Abstract
Transient receptor potential melastatin 2 (Trpm2) channels are nonvoltage-activated channels permeable to monovalent and divalent cations, and are expressed in heart, brain, kidney, vasculature, and hematopoietic cells. Trpm2 is overexpressed in bladder, lung, breast, liver, head, and neck cancers. Classically, Trpm2 activation induces cell injury and death by Ca2+ overload or enhanced inflammatory response. Recent studies show that Trpm2 protects lungs from endotoxin-induced injury by reducing reactive oxygen species production in phagocytes; and improves cardiac function after ischemia-reperfusion injury by preserving mitochondrial respiration and cellular adenosine triphosphate levels while decreasing reactive oxygen species levels. In neuroblastoma xenografts, Trpm2 overexpression promotes tumor growth through modulation of hypoxia-inducible transcription factor expression and cellular bioenergetics; whereas Trpm2 inhibition results in enhanced sensitivity to doxorubicin. The robust expression in cancer cells and its pro-survival and proliferative properties make Trpm2 a rational target for cancer therapy. Indiscriminate Trpm2 inhibition, however, may engender serious untoward side effects in other vital organs.
TRANSIENT RECEPTOR POTENTIAL CHANNEL SUPERFAMILY
Transient receptor potential (Trp) channels consist of a superfamily of monovalent and divalent cation-permeable ion channels with 6 transmembrane (TM) domains and are homologues of the Drosophila melanogaster Trp channel, a Ca2+-permeable channel that is essential for phototransduction (1). To date, the mammalian Trp superfamily consists of 28 members grouped into 6 subfamilies based on amino acid sequence homology: canonical (C), vanilloid (V), melastatin (M), polycystin (P), mucolipin (ML), and ankyrin (A). To form a functional channel, Trp proteins assemble into either homo- or hetero-tetramers (2–4).
TRANSIENT RECEPTOR POTENTIAL – MELASTATIN CHANNEL FAMILY MEMBER 2 (Trpm2)
The Trpm subfamily is named after the first member to be cloned, Trpm1 (M, melastatin), which is a putative tumor suppressor protein (5). The Trpm subfamily contains 8 mammalian members (Trpm1 to Trpm8); some of them have short splice variants (6,7). Trpm channels have important roles in cell proliferation and survival (5–8).
The human Trpm2 (previously LTrpc2) gene is located in chromosome 21q22.3, consists of 32 exons, and spans ~90 kb, its transcript is ~6.5kb and encodes a protein of 1503 amino acids with a predicted molecular mass of ~170 kDa (9). The mouse Trpm2 gene contains 34 exons, spans ~61 kb and encodes a protein of 1,507 amino acids (10). Trpm2 channels are expressed in many cell types including brain, hematopoietic cells, heart, vascular smooth muscle and endothelial cells (11,12).
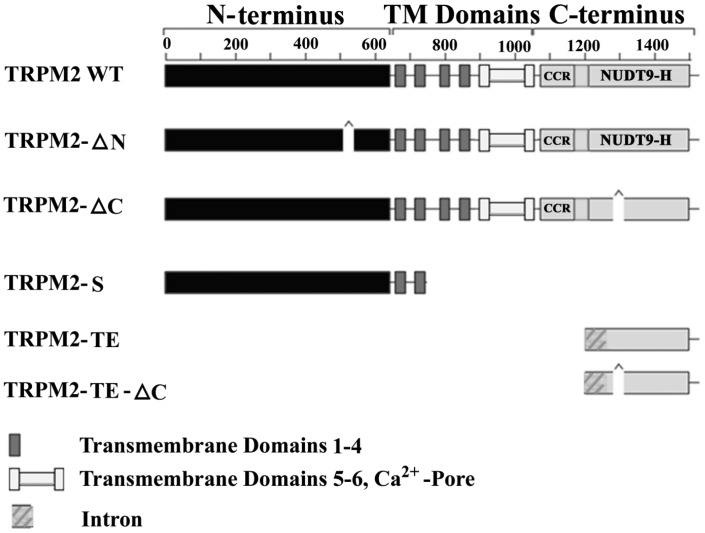
In addition to full length Trpm2 (Trpm2-L), physiological splice variants include Trpm2-S (short) (13), Trpm2-∆N (14), Trpm2-∆C (14), Trpm2-AS (anti-sense), Trpm2-TE (tumor enriched) (15) and Trpm2-TE-∆C (Figure 1). Trpm2-S has a deletion of four of six C-terminal TM domains, the putative calcium pore, and the entire C-terminus. Trpm2-S suppresses Ca2+ influx through Trpm2-L and thus acts as a dominant negative (13). When exposed to low, physiological concentrations of H2O2, cells co-expressing Trpm2-L andTrpm2-S had reduced cell viability as compared to cells expressing Trpm2-L alone (16). This is the first observation to suggest that inhibition of Ca2+ influx via Trpm2 may be detrimental to cells. Trpm2-ΔN has deletion of amino acids 538-557 from the N-terminus, and does not respond to H2O2 or adenosine diphosphate-ribose (ADPR), suggesting that this deletion disrupts channel gaiting or assembly (14). Trpm2-ΔC results from deletion of nucleotides encoding amino acids 1292-1325 in the C-terminus of Trpm2-L (14). Trpm2-ΔC has decreased affinity for ADPR, and cells expressing this deletion splice variant do not respond to ADPR. However, they do respond to H2O2, suggesting that oxidative stress may activate Trpm2 through mechanisms independent of ADPR. Trpm2-TE was identified by Orfanelli et al (15) when they were searching for antisense transcripts in melanoma to identify new tumor suppressor genes, using the software program Anti-Hunter (freely available at http://bioinfo.crs4.it/AH2.0). Trpm2-TE transcripts encode either a 218–amino acid protein or a 184–amino acid protein including the ΔC deletion (Trpm2-TE-ΔC). Expression of Trpm2-TE is thought to result following hypomethylation of a CpG island in the Trpm2 C-terminus, and Trpm2-TE is highly expressed in tumor cells including melanoma and lung compared to normal tissue. Co-expression of Trpm2-TE and Trpm2-L protected cells from apoptosis. At present, little is known about the mechanisms through which alternative splicing is regulated in vivo, or the physiological functions and interactions of these isoforms.
