notably observed during infection trials 4 and 5 when eggs had 0.53 and 0.59 times the odds of being ingested compared with eggs observed during other infection trials (P = 0.002 and P = 0.01, Fig. 4A, Table 3). Eggs held at 35°C had 0.63 times the odds of being ingested compared with eggs observed under other temperature conditions (P = 0.005, Fig. 4A).
Fig. 4.
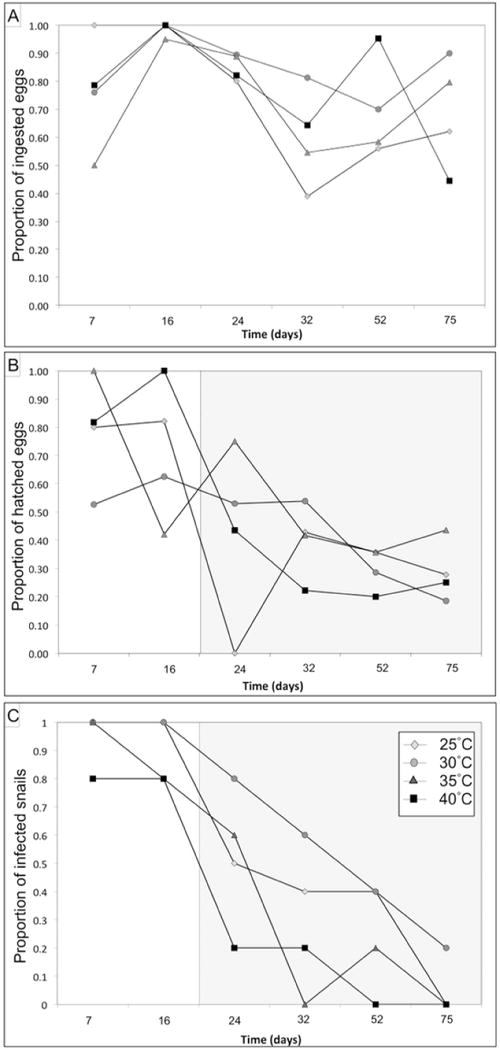
Graphical representations of experimental ingestion and hatching of Opisthorchis viverrini eggs and infection of snails. (A) Proportion of O. viverrini eggs ingested; (B) proportion of eggs which hatched; and (C) proportion of snails infected over time and across temperatures. Different background shades represent temporal thresholds beyond which significant differences were observed.
The model that best fit the hatching data was composed of temperature, time and replicate factors. No interaction terms between the factors significantly improved model fit and were therefore not included in the analysis (Table 1). According to the relatively simple additive model selected, time had a strong and significant influence on hatching success (P < 0.001, Table 2). Each following time period had 0.71 times the odds of hatching of the preceding time period, reflecting a relatively sharp decline over time (ORcrude=0.71, P <0.001, 95% CI: 0.62, 0.81). Temperature also had a significant influence on hatching (P = 0.023, Table 3), with hatching success decreasing with increasing temperature. While miracidia hatching success declined over time, it is noteworthy that there were two distinct periods when hatching success was remarkably different. Hatching rate was much higher during the two first infection trials (79% and 72%) than the last four infection trials (43%, 40%, 30% and 29%). Translated into ORs, the last four infection trials had 0.26 times the odds of eggs hatching during the first two infection trials (ORcrude=0.268, P = 0.001, 95% CI: 0.163, 0.431; Fig. 4B). Hatching success did not vary across replicates (P = 0.146).
The model that best fit the data for infection rate was composed of time and temperature (Table 1). Infection rate significantly decreased over time (P <0.0001; Table 2) particularly after trial 2, 2 weeks after the start of the experiment, when infection odds were 0.06, 0.02, 0.01 and 0.002 times the odds of being infected in trials 1 and 2 (P = 0.001, P < 0.001, P < 0.001, and P < 0.001 respectively, Fig. 4C, Table 3). Temperature had a moderate but significant effect on infection rate, with increasing temperature being associated with lower infection rates, notably at 40°C (P = 0.003, Fig. 4C, Table 3).
3.2. Modeling TRI and its seasonal variation
3.2.1. Simulations of the deterministic model and assessment of the TRI
Fig. 5 shows eight simulations of the deterministic model run as a basis for constructing the TRI. All of the simulations predicted higher infection success at lower temperatures (20°C or 25°C) initially, although slopes vary depending upon simulation type. Generally, lower temperatures show a faster decrease in TRI over time compared with higher temperatures. At the 1 month mark, higher temperatures (40°C and 45°C) predict a higher TRI despite egg degradation. The simulations forecast the maximum time of viability: in most cases approximately 1 month, and up to 2–3 months in the most conservative simulation (Fig. 5G). The grouped scenarios Fig. 5G, H, demonstrate a steep decline between 30°C and 35°C based on the data, with markedly different curves emerging. Fig. 5D–F extrapolate the TRI at 20°C and 45°C (temperatures not used in the laboratory experiment).
Fig. 5.
