Abstract
Stem cells respond to environmental signals that induce their differentiation to cells that make up specialized tissues and organs. Our laboratory has focused on bone marrow mesenchymal stem cells (MSCs) that supply bone osteoblasts and marrow adipocytes, an output that appears to be reciprocal. Case in point: exercise promotes osteogenesis and bone formation, and inhibits marrow adipose accrual. A mechanically induced signal pathway concentrating on preserving β-catenin also causes increased structure of the actin cytoskeleton, both of which inhibit adipogenesis. Recently we showed that intranuclear actin is as important to MSC lineage decisions as cytoplasmic actin. This opens up new areas for understanding gene expression in stem cells.
INTRODUCTION
Cells respond to both chemical and physical information to guide their differentiation and function. In the case of stem cells, signals from the micro- and macro-environments coalesce to either preserve stemness or to initiate a specific process of differentiation with overall goals of generating and adapting functional tissues. In our laboratory, we have focused on bone marrow mesenchymal stem cells (MSCs), progenitors for the differentiated cell types required for construction and maintenance of the skeleton. The primary output of bone marrow MSCs include the bone osteoblast of which a small proportion when enclosed in mineralized matrix become bone osteocytes (1), and the bone marrow adipocyte, which serves an as yet unclear function that might include provision for local energy storage (2). Bone marrow MSC output appears to reflect a reciprocal relationship between osteoblast and adipocyte lineages. The reciprocity of fat and bone in the marrow is apparent in the case of the constitutive low-density lipoprotein receptor- related protein 5 (Lrp5) activation associated with high bone mass (3), where increased trabecular bone is accompanied by decreased fat in bone marrow (4). Furthermore, possessing a single allele for the fat master transcription factor peroxisome proliferator activated receptor γ (PPARγ) results in an increased bone mass as adipocytes are diminished (5). Such reciprocity also pertains in the increased fat phenotype in the leptin null obese (ob/ob) mouse as well as in the fatless A-ZIP/F-1 mouse, with decreased and increased bone mass, respectively (6). It thus seems reasonable to equate increased marrow adipose cells with decreased bone formation.
In pathological states, a proportionality ratio between osteoblasts and adipocytes appears to predict bone and fat mass in the skeleton. Such a zero sum game includes the osteoporosis that accompanies aging (7), estrogen deficiency (8), and likely that of anorexia nervosa (9). PPARγ agonists lead to increases in marrow adipocytes while diminishing bone strength in aged mice and humans (10). Furthermore, in rodents, high fat diets which increase total fat mass may lead to reduced bone mineral density (BMD), at least insofar as adjusted for the bone mass needed to adequately support the increased body weight (11,12). Finally, a lack of exercise restrains osteogenesis and favors marrow adipose accrual (13,14). In this way, the output of the marrow MSC suggests a response to signals that promote one output while suppressing the other.
EXERCISE STIMULATES BONE AND REPRESSES MARROW FAT IN VITRO AND IN VIVO
Our laboratory has concentrated on understanding how exercise, or dynamic loading, regulates MSC differentiation both by inhibiting adipogenesis and stimulating osteogenesis. Over the last few years we have shown that mechanical signals directly regulate MSC lineage. Daily mechanical input delivered via substrate stretch (resulting in cell strain) effectively counteracts an adipogenic stimulus, inhibiting adipogenesis of primary marrow-derived MSCs and embryonic C3H10T1/2 MSCs (15,16) as evidenced by reduction in lipid and expression of PPARγ and adiponectin. Mechanical repression of adipogenesis depends on preservation of β-catenin activity which is affected via inhibition of GSK3β (17). Mechanical activation of β-catenin preserves MSC multipotentiality (18) in the face of adipogenic stimuli, and promotes accelerated osteogenesis in response to bone morphogenetic protein-2 (BMP2) (16). The anti-adipogenic effect of mechanical force is β-catenin dependent: β-catenin knockdown limits the ability of strain to prevent adipogenesis (Figure 1A).