Fig. 1.
Schematic representation of Trpm2 isoforms. Wild-type (WT) transient receptor potential melastatin 2 (Trpm2) is also known as Trpm2-L. Membrane spanning domains 1–4 and the putative pore region including transmembrane domains 5–6 are indicated. CCR represents the coiled-coil region which may mediate protein/protein interactions. NUDT9-H represents the NUDT9 adenosine diphosphate–ribose hydrolase (also known as NUDIX) domain.
In many malignancies including bladder, lung, breast, liver, head, and neck cancers (17), melanoma (15), and neuroblastoma (16), Trpm2 channels and/or its splice variants (Trpm2-AS and Trpm2-TE) are overexpressed. Because Trpm2, similar to other Trp channels, are tetramers, association of splice variants with Trpm2-L may modulate Trpm2 structure and function. For example, the dominant-negative Trpm2-S inhibited Ca2+ influx through Trpm2-L (13) and suppressed neuroblastoma xenograft growth (16).
Trpm2 MOLECULAR STRUCTURE
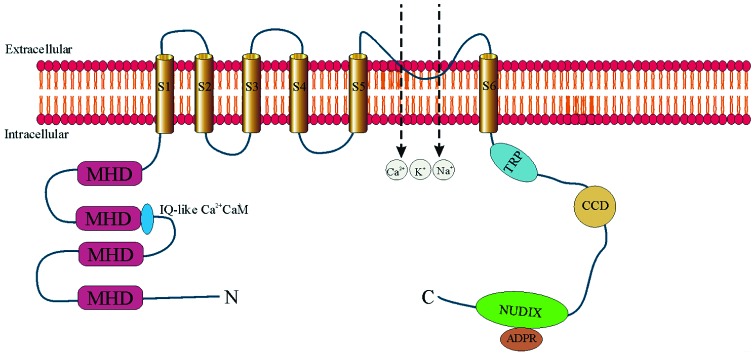
The Trpm2 channel consists of 6 TM domains (S1–S6) with both N- and C-termini located in the cytosol (Figure 2). The N-terminus has 4 Trpm subfamily homologous domains (MHD) and a calmodulin-binding IQ-like motif (18,19). The pore-forming loop domain is located between S5 and S6 (18,20). The C-terminus contains the Trp box and a coiled-coil domain (21), and the unique ADPR pyrophosphatase domain (NUDIX-like domain or NUDT9 homology domain) (18,20). Because of co-existing ion channel function and enzymatic function, Trpm2 is known as a “chanzyme.” Similar to Trp channels, Trpm2 is a homo-tetrameric nonvoltage activated and nonselective cation channel.
Fig. 2.
Topology model of transient receptor potential melastatin 2 (Trpm2) channel. Trpm2 monomer is depicted as having 6 transmembrane domains (S1 to S6) with the putative pore-forming loop situated between S5 and S6. Four monomers associate to form a functional Trpm2 channel. Both N- and C-termini are in the cytosol. The N-terminus contains 4 sections of Trpm subfamily homology domain (MHD). In the second MHD, there is an IQ-like motif which binds Ca2+-calmodulin. The C-terminus contains a Trp box (TRP), a coiled-coil domain (CCD), and the adenosine diphosphate ribose (ADPR) pyrophosphatase homolog domain (NUDIX). Trpm2 is a non-specific cation-permeable channel which allows entry of Ca2+, Na+, and K+.
Tprm2 REGULATION
Extracellular signals which activate Trpm2 include oxidative stress, tumor necrosis factor α (TNFα), amyloid β-peptide, and concanavalin A (14, 22–24). Stimulation with these extracellular signals results in production of ADPR which binds to the Trpm2 C-terminal NUDT9-H domain (Figure 2) and Trpm2 is activated (11, 24–27). Cyclic adenosine diphosphoribose (cADPR) potentiates the effects of ADPR on Trpm2 at low concentrations and can also gate Trpm2 by itself (25). ADPR may arise from mitochondria (26) or through activation of poly(ADPR)polymerases (PARP) or poly(ADPR)glycohydrolases (PARG) (28,29). Trpm2 is also positively regulated by intracellular Ca2+ (19,30,31). Interaction of ADPR with Trpm2 supports initial Ca2+ entry through Trpm2. The subsequent increase in Ca2+-bound calmodulin enhances calmodulin binding to an IQ-motif in the N-terminus of Trpm2 (Figure 2), providing positive feedback for Trpm2 activation and increased Ca2+ influx (19). Trpm2 with mutant ADPR binding sites can be directly activated by an increased in [Ca2+]i, and Trpm2 may be activated in a wide range of physiological situations through this mechanism (31). In the absence of either external or internal Ca2+, ADPR is ineffective in activating Trpm2 channels (30). Trpm2 has also been reported to be temperature sensitive (32) and inhibited by acidification (33–35), thereby providing a mechanism for limiting Ca2+ entry during ischemia.
ELECTROPHYSIOLOGY OF Trpm2 IN ADULT CARDIAC VENTRICULAR MYOCYTES
In freshly isolated adult mouse left ventricular (LV) myocytes subjected to voltage-clamp, ADPR (300 µM) in the pipette elicited a large inward and outward current in wild-type (WT) but not in global Trpm2 knockout (gKO) or cardiac-specific Trpm2 knockout (cKO) myocytes (36,37). The ADPR-activated current in adult LV myocytes was inhibited by flufenamic acid, displayed a linear current-voltage relationship, did not inactivate, and had a reversal potential close to 0 mV (36,37): characteristics of Trpm2 currents (18). The ratio of Ca2+ conductance to Na+ conductance was 0.56 ± 0.02 in adult cardiac myocytes (36) and 0.65 ± 0.08 in transiently transfected HEK293 cells (37).