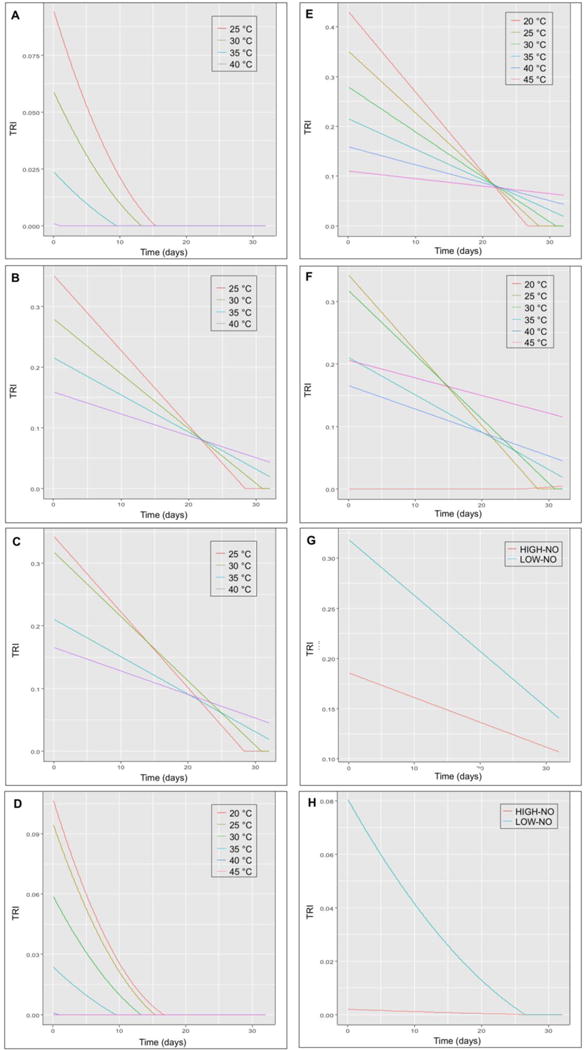
Simulations of the deterministic model run as a basis for constructing the transmission risk index (TRI). Different input characteristics and resulting outcomes are represented. (A) Constant ingestion rate with motility. (B) Constant ingestion rate without motility. (C) Variable ingestion rate with motility. (D) Constant ingestion rate with motility, including predicted (not tested experimentally) maximum low (20°C) and high (45°C) temperatures. (E) Constant ingestion rate without motility, including predicted maximum low and high temperatures. (F) Variable ingestion rate with motility, including predicted maximum low and high temperatures. (G) Combined low (25°C and 30°C) and high (35°C and 40°C) temperatures with motility and constant ingestion rate. (H) Combined low and high temperatures without motility and constant ingestion rate.
When considering the four parameters separately, the following observations were made. For miracidia mortality, there was very clear agreement between 25°C and 30°C, and between 35°C and 40°C. This partially explains the similarity between those respective curves. Because the inclusion of motility drops the maximum likelihood of hatching success from approximatley one in three successful infection events to one in 10 when included, the simulations with motility as an input factor (i.e. simulations A, C, D, F, H; Fig. 5) predict significantly decreased the TRI compared with those without motility (i.e. simulations B, E, G; Fig. 5). Considering ingestion by comparing Fig. 5A and Fig. 5D with Fig. 5C and Fig. 5F, the non-linear behavior also causes some convergence between the lower temperatures and the higher temperatures.
Fig. 6 compares the curves between predicted infection likelihood for the “low” and “high” scenarios, based on the ingestion and hatching parameters found with the experimental data and the actual infection rates observed. Although working with limited data points, statistical significance was demonstrated for both low (P = 0.029) and high (P = 0.0017) models.
Fig. 6.
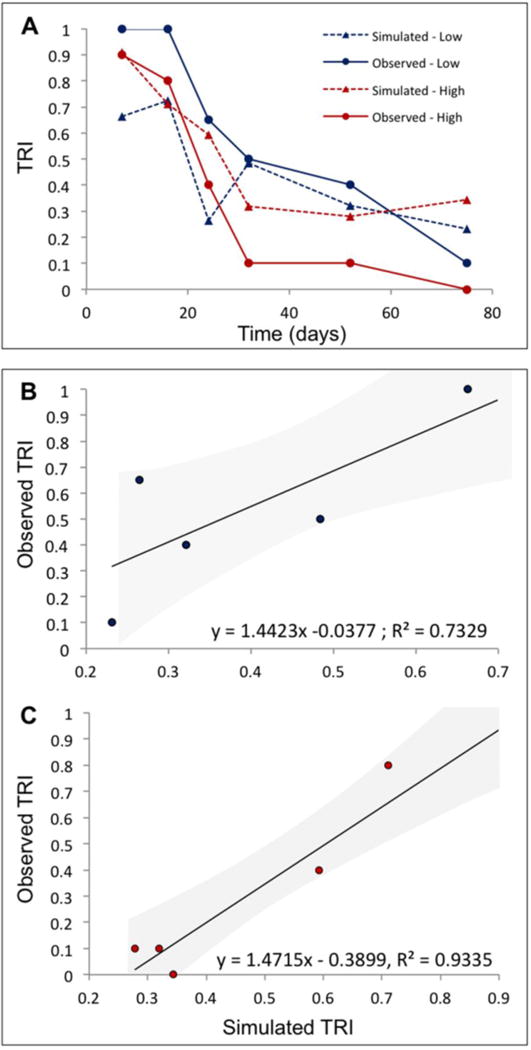
Experimental versus simulated infection risk. (A) Experimental (circles) and simulated (triangles) Transmission Risk Index (TRI) over time. The blue (black) lines represent colder (25°C and 30°C) conditions, the red (grey) lines warmer (35°C and 40°C) conditions. (B) Linear relationship between experimental and simulated TRI for low (25°C and 30°C) temperature conditions. (C) Linear relationships between experimental and simulated TRI for high (35°C and 40°C) temperature conditions.
3.2.2. Seasonal variation in TRI
Using the previously described simulations as a basis, we combined the TRI model produced with seasonal data on precipitation, temperature, egg abundance, and snail availability, normalized the output data by month, and estimated TRI seasonal variation (seasonal TRI). Multiple seasonal variation curves were produced by incorporating different sources of input data (see Supplementary Data S1). For each input, at least two different data sources were tested independently and combined for use in the model. Based on what were perceived to be the best choices for data, modeling technique, and biological plausibility, a final curve was chosen to represent seasonal TRI (Fig. 7). This curve is in alignment with the seasons experienced in northeastern Thailand: dry season from November through February, hot season from February through May, and wet season from May through November (Lacombe et al., 2013). We see the seasonal TRI coincide with the beginning of the wet season and reach its peak in September, when precipitation is at its maximum. As the dry season starts, the risk drops off and remains low during the hot season.