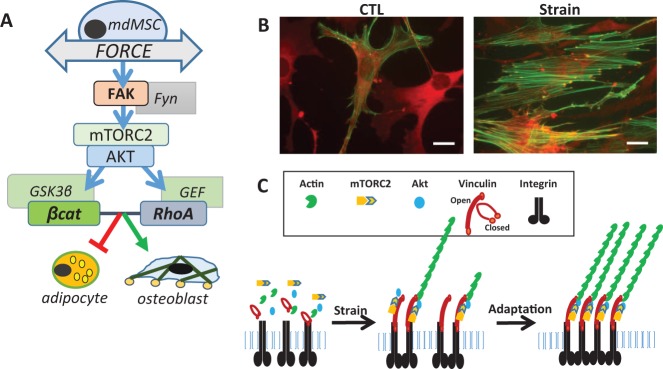
Fig. 1.
Force activated signaling prevents adipogenesis of mesenchymal stem cells (MSCs). (A) Cartoon showing signaling pathways important for reciprocal differentiation of adipocytes and osteoblasts. (B) Strain induces cytoskeletal connectivity with F-actin (green) linking focal adhesions (vinculin in orange). (C) Graphic showing that strain causes the recruitment of focal adhesion molecules into clusters which support enhanced signal density.
In vivo, exercise also prevents not only generalized adipogenesis, likely by promoting energy use, but also prevents expansion of marrow adipose tissue mass. Marrow adipose tissue (MAT) accumulation due to high fat diet or PPARγ agonist can be suppressed by daily exercise in mice (2,19), and exercise suppression of MAT expansion occurs simultaneously with new bone formation (20,21). Our findings that running exercise decreases marrow fat in young and old mice fed either a control or high fat diet (2) indicates that exercise might shrink marrow adipocyte size by energy use, and also suggests that force experienced in local skeletal bone during exercise might bias MSCs away from environmental stimuli that promote adipogenesis. That exercise can also decrease marrow fat induced by the strong PPARγ agonist, rosiglitazone, which promotes fat cell differentiation in vitro (18,22), supports that mechanical signaling inhibits fat cell development in the whole animal (19). In this way, exercise may represent a non-energy based mechanism to prevent fat development.
To fully understand the signaling responsible for preventing adipogenesis, our studies moved proximally from the GSK3β/β-catenin signaling node toward the plasma membrane, where the tug of substrate stretch is transmitted into the cell through integrins clustered into focal adhesions. Such focal adhesions connect the external substrate to bundled actin inside the cell (23). We were able to show that the strain-activated β-catenin signaling cascade is propagated at focal adhesions through recruitment of focal adhesion kinase operating in conjunction with Fyn to activate mTORC2, which then initiates the signal cascade of ↑Akt→ ↓GSK3β→ ↑β-catenin (17,24) (Figure 1A). The predominant effect of maintaining β-catenin in marrow derived MSC is preservation of multipotentiality, thus increasing osteogenic potential (18).
Occurring within the same time frame required for β-catenin activation, we showed that strained cells develop significant bundled actin struts (F-actin) that connect new focal adhesions to each to span the cell area (25) (Figure 1B). It has been reported that greater cytoskeletal structure enhances MSC differentiation into lineages supplying tissues with greater mechanical competence (i.e., bone, cartilage). As such, substrate stiffness, translated into enhanced cytoskeletal structure, promotes osteoblastic differentiation (26). This suggested that a complementary mechanical regulator of cell differentiation, along with β-catenin, might be the structural cytoskeleton itself.