OXIDATIVE STRESS
Oxidative stress results from imbalance between the amount of oxidants produced and antioxidants, leading to tissue injury depending on the severity and duration (11). Reactive oxygen species (ROS) are produced physiologically during respiration by the mitochondrial electron transport chain, and pathologically by neutrophils and phagocytes involved in inflammation and infection. Reduced mitochondrial electron chain transport activity, a consequence of partial inhibition of cytochrome-c oxidase activity, can also result in increased ROS production. In the heart, after ischemia-reperfusion (I/R) (38) or doxorubicin exposure (39,40), ROS production is increased. ROS plays a major role in myocyte injury through protein oxidation, lipid peroxidation, nitrosylation, and DNA oxidation and mutagenesis. During reperfusion, ROS can trigger mitochondrial permeability transition pore (mPTP) opening (41), leading to irreversible cell death. In addition, ROS-induced ROS release has been proposed as the mechanism underlying the wave of mPTP opening in myocytes (42).
Physiological levels of ROS can activate transcription factors and signaling kinases including nuclear factor-activated thymocytes, calmodulin kinase, and serine-threonine and tyrosine kinases (43). Aberrant increases in ROS are associated with damage to mitochondria, DNA, proteins, and lipids which ultimately leads to cell loss and organ failure (44–47). Ca2+ is also an important signaling molecule and a mediator of signaling pathways. ROS and Ca2+ appear to participate as partners in pathophysiological settings, but their interaction and signaling mechanisms associated with the development of acute organ injury are poorly understood.
CONTROVERSIES OF Trpm2 IN INFLAMMATION, OXIDATIVE STRESS, AND CELL DEATH
Early studies using moderately high doses of H2O2 in cultured cells suggest that activation of Trpm2 channels by oxidative stress induced cell death by sustained elevation in cytosolic Ca2+ concentration ([Ca2+]i) (12,22,48,49) or by increased cytokine production and release, resulting in enhanced inflammation and cell injury (50,51). Indeed, in dextran sulfate sodium-induced colitis in mice (simulating human inflammatory bowel disease), Trpm2-mediated Ca2+ influx stimulated chemokine (chemokine [C-X-C motif] ligand 2 or CXCL2) production in monocytes, increased recruitment of neutrophils thereby exacerbating the inflammatory response and enhancing tissue injury (52). Trpm2 in microglia and macrophages was shown to exacerbate ischemic brain injury by enhancing migration of inflammatory cells to the ischemic brain (53) and by controlling nitric oxide release-mediated cerebral ischemic injury (54). Trpm2 has been implicated in bipolar disorder type I in which patients have high basal [Ca2+]i in B-lymphoblasts (55,56), and in diabetes mellitus by affecting insulin secretion mediated by increase in [Ca2+]i (57,58). In kidneys subjected to I/R injury, either knocking out Trpm2 or pharmacological inhibition of Trpm2 by 2-aminoethoxydiphenyl borate (2-APB) was found to be protective (59). Unlike cardiac myocytes (60,61) and phagocytes (62) in which Trpm2 was expressed on the plasma membrane, Trpm2 was detected in the cytoplasm and intracellular organelles but not in the plasma membrane of renal tubular epithelial cells (59). In post-I/R kidneys, Trpm2 physically interacted with Ras-related C3 botulinum toxin substrate 1 (Rac1), which recruited nicotinamide-adenine dinucleotide phosphate-oxidase (NADPH oxidase or NOX) to the plasma membrane, increased ROS production and resulted in cell injury and death. The role of Ca2+ permeation through activated Trpm2 channels (presumably the mechanism of action of 2-APB) to exacerbate renal I/R injury is not clear.
In sharp contrast to these observations are recent reports showing beneficial, as opposed to detrimental, effects of Trpm2 activation under physiological and pathological conditions. For example, in WT mice subjected to intraperitoneal injection of endotoxin, cation entry via activated Trpm2 channels depolarized plasma membrane of phagocytes, leading to reduced NOX-mediated ROS production in phagocytes, lessened the inflammatory response, preserved tissue viability, and resulted in animal survival that was five times higher than that observed in endotoxin-treated Trpm2-KO mice (62). In addition, in a subtype of Guamanian amyotropic lateral sclerosis and Parkinson dementia patients, a Trpm2 mutant (P1018L) was identified (63). Unlike WT Trpm2 currents which do not inactivate, the P1018L mutant channel inactivates after channel opening by ADPR (64), thereby limiting Ca2+ entry. This observation suggests that sustained Ca2+ entry via activated Trpm2 channels was necessary to maintain normal neuronal function. Another report showed that in pyramidal neurons subjected to oxidant injury, inhibition of Trpm2 results in enhanced cellular damage (65), confirming that Trpm2 can protect against oxidative stress. In neuroblastoma cells subjected to low doses of H2O2, expression of the dominant-negative Trpm2-S mutant reduced Ca2+ influx and enhanced ROS levels, leading to decreased cell viability (66). In vivo, growth of neuroblastoma xenografts expressing Trpm2-S was also significantly reduced compared to tumors expressing WT Trpm2, particularly after doxorubicin treatment (16). This cursory review indicates that Ca2+ entry via Trpm2 channels in disease states can either be friend (reduced ROS production) (16,36,60,62,63,65) or foe (increased Ca2+ influx) (13,49,52,61), depending on the tissue type, experimental model, and conditions. In the following discussion, we will focus on the role of Trpm2 in oxidative stress-induced injury, cellular bioenergetics, and ROS production in the heart.