Fig. 7.
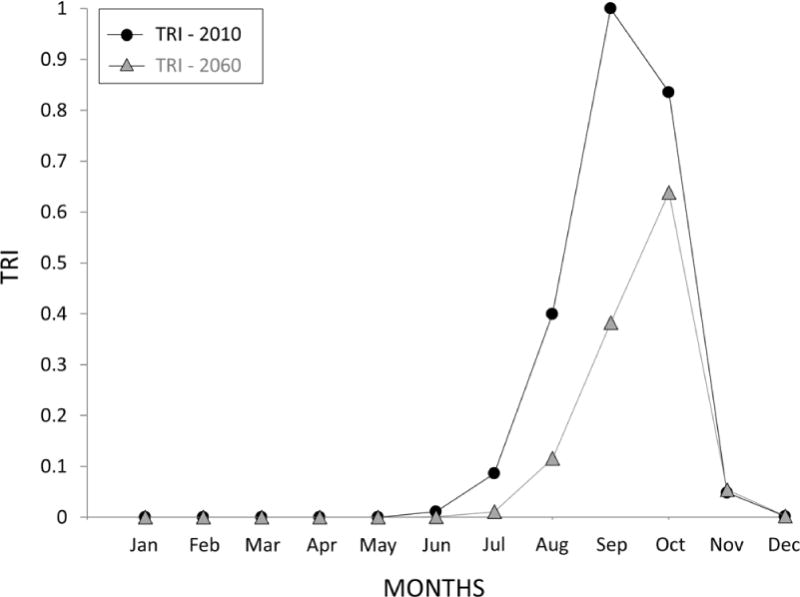
Seasonal variation in the Transmission Risk Index (TRI). The black line and circles simulate the current seasonal transmission risk. The grey line and triangles simulate the future transmission risk based on climate predictions for 2060.
Fig. 7 also includes predicted temperature and precipitation data from 50 years in the future (2060) based on climate models and predictions within Thailand, specifically Khon Kaen Province (Babel et al., 2011; Kawasaki and Herath, 2011). The changes in precipitation and temperature are counterbalancing; while climate change is likely to increase extreme precipitation events that would lengthen the rainy season and the extent of suitable habitat for snails, rising temperatures would mitigate transmission success by reducing egg viability. As a consequence of these combined changes, we see an overall decrease in the TRI in 2060 compared with 2010 and a shift in peak risk to October, 1 month later than the 2010 scenario. Similar trends were produced across several variations of input parameters in the model (Supplementary Data S1; Supplementary Figs. S2 and S3).
4. Discussion
The capacity of parasite eggs to remain viable in the environment for extended periods of time is a remarkable adaptation to cope with the uncertainty of host availability and to maximize transmission success in variable environments (Poulin, 2011). Our results indicate that O. viverrini eggs can remain morphologically viable in the water under our controlled experimental conditions for up to 12 weeks, as a small subset of the eggs observed (8%) appeared viable at the end of the experiment. This observation also suggests that within an egg cluster released into the environment, some eggs may survive more than 12 weeks. More experiments are needed to test this hypothesis as well as to assess the role of water quality variation in influencing miracidia survival. In this study, we used distilled water, in which hypotonicity may have induced an osmotic gradient leading water to travel into the egg shell and trigger the opening of the shell and death of the miracidia. The majority (80%) of the eggs remained morphologically viable up to 3 weeks before we observed a steady decline during the subsequent 8 weeks. After three weeks, many eggs had lost their biological integrity, with an increasing number of open opercula. Similarly, miracidia motility was observed regularly up to 3 weeks after the start of our experiment, indicating functional viability and infective potential during this period. The sharp decline in miracidia motility and overall loss of O. viverrini egg viability observed after 3 weeks corroborates our observations of hatching and infection success. Both hatching and infection rates decreased significantly over time, particularly after 2 weeks, indicating that observations of egg morphology and miracidia motility are useful proxies for assessing the infective potential of O. viverrini eggs.
The observation that O. viverrini eggs can remain infective for several weeks in the environment corroborates what is generally observed for other major helminths (Smith, 1999). Field investigations in Russia from the 1980s indicate that Opisthorchis felineus eggs can remain viable in cold environments for several months (Krivenko, 1979; Krivenko et al., 1981), although the authors did not assess the infectivity of the miracidia in subsequent infection experiments that may have shown shorter infective time periods.
From a disease prevention and control perspective, understanding the biological mechanisms responsible for egg persistence and sustained infective potential in the environment, and their biotic and abiotic modifiers, is a promising avenue of research. Our results revealed a strong detrimental influence of elevated water temperatures on O. viverrini miracidia survival and infectivity. This finding confirms the promising role of heat treatment in septic systems for control (Dr. Trevor Petney, personal communication), as ineffective on-site sanitation systems are still prevalent in the region. These sanitation systems enable wastewater, potentially containing O. viverrini eggs, to enter the environment through interaction with subsurface water over short time periods during annual floods. Although the significance of these transmission events has not been assessed, they may allow viable O. viverrini eggs to reach susceptible host snails.
Detrimental temperature effects were strongest during the first 3 weeks for eggs held under higher temperature regimens (>30°C). These miracidia had a higher likelihood of becoming non-viable as evidenced by lower rates of motility and deterioration of their morphological appearance. Interestingly, the differences in egg morphological viability observed between higher (> 30°C) and lower (< 30°C) temperatures disappeared after 3 weeks. These trends may reflect the convergence of two processes, energy resource depletion and osmotic balance disruption, which may lead to the observed time-temperature interaction. Briefly, the amount of energy reserves available during embryogenesis will condition miracidia survival (Khampoosa et al., 2012), which, based on our results, averages 3 weeks. Increased temperature is known to alter lipid membrane integrity of helminth eggs, which renders the eggs vulnerable to osmotic effects and chemicals present in the external environment (Barrett, 1976). This creates a stressful situation in which the miracidia may deplete their energy reserves at higher rates and die faster, possibly explaining the much higher miracidia mortality rate observed under higher temperature treatments. After 3 weeks, the detrimental influence of elevated temperature observed during the early phase of the experiment disappeared, as miracidia across all temperature treatments exhibiting higher mortality due to the natural depletion of energy resources predominated.
