Abstract
The hallmark of rheumatoid arthritis is synovitis, or inflammation of synovial tissues lining joints. Synovitis in rheumatoid arthritis promotes destruction of articular bone by inducing the differentiation and function of osteoclasts, leading to significant patient morbidity. The cell types and pathways mediating articular bone destruction have now been elucidated and the critical role of receptor activator of nuclear factor-kappa B ligand has been recognized, leading to the identification of new targets for the protection of articular bone. Synovitis not only promotes bone destruction, but also inhibits the ability of bone-forming osteoblasts to repair bone. In stark contrast, inflammation in spondyloarthritis, including ankylosing spondylitis, promotes bone formation at periosteal sites, resulting in pain and decreased motion of the spine and joints. Local anatomic factors contribute to these distinct outcomes for bone and anabolic pathways regulating bone formation are now being investigated to identify novel targets for prevention of abnormal bone formation.
INTRODUCTION
Since the advent of early radiography, it has been known that the rheumatic diseases have a significant impact on bone that varies tremendously depending upon the specific rheumatic disease. Rheumatoid arthritis (RA) is the most common form of inflammatory arthritis that is accompanied by destruction of bone. Patients with RA suffer erosion of articular bone and cartilage. Plain radiographs are widely used to detect and quantify bone erosion, to assess joint structural damage, and to monitor the efficacy of therapy. Articular erosions are closely correlated with disability in RA patients (1), and the importance of articular erosions is highlighted by their inclusion in the Food and Drug Administration’s core outcomes for this disease (2). In stark contrast, ankylosing spondylitis (AS) is a rheumatic disease in which inflammation promotes bone formation, leading ultimately to fusion of the spine and loss of spinal motion. Diseases such a psoriatic arthritis (PsA) represent a middle ground, with some joints showing articular erosion and others showing periarticular bone formation, particularly at the sites of tendon and ligament insertion into bone known as entheses.
Work in our laboratory has focused on defining the pathophysiologic mechanisms by which inflammation in the rheumatic diseases impacts bone. The anatomic site of inflammation plays an important role in the differential effects of the rheumatic diseases on bone. Inflammation in RA occurs initially in the synovium lining diarthrodial joints, and progresses to an intense immune-mediated process that leads to the production of proinflammatory cytokines, as well as the proliferation of synovial tissue. This inflamed synovial tissue ultimately enters in the bone marrow space deep to the joint surface and erodes articular bone. In the spondyloarthritis diseases, of which AS is the prototype, synovial inflammation is also present in many cases. However, the initial site of inflammation in these diseases is the enthesis, including enthesial sites around the spine. The cell types, mediators, and pathways regulating bone in these distinct anatomic sites are different, resulting in unique outcomes for bone.
Importantly, what has emerged from this work is the realization that many cytokines and factors that are known to regulate inflammation simultaneously play a critical role in bone homeostasis. This work has led to the birth of a new field, termed “Osteoimmunology,” the study of factors that affect both the immune system and bone (3). This review summarizes our knowledge of these factors and highlights the pathways that lead to such dramatically different outcomes for bone in the inflammatory rheumatic diseases.
BONE LOSS IN RHEUMATOID ARTHRITIS
Articular Bone Erosions
Several forms of bone loss are seen in RA, including periarticular demineralization, articular bone erosion, and systemic osteopenia/osteoporosis. Erosions are breaks in the cortical surface of articular bone, and adjacent subchondral and trabecular bone are also often destroyed. The term “bone erosion” is a radiologic one, showing that imaging is required for detection (4). Although erosions are seen in other forms of arthritis, their severity and the almost complete absence of associated new bone formation and erosion repair are unique to RA. Technologic advances in joint imaging have improved the ability to detect and quantify erosion number and volume, and modalities including computed tomography, high-resolution ultrasound, and magnetic resonance imaging are all used currently to detect early bone erosions in RA patients (5,6).
The Role of Osteoclasts
RA is the prototype of a systemic rheumatic disease that results in inflammation of synovial tissues and subsequent destruction of bone. Early work investigating the cells that regulate bone loss in RA suggested that the synovial fibroblast, a cell type lining the surface of the synovium, was responsible for bone erosion through the generation of a mildly acidic environment that led to slow dissolution of bone. However, in physiologic conditions, the only cell type that is capable of resorbing bone is the osteoclast. Osteoclasts are terminally differentiated, multinucleated cells derived from cells of the monocyte/macrophage lineage. These cells are uniquely capable of resorbing bone through their ability to form an adherent seal on the bone surface and to generate a significantly acidic environment (pH of 4-5) deep to the cell surface, thus leaching mineral from bone. In addition, lysosomes within osteoclasts contain enzymes, including cathepsin K and gelatinases, that are secreted across the cell’s ruffled boarder onto the bone surface and that are capable of degrading the organic bone matrix.
We obtained tissues from orthopedic joint replacement surgeries in RA patients and observed that the interface between the inflamed synovially derived tissues and bone was lined by sites of bone resorption containing large, multinucleated cells that we hypothesized were osteoclasts. Using molecular and immunologic techniques to identify surface markers, we showed definitively the identity of these cells as osteoclasts (7). Because of loss of subchondral bone in the joint that provides a scaffold for cartilage, osteoclastic bone resorption in RA also results in significant loss of articular cartilage (8,9), contributing to disability.