FROM FOCAL ADHESIONS TO BUNDLED ACTIN FIBERS
Rearrangement of focal adhesions and their interconnecting filamentous actin (F-actin) struts induced by dynamic strain leads to amplification of signal generation (27). At the interface between the basal substrate and adjacent cells, the plasma membrane uses specific structural proteins to transduce force into biochemical signals. These structural proteins gather together to form focal adhesions, which span the cell membrane and connect the extracellular matrix to the actin cytoskeleton. Focal adhesions also serve as hubs for the recruitment and clustering of signaling molecules, where the combination of force transmission and signal transduction result in further cytoskeletal remodeling (25). Focal adhesion development requires activation of RhoA, a process that we found used the same proximal signaling tool box as that resulting in GSK3β inhibition and β-catenin preservation: here FAK/Fyn activation of mTORC2 and subsequently Akt was necessary for strain induce RhoA activation (27,28). Importantly, the absence of Rictor, the regulatory protein that differentiates mTORC2 from mTORC1 (29), affects both the basal cell cytoskeleton (30) and the ability of the MSC cytoskeleton to respond to mechanical load (27). Investigating a potential role of mTORC2 in marrow-derived MSC cell differentiation, we found that deleting mTORC2 function in cells by knocking down Rictor leads to adipogenesis. Accordingly, Rictor knockout mice were recently shown to have reduced skeletal mass (31).
An important result of focal adhesion development through dynamic strain is the enhancement of signaling. We found that a second daily bout of mechanical strain caused an augmented signal pathway as shown by increased phospho-Akt, GSK3β inhibition, β-catenin preservation, and, when repeated twice daily over the 4 days necessary for adipocyte differentiation, significantly enhanced the ability of strain delivery to repress adipogenesis (25). The source of such enriched signaling is dependent on the generation of focal adhesions: the subsequent recruitment of FAK and Akt to the focal adhesions allows a second or third bout of strain to respond to force with increased signal density (Figure 1C).
CYTOSKELETAL DETERMINANTS OF MSC DIFFERENTIATION CHOICES
The cell cytoskeleton, which participates in signal transduction, protein transport, and signal compartmentalization, undergoes reorganization in response to its microenvironment. Cytoskeletal reorganization in response to static physical cues from substrate attachment influences lineage allocation of stem cells (32,33). In the case of marrow-derived MSC differentiation, greater F-actin cytoskeletal structure enhances osteoblastic differentiation while limiting adipocyte differentiation (26,34). As such, cytoskeletal remodeling after attachment on hard bone mineral surfaces promotes osteogenic differentiation. Dynamic physical exercise also reinforces the skeleton (35,36) and its withdrawal promotes bone resorption (37).
These findings led to our interest in actin structural dynamics. In a study aimed at separating effects of b-catenin and cytoskeletal regulation of differentiation, we disrupted the MSC cytoskeleton using continuous cytochalasin D over the several days necessary to induce differentiation from the multipotential state (25,38). Expecting that this would induce adipogenesis, we were instead surprised to find our cultured MSC rapidly and robustly entering the osteogenic lineage (32). Such osteogenesis occurred even in the absence of osteogenic medium which promotes the osteogenic gene program through ascorbate-directed formation of an extracellular matrix. Finally, 10 days after cytochalasin D actin disruption, cultured cells formed bone nodule that intensely stained with alizarin red (32). Sustained F-actin disruption similarly induces osteogenic differentiation of human marrow-derived (32) and adipose-derived MSC (unpublished data from my lab, 2017).
What was even more surprising was that injection of the murine tibial space with cytochalasin D led to abundant formation of trabecular and cortical bone by 1 week (32) (Figure 2A). Along with structural and histological evidence of bone formation, we also noticed some degree of adipogenesis in the tibial marrow compartment, suggesting that not all extant MSCs responded to cytoskeletal disruption with osteogenesis, differentiating instead into adipocytes. Such a graded response might be expected in a marrow cavity possessing MSCs in varying stages of differentiation.
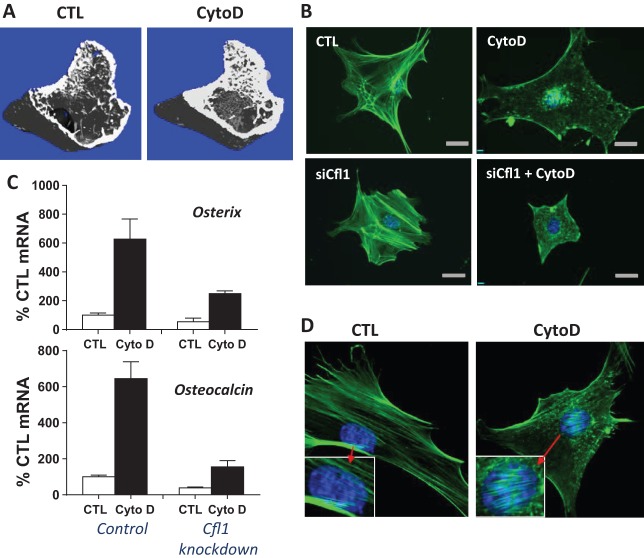
Fig. 2.