Trpm2 IN CARDIAC OXIDATIVE STRESS–INDUCED INJURY
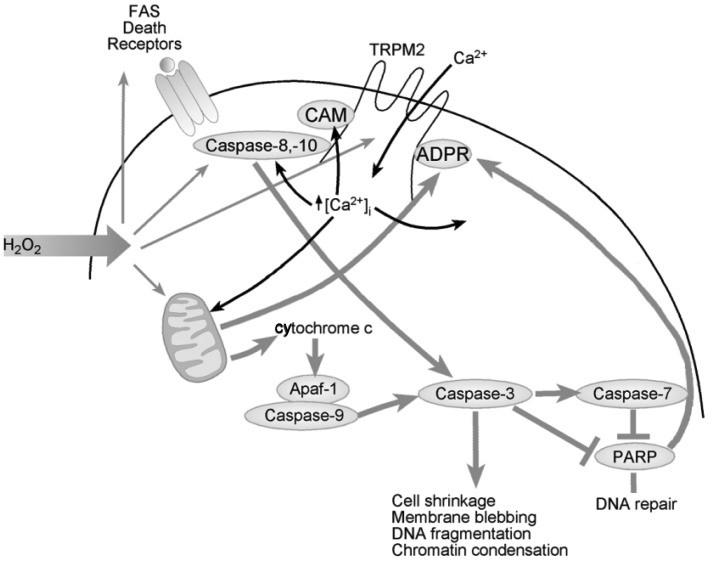
In the heart, low levels of H2O2 emission which occurs in normal respiring mitochondria (67) may tonically activate Trpm2 channels and provide the necessary Ca2+ for bioenergetics maintenance (vide infra). After hypoxia-reoxygenation (H/R; in vitro experimental model to simulate I/R), β-adrenergic stimulation or doxorubicin exposure, increases in ADPR may further activate Trpm2 channels and leads to elevation in [Ca2+]i. According to the classical paradigm, sustained [Ca2+]i elevation results in Ca2+ overload which disrupts mitochondrial bioenergetics maintenance, leading to enhanced ROS production, opening of the mitochondrial permeability transition pore (mPTP), activation of the caspase cascade, and irreversible cell death (Figure 3). Viewed in this framework, activation of Trpm2 channels under pathological conditions has been proposed to be detrimental to the heart. Evidence supporting the classical paradigm is briefly summarized below.
Fig. 3.
Proposed classical paradigm by which transient receptor potential melastatin 2 (Trpm2) activation induces cell injury and death. H2O2 increases production of adenosine diphosphate–ribose (ADPR) in the mitochondria through activation of poly(ADPR), polymerases (PARP), and poly(ADPR) glycohydrolases (PARG). ADPR activates Trpm2 by binding to the C-terminal NUDIX domain (Figure 2). Ca2+ influx ensues, which enhances calmodulin (CAM) binding to the IQ-like motif in the N-terminus of Trpm2 and further promotes channel opening. [Ca2+]i rises, and in association with other oxidative stress-induced signals, results in activation of extrinsic and intrinsic cell death pathways, leading to caspase-3 activation and PARP cleavage and inactivation. Abbreviation: Apaf-1, Apoptotic protease activating factor 1.
In cultured neonatal rat ventricular myocytes (NRVMs), exposure to H2O2 (20-100 µM) led to increases in both [Ca2+]i and [Na+]i, which in turn caused mitochondrial Na+ and Ca2+ overload, resulting in mitochondrial membrane disruption, cytochrome c release, and caspase 3-dependent apoptosis (61). Inhibition of Trpm2 by clotrimazole almost completely abolished H2O2-induced cytochrome c release, caspase 3 activation and chromatin condensation/fragmentation, suggesting that Trpm2 activation was involved in oxidative stress-induced apoptosis.
In isolated adult mouse ventricular myocytes, TNF-α induced a nonspecific cation current that was inhibited by Trpm2 inhibitors clotrimazole and flufenamic acid (68). TNF-α induced caspase 8 activation led to increased mitochondrial ROS production, PARP-1 activation and ADPR production, and increased [Ca2+]i spike frequency and cardiomyocyte cell death. Clotrimazole or blocking Trpm2 antibody significantly reduced TNF-α induced [Ca2+]i spikes and cell death, thereby implicating that Trpm2 activation was involved in TNF-α induced cardiomyocyte cell death.
In global Trpm2 knockout (gKO) mice subjected to 45 minutes of ischemia followed by 24 hours of reperfusion, neutrophil infiltration was less, infarct size was smaller, and first time derivative of left ventricular pressure rise (+dP/dt) was higher compared to WT mice subjected to I/R (69). In addition, in both ex vivo and in vivo hearts perfused with either WT or gKO neutrophils and subjected to I/R, WT neutrophils produced the largest infarcts in either WT or gKO hearts, whereas gKO neutrophils were protective in terms of smaller infarct size. Finally, the Trpm2 inhibitor econazole was efficacious in reducing infarct size in gKO hearts perfused with WT neutrophils and subjected to I/R. Collectively, these observations suggest that Trpm2 channels in WT neutrophils promoted adhesion in the I/R regions, leading to exacerbation of myocardial reperfusion injury.
To investigate the role of Trpm2 in mediating cardiac oxidative stress induced injury, we generated both gKO (60) and cardiac-specific Trpm2 knockout (cKO) mice (37) and subjected them to 30 minutes of cardiac ischemia followed by 72 hours of reperfusion. At baseline, there were no differences in body weights, LV masses, heart rates, fractional shortening (FS), +dP/dt, and responsiveness to β-adrenergic stimulation between gKO and WT animals (60). Despite similar infarct sizes, both gKO-I/R and cKO-I/R hearts exhibited lower FS and +dP/dt when compared to WT-I/R hearts. To simulate I/R in vitro, freshly isolated myocytes were subjected to H/R (30 minutes of hypoxia followed by 2 hours of reoxygenation). Under normoxic conditions, there were no differences in ROS levels between WT and gKO myocytes. After H/R, gKO myocytes had significantly higher ROS levels compared to WT myocytes. Our observations indicate that Trpm2 deficiency in the cardiac myocyte was associated with elevated ROS levels post-H/R and worse myocardial function post-I/R. In addition, the results obtained from our cKO-I/R hearts, together with the weight of evidence in the literature (70), argue against an important role of neutrophils in causing cardiac I/R injury. Furthermore, the role of Trpm2 in angiogenesis and ischemic neovascularization in protection against cardiac I/R injury (71) is likely to be small based on results from cKO hearts. In another model of oxidative stress-induced cardiac injury (doxorubicin cardiotoxicity), gKO mice had lower +dP/dt and worse survival compared to WT animals (37).