Receptor Activator of Nuclear Factor-Kappa B Ligand (RANKL) as a Critical Cytokine in the Pathogenesis of Articular Erosions
To define the mechanisms by which osteoclasts are generated at the interface of inflamed synovial tissues and bone, we considered a number of cytokines, chemokines, and factors known to be produced in the inflamed RA synovium that can promote the differentiation of monocyte/macrophage lineage cells to osteoclasts. These include tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, IL-15, IL-17, and prostaglandins, among others. However, none of these factors is required for osteoclastogenesis. As these studies were being performed, the essential factor for osteoclastogenesis was cloned and identified, which is RANKL. In mice deficient in RANKL, osteoclasts cannot be generated and thus bone cannot be remodeled. RANKL-deficient mice show an osteopetrotic phenotype, with thickened, trumpet-like bones (10,11). Interestingly, RANKL was cloned and identified almost simultaneously in four different laboratories and was also named tumor necrosis factor-related activation-induced cytokine (TRANCE), osteoprotegerin ligand (OPGL), and osteoclast differentiation factor (ODF). One of these laboratories, that of Dr. Yongwon Choi, identified RANKL as a factor that allowed for the augmentation of dendritic cell-T cell interactions (12). Thus, RANKL was the first of many factors in the field of osteoimmunology that was found to play a dual role in both the immune system and bone.
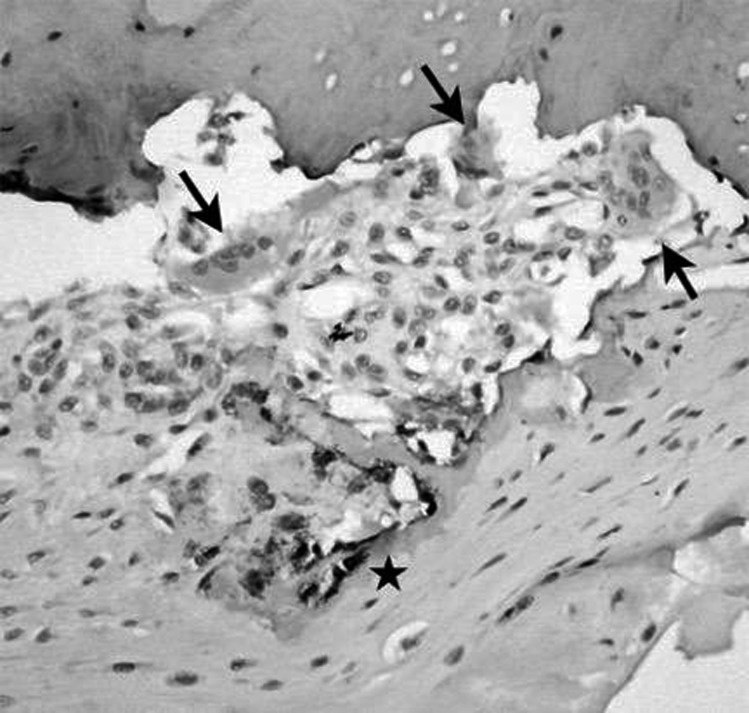
Attention was thus focused on the possibility that cells within the inflamed synovial tissues might provide an alternative source of RANKL that was brought into the bone microenvironment with the invading synovium. We first generated total RNA from whole synovial tissues from RA joint replacement tissue samples and showed that all of these samples expressed messenger RNA (mRNA) for RANKL, whereas no joint tissues from normal controls expressed mRNA for this factor. We then isolated specific cell types from RA synovial tissues and identified that RANKL was expressed by RA synovial fibroblasts and by activated CD4+ T cells (13). We followed these studies by observations using immunohistochemistry and showed RANKL protein expression specifically and focally at the synovial/bone interface at sites of osteoclastic resorption of bone (Figure 1). These findings were corroborated in other laboratories (14) and it has subsequently been shown that B cells also provide a source of RANKL (15).
Fig. 1.
RANKL is expressed locally at sites of articular erosion in rheumatoid arthritis (RA). Tissue obtained from the interface of inflamed synovium (pannus) and bone at the time of joint replacement surgery in a patient with RA. Arrows show multinucleated osteoclasts that have actively resorbed bone locally. Immunohistochemical staining using an antibody to RANKL shows the local production of RANKL (dark staining, starred region) at sites of osteoclastic bone resorption.
The mere expression of a factor at a site of pathology may implicate that factor in pathogenesis, but does not, of course, prove causality. To show mechanism, we obtained the RANKL-deficient mice from Dr. Choi and generated serum transfer arthritis in these mice and in controls. This model was kindly shared with my laboratory by Drs. Christophe Benoist and Diane Mathis. The serum transfer model is a well-established model of RA in which K/BxN mice develop a spontaneous arthritis due to the production of an arthritogenic antibody, anti-glucose-6-phosphate isomerase (anti-GPI). Serum from these arthritic mice containing anti-GPI antibody can be transferred to recipient mice to induce arthritis, and recipient mice develop articular bone erosions as a prominent feature (16). The RANKL-deficient recipient mice developed a level of inflammatory arthritis that was equivalent to that seen in controls, and also developed cartilage destruction. However, they were protected from articular bone destruction in the absence of RANKL and thus in the absence of osteoclasts (8). Similar findings were seen in several other mouse models of RA subjected to treatment with osteoprotegerin (OPG), the decoy receptor for RANKL that blocks the biologic function of RANKL. Additionally, in a model of RA driven by TNF that was crossed to another osteoclast-deficient mouse, the c-fos–deficient mouse (9), bone erosion was also absent, confirming the requirement for osteoclasts in the generation of articular bone destruction in RA.
Clinical trials have subsequently been performed to show a critical role for osteoclasts in bone destruction in human RA. In a trial using the potent bisphosphonate zoledronic acid, slowing of the progression of articular erosions was shown (17,18). Subsequently, an antibody targeting RANKL, Denosumab, was developed and used in a randomized controlled trial in RA and compared with placebo (19). This trial showed a statistically significant decrease in the number of articular erosions compared with placebo, and these findings were confirmed in a later follow up trial (20). The latter trial also assessed the possibility of healing erosions with Denosumab and showed partial healing of erosions in RA patients.