Nuclear actin transport stimulates osteogenesis in mesenchymal stem cells (MSCs). (A) X-ray microtomography (micro-CT) showing that trabecular and cortical bone forms 1 week after cytochalasin D injection into mouse tibial marrow cavity. (B) MSC stained for actin (green) and nucleus (blue) show that cytochalasin D causes mass transport of actin into the nucleus, a process dependent on the cofilin-1 actin transporter. (C) Preventing actin transport with cofilin-1 knockdown (right bars on each graph) inhibits cytochalasin stimulation of osteogenesis (black bars) as measured by expression of the bone specific genes osterix and osteocalcin. (D) After cytochalasin D treatment, intranuclear actin forms filaments which stain with phalloidin.
NUCLEAR ACTIN PROMOTES MSC DIFFERENTIATION
We then showed that cytochalasin D actin disruption results in mass transport of cellular actin into the nucleus as shown by the top two panels in Figure 2B. After cytochalasin D treatment, stress fibers disassemble and accumulate in the nucleus within 30 minutes (32). Actin transport into the nucleus is dependent on the level of monomeric actin substrate and the co-transporters importin-9 and the actin binding protein cofilin-1 (39). Knock out of either importin-9 or cofilin-1 prevented actin translocation into the nucleus, shown in the bottom panel of Figure 2B in the case of cofilin-1 silencing. Importantly, preventing actin transport to the nucleus prevents osteogenesis altogether, including both cytochalasin D–stimulated osteogenesis and the much less robust osteogenesis stimulated by osteogenic medium, shown in Figure 2C. Thus, differentiation can be effected not only by external cytoskeletal cues, but by changes in gene expression induced by mass actin transport into the nucleus.
NUCLEAR ACTIN STRUCTURE IS CRITICAL TO MSC FATE DECISIONS
What are the mechanisms by which nuclear actin might induce MSC osteoblast differentiation? Actin is known to play a role in gene transcription, at the very least through altering chromatin architecture (40), and altering transcriptional processes (41,42). This is certainly part of why actin nuclear transport is tightly regulated (39). As well, nuclear shape is controlled through the pinning of the nuclear membrane by cytoplasmic cytoskeletal connections with the linker of nucleoskeleton and cytoskeleton (LINC) complex, a complex and dynamic association of proteins that link actin and microtubules through Giant Nesprin on the outer nuclear membrane, to SUN proteins within the nuclear membrane leaflet (43). Importantly, the LINC complex connects to internal nuclear chromatin, such that changes in nuclear shape are thought to be able to alter gene silencing and activation through regulating the internal nucleoskeleton, largely made up of lamin (40). The possibility thus presents itself that intranuclear actin might also participate in structural rearrangements of chromatin and heterochromatin.
Recent work also identifies the nucleus and its membrane as mechanosensory organelles, where anchoring to the cytoskeleton via LINC complexes enables transmission of mechanical force from the outside in (44). We have further shown that force can be transmitted from the nucleus to focal adhesions to activate FAK from the inside to the outside of the cell (45). In this way, mechanical signals have the potential to further regulate connections between the nucleus and the cell cytoskeleton, generating another level whereby mechanical input can control cell behavior. Thus, while it has become accepted that genetic elements within the nucleus respond to mechanical challenges indirectly through their transduction into intermediary biochemical cascades, it has only recently been considered that applied forces might also directly alter chromosomal conformations, thus influencing the accessibility of genetic information for binding of transcriptional enhancers or repressors (46,47).