Both hatching success and infection rates were negatively influenced by elevated temperatures, which corroborates our observations of morphological deterioration and motility trends. As we used a single “neutral” temperature (34°C) during our infection trials, the reduction in hatching and infection rates associated with elevated temperature indicates that temperature directly influences O. viverrini biological integrity during its “free-living” stages, and ultimately transmission success, independently of temperature–mediated snail susceptibility. A recent study also showed that temperature mediates O. viverrini infection success when snails are exposed to O. viverrini eggs under various temperature conditions (Prasopdee et al., 2015). While the authors of this study found that elevated temperatures (34°C) were associated with the highest O. viverrini infection rate, these trends reflect the influence of temperature on ectothermic snails, including their feeding behavior, rather than on the persistent effects of temperature on parasite physiology. These results therefore are not directly comparable with the results presented in our study. Taken together however, the results of our study and of Prasopdee et al. (2015) suggest that both O. viverrini miracidia and their snail hosts are influenced by environmental parameters that create complexity through non-linear temperature-dependent processes and time-dependent metabolic trade-offs favoring specific and narrow windows of effective transmission. This influence of temperature on transmission outcomes via host-parasite encounter modulation has been similarly reported in several other host-parasite systems (Tubbs et al., 2005; van Dijk and Morgan, 2008; Tinsley et al., 2011), including other parasites of medical and veterinary significance such as Fasciola hepatica (Mas-Coma et al., 2009) and Schistosoma spp. (McCreesh and Booth, 2013, 2014a, b).
The experimental investigations and the results described above highlight temperature-dependent thresholds of O. viverrini miracidia infective potential and degradation over time, but these data alone do not provide an integrated transmission risk assessment which can be used for control initiatives. In order to identify windows of transmission risk for the snail first intermediate host in O. viverrini endemic regions and provide practical suggestions for control, we integrated the present experimental data in a transmission model and ran simulations incorporating regional temperature and precipitation records as well as documented snail abundance seasonal patterns and estimated egg availability in the environment.
Consistent with our interpretation of the experimental data, simulations of our integrated risk index (TRI) suggest a fairly short window of high infective potential, in the order of 1 month. This is an important finding with implications for how O. viverrini egg control is practiced. It strengthens the case that control can and should be practiced seasonally during the year rather than year-round in order to maximize resource utility. Hinz et al. (1994) suggested coupling the timing of praziquantel treatment with the period of the year known to present minimal risk of infection, from March to June, to reduce re-infection rates and subsequent transmission events. The most conservative simulation predicts one out of every 10 eggs having infective potential at peak viability and temperature. However, the model did not account for numerous unknown and unmeasured environmental factors that further decrease this likelihood by orders of magnitude. This low probability of infection success is counteracted by the large numbers of O. viverrini eggs released into the environment by a single infected host. This finding also sheds some light on the extremely low snail prevalence observed, generally ranging between 0.2% and 1.2% in Thailand (reviewed in Petney et al., 2012, but see Kiatsopit et al., 2012). Understanding why snail prevalence is so low, by means of characterizing the encounter and compatibility filters (Combes, 2005) between snails and eggs will be essential for improving our understanding of transmission dynamics, improving current modeling investigations and further informing environmental control strategies. The changes in simulated infection success over time as they converge across temperatures also suggest that temperature becomes superseded by other factors as primary determinants of infection likelihood and as other biological processes render the eggs unviable, warranting additional investigations from an ecological perspective (cf. Kiatsopit et al., 2014; Thieltges et al., 2008).
When incorporating local environmental and climate parameters in our model, transmission risk varies greatly over the year, with very low transmission risk during the dry seasons (both hot and cool) and increasing transmission risk at the onset of the rainy season in June-July that peaks in September. This outcome indicates a strong seasonal pattern of transmission in snails and the crucial, although indirect, role of environmental and climatic parameter variation in modulating the transmission process. Field studies investigating snail population dynamics are relatively scarce but indicate that snail abundance varies significantly throughout the year depending not only on the local climate but also on other ecological factors. Brockelman et al. (1986) found rapid Bythinia reproduction after spring rains and monsoon flooding, with population doubling time estimated at around 7 weeks (Brockelman et al., 1986). Higher average abundances are generally observed between August and December (reviewed in Petney et al., 2012), suggesting the dominant influence of snail abundance in determining the patterns of seasonal transmission risk in our model output. In contrast, a recent study found snail infection prevalence to be highest during the cool dry season (Kiatsopit et al., 2014), although cercariae release can be high throughout the year (Kiatsopit et al., 2012). Human activity in the environment is usually high during the rainy season, as rain-fed rice farming peaks and coincides with increased fecal bacterial contamination and potentially O. viverrini egg presence in natural water reservoirs (Kaewkes et al., 2012). After the snails acquire infection, the parasite requires approximately 2 months for larval development before the emergence of free-swimming cercariae, and high infection rates in fish generally occur during the late rainy season and winter (July to January) (Sithithaworn et al., 1997). From a temporal dynamics standpoint, these observations support our model-based hypothesis that the bulk of successful snail infections would take place near the end of the rainy season, likely around September.