Cytokines that Regulate Osteoclastogenesis in RA
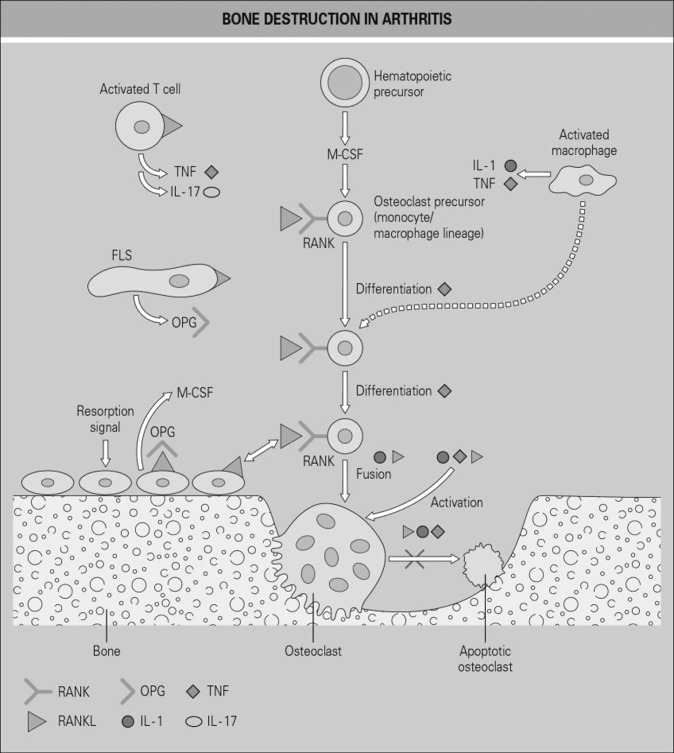
Inflamed RA synovial tissues are also a source of a host of cytokines that are pro-inflammatory and also augment the action of RANKL in promoting osteoclastogenesis and articular bone destruction, either independently or synergistically with RANKL. These include TNF, IL-1, IL-6, IL-17, and others. Many of these cytokines can also induce the expression of RANKL by synovial fibroblasts and/or osteoblasts, and it has been shown that RA patients with more active disease show a higher expression of RANKL (21–23). One of the most important pro-inflammatory cytokines that promotes articular bone destruction is TNF, which is a critical regulatory cytokine in inflammation in RA. TNF also promotes osteoclast precursor cell proliferation (24) and induces nuclear factor-kappa B (NF-кB), which in turn promotes the nuclear factor of activated T-cells (NFAT)c1 signaling central to osteoclast differentiation (25) and acts both independently and synergistically with RANKL in promoting osteoclast differentiation. Finally, TNF induces IL-1, which promotes TNF-induced osteoclastogenesis (26). Figure 2 provides a summary of the RANKL/RANK pathway and the key cytokines that interact with this pathway.
Fig. 2.
Schematic view of osteoclast differentiation and the role of the receptor activator of nuclear factor kappa-B ligand (RANKL) in rheumatoid arthritis (RA). Several cell types express RANKL at sites of bone erosion in RA. These include activated T cells and synovial fibroblasts (FLS), as well as osteoblasts lining the bone surface. Production of RANKL by these cells and its interaction with the RANK receptor on osteoclast precursor cells promotes the differentiation of these hematopoietic precursors into multinucleated bone-resorbing osteoclasts. Many locally produced pro-inflammatory cytokines contribute to this process, including interleukin 1 (IL-1) and tumor necrosis factor (TNF) derived from activated macrophages and T cells, T cell–derived IL-17 and macrophage colony-stimulating factor (M-CSF), which promotes expansion of the precursor cell pool. Osteoprotegerin (OPG) is the naturally occurring decoy receptor for RANKL, which is produced by several local cell types and blocks the biologic functions of RANKL. Pro-inflammatory cytokines also play a role in fusion (multinucleation) and activation of osteoclasts, as well as inhibition of their apoptosis. (From Gravallese EM, Monarch PA. “The rheumatoid joint: synovitis and tissue destruction.” In Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology, 6th ed. Philadelphia, PA: Elsevier; 2015. © Mosby, an imprint of Elsevier Ltd.)
IL-6 is another important cytokine in the induction of inflammation in RA. However, the effects of IL-6 on osteoclastogenesis in vitro are conflicting. IL-6 induces RANKL expression, but it can also inhibit osteoclast precursor cell differentiation by interfering with downstream signaling pathways for RANK, the cognate receptor for RANKL. Blocking IL-6 decreases the number of osteoclasts (27) and inhibits joint damage in an in vivo murine model of RA (28), as well as in human RA (29). Finally, IL-6 enhances the expression of IL-17, another important cytokine in both inflammation and bone destruction in RA. IL-17 induces the production of several pro-inflammatory mediators, including prostaglandins, nitric oxide, cytokines, and chemokines. IL-17 induces IL-1 and TNF production in macrophages and fibroblasts (30,31) and acts synergistically with IL-1 to induce the production of inflammatory mediators by synovial fibroblasts. IL-17 is produced by several cell types within the inflamed synovium, including a subset of T cells known as Th17 cells (32). Importantly, IL-17 induces the expression of RANKL on both osteoblasts and synovial fibroblasts, and induces prostaglandin E2 production by these cells to induce osteoclastogenesis. IL-17 blockade has been shown to be effective in murine models of RA in inhibiting inflammation and preventing articular bone erosion (33), but blockade of IL-17 or the IL-17 receptor in patients with RA has been disappointing (34). Finally, anti-inflammatory cytokines such as IL-4 and IL-10 are also expressed in inflamed RA synovial tissues and inhibit the development of articular erosions in murine models of RA (35). However, the effects of the above described pro-inflammatory cytokines and other pro-osteoclastogenic factors overcome any protective effect these anti-inflammatory factors may show.
INHIBITION OF BONE FORMATION IN RA
Impact of Inflammation on Osteoblasts and Bone Formation
Current therapies for RA are extremely effective in preventing the progression of articular bone destruction. Using a combination of methotrexate and biologic agents that block the function of key pro-inflammatory cytokines, it is often possible to halt this progression entirely. Surprisingly, however, existing bone erosions typically do not heal, as would be expected in settings of physiologic bone loss. Thus, our laboratory hypothesized that in RA, the inflammatory environment may not only promote the generation of osteoclasts that resorb bone, but also inhibit the function of the cell that forms bone, the osteoblast.