The controversial roles of Trpm2 in oxidative stress-induced injury between NRVMs (deleterious) (61) and adult mouse ventricular myocytes (beneficial) (36,37,60) can be partially explained by the following observations. In culture, NVRMs proliferate whereas adult mouse cardiac myocytes do not divide. Cultured NVRMs spontaneously beat in culture whereas healthy adult mouse ventricular myocytes are quiescent and do not beat unless externally paced. Unlike adult cardiac myocytes, NRVMs do not possess a well-developed t-tubular system. Trpm2 was detected in intracellular locations (peri-nuclear region and endoplasmic reticulum) in addition to the sarcolemma in NRVMs (61), in contrast to its exclusive localization to the sarcolemma and t-tubules in adult cardiac myocytes (60). NVRMs express α1- and α3-isoforms (72) whereas adult mouse cardiac myocytes express α1- and α2-isoforms of Na+-K+-ATPase (73). The lower Na+ affinity of the α3-isoform, when compared to the α2-isoform, may account for slower Na+ transport rate, resulting in [Na+]i overload in NRVMs (61) but not in adult mouse cardiac myocytes (37) when Trpm2 channels were activated with H2O2. These fundamental differences in physiology and Trpm2 localization between NVRMs and adult cardiac myocytes may very well account for the divergent experimental observations.
Perhaps it is more challenging to reconcile our results that Trpm2 was protective against cardiac I/R injury (36,37,60) with those of Hiroi et al (69) who reported that Trpm2 exacerbated cardiac I/R injury. Both groups used globally Trpm2 knockout mice for the experiments although Trpm2 exons targeted for deletion were different. The I/R conditions and anesthetic used [45 minutes of ischemia followed by 24 hours of reperfusion and pentobarbital (69) versus 30 minutes of ischemia followed by 72 hours of reperfusion and isoflurane (60), the significantly larger infarct sizes (45% vs. 27% area-at-risk) in the study of Hiroi et al (69), the differences in the surgical techniques and anesthetics used to measure in vivo hemodynamics [opening the chest followed by LV puncture and pentobarbital (69) vs. right carotid artery catheterization in a closed-chest animal and avertin (37,60)], and increased heat dissipation during hemodynamic measurements in open-chest (69) versus closed-chest (37,60) model, may all contribute to the differences in results obtained. More importantly, we note that the protective effects of Trpm2 were also shown in the cKO hearts subjected to I/R and gKO hearts exposed to doxorubicin treatment (37). Finally, the hypothesis advanced by Hiroi et al (69) that Trpm2 channels in neutrophils exacerbated cardiac I/R injury is not supported by our results obtained from cKO mice subjected to I/R; nor is it consistent with the report that cation entry via Trpm2 channels reduced ROS production in phagocytes (62), thereby lessening the inflammatory response.
Trpm2 AND MAINTENANCE OF CELLULAR BIOENERGETICS IN THE HEART
As a first step to identify the mechanisms by which Trpm2, a sarcolemmal cation-permeable channel, afforded protection to hearts subjected to oxidative stress-induced injury, we compared proteomes of WT-I/R and gKO-I/R hearts (36). Global label-free proteomics analysis using in-gel tryptic digestion followed by liquid chromatography-tandem mass spectroscopy showed that the largest differences occurred in canonical pathways involving mitochondrial function and tricarboxylic acid cycle. Specifically, Complexes I, III, and IV were down-regulated but Complexes II and V were upregulated in gKO-I/R compared to WT-I/R hearts. Functionally, mitochondrial membrane potential (Δψm), mitochondrial Ca2+ uptake, and oxygen consumption rate were lower in gKO myocytes under basal conditions and mitochondrial dysfunction in gKO myocytes was exacerbated after H/R (36). Reduced mitochondrial Ca2+ uptake in gKO myocytes was due to a combination of lower driving force (Δψm) and reduced intrinsic mitochondrial Ca2+ uniporter activity.
As a consequence of mitochondrial dysfunction, ATP levels were lower in gKO myocytes under basal conditions (36). Aberrant mitochondrial bioenergetics and increased electron leakage through the electron transport chain promoted increased generation of superoxide and ROS in gKO myocytes (36). Defenses against ROS such as superoxide dismutase (SOD) and thioredoxin 2, and key mitochondrial components such as NADH dehydrogenase (ubiquinone) 1α subcomplex 4-like 2 (NDUFA4L2) and Bcl2/adenovirus E1B 19 KDa-interacting protein 3 (BNIP3), both of which limit ROS production (74–76), were decreased in gKO when compared to WT myocytes (37). These changes, made worse after H/R, conspired to elevate mitochondrial superoxide and cellular ROS levels in Trpm2-deficient myocytes, thereby increasing oxidative stress and cell injury.
Trpm2-MEDIATED Ca2+ ENTRY IS ESSENTIAL FOR NORMAL MITOCHONDRIAL FUNCTION
Having identified mitochondrial dysfunction as a key disturbance associated with loss of Trpm2 in hearts, we next addressed whether cation entry, specifically Ca2+ influx via activated Trpm2 channels, was necessary for maintenance of normal mitochondrial bioenergetics. In this context, recent studies showed that constitutive low level mitochondrial Ca2+ uptake is essential in maintaining cellular bioenergetics (77). It is also important to note that normal respiring mitochondria emit low levels of H2O2 (67), which may tonically activate Trpm2 channels and provide the necessary Ca2+ for mitochondrial bioenergetics maintenance in WT but not gKO or cKO myocytes.
Because ROS and superoxide levels were higher in gKO compared to WT hearts (36), and because superoxide is exclusively derived from the mitochondria, we used ROS and superoxide levels as indicators of mitochondrial health to test whether reconstitution with WT Trpm2 (or its mutants) in gKO hearts could lower ROS and superoxide levels back to those observed in WT hearts. WT Trpm2, loss-of-function mutant (E960D) (64), inactivating mutant (P1018L) which allowed transient but not sustained Ca2+ entry (63), or control green fluorescent protein (GFP) were expressed in gKO hearts by adenovirus-mediated gene transfer (37). As expected, gKO-GFP hearts had significantly higher ROS levels compared to WT-GFP hearts (37). WT Trpm2 but not the loss-of-function E960D mutant decreased the elevated ROS and superoxide levels and increased oxygen consumption rate in gKO hearts to normal (37). This important observation indicates that Ca2+ entry via Trpm2 was necessary for normal mitochondrial function.