Although the model outputs need to be interpreted with caution, both field and model-based-evidence suggest transmission risk to peak during September and be markedly high during the preceding period of seasonal rice farming, with rice fields known to be a prime habitat for Bythinia snails (Petney et al., 2012; Wang et al., 2014). Control interventions targeting snail transmission interruption should therefore mostly be targeted during the rainy season and participatory in nature with farmer involvement in the control process. Farmers could be consulted for information on their defecation practices and spatially disconnected defecation spots could be designated to reduce snail-egg encounters.
The seasonal patterns of transmission risk described and the insights they provide regarding control initiatives may be subject to change or nuance as temperature and precipitation regimes in the central Mekong Basin are forecast to change (Babel et al., 2011; Lacombe et al., 2013). Between 1953 to 2004, dry season rainfall significantly increased in frequency (more rainy days) and intensity (higher cumulative rainfall depths) in the central part of the Mekong Basin (Lacombe et al., 2013). Our predictive model for Khon Kaen Province suggests that the transmission risk seasonal peak will shift to later in the year, in October rather than September, in response to precipitation and seasonal timing changes. However, the overall transmission risk throughout the year may decrease as increasing temperatures become less favorable to snails and less conducive to successful O. viverrini egg hatching based on our experiments. The modification of precipitation/temperature regimes in southeastern Asia may induce changes in rain-dependent rice farming schedules, growing season duration and irrigation schemes that will influence patterns of snail distribution, human activity in rice fields and, indirectly, egg prevalence in the environment. Climate change will also modify infection risk for cyprinid fish and the direct risk for infection in human populations. Although our model does not incorporate data from fish population trends, human socio-economic/demographic predicted changes, or agriculture mechanization trends (i.e. further reduction of human presence in the fields), these are likely to modulate snail-egg encounter rates and will need to be accounted for in future modeling investigations.
Our models are limited by the available data and what they do not take into account. During the creation of the initial TRI model, we ignored all input factors other than temperature and time, including density-dependent processes, random effects, water availability and quality. The interpretation of our assessment of infectivity under experimental conditions is limited because a small number of snails were used during infection trials. More experimental work is needed to confirm the trends documented in our experimental study and strengthen the transmission model.
Due to the absence of modeling literature related to O. viverrini, we examined modeling techniques and strategies used in other helminths (i.e. Schistosoma; McCreesh and Booth, 2014a, b) and used these to inform experimental design, parameter choice, and model fit. Future models would incorporate greater complexity, including some of the aforementioned foregone elements, to better represent and reflect reality. However, as a simple model to predict infection success based on experimentally acquired parameters, this initial effort produces useful preliminary results and provides the necessary foundations for subsequent modeling work.
The seasonal transmission risk index goes beyond the TRI in its results and conclusions but is also more limited by data availability and assumptions necessary to make the model function. Because it uses many types of disparate input data collected and collated from multiple sources, each data source introduces its own uncertainty and error into the model with issues of data quality and protocol suitability. The input data were collected over the span of many decades and averaged whenever possible. Most of the studies from which we derived parameters are from separate sites, although all are in Khon Kaen Province, Thailand, or the immediate vicinity, controlling to a degree for confounding effects. As a consequence, the results of this model are meant to be applied to Khon Kaen Province and are not meant to be generalizable beyond northeastern Thailand, although the same framework could be used to predict seasonal TRIs in different settings if data is available.
A major limitation in this model was the data representing O. viverrini egg abundance and snail availability. Studies documenting these quantities are non-existent or very limited in scope. The lack of ability to measure O. viverrini eggs or cercariae in the environment is a major limitation that warrants further research. Using metacercarial burden as a proxy introduces many sources of error and requires an approximated time shift to address seasonality. Updating this input with O. viverrini egg or cercarial burden data in the future should be practiced in modeling as soon as the data is available. Additionally, it is possible that evolutionary processes may modify parasite transmission strategies, including adaptations to changing temperatures, making accurate predictions regarding seasonal transmission dynamics for the future difficult. Finally, snail availability data in Thailand in published literature was very limited, and we found it necessary to use two studies over 20 years apart (see Supplementary Table S2). However, we ran the model with both sets of data and found similar results when used alone or together.
Parasite transmission is a complex multi-agent process involving hosts, infective stages of the parasite and their environment. Environmental influence has been increasingly recognized as critically important, as both biotic and abiotic factors can modulate the rate of host-parasite encounters through environmentally mediated mechanisms and impact morbidity and mortality in human populations. Transmission processes need to be understood as highly dynamic through space and time as well as context-dependent.
Forecasting spatial and seasonal hotspots of parasite transmission for effective control is difficult and requires the concerted effort of parasitologists, ecologists and epidemiologists, as well as other stakeholders, to collect various types of relevant information. The multi-faceted data collected by these parties then needs to be integrated and used to inform practical intervention tools. Such integration can be done using mathematical models, whose analytical abilities have recently increased with the development of more efficient programming environments and increased computing power.
Notwithstanding the global effort to control human parasitic infections and the surge of control program development in tropical countries where parasitic infections are endemic, the role of mathematical models in evaluating these programs and furthering our understanding of disease transmission dynamics remains limited (Basáñez et al., 2012). In particular, the implementation of quantitative approaches coupling controlled experiments and modeling to gain insights regarding O. viverrini transmission dynamics are to our knowledge non-existent despite their significance.
Our experimental results indicate a relatively short (i.e 1–2 months) infectivity period of O. viverrini eggs once released in the environment, and further suggest that higher temperatures contribute to a faster deterioration of O. viverrini eggs, reducing their infective potential. When incorporating environmental and climatic regional parameters in our deterministic transmission model, we observed non-trivial feedback of environmental influences that create marked seasonal patterns of transmission risk to snails and identify prime seasonal windows for interventions.