Once intranuclear, actin can be found in filamentous forms (48,49), and as actin-cofilin rods (50). The role of intranuclear actin structures in controlling gene transcription is poorly understood. Whether intranuclear actin forms serve specific regulatory roles in monomeric or polymerized forms is unknown (51). Interestingly, we were able to observe phalloidin staining within the nucleus, a sign of actin bundling (Figure 2D); this is possible because cytochalasin is not transferred into the nucleus (52). Moreover, an increase in intra-nuclear actin concentration should promote substrate-regulated polymerization (53). Importantly, within the nucleus are to be found all the generally accepted members of the actin tool box that allow polymerization and depolymerization of actin monomers. These components include formins necessary for end-on-end polymerization and key members of the Arp2/3 complex that initiate secondary branching (54).
How might such intra-nuclear actin structures influence gene transcription? Progression in the osteogenic lineage requires the master osteogenic transcription factor, Runx2, which although present, is not active, or interacting with its target cistrome until the MSC is induced to leave the multipotential state and enter osteogenesis (55). The PY motif of Runx2 has been previously shown to recruit YAP to Runx2 binding sites at heterochromatin, where its presence represses Runx2 activity (56). Our data suggest that Runx2 activation, consistent with the report by Zaidi, may be regulated through nuclear availability of YAP (32). Another possibility is that internal nuclear structure itself controls heterochromatization, a mechanism supported by the binding of lamins A and C to DNA causing specific silencing, perhaps through recruiting polycomb complexes (57,58). Our findings that actin polymerization occurs within the nucleus, and our unpublished data that osteoblastogenesis depends on Arp2/3-induced secondary branching, suggests that control of actin polymerization is a strategic area to explore regulation of gene expression.
PROPOSAL: “ACTIN UP” IN THE NUCLEUS
There are many unanswered questions arising from our work on nuclear actin and its involvement in specific gene expression. Some questions which we hope to address in future research include asking if nuclear actin can regulate transcription factor localization within the nucleus, or transcription factor access to previously silenced cistromes. We will also be interested in whether nuclear structure itself can define processes of heterochromatization, either by holding cistrome targets in silenced or opened configurations, or by regulating polycomb activity. We look forward to being part of emerging research into how nuclear structure regulates gene expression.
ACKNOWLEDGEMENTS
We would like to thank our valued colleagues in the laboratory, especially Drs. Gunes Uzer (Boise State University), Zhihui Xie (UNC SOM), and Maya Styner (UNC SOM), as well as the many other collaborators who have made our daily scientific experiences so very rewarding.
Footnotes
Potential Conflicts of Interest: Dr. Rubin has received National Institutes of Health (NIH) Grants AR066616, AR042360, and AR056655.
DISCUSSION
Reiser, Chicago: This is a wonderful talk on cytoskeletal regulation. Two questions: One is that cytochalasin depolymerizes actin as you explained to us — what if you use something that inhibits polymerization such as latrunculin, for example?
Rubin, Chapel Hill: So, it’s interesting....so we have not come back to latrunculin when we started studying actin inside the nucleus. Latrunculin has off-target effects because it inhibits protein transport out of the nucleus — so as a group, de-polymerization agents do not have exactly similar effects on controlling MSC fate.
Reiser, Chicago: Which leads me to the second question — might this give you some hint into the sorts of physiological stimuli that lead to a physiological de-polymerization effect and with that the reprogramming — gene programs?
Rubin, Chapel Hill: That is, of course, where we have every intention of going, thank you!
Gravallese, Worcester: That was a beautiful talk Janet, thank you very much. You alluded to this in your last slide, but I wanted you to elaborate a little bit on the types of mechanical forces that you are looking at here. Is this stretch, or is this pressure? Is that going to matter in terms of how this might translate to the in vivo setting?