We next addressed whether Trpm2-mediated Ca2+ influx was necessary for the protection against cardiac I/R injury. Cardiac myocytes isolated from gKO hearts and expressing either GFP, WT Trpm2, E960D, or P1018L mutants were subjected to H/R. Only WT Trpm2, but not E960D or P1018L mutants, was able to reduce superoxide levels, and by inference, improve mitochondrial function in gKO myocytes post-H/R (37). Importantly, protection by WT Trpm2 against H/R injury was lost when extracellular Ca2+ was removed. Collectively, these observations indicate that sustained Ca2+ entry via activated Trpm2 was necessary to reduce ROS levels and protect myocytes against H/R injury. Interestingly, attempts to double the amount of Ca2+ entry by expressing the Q981E/P983Y mutant (2x Ca2+ permeability compared to WT Trpm2) did not afford additional protection in gKO myocytes against H/R injury (37).
Trpm2, Ca2+ ENTRY AND I/R INJURY
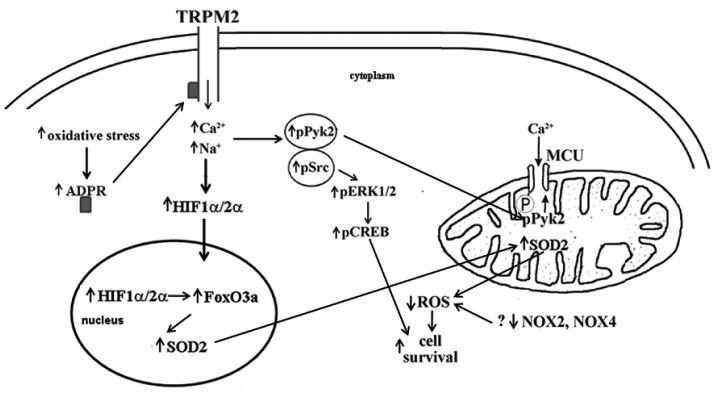
The paradigm that unrestrained Ca2+ entry secondary to ATP depletion during I/R leading to progressive elevation in diastolic [Ca2+]i (78), caspase activation, mitochondrial Ca2+ overload, mitochondrial permeability transition pore (mPTP) opening and irreversible cell death has enjoyed widespread popularity although as far back as 1986, the concept that Ca2+ is the mediator of ischemic injury has been challenged (79). Our data clearly indicate that Trpm2-mediated Ca2+ entry supported mitochondrial function and reduced mitochondrial superoxide and cellular ROS under both basal and H/R conditions (37). How then, can Trpm2-mediated Ca2+ entry during I/R be beneficial? Recent data suggest that in isolated cardiac myocytes subjected to H/R, Ca2+ overload was the consequence of bioenergetic failure after mPTP opening rather than its cause (80). In addition, in isolated perfused mouse hearts subjected to H/R, the mitochondrial-targeted SOD-mimetic MitoQ dramatically reduced Ca2+-wave associated mPTP opening (81). In support of this, we have preliminary data that treatment with MitoTempo, a specific mitochondrial superoxide scavenger, not only reduced mitochondrial superoxide levels as expected, but also restored Δψm to normal levels in gKO myocytes post-H/R (Cheung lab, unpublished observations). We hypothesize that maintenance of bioenergetics by Trpm2-mediated Ca2+ entry may prevent mPTP opening and protect hearts from oxidative injury. Trpm2-mediated Ca2+ entry may also activate Ca2+-dependent signaling pathways [e.g., proline-rich tyrosine kinase 2 (Pyk2)] and results in reduction in ROS production and enhances pro-survival signals such as phosphorylated protein kinase B (pAkt) and phosphorylated extracellular signal-regulated protein kinases 1 and 2 (pERK1/2) (Figure 4). Finally, absence of Trpm2 results in a tenuous bioenergetics condition and sensitizes KO myocytes to further oxidative stress brought about by I/R or doxorubicin treatment.
Fig. 4.
Potential mechanisms by which Ca2+ influx via activated transient receptor potential melastatin 2 (Trmp2) channels protects against oxidative injury. Under oxidative stress, Trpm2 channels are activated which allows Ca2+ entry. Proline-rich tyrosine kinase 2 (Pyk2) is phosphorylated which activates downstream pro-survival signals such as phosphorylated extracellular signal-regulated protein kinases 1 and 2 (pERK1/2), phosphorylated proto-oncogene tyrosine protein kinase (pSrc), phosphorylated protein kinase B (pAkt), and phosphorylated cAMP response element binding protein (pCREB), leading to enhanced cell survival. In addition, phosphorylated Pyk2 translocates to mitochondria, phosphorylates mitochondrial Ca2+ uniporter (MCU) which enhances mitochondrial Ca2+ uptake, thereby preserving cellular bioenergetics and reducing mitochondrial reactive oxygen species (ROS) production. Another potential protective mechanism is that Ca2+ entry via activated Trpm2 channels enhances calcineurin activity, resulting in increased levels of hypoxia inducible factors (HIF 1α/2α) which enhance cell viability through regulation of a large number of genes including forkhead box transcription factor 3a (FoxO3a) and Mn2+ superoxide dismutase (SOD2).
PROPOSED MECHANISMS BY WHICH Trpm2 PROTECTS AGAINST OXIDATIVE INJURY
Little is known about Trpm2 function in the heart, much less about its signaling pathways. To link Trpm2-mediated Ca2+ influx with cardiac protection, four major Ca2+-dependent signaling pathways in the heart are potential candidates: Ca2+-calmodulin kinase, protein kinase C (PKC), calcineurin, and Pyk2. Pyk2 is activated by autophosphorylation (Y402) after cerebral (82) and limb muscle (83) ischemia. In addition, pPyk2/Pyk2 was increased in end-stage human nonischemic cardiomyopathy and has been postulated to protect against arrhythmias (84). Ca2+ influx through Trpm2 activated Pyk2 and amplified ERK signaling in human U937 cells (52). We have preliminary data that pPyk2/Pyk2 and its downstream pro-survival signals pERK/ERK and pAkt/Akt were higher in WT-I/R compared to gKO-I/R and cKO-I/R hearts (Cheung lab, unpublished observations). In addition, after H2O2 exposure, the ratio pPyK2/PyK2 increased in WT but not gKO myocytes (Cheung lab, unpublished observations). Incorporating our preliminary data with the recent observation that under stress, pPyk2 translocated to the mitochondrial matrix, phosphorylated MCU and enhanced mitochondrial Ca2+ uptake (85) (Figure 4), we postulate that pPyk2 provides a critical link between Trpm2-mediated Ca2+ influx and preserving mitochondrial Ca2+ uptake and bioenergetics. Better mitochondrial bioenergetics will not only preserve ATP levels but also reduce ROS production, thereby enhancing cell survival.