Although our model is limited and is only preliminary at this stage, we contend that our approach, combining experimental transmission studies and modeling, encourages the implementation of more research at the interface between environment and disease transmission and advocates for the inclusion of population biology, ecology and systems sciences in existing research programs to address environment-mediated issues related to transmission. The relevance of this amended conceptual and methodological framework for an improved understanding of O. viverrini transmission dynamics aligns with recent propositions advocating the development of interdisciplinary research agendas and transdisciplinary actions for more sustainable disease control (Echaubard et al., 2015, 2016; Ziegler et al., 2016, Wilcox and Echaubard, in press).
Supplementary Material
Supplementary Fig. S1. Model Diagram representing how the models generate the Opisthorchis viverrini Transmission Risk Index (TRI) and seasonal TRI.
Supplementary Fig. S2. Graphical representation of Opisthorchis viverrini seasonal Transmission Risk Index (TRI) under current (2010) weather conditions. Each curve represents a different combination of input data sources described in Supplementary Table S3 and Supplementary Table S4.
Supplementary Fig. S3. Graphical representation of Opisthorchis viverrini seasonal Transmission Risk Index (TRI) in projected (2060) weather conditions. Each curve represents a different combination of input data sources described in Supplementary Table S6 and Supplementary Table S7.
Highlights.
Opisthorchis viverrini miracidium transmission success decreases after 3 weeks and at temperatures above 30°C
Our model suggests that O. viverrini transmission to snails is seasonal with a peak at the onset of the rainy season
Climate predictions suggest a seasonal shift in transmission risk to later in the year
Heating the septic system can be an efficient tool for control
Acknowledgments
We would like to thank Trevor Petney for comments and suggestions during the preparation of the manuscript as well as Manop Sripa for technical advice during data collection. We gratefully acknowledge support from the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS), Khon Kaen University, from the Thailand Research Fund (TRF) grant number RTA 5680006 and from the US National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), grant number P50AI098639. PE is a post-doctoral research scholar of Khon Kaen University. BS is a TRF Senior Research Scholar. Other financial support was provided by the Department of Biology, Laurentian University, Sudbury, Ontario, Canada. The content is solely the responsibility of the authors and does not necessarily represent the official views of the TRF, NIAID or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RM, May RM. Coevolution of hosts and parasites. Parasitology. 1982;85:411–426. doi: 10.1017/S0031182000055360. [DOI] [PubMed] [Google Scholar]
- Babel M, Agarwal A, Swain D, Herath S. Evaluation of climate change impacts and adaptation measures for rice cultivation in Northeast Thailand. Clim Res. 2011;46:137–146. doi: 10.3354/cr00978. [DOI] [Google Scholar]
- Barrett J. Studies on the induction of permeability in Ascaris lumbricoides eggs. Parasitology. 1976;73:109–121. doi: 10.1017/s0031182000051374. [DOI] [PubMed] [Google Scholar]
- Basáñez MG, McCarthy JS, French MD, Yang GJ, Walker M, Gambhir M, Prichard RK, Churcher TS. A research agenda for helminth diseases of humans: modelling for control and elimination. PLoS Negl Trop Dis. 2012;6:e1548. doi: 10.1371/journal.pntd.0001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanawong A, Waikagul J. Laboratory studies on host-parasite relationship of Bithynia snails and the liver fluke, Opisthorchis viverrini. SE Asian J Trop Med Publ Hlth. 1965;22:235–239. [PubMed] [Google Scholar]
- Combes C. The Art of Being a Parasite. University of Chicago Press; Chicago, USA: 2005. [Google Scholar]
- Crofton HD. A model of host-parasite relationships. Parasitology. 1971;63:343–364. doi: 10.1017/s0031182000079890. [DOI] [PubMed] [Google Scholar]
- Day T. Parasite transmission modes and the evolution of virulence. Evolution. 2001;55:2389–2400. doi: 10.1111/j.0014-3820.2001.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Echaubard P, Wilcox BA, Smith JF, Sripa B, Mallory FF. News from the IAEH: The Importance of Socio-Ecological Context in Ecohealth Initiatives. EcoHealth. 2015;12:4–7. doi: 10.1007/s10393-014-0999-7. [DOI] [Google Scholar]
- Echaubard P, Sripa B, Mallory FF, Wilcox BA. The role of evolutionary biology in Liver fluke research and control in Southeast Asia. Infection Genetics and Evolution. 2016;43:381–397. doi: 10.1016/j.meegid.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald PW. Host-Parasite Relations, Vectors, and the Evolution of Disease Severity. Annu Rev Ecol Syst. 1983;14:465–485. doi: 10.1146/annurev.es.14.110183.002341. [DOI] [Google Scholar]
- Grundy-Warr C, Andrews RH, Sithithaworn P, Petney TN, Sripa B, Laithavewat L, Ziegler AD. Raw attitudes, wetland cultures, life-cycles: Socio-cultural dynamics relating to Opisthorchis viverrini in the Mekong Basin. Parasitol Int. 2012;61:65–70. doi: 10.1016/j.parint.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Hinz E, Saowakontha S, Pipitgool V. Opisthorchiasis control in northeast Thailand: proposal for a new approach. Appl Parasitol. 1994;35:118–124. [PubMed] [Google Scholar]