Rubin, Chapel Hill: We study many mechanical forces in our lab. Most of our publications have used stretch — we stretch at 1% to 2%. That’s probably within the domain of what an MSC might see sitting in a bone niche. We have also used flow — we don’t like to use flow very much cause it’s messy; and we have also used vibration. When you ask what might, what does mechanical force do to MSCs, it gets a little hard. One of the things is really true is that no one has shown that force causes MSCs to become osteoblasts. They need other factors —so anybody who has published this, they are wrong! We actually think we might know why that is. Now stretch, for instance, turns on EZH2 as Richard Mauck at U Penn has shown. EZH2 is a part of the Polycomb Repressive Complex and it’s necessary for EZH2 activity to be turned off for osteogenesis to occur. So this might explain why we’re seeing unexpected negative repression of osteogenesis with stretch. That probably wasn’t what you were asking — now that I think about it.
Hochberg, Baltimore: So this is probably a naïve question and it’s coming from a clinician. Colchicine affects microtubule assembly. So, the first part is, does colchicine have an effect in your system? Part two is that there are patients who take chronic colchicine therapy. I’m thinking particularly patients with Familial Mediterranean fever and other hereditary febrile syndromes. Because it prevents not only the occurrences of the attacks, but also prevents secondary amyloidosis. So, I wonder if does affect the system. Are there any clinical data which suggest differences in body composition based on the use of chronical colchicine therapy?
Rubin, Chapel Hill: I would be wrong to say that microtubules had no role to play because of course they have lots of roles to play. But in terms of the way that we strain cells — we are stimulate signaling through focal adhesions that are strutted by F-actin fibers. When we have used colchicine to break up the microtubules we have found no effect on the signals that we generate to either cause new F-actin bundling or Akt signaling through to beta-catenin. There are lots of structures in the cell including lamina inside the nucleus with which the cytoskeleton all interacts. There are microtubules that also bind to the nuclear envelope at nesprin LINC connections, just like actin does. So clearly microtubules are part of the cytoskeleton. I have just not found, in the assays we perform in the lab, that colchicine has effects.
Hasday, Baltimore: So, our lab’s interested in stress and we’re interested in the P38 kinase and the HSP27 pathway which we’ve always looked at outside the nucleus. Because this pathway causes stress fibers and depolarization and new actin structures. We never thought about looking in the nucleus; have you looked in all of the HSP27-dependent possible effects in the nucleus?
Rubin, Chapel Hill: I haven’t, but people who study signal transduction know that a lot of these enzymes are moving into the nucleus, like ERK1/2 and NFAT1 move into the nucleus. Everything is moving in and out all the time — so I think that nuclear biology is just beginning to have its day: lots is happening in the nucleus.
REFERENCES
- 1.Thompson WR, Uzer G, Brobst KE, et al. Osteocyte specific responses to soluble and mechanical stimuli in a stem cell derived culture model. Sci Rep. 2015;5:11049. doi: 10.1038/srep11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Styner M, Thompson WR, Galior K, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. doi: 10.1016/j.bone.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–21. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 4.Qiu W, Andersen TE, Bollerslev J, Mandrup S, et al. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res. 2007;22:1720–31. doi: 10.1359/jbmr.070721. [DOI] [PubMed] [Google Scholar]
- 5.Akune T, Ohba S, Kamekura S, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–55. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wren TA, Chung SA, Dorey FJ, Bluml S, et al. Bone marrow fat is inversely related to cortical bone in young and old subjects. J Clin Endocrinol Metab. 2011;96:782–6. doi: 10.1210/jc.2010-1922. [DOI] [PubMed] [Google Scholar]
- 8.Cohen A, Dempster DW, Stein EM, et al. Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. J Clinical Endocrinol Metab. 2012;97:2782–91. doi: 10.1210/jc.2012-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fazeli PK, Bredella MA, Freedman L, et al. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Mineral Res. 2012;27:1864–71. doi: 10.1002/jbmr.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harslof T, Wamberg L, Moller L, et al. Rosiglitazone decreases bone mass and bone marrow fat. J Clin Endocrinol Metab. 2011;96:1541–8. doi: 10.1210/jc.2010-2077. [DOI] [PubMed] [Google Scholar]