Osteoblasts are cells derived from precursor cells of the mesenchymal lineage. Committed osteoblast progenitors differentiate to cells capable of the production and mineralization of organic bone matrix. Fully differentiated osteoblasts can go on to become osteocytes, cells embedded within the matrix of bone that act as critical mechanosensors. Osteocytes are fully interconnected to one another as well as to local blood vessels. These cells send signals to osteoclasts to promote resorption of bone when bone is unloaded and, in contrast, induce osteoblasts to form bone in the setting of mechanical loading. Two pathways have been identified that are required for osteoblast differentiation from mesenchymal precursors: the bone morphogenetic protein (BMP) and the canonical Wingless (Wnt) pathways. These pathways are anabolic and strongly promote bone formation. These will be discussed in greater detail in later sections. It has been shown by the work of our group and many others that not only do the pro-inflammatory cytokines central to the inflammatory process in RA promote osteoclastogenesis and bone loss, but they also act on osteoblasts to inhibit their differentiation and function (36). For example, TNF inhibits the expression of a key transcription factor for osteoblast differentiation, Runx2 (37), as well as inducing the apoptotic cell death of osteoblasts (38).
The Potential to Repair Articular Erosions in RA
To better understand the potential of osteoblasts within sites of articular bone erosion in RA to form bone, we used a modification of the serum transfer model of RA in which we induced inflammation and articular bone erosion by the transfer of serum to naïve mice, and subsequently discontinued serum administration and followed mice over time as inflammation waned and eventually resolved. In addition, at serial intervals over the inflammatory and resolution phases of disease, we administered fluorochromes, fluorescent molecules that become incorporated into newly formed bone to allow the calculation of a bone formation rate. The results were striking — during the inflammatory phase of disease, bone formation rates at erosion sites were extremely low and were in the range of the rates in non-arthritic mice. In contrast, as inflammation resolved, bone formation rates increased significantly, resulting in complete healing of existing erosions (39). Furthermore, at peak inflammation, only immature osteoblast precursor cells were identified, showing that the differentiation of these cells is indeed inhibited in the presence of inflammation.
In contrast, with resolution of inflammation, differentiation of osteoblast precursor cells was shown (40), and mature osteoblasts populated sites of erosion healing. These data show that as long as inflammation is present in the joint, repair or healing of erosions will not occur, and further suggest that in patients with RA in whom erosion healing is not seen with therapy, there may be sub-clinical, residual inflammation within joints. This residual inflammation has now been shown using advanced imaging techniques (41) in patients who were deemed to be in clinical remission. The importance of the presence of residual inflammation is multifold. Inflammation in joints in RA has been shown to promote a significant downstream impact on health, including increasing the risk of cardiovascular disease and systemic osteoporosis. Current studies are addressing the degree to which inflammation must be controlled to mitigate these damaging downstream events.
Additional mechanisms by which erosion repair is limited in RA involve the inhibition of pathways that promote new bone formation. Factors that have been shown to limit bone formation and erosion repair include antagonists of the anabolic Wnt signaling pathway, such as Dickkopf (DKK)-1 and members of the secreted frizzled related protein (sFRP) family. DKK1 is induced by TNF in synovial fibroblasts in both animal models and in cells from RA patients and potently blocks Wnt signaling by binding to the receptor for Wnt ligands (42). We showed in the serum transfer model of arthritis that inflamed synovial tissues also express sFRP-1 and -2, and expression of this family of Wnt inhibitors diminishes as inflammation wanes and resolves (40). It is still not known whether blockade of Wnt antagonists will trigger healing of bone erosion, but studies are currently underway to address this question.
ANKYLOSING SPONDYLITIS AND BONE FORMATION
In stark contrast to the effects of inflammation on bone in RA, inflammation in the spondyloarthritis syndromes, including AS, PsA, juvenile arthritis, and the arthritis associated with inflammatory bowel disease, leads to bone formation on the outer, or periosteal surface of bone. This bone formation can result in significant disability, with the formation of new bone at sites of tendon and ligament insertions (entheses), and bone formation around the spine, leading to decreased spinal mobility, pain and in severe cases, to fusion and spinal immobility (43). AS is also characterized by synovitis of peripheral and sacroiliac joints that show many of the same histologic characteristics as synovitis in RA, including inflammatory cell infiltration, synovial lining hyperplasia, and the formation of pannus. Within the joint, articular erosions are common, similar to those seen in patients with RA. However, inflammation in AS at the enthesial sites is typically accompanied bone formation (44).
The differential mechanisms responsible for the effects on bone in RA versus spondyloarthritis have been the subject of intense investigation in our laboratory and others. Synovial tissues derived from the facet joints of AS patients have been studied (45) and foci of new bone formation have been identified at contacts points between the cartilage and inflamed synovial tissues. These regions of bone formation contained Runx2 and type I collagen–expressing cells, markers that are consistent with immature cells of osteoblast lineage. Lories et al (46) have also analyzed inf lamed synovial tissues from patients with AS versus RA and identified the induction of bone morphogenetic proteins BMP-2 and -6 in tissues from patients with both diseases. They speculated that one of the major differences in AS and RA that could account for differential effects on bone is the anatomic site of inflammation, with inflammatory infiltrates in RA being exclusively within the joint, and in AS being located both within joints and also at entheses. This group extended these observations to animal models of AS, including the DBA/1 mice that develop both arthritis and enthesial bone formation spontaneously. The excessive bone formation seen in these mice mimics that characteristically seen in patients with AS. They showed in vivo that treatment of these mice with noggin, an antagonist of the BMP pathway, resulted in attenuation of enthesial bone formation (47). This placed the BMP pathway as a central pathway in the process of bone formation in spondyloarthritis. Immunohistochemical analysis of biopsy specimens from enthesial sites in patients with AS has also shown expression of phosphorylated Smad 1/5, downstream signaling molecules that are consistent with activation of the BMP signaling pathway at these sites (47).