- Kaewkes S. Taxonomy and biology of liver flukes. Acta Trop. 2003;88:177–186. doi: 10.1016/j.actatropica.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Kaewkes W, Kaewkes S, Tesana S, Laha T, Sripa B. Fecal bacterial contamination in natural water reservoirs as an indicator of seasonal infection by Opisthorchis viverrini in snail intermediate hosts. Parasitol Int. 2012;61:49–51. doi: 10.1016/j.parint.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Kawasaki J, Herath S. Impact assessment of climate change on rice production in Khon Kaen province, Thailand. J ISSAAS. 2011;17:14–28. [Google Scholar]
- Khampoosa P, Jones MK, Lovas EM, Srisawangwong T, Laha T, Piratae S, Thammasiri C, Suwannatrai A, Sripanidkulchai B, Eursitthichai V, Tesana S. Light and electron microscopy observations of embryogenesis and egg development in the human liver fluke, Opisthorchis viverrini (Platyhelminths, Digenea) Parasitol Res. 2012;110:799–808. doi: 10.1007/s00436-011-2557-3. [DOI] [PubMed] [Google Scholar]
- Kiatsopit N, Sithithaworn P, Kopolrat K, Andrews RH, Petney TN. Seasonal cercarial emergence patterns of Opisthorchis viverrini infecting Bithynia siamensis goniomphalos from Vientiane Province, Lao PDR. Parasit Vectors. 2014;7:551. doi: 10.1186/s13071-014-0551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiatsopit N, Sithithaworn P, Saijuntha W, Boonmars T, Tesana S, Sithithaworn J, Petney TN, Andrews RH. Exceptionally High Prevalence of Infection of Bithynia siamensis goniomphalos with Opisthorchis viverrini Cercariae in Different Wetlands in Thailand and Lao PDR. Am J Trop Med Hyg. 2012;86:464–469. doi: 10.4269/ajtmh.2012.11-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivenko VV. Action of environmental factors on the survivability of Opisthorchis eggs in reservoirs and in soil. Gig Sanit. 1979:80–81. [PubMed] [Google Scholar]
- Krivenko VV, Filatov VG, Kuzovlev AP, Glazkov GA. Significance of Opisthorchis egg survival in the environment in the transmission cycle in opisthorchiasis. Gig Sanit. 1981:62–64. [PubMed] [Google Scholar]
- Lacombe G, Smakhtin V, Hoanh CT. Wetting tendency in the Central Mekong Basin consistent with climate change-induced atmospheric disturbances already observed in East Asia. Theor Appl Climatol. 2013;111:251–263. doi: 10.1007/s00704-012-0654-6. [DOI] [Google Scholar]
- Lafferty KD. Environmental parasitology: What can parasites tell us about human impacts on the environment? Parasitol. Today. 1997;13:251–255. doi: 10.1016/S0169-4758(97)01072-7. [DOI] [PubMed] [Google Scholar]
- Liang S, Seto EY, Remais JV, Zhong B, Yang C, Hubbard A, Davis GM, Gu X, Qiu D, Spear RC. Environmental effects on parasitic disease transmission exemplified by schistosomiasis in western China. Proc Natl Acad Sci. 2007;104:7110–7115. doi: 10.1073/pnas.0701878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustigman S, Geldhof P, Grant WN, Osei-Atweneboana MY, Sripa B, Basáñez M-G. A Research Agenda for Helminth Diseases of Humans: Basic Research and Enabling Technologies to Support Control and Elimination of Helminthiases. PLoS Negl Trop Dis. 2012;6:e1445. doi: 10.1371/journal.pntd.0001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas-Coma S, Valero MA, Bargues MD. Chapter 2. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv Parasitol. 2009;69:41–146. doi: 10.1016/S0065-308X(09)69002-3. [DOI] [PubMed] [Google Scholar]
- McCreesh N, Booth M. The Effect of Increasing Water Temperatures on Schistosoma mansoni Transmission and Biomphalaria pfeifferi Population Dynamics: An Agent-Based Modelling Study. PLoS ONE. 2014a;9:e101462. doi: 10.1371/journal.pone.0101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreesh N, Booth M. The Effect of Simulating Different Intermediate Host Snail Species on the Link between Water Temperature and Schistosomiasis Risk. PLoS ONE. 2014b;9:e87892. doi: 10.1371/journal.pone.0087892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreesh N, Booth M. Challenges in predicting the effects of climate change on Schistosoma mansoni and Schistosoma haematobium transmission potential. Trends Parasitol. 2013;29:548–555. doi: 10.1016/j.pt.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Petney T, Sithithaworn P, Andrews R, Kiatsopit N, Tesana S, Grundy-Warr C, Ziegler A. The ecology of the Bithynia first intermediate hosts of Opisthorchis viverrini. Parasitol Int. 2012;61:38–45. doi: 10.1016/j.parint.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Pietrock M, Marcogliese DJ. Free-living endohelminth stages: at the mercy of environmental conditions. Trends Parasitol. 2003;19:293–299. doi: 10.1016/S1471-4922(03)00117-X. [DOI] [PubMed] [Google Scholar]
- Poulin R. Evolutionary Ecology of Parasites. Second. Princeton University Press; Princeton, USA: 2011. [Google Scholar]
- Poulin R. Toxic pollution and parasitism in freshwater fish. Parasitol Today Pers Ed. 1992;8:58–61. doi: 10.1016/0169-4758(92)90090-o. [DOI] [PubMed] [Google Scholar]
- Prasopdee S, Kulsantiwong J, Piratae S, Khampoosa P, Thammasiri C, Suwannatrai A, Laha T, Grams R, Loukas A, Tesana S. Temperature dependence of Opisthorchis viverrini infection in first intermediate host snail, Bithynia siamensis goniomphalos. Acta Trop. 2015;141:112–117. doi: 10.1016/j.actatropica.2013.10.011. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- Salter RM. Nova: A Modern Platform for System Dynamics, Spatial, and Agent-based Modeling. Procedia Comput Sci. 2013;18:1784–1793. doi: 10.1016/j.procs.2013.05.347. [DOI] [Google Scholar]
- Sithithaworn P, Pipitgool V, Srisawangwong T, Elkins DB, Haswell-Elkins MR. Seasonal variation of Opisthorchis viverrini infection in cyprinoid fish in north-east Thailand: implications for parasite control and food safety. Bull World Health Organ. 1997;75:125–131. [PMC free article] [PubMed] [Google Scholar]