- 11.Halade GV, Rahman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem. 2010;21:1162–9. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao JJ, Gregoire BR, Gao H. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone. 2009;44:1097–104. doi: 10.1016/j.bone.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Casazza K, Hanks LJ, Hidalgo B, Hu HH, et al. Short-term physical activity intervention decreases femoral bone marrow adipose tissue in young children: a pilot study. Bone. 2012;50:23–7. doi: 10.1016/j.bone.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trudel G, Coletta E, Cameron I, et al. Resistive exercises, with or without whole body vibration, prevent vertebral marrow fat accumulation during 60 days of head-down tilt bed rest in men. J Appl Physiol. 2012;112:1824–31. doi: 10.1152/japplphysiol.00029.2012. [DOI] [PubMed] [Google Scholar]
- 15.Case N, Xie Z, Sen B, et al. Mechanical activation of beta-catenin regulates phenotype in adult murine marrow-derived mesenchymal stem cells. J Orthop Res. 2010;28:1531–8. doi: 10.1002/jor.21156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sen B, Xie Z, Case N, Ma M, et al. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–75. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sen B, Styner M, Xie Z, Case N, et al. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chemistry. 2009;284:34607–17. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Case N, Thomas J, Xie Z, et al. Mechanical input restrains PPARgamma2 expression and action to preserve mesenchymal stem cell multipotentiality. Bone. 2013;52:454–64. doi: 10.1016/j.bone.2012.08.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Styner M, Pagnotti GM, Galior K, et al. Exercise regulation of marrow fat in the setting of PPARgamma agonist treatment in female C57BL/6 mice. Endocrinology. 2015;156:2753–61. doi: 10.1210/en.2015-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivasan S, Agans SC, King KA, Moy NY, et al. Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. Bone. 2003;33:946–55. doi: 10.1016/j.bone.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan S, Ausk BJ, Bain SD, Gardiner EM, et al. Rest-intervals reduce the number of loading bouts required to enhance bone formation. Med Sci Sports Exerc. 2015 May;47((5)):1095–103. doi: 10.1249/MSS.0000000000000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Styner M, Meyer MB, Galior K, et al. Mechanical strain downregulates C/EBPbeta in MSC and decreases endoplasmic reticulum stress. PloS ONE. 2012;7:e51613. doi: 10.1371/journal.pone.0051613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burridge K, Chrzanowska-Wodnicka M, Zhong C. Focal adhesion assembly. Trends Cell Biol. 1997;7:342–7. doi: 10.1016/S0962-8924(97)01127-6. [DOI] [PubMed] [Google Scholar]
- 24.Case N, Thomas J, Sen B, et al. Mechanical regulation of glycogen synthase kinase 3beta (GSK3beta) in mesenchymal stem cells is dependent on Akt protein serine 473 phosphorylation via mTORC2 protein. J Biol Chem. 2011;286:39450–6. doi: 10.1074/jbc.M111.265330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sen B, Guilluy C, Xie Z, et al. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem Cells. 2011;29:1829–36. doi: 10.1002/stem.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 27.Sen B, Xie Z, Case N, et al. mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells. J Bone Miner Res. 2014;29:78–89. doi: 10.1002/jbmr.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson WR, Guilluy C, Xie Z, et al. Mechanically activated Fyn utilizes mTORC2 to regulate RhoA and adipogenesis in mesenchymal stem cells. Stem Cells. 2013;31:2528–37. doi: 10.1002/stem.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber JD, Gutmann DH. Deconvoluting mTOR biology. Cell Cycle. 2012;11:236–48. doi: 10.4161/cc.11.2.19022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Holguin N, Shi Y, Silva MJ, et al. mTORC2 signaling promotes skeletal growth and bone formation in mice. J Bone Miner Res. 2015 Feb;30((2)):369–78. doi: 10.1002/jbmr.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sen B, Xie Z, Uzer G, et al. Intranuclear actin regulates osteogenesis. Stem Cells. 2015;33:3065–76. doi: 10.1002/stem.