Another potential protective mechanism is that Ca2+ entry via activated Trpm2 channels stimulates calcineurin, which dephosphorylates receptor for activated C kinase 1 (RACK1) and blocked RACK1 dimerization (86). This results in increased levels of hypoxia inducible factors (HIF 1α/2α) by impeding their ubiquitination and degradation (Figure 4). HIFs are transcription factors that enhance cell viability through regulation of a large number of genes involved in angiogenesis, glycolysis, and energy and redox homeostasis. These include forkhead box transcription factor 3a (FoxO3a) (87), Mn2+ superoxide dismutase (SOD2) (88), and genes involved in mitochondrial electron transport chain (NDUFA4L2) (74) and mitochondrial autophagy (BNIP3) (75). Both NDUFA4L2 and BNIP3 are necessary for limiting ROS production (74–76). In support of this hypothesis, we have shown that compared to WT-I/R hearts, gKO-I/R hearts had lower HIF1α, FoxO1, FoxO3a, SOD1, SOD2, NDUFA4L2, and BNIP3 levels (37,60).
Trpm2 ENHANCES CELL SURVIVAL IN CANCER
Trpm2 recently was observed to be overexpressed in most tumors (17) including melanoma (15), breast cancer (unpublished observations, Miller Lab), and neuroblastoma (16,66). The mechanisms regulating expression are not known, but the Trpm2 promoter has a number of methylation sites which could be modulated in malignancy. We showed that Trpm2 protected neuroblastoma cells from moderate oxidative stress through increased levels of FoxO3a and SOD2, whereas cells in which Trpm2 was inhibited by the dominant-negative isoform Trpm2-S showed reduced FoxO3a and SOD2, and increased ROS and susceptibility to cell death (66). In vivo, growth of tumor xenografts was also inhibited by Trpm2-S (16). This finding was confirmed with two different neuroblastoma cell lines (SH-SY5Y, SK-N-AS) and a breast cancer cell line (MCF-7, Miller lab, unpublished observations). HIF-1α/2ααtranscription factors are upregulated in many cancers and mediate expression of a number of genes involved in cancer growth including glycolysis, oxidant stress, angiogenesis, mitochondrial function, and mitophagy. In neuroblastoma, increased expression of HIF-2α is particularly associated with disseminated disease and a poor outcome (89). In Trpm2-S expressing neuroblastoma xenografts, HIF-1α/2ααand downstream target proteins involved in oxidative stress (FoxO3a), glycolysis, angiogenesis [vascular endothelial growth factor (VEGF)], and mitochondrial function (BNIP3, NDUFA4L2, cytochrome oxidase 4.1/4.2 in complex IV) were significantly reduced compared to cells expressing Trpm2-L. Potential mechanisms for the reduced HIF-1α/2α expression in cells in which Trpm2 function is inhibited are discussed above and previously (16). Trpm2 inhibition in neuroblastoma resulted in increased ROS, reduced mitochondrial function, ATP production, cell viability, and tumor growth, particularly after doxorubicin. The importance of Trpm2 activity in malignant growth and the potential of inhibition as a therapeutic modality are being recognized in an increasingly wide range of tumors (17). For example, targeting Trpm2 was recently shown to promote cell death in T cell leukemia (90).
CLINICAL SIGNIFICANCE
We have shown that Trpm2 channels protect the heart from oxidative stress, specifically I/R injury (36,60) and doxorubicin cardiotoxicity (37). Thus therapy designed to promote Trpm2 activation may be beneficial in acute coronary syndromes, ischemic cardiomyopathy, and doxorubicin cardiotoxicity. On the other hand, Trpm2 channels are involved in cell proliferation and differentiation and sustained indiscriminate Trpm2 activation may not only protect cells from injury including chemotherapy but also promote survival of occult malignant cells. Therefore, therapy must be thoughtful and designed to be organ- and tissue-specific.
As discussed above, Trpm2 channels are overexpressed in a large number of cancers (15,17) including neuroblastoma (66). In neuroblastoma cells, Trpm2 was pro-survival via mechanisms (improved cellular bioenergetics, decreased ROS and increased HIF-1α levels, etc.) very similar to those observed in the heart (16,66). Inhibiting Trpm2 by expressing the dominant-negative Trpm2-S resulted in slowing of neuroblastoma xenograft growth in nude mice and enhanced tumor cell killing by doxorubicin (16). Trpm2 is therefore a novel and rational target for cancer therapy (91). In this light, we have shown that gKO hearts exposed to the chemotherapeutic agent doxorubicin had significantly decreased contractile function when compared to WT hearts treated with doxorubicin (37). More importantly, animal survival was much worse in gKO mice exposed to doxorubicin compared to equivalently treated WT mice (37). Targeting Trpm2 without adequate cardiac protection or sparing cardiac Trpm2 channels, or using a cocktail of Trpm2 inhibitors and anthracyclines for chemotherapy, may have serious untoward cardiac consequences including decreased patient survival. An in-depth study of Trpm2 is thus warranted in the emerging field of onco-cardiology.
CONCLUSIONS
Trpm2 channels are pro-survival by modulating HIF-1α expression, preserving mitochondrial bioenergetics, decreasing mitochondrial ROS production, and increasing ROS scavenging in cells subjected to oxidative stress. Trpm2 channels protected hearts against I/R injury and tumor cells from doxorubicin toxicity. Targeting Trpm2 channels in the treatment of diseases may result in benefit, e.g., ameliorating cardiac ischemia-reperfusion injury (friend), or harm, e.g., aggravating doxorubicin cardiotoxicity (foe). Therapy with Trpm2 inhibitors may require specific targeting to cancer cells. More detailed investigation needs to be performed before thoughtful and safe therapy centering on Trpm2 channels can be devised.