- Sithithaworn P, Andrews RH, Van De N, Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley PJ, Sripa B. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int. 2012a;61:10–16. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithithaworn P, Andrews RH, Petney TN, Saijuntha W, Laoprom N. The systematics and population genetics of Opisthorchis viverrini sensu lato: Implications in parasite epidemiology and bile duct cancer. Parasitol Int. 2012b;61:32–37. doi: 10.1016/j.parint.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Sithithaworn P, Yongvanit P, Duenngai K, Kiatsopit N, Pairojkul C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepato-Biliary-Pancreat Sci. 2014;21:301–308. doi: 10.1002/jhbp.62. [DOI] [PubMed] [Google Scholar]
- Smith HV. Detection of parasites in the environment. Parasitology. 1999;117:113–141. [PubMed] [Google Scholar]
- Smout MJ, Sripa B, Laha T, Mulvenna J, Gasser RB, Young ND, Bethony JM, Brindley PJ, Loukas A. Infection with the carcinogenic human liver fluke, Opisthorchis viverrini. Mol Biosyst. 2011;7:1367. doi: 10.1039/c0mb00295j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A. The tumorigenic liver fluke Opisthorchis viverrini – multiple pathways to cancer. Trends Parasitol. 2012;28:395–407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010;72:305–350. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Tangkawattana S, Laha T, Kaewkes S, Mallory FF, Smith JF, Wilcox BA. Toward integrated opisthorchiasis control in northeast Thailand: The Lawa project. Acta Trop. 2015;141:361–367. doi: 10.1016/j.actatropica.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwannatrai A, Suwannatrai K, Haruay S, Piratae S, Thammasiri C, Khampoosa P, Kulsantiwong J, Prasopdee S, Tarbsripair P, Suwanwerakamtorn R, Sukchan S, Boonmars T, Malone JB, Kearney MT, Tesana S. Effect of soil surface salt on the density and distribution of the snail Bithynia siamensis goniomphalos in northeast Thailand. Geospatial Health. 2011;5:183–190. doi: 10.4081/gh.2011.170. [DOI] [PubMed] [Google Scholar]
- Thieltges DW, Jensen KT, Poulin R. The role of biotic factors in the transmission of free-living endohelminth stages. Parasitology. 2008;135 doi: 10.1017/S0031182007000248. [DOI] [PubMed] [Google Scholar]
- Tinsley RC, York JE, Everard ALE, Stott LC, Chapple SJ, Tinsley MC. Environmental constraints influencing survival of an African parasite in a north temperate habitat: effects of temperature on egg development. Parasitology. 2011;138:1029–1038. doi: 10.1017/S0031182011000461. [DOI] [PubMed] [Google Scholar]
- Tubbs LA, Poortenaar CW, Sewell MA, Diggles BK. Effects of temperature on fecundity in vitro, egg hatching and reproductive development of Benedenia seriolae and Zeuxapta seriolae (Monogenea) parasitic on yellowtail kingfish Seriola lalandi. Int J Parasitol. 2005;35:315–327. doi: 10.1016/j.ijpara.2004.11.008. [DOI] [PubMed] [Google Scholar]
- van Dijk J, Morgan ER. The influence of temperature on the development, hatching and survival of Nematodirus battus larvae. Parasitology. 2008;135:269–283. doi: 10.1017/S0031182007003812. [DOI] [PubMed] [Google Scholar]
- Wang YC, Ho RCY, Feng CC, Namsanor J, Sithithaworn P. An ecological study of Bithynia snails, the first intermediate host of Opisthorchis viverrini in northeast Thailand. Acta Trop. 2014 doi: 10.1016/j.actatropica.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Wilcox BA, Echaubard P. Balancing Biomedical and Ecological Perspectives in Research Framing of Liver Fluke and Cholangiocarcinoma in NE Thailand. Parasitol Int. doi: 10.1016/j.parint.2016.10.002. In press. [DOI] [PubMed] [Google Scholar]
- Winnepenninckx B, Backeljau T, De Wachter R. Extraction of high molecular weight DNA from molluscs. Trends Genet. 1993;9:407. doi: 10.1016/0168-9525(93)90102-N. [DOI] [PubMed] [Google Scholar]
- Wongratanacheewin S, Pumidonming W, Sermswan RW, Maleewong W. Development of a PCR-based method for the detection of Opisthorchis viverrini in experimentally infected hamsters. Parasitology. 2001;122:175–180. doi: 10.1017/s0031182001007235. [DOI] [PubMed] [Google Scholar]
- Wykoff DE, Harinasuta C, Juttijudata P, Winn MM. Opisthorchis viverrini in Thailand: The Life Cycle and Comparison with O felineus. J Parasitol. 1965;51:207–214. doi: 10.2307/3276083. [DOI] [PubMed] [Google Scholar]
- Ziegler AD, Echaubard P, Lee YT, Chuah CJ, Wilcox BA, Grundy-Warr C, Sithithaworn P, Petney TN, Laithevewat L, Ong X, Andrews RH, Ismail T, Sripa B, Khuntikeo N, Poonpon K, Tungtang P, Tuamsuk K. Untangling the Complexity of Liver Fluke Infection and Cholangiocarcinoma in NE Thailand Through Transdisciplinary Learning. EcoHealth. 2016 doi: 10.1007/s10393-015-1087-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. Model Diagram representing how the models generate the Opisthorchis viverrini Transmission Risk Index (TRI) and seasonal TRI.
Supplementary Fig. S2. Graphical representation of Opisthorchis viverrini seasonal Transmission Risk Index (TRI) under current (2010) weather conditions. Each curve represents a different combination of input data sources described in Supplementary Table S3 and Supplementary Table S4.
Supplementary Fig. S3. Graphical representation of Opisthorchis viverrini seasonal Transmission Risk Index (TRI) in projected (2060) weather conditions. Each curve represents a different combination of input data sources described in Supplementary Table S6 and Supplementary Table S7.