The BMP and Canonical Wnt Signaling Pathways
BMPs are members of the transforming growth factor–β (TGF-β) superfamily and are critical factors for skeletal development and bone homeostasis. Mutations in the BMP signaling pathway result in abnormal bone mass. In bone, BMPs are produced most abundantly by osteoblasts, but are also made by chondrocytes, and endothelial cells. Most of the members of the BMP ligand family, including BMP-2, -4, -6, and -7, promote bone formation and are anabolic (48,49). These BMP ligands bind to BMP receptors on cell membranes, phosphorylating downstream Smad proteins 1/5/8 that in turn form complexes with Smad4 to activate transcription of genes known to be responsive to BMPs. Noggin (mentioned above) and chordin are inhibitors of this pathway that act by binding directly to BMP ligands to prevent receptor interaction and signaling.
Another critical pathway regulating osteoblast differentiation and function is the canonical Wnt signaling pathway that is activated by secreted Wnt ligand proteins. The receptor for this pathway is actually a co-receptor complex of a Frizzled and lipoprotein receptor related protein (LRP) 5/6. Once activated by Wnt ligands, downstream signaling inhibits glycogen synthase kinase (GSK)3B, preventing its interaction with cytosolic β-catenin, which then translocates to the nucleus to activate transcription of a host of genes controlling osteoblast differentiation. Crosstalk also exists between the BMP and Wnt signaling pathways. For example, BMP-2, an anabolic ligand in the BMP pathway, induces the expression of Wnt ligands that promote Wnt signaling (such as Wnt-1 and Wnt-3a) in mesenchymal stem cell lines (50).
Inhibition of the Wnt signaling pathway occurs mainly through the action of secreted antagonists, including inhibitors in the sFRP and DKK families, as well as the secreted Wnt antagonist sclerostin. The sFRP family includes soluble decoy receptors that bind Wnt ligands and prevent their interaction with the Frizzled-LRP5/6 receptor. On the other hand, the DKK family of antagonists and sclerostin bind to the LRP5/6 co-receptor directly to inhibit signaling. It has also been shown that DKK1 can induce the expression of sclerostin to further inhibit signaling (51). Our laboratory hypothesized that Wnt signaling antagonists might play a role in the inhibition of bone formation in RA, and showed that in arthritic synovium in the K/BxN murine model of RA, expression of members of both the DKK and sFRP families was upregulated (39). Importantly, as synovial inflammation resolved, expression of the Wnt antagonists of the sFRP family was downregulated, but significant resolution of inflammation was needed to promote Wnt signaling and erosion repair in this model. Thus, factors are expressed in inflamed synovial tissues that not only promote bone resorption, but also inhibit bone formation through inhibition of Wnt signaling and thus of osteoblast differentiation and function. Other laboratories have also shown a critical role for DKK1 in the inhibition of bone formation in RA, and have shown that DKK1 is expressed by synovial fibroblasts in human RA synovium and that this factor is upregulated by TNF (42).
A Newly Identified T Cell Subset and its Potential Role in the Pathogenesis of Bone Formation in Spondyloarthritis
Recent data suggests that inflammation in spondyloarthritis is driven by innate immune mechanisms, leading to the production of IL-23 and IL-17, cytokines that have been implicated as central to disease pathogenesis (52,53). In a landmark study, Sherlock et al showed that overexpression of IL-23 in a murine model resulted in enthesial inflammation similar to that seen in spondyloarthritis. Arthritis followed enthesitis, showing that the earliest site of pathology in spondyloarthritis is the enthesis, with subsequent transfer of inflammation to joints (52,54). In this model, IL-23 was found to act on a unique cell population to induce the production of IL-17A and F and IL-22. The cell type acted upon was found to be a newly described enthesial resident T cell, identified as RORgammat +CD3+CD4-CD8-, also expressing the IL-23R. In turn, IL-22 produced by these cells induces osteoblast differentiation from mesenchymal cells present on the periosteal surface of bone locally, with subsequent bone formation. However, it has been shown that IL-23 also induces osteoclastogenesis when placed in co-culture systems with osteoblasts (55) and appears to act through IL-17 to induce the formation of osteoclasts with subsequent bone resorption (56). IL-23 therefore exerts differential effects on bone resorption and formation, suggesting that other mechanisms may also promote periosteal formation in spondyloarthritis. The source of IL-23 in spondyloarthritis is a topic of great interest. Current evidence suggests that there may be increased production of IL-23 in the gut in patients with spondyloarthritis, and these patients have been shown to develop microscopic gut inflammation (57). In addition, HLA-B27 is a risk allele in spondyloarthritis, and this molecule has been shown to induce IL-23 production through activation of pathways involved in the process of autophagy (58). Finally, IL-17 has been shown to play a role in inflammation in spondyloarthritis, and new therapies blocking IL-17 are effective in decreasing inflammation in this disease. However, the role of IL-17 in osteoblast biology is complex, with studies showing that IL-17 induces osteoblast differentiation in mesenchymal cells, but inhibits osteoblast differentiation in more mature osteoblasts (59,60). The outcome for bone in the setting of IL-17 inhibition remains to be determined.
CONCLUSION
RA is the prototype of an inflammatory arthritis that results in articular bone loss (erosion) and osteoclasts are the critical cell type leading to bone resorption in this disease. Cytokines critical to the bone resorption seen in RA include RANKL, which is essential for this process and is produced by cells within inflamed synovial tissues. In addition, a host of pro-inflammatory cytokines also produced locally within the joint contribute to bone erosion by either inducing RANKL on local cell types, or by acting synergistically with RANKL to promote the formation of bone-resorbing osteoclasts. Inflammation in RA also impairs bone formation by osteoblasts, enhancing the loss of bone and preventing erosion repair. Thus, even when patients are treated with potent disease modifying agents, erosion progression can be halted, but repair of erosions is rare, suggesting that there may be residual, subclinical inflammation remaining in the joints. In contrast, patients with spondyloarthritis develop disabling bone formation at enthesial sites both in peripheral joint and in the spine, leading to pain and decreased mobility. Mechanisms of bone formation in these diseases include activation of the BMP and Wnt signaling pathways, and cytokines including IL-17A, IL-22 and IL-23 that promote inflammation and osteoblast differentiation and bone formation. Knowledge of these pathways has led to novel therapeutic strategies for the treatment of patients with rheumatic diseases and for the protection from disabling alterations in bone homeostasis.