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swift J, Ivanovska IL, Buxboim A, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Develop Cell. 2004;6:483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 35.Gross TS, Srinivasan S, Liu CC, Clemens TL, et al. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res. 2002;17:493–501. doi: 10.1359/jbmr.2002.17.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warden SJ, Mantila Roosa SM, Kersh ME, et al. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci U S A. 2014;111:5337–42. doi: 10.1073/pnas.1321605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gross TS, Rubin CT. Uniformity of resorptive bone loss induced by disuse. J Orthop Res. 1995;13:708–14. doi: 10.1002/jor.1100130510. [DOI] [PubMed] [Google Scholar]
- 38.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 39.Dopie J, Skarp KP, Rajakyla EK, Tanhuanpaa K, et al. Active maintenance of nuclear actin by importin 9 supports transcription. Proc Natl Acad Sci U S A. 2012;109:E544–52. doi: 10.1073/pnas.1118880109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martins RP, Finan JD, Guilak F, Lee DA. Mechanical regulation of nuclear structure and function. Ann Rev Biomed Eng. 2012;14:431–55. doi: 10.1146/annurev-bioeng-071910-124638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapoor P, Chen M, Winkler DD, Luger K, et al. Evidence for monomeric actin function in INO80 chromatin remodeling. Nat Struct Mol Biol. 2013;20:426–32. doi: 10.1038/nsmb.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–52. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- 43.Crisp M, Liu Q, Roux K, et al. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, et al. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem. 2011;286:26743–53. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uzer G, Thompson WR, Sen B, et al. Cell mechanosensitivity to extremely low- magnitude signals is enabled by a LINCed nucleus. Stem Cells. 2015;33:2063–76. doi: 10.1002/stem.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le HQ, Ghatak S, Yeung CC, et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat Cell Biol. 2016;18((8)):864–75. doi: 10.1038/ncb3387. [DOI] [PubMed] [Google Scholar]
- 47.Makhija E, Jokhun DS, Shivashankar GV. Nuclear deformability and telomere dynamics are regulated by cell geometric constraints. Proc Natl Acad Sci U S A. 2016;113:E32–40. doi: 10.1073/pnas.1513189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baarlink C, Wang H, Grosse R. Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science. 2013;340:864–7. doi: 10.1126/science.1235038. [DOI] [PubMed] [Google Scholar]
- 49.Belin BJ, Cimini BA, Blackburn EH, Mullins RD. Visualization of actin filaments and monomers in somatic cell nuclei. Mol Biol Cell. 2013;24:982–94. doi: 10.1091/mbc.E12-09-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munsie LN, Desmond CR, Truant R. Cofilin nuclear-cytoplasmic shuttling affects cofilin-actin rod formation during stress. J Cell Sci. 2012;125:3977–88. doi: 10.1242/jcs.097667. [DOI] [PubMed] [Google Scholar]
- 51.Baarlink C, Grosse R. Formin’ actin in the nucleus. Nucleus. 2014;5:15–20. doi: 10.4161/nucl.28066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munter S, Enninga J, Vazquez-Martinez R, et al. Actin polymerisation at the cytoplasmic face of eukaryotic nuclei. BMC Cell Biol. 2006;7:23. doi: 10.1186/1471-2121-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holaska JM, Kowalski AK, Wilson KL. Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLoS Biol. 2004;2:E231. doi: 10.1371/journal.pbio.0020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–94. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyer MB, Benkusky NA, Sen B, Rubin J, et al. Epigenetic plasticity drives adipogenic and osteogenic differentiation of marrow-derived mesenchymal stem cells. J Biol Chem. 2016;291:17829–47. doi: 10.1074/jbc.M116.736538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaidi SK, Sullivan AJ, Medina R, et al. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J. 2004;23:790–9. doi: 10.1038/sj.emboj.7600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramdas NM, Shivashankar GV. Cytoskeletal control of nuclear morphology and chromatin organization. J Mol Biol. 2015;427:695–706. doi: 10.1016/j.jmb.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Verboon JM, Rincon-Arano H, Werwie TR, et al. Wash interacts with lamin and affects global nuclear organization. Curr Biol. 2015;25:804–10. doi: 10.1016/j.cub.2015.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]