ACKNOWLEDGEMENTS
The work presented here would not have been possible without our generous collaborators, especially Christophe Benoist and Diane Mathis, Yongwon Choi, Steven Goldring, David Burr, and our Orthopedic colleagues Benjamin Bierbaum and David Mattingly. My deepest thanks go also to the tremendous efforts of all of the postdoctoral fellows, MD, and PhD students and technicians who have worked in my laboratory over the years. In particular, I would like to acknowledge: Rebecca Baum, Yukiko Maeda, Catherine Manning, Melissa Matzelle, Anita Shaw, Allison Pettit, and Nicole Walsh. I am grateful as well to Ruth Lopriore for longstanding graphic assistance.
Footnotes
Potential Conflicts of Interest: Dr. Gravallese has received research grant funding from Abbvie, Inc., and Eli Lilly and Company. She has served as a consultant for: Abbvie, Inc.; Amgen; Eli Lilly and Company; Flexion; Sanofi; GlaxoSmithKline; Merck; Novo Nordisk and SeraCare. She holds stock in Johnson and Johnson and receives royalties from Elsevier (textbook editor, Rheumatology) and UptoDate.
DISCUSSION
Loeser, Chapel Hill: Really nice talk, Ellen. I am glad you mentioned sclerostin at the end because I was wondering in terms of inflammatory erosions if there is any pre-clinical evidence that inhibiting sclerostin would have a benefit.
Gravallese, Worcester: That’s a very good question. There is an interesting article in Science Translational Medicine just recently published looking at the anti-sclerostin antibody in animal models of arthritis. And what they showed was very surprising. If you block sclerostin, you actually increase inflammation in the models that are TNF driven. And they demonstrated that sclerostin is an inhibitor of some of the downstream pathways from TNF. So I think this is going to limit use of the anti-sclerostin antibody in our diseases where TNF is such a prominent player in inflammation.
Collier, Newark: Ellen, isn’t it important to heal erosions in rheumatoid arthritis?
Gravallese, Worcester: That’s a great question Ginger. Thanks for that question. I am not sure that it’s important to heal erosions. I don’t think that we are going to be able to completely reconstruct a normal joint by healing erosions. But I think that this information is very important because healing of erosions, I believe, is a new biomarker for how well we are controlling inflammation. If we study rheumatoid arthritis patients in whom we think disease activity is very well controlled, and we do ultrasound or high-end MRI, we can show that there is still residual inflammation in some of these joints ....so perhaps healing of erosions is a marker for excellent control of inflammation in those joints and I think that’s why it’s important.
Collier, Newark: Thank you.
Harrison, Nashville: Really nice talk.... We have observed these double-negative T cells, CD3 positive cells in the kidneys, and vessels of hypertensive models for several years. It turns out that about half of them are gamma delta T-cells and they are big producers of interleukin 17 which I noticed your cells are. Could your cells be gamma delta cells? Have you looked for that?
Gravallese, Worcester: That has not been looked at but that’s a really great question. I’d be interested to talk to you further about that. IL-17 is actually a very important molecule, and we have agents now to block IL-17....they are very effective in controlling inflammation in the spondyloarthritis syndromes, but we are concerned because IL-17 inhibits osteoblastic differentiation so if you block it you may actually promote bone formation. I don’t know what would happen in the kidney and in vessels.
Moore, New York City: A clinical question: Are you introducing Denosumab early after a diagnosis of rheumatoid arthritis to try to prevent the bone loss?
Gravallese, Worcester: Interestingly Amgen did not pursue Denosumab for this indication. They went for the bigger indications of osteoporosis and cancer. Really that was just a proof of principle study. Interestingly, when we used agents such as bisphosphonates that are used for osteoporosis, at the doses that we typically use, they don’t really prevent the progression of joint destruction. At very high doses there have been several studies demonstrating that you can block erosion progression. But many of our patients do have osteoporosis and end up on these drugs. We are in the process of doing studies looking at adding anabolic agents to see if we can heal erosions in that setting. I think if inflammation is fully controlled, that may be possible.
Weinblatt, Boston: Lovely talk Ellen. I wanted to ask about two older drugs that impact radiographic progression — higher doses of corticosteroids and methotrexate. We’ve previously reported that methotrexate can actually heal the erosions in rheumatoid arthritis. Is that related to its primary effect on inflammation or related to this pathway?
Gravallese, Worcester: So there are a couple of questions, I think, mixed in there. As far as methotrexate, I remember being in the radiology reading room when we first looked at the healing of erosions with methotrexate. And I think that the major impact of methotrexate is not through bone specifically, although we know it can affect osteoclast differentiation. But probably it works more through its effects on inflammation.... and the first part of your question?
Weinblatt, Boston: Corticosteroids?
Gravallese, Worcester: Corticosteroids are very mixed bag because they actually lead to osteoblast death. And so although they control inflammation, they are going to decrease the ability of osteoblasts to form bone. So, a very mixed bag there.
Hochberg, Baltimore: So, regarding the bone forming agents, are there any data either pre-clinical or in humans from teriparatide or the new abaloparatide?
Gravallese, Worcester: We are doing that study right now. We’re looking at patients with rheumatoid arthritis who are well controlled on an anti-TNF agent, the best way that we can control inflammation, and then adding teriparatide — intermittently administered PTH — and we’ll see.
REFERENCES
- 1.Scott DL. Prognostic factors in early rheumatoid arthritis. Rheumatology (Oxford) 2000;39((Suppl 1)):24–9. doi: 10.1093/oxfordjournals.rheumatology.a031490. [DOI] [PubMed] [Google Scholar]
- 2.Administration FDA. Guidance for Industry Clinical Development Programs for Drugs, Devices, and Biological Products for the Treatment of Rheumatoid Arthritis (RA) Rockville, MD: Services DoHaH; 1999. [Google Scholar]
- 3.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8((11)):656–64. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharp JT, Lidsky MD, Collins LC, et al. Methods of scoring the progression of radiologic changes in rheumatoid arthritis. Correlation of radiologic, clinical and laboratory abnormalities. Arthritis Rheum. 1971;14((6)):706–20. doi: 10.1002/art.1780140605. [DOI] [PubMed] [Google Scholar]
- 5.Szkudlarek M, Terslev L, Wakefield RJ, et al. Summary findings of a systematic literature review of the ultrasound assessment of bone erosions in rheumatoid arthritis. J Rheumatol. 2016;43:12–21. doi: 10.3899/jrheum.141416. [DOI] [PubMed] [Google Scholar]
- 6.Dohn UM, Ejbjerg B, Boonen A, et al. No overall progression and occasional repair of erosions despite persistent inflammation in adalimumab-treated rheumatoid arthritis patients: results from a longitudinal comparative MRI, ultrasonography, CT and radiography study. Ann Rheum Dis. 2011;70((2)):252–8. doi: 10.1136/ard.2009.123729. [DOI] [PubMed] [Google Scholar]
- 7.Gravallese EM, Harada Y, Wang JT, et al. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998;152((4)):943–51. [PMC free article] [PubMed] [Google Scholar]
- 8.Pettit AR, Ji H, von Stechow D, Muller R, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159((5)):1689–99. doi: 10.1016/S0002-9440(10)63016-7. doi: doi: S0002-9440(10)63016-7 [pii]10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redlich K, Hayer S, Ricci R, et al. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest. 2002;110((10)):1419–27. doi: 10.1172/JCI15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93((2)):165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95((7)):3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong BR, Josien R, Lee SY, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186((12)):2075–80. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gravallese EM, Manning C, Tsay A, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43((2)):250–8. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 14.Romas E, Bakharevski O, Hards DK, et al. Expression of osteoclast differentiation factor at sites of bone erosion in collagen-induced arthritis. Arthritis Rheum. 2000;43((4)):821–6. doi: 10.1002/1529-0131(200004)43:4<821::AID-ANR12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 15.Meednu N, Zhang H, Owen T, et al. Production of RANKL by memory B cells: a link between B cells and bone erosion in rheumatoid arthritis. Arthritis Rheumatol. 2016;68((4)):805–16. doi: 10.1002/art.39489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangialaio S, Ji H, Korganow AS, et al. The arthritogenic T cell receptor and its ligand in a model of spontaneous arthritis. Arthritis Rheum. 1999;42((12)):2517–23. doi: 10.1002/1529-0131(199912)42:12<2517::AID-ANR3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 17.Jarrett SJ, Conaghan PG, Sloan VS, et al. Preliminary evidence for a structural benefit of the new bisphosphonate zoledronic acid in early rheumatoid arthritis. Arthritis Rheum. 2006;54((5)):1410–4. doi: 10.1002/art.21824. [DOI] [PubMed] [Google Scholar]
- 18.Goldring SR, Gravallese EM. Bisphosphonates: environmental protection for the joint? Arthritis Rheum. 2004;50((7)):2044–7. doi: 10.1002/art.20383. Epub 2004/07/13. doi: 10.1002/art.20383. [DOI] [PubMed] [Google Scholar]
- 19.Cohen SB, Dore RK, Lane NE, et al. Denosumab Rheumatoid Arthritis Study G. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58((5)):1299–309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 20.Yue J, Griffith JF, Xiao F, et al. Arthritis Care Res (Hoboken) 2016. Repair of bone erosion in rheumatoid arthritis by denosumab: a high-resolution peripheral quantitative computed tomography study. [DOI] [PubMed] [Google Scholar]
- 21.Crotti TN, Smith MD, Weedon H, et al. Receptor activator NF-kappaB ligand (RANKL) expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathy, osteoarthritis, and from normal patients: semiquantitative and quantitative analysis. Ann Rheum Dis. 2002;61((12)):1047–54. doi: 10.1136/ard.61.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YH, Heulsmann A, Tondravi MM, et al. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem. 2001;276((1)):563–8. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]
- 23.Wong PK, Quinn JM, Sims NA, et al. Interleukin-6 modulates production of T lymphocyte-derived cytokines in antigen-induced arthritis and drives inflammation- induced osteoclastogenesis. Arthritis Rheum. 2006;54((1)):158–68. doi: 10.1002/art.21537. [DOI] [PubMed] [Google Scholar]
- 24.Yao Z, Li P, Zhang Q, et al. Tumor necrosis factor-alpha increases circulating osteoclast precursor numbers by promoting their proliferation and differentiation in the bone marrow through up-regulation of c-Fms expression. J Biol Chem. 2006;281((17)):11846–55. doi: 10.1074/jbc.M512624200. [DOI] [PubMed] [Google Scholar]
- 25.Yarilina A, Xu K, Chen J, et al. TNF activates calcium-nuclear factor of activated T cells (NFAT)c1 signaling pathways in human macrophages. Proc Natl Acad Sci U S A. 2011;108((4)):1573–8. doi: 10.1073/pnas.1010030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei S, Kitaura H, Zhou P, et al. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115((2)):282–90. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Axmann R, Bohm C, Kronke G, et al. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 2009;60((9)):2747–56. doi: 10.1002/art.24781. [DOI] [PubMed] [Google Scholar]
- 28.Takagi N, Mihara M, Moriya Y, et al. Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum. 1998;41((12)):2117–21. doi: 10.1002/1529-0131(199812)41:12<2117::AID-ART6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 29.Smolen JS, Avila JC, Aletaha D. Tocilizumab inhibits progression of joint damage in rheumatoid arthritis irrespective of its anti-inflammatory effects: disassociation of the link between inflammation and destruction. Ann Rheum Dis. 2012;71((5)):687–93. doi: 10.1136/annrheumdis-2011-200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fossiez F, Djossou O, Chomarat P, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183((6)):2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jovanovic DV, Di Battista JA, Martel-Pelletier J, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160((7)):3513–21. [PubMed] [Google Scholar]
- 32.Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203((12)):2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenders MI, Lubberts E, Oppers-Walgreen B, et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167((1)):141–9. doi: 10.1016/S0002-9440(10)62961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genovese MC, Greenwald M, Cho CS, et al. A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol. 2014;66((7)):1693–704. doi: 10.1002/art.38617. [DOI] [PubMed] [Google Scholar]
- 35.van Roon JA, Lafeber FP, Bijlsma JW. Synergistic activity of interleukin-4 and interleukin-10 in suppression of inflammation and joint destruction in rheumatoid arthritis. Arthritis Rheum. 2001;44((1)):3–12. doi: 10.1002/1529-0131(200101)44:1<3::AID-ANR2>3.0.CO;2-U. doi: 10.1002/1529-0131(200101)44:1<3::AID-ANR2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 36.Huang H, Zhao N, Xu X, et al. Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2011;44((5)):420–7. doi: 10.1111/j.1365-2184.2011.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilbert L, He X, Farmer P, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277((4)):2695–701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 38.Jilka RL, Weinstein RS, Bellido T, et al. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13((5)):793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 39.Walsh NC, Reinwald S, Manning CA, et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res. 2009;24((9)):1572–85. doi: 10.1359/jbmr.090320. Epub 2009/04/03. [DOI] [PubMed] [Google Scholar]
- 40.Matzelle MM, Gallant MA, Condon KW, et al. Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum. 2011;64((5)):1540–50. doi: 10.1002/art.33504. Epub 2011/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown AK, Conaghan PG, Karim Z, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58((10)):2958–67. doi: 10.1002/art.23945. [DOI] [PubMed] [Google Scholar]
- 42.Diarra D, Stolina M, Polzer K, et al. Nat Med. 2007. Dickkopf-1 is a master regulator of joint remodeling. [DOI] [PubMed] [Google Scholar]
- 43.Kehl AS, Corr M, Weisman MH. Review: enthesitis: new insights into pathogenesis, diagnostic modalities, and treatment. Arthritis Rheumatol. 2016;68((2)):312–22. doi: 10.1002/art.39458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lories RJ, Schett G. Pathophysiology of new bone formation and ankylosis in spondyloarthritis. Rheum Dis Clin North Am. 2012;38((3)):555–67. doi: 10.1016/j.rdc.2012.08.003. doi: 10.1016/j.rdc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Bleil J, Sieper J, Maier R, et al. Cartilage in facet joints of patients with ankylosing spondylitis (AS) shows signs of cartilage degeneration rather than chondrocyte hypertrophy: implications for joint remodeling in AS. Arthritis Res Ther. 2015;17:170. doi: 10.1186/s13075-015-0675-5. doi: 10.1186/s13075-015-0675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lories RJ, Derese I, Ceuppens JL, et al. Bone morphogenetic proteins 2 and 6, expressed in arthritic synovium, are regulated by proinflammatory cytokines and differentially modulate fibroblast-like synoviocyte apoptosis. Arthritis Rheum. 2003;48((10)):2807–18. doi: 10.1002/art.11389. [DOI] [PubMed] [Google Scholar]
- 47.Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115((6)):1571–9. doi: 10.1172/JCI23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosen V. BMP and BMP inhibitors in bone. Ann N Y Acad Sci. 2006;1068:19–25. doi: 10.1196/annals.1346.005. [DOI] [PubMed] [Google Scholar]
- 49.Daluiski A, Engstrand T, Bahamonde ME, et al. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet. 2001;27((1)):84–8. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- 50.Rawadi G, Vayssiere B, Dunn F, et al. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18((10)):1842–53. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 51.Heiland GR, Zwerina K, Baum W, et al. Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann Rheum Dis. 2010;69((12)):2152–9. doi: 10.1136/ard.2010.132852. [DOI] [PubMed] [Google Scholar]
- 52.Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18((7)):1069–76. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 53.Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol. 2014;57((1)):44–51. doi: 10.1016/j.molimm.2013.07.013. Epub 2013/09/03. doi: 10.1016/j.molimm.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199((Pt 5)):503–26. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang YK, Zhang MC. IL-23 promotes osteoclastogenesis in osteoblast-osteoclast co-culture system. Genet Mol Res. 2014;13((2)):4673–9. doi: 10.4238/2014.June.18.10. [DOI] [PubMed] [Google Scholar]
- 56.Yago T, Nanke Y, Kawamoto M, et al. IL-23 induces human osteoclastogenesis via IL-17 in vitro, and anti-IL-23 antibody attenuates collagen-induced arthritis in rats. Arthritis Res Ther. 2007;9((5)):R96. doi: 10.1186/ar2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Praet L, Van den Bosch FE, Jacques P, et al. Microscopic gut inflammation in axial spondyloarthritis: a multiparametric predictive model. Ann Rheum Dis. 2013;72((3)):414–7. doi: 10.1136/annrheumdis-2012-202135. [DOI] [PubMed] [Google Scholar]
- 58.Ciccia F, Accardo-Palumbo A, Rizzo A, et al. Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann Rheum Dis. 2014;73((8)):1566–74. doi: 10.1136/annrheumdis-2012-202925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaw AT, Maeda Y, Gravallese EM. IL-17A deficiency promotes periosteal bone formation in a model of inflammatory arthritis. Arthritis Res Ther. 2016;18((1)):104. doi: 10.1186/s13075-016-0998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uluckan O, Wagner EF. Role of IL-17A signalling in psoriasis and associated bone loss. Clin Exp Rheumatol. 2016;34((4 Suppl 98)):17–20. [PubMed] [Google Scholar]