Footnotes
Potential Conflict of Interest: This work was supported in part by National Institutes of Health Grants RO1-HL-58672, RO1-HL74854, RO1-HL123093, RO1-DK46778, R01-GM117014, American Heart Association (Great Rivers Affiliate) Grant-in-Aid 15GRNT25680042, PA CURE grant (Project 5; SAP#4100062220), Hyundai Hope on Wheels, and the Four Diamonds Fund.
DISCUSSION
Kaelin, Boston: I would just be very cautious about using knockouts to predict the toxicity of a future drug. Most knockouts are models of loss of a target 24/7, with complete inhibition and most drugs are drugs precisely because you don’t achieve 100% inhibition 24/7. You can titrate them to the desired effect. So I think it’s always important to just keep that in mind when trying to think about what a knockout means in terms of future toxicity.
Cheung, Philadelphia: Thank you very much for that point. We are using the knockout as a model to look at signaling pathways of Trpm2.
Kaelin, Boston: Yes, but the target is completely ablated.
REFERENCES
- 1.Ramsey IS, Delling M, Clapham DE, et al. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–47. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 2.Goel M, Sinkins WG, Schilling WP, et al. Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem. 2002;277:48303–10. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann T, Schaefer M, Schultz G, Gudermann T, et al. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A. 2002;99:7461–6. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Jiang J, Yue L, et al. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–37. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan LM, Deeds J, Hunter J, et al. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998;58:1515–20. [PubMed] [Google Scholar]
- 6.Venkatachalam K, Montell C, et al. TRP channels. Ann Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vazquez E, Valverde MA, et al. A review of TRP channels splicing. Semin Cell Develop Biol. 2006;17:607–17. doi: 10.1016/j.semcdb.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Aarts M, Iihara K, Wei WL, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–77. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 9.Nagamine K, Kudoh J, Minoshima S, et al. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics. 1998;54:124–31. doi: 10.1006/geno.1998.5551. [DOI] [PubMed] [Google Scholar]
- 10.Uemura T, Kudoh J, Noda S, Kanba S, Shimizu N, et al. Characterization of human and mouse TRPM2 genes: identification of a novel N-terminal truncated protein specifically expressed in human striatum. Biochem Biophys Res Commun. 2005;328:1232–43. doi: 10.1016/j.bbrc.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 11.Miller BA, Zhang W, et al. TRP channels as mediators of oxidative stress. Adv Exp Med Biol. 2011;704:531–44. doi: 10.1007/978-94-007-0265-3_29. [DOI] [PubMed] [Google Scholar]
- 12.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB, et al. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res. 2008;102:347–55. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Chu X, Tong Q, et al. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem. 2003;278:16222–9. doi: 10.1074/jbc.M300298200. [DOI] [PubMed] [Google Scholar]
- 14.Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, Luckhoff A, et al. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem. 2002;277:23150–6. doi: 10.1074/jbc.M112096200. [DOI] [PubMed] [Google Scholar]
- 15.Orfanelli U, Wenke AK, Doglioni C, Russo V, Bosserhoff AK, Lavorgna G, et al. Identification of novel sense and antisense transcription at the TRPM2 locus in cancer. Cell Res. 2008;18:1128–40. doi: 10.1038/cr.2008.296. [DOI] [PubMed] [Google Scholar]
- 16.Chen SJ, Hoffman NE, Shanmughapriya S, et al. A splice variant of the human ion channel TRPM2 modulates neuroblastoma tumor growth through hypoxia-inducible factor (HIF)-1/2α. J Biol Chem. 2014;289:36284–302. doi: 10.1074/jbc.M114.620922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park YR, Chun JN, So I, et al. Data-driven analysis of TRP channels in cancer: linking variation in gene expression to clinical significance. Cancer Genomics Proteomics. 2016;13:83–90. [PubMed] [Google Scholar]
- 18.Perraud AL, Fleig A, Dunn CA, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–9. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 19.Tong Q, Zhang W, Conrad K, et al. Regulation of the transient receptor potential channel TRPM2 by the Ca2+ sensor calmodulin. J Biol Chem. 2006;281:9076–85. doi: 10.1074/jbc.M510422200. [DOI] [PubMed] [Google Scholar]
- 20.Sano Y, Inamura K, Miyake A, et al. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–30. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- 21.Jiang LH, et al. Subunit interaction in channel assembly and functional regulation of transient receptor potential melastatin (TRPM) channels. Biochem Soc Trans. 2007;35:86–8. doi: 10.1042/BST0350086. [DOI] [PubMed] [Google Scholar]
- 22.Hara Y, Wakamori M, Ishii M, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–73. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 23.Fonfria E, Marshall IC, Boyfield I, et al. Amyloid β-peptide(1-42) and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures. J Neurochem. 2005;95:715–23. doi: 10.1111/j.1471-4159.2005.03396.x. [DOI] [PubMed] [Google Scholar]
- 24.Gasser A, Glassmeier G, Fliegert R, et al. Activation of T cell calcium influx by the second messenger ADP-ribose. J Biol Chem. 2006;281:2489–96. doi: 10.1074/jbc.M506525200. [DOI] [PubMed] [Google Scholar]
- 25.Kolisek M, Beck A, Fleig A, Penner R, et al. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell. 2005;18:61–9. doi: 10.1016/j.molcel.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 26.Perraud AL, Takanishi CL, Shen B, et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem. 2005;280:6138–48. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- 27.Toth B, Csanady L, et al. Identification of direct and indirect effectors of the transient receptor potential melastatin 2 (TRPM2) cation channel. J Biol Chem. 2010;285:30091–102. doi: 10.1074/jbc.M109.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonfria E, Marshall IC, Benham CD, et al. TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol. 2004;143:186–92. doi: 10.1038/sj.bjp.0705914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buelow B, Song Y, Scharenberg AM, et al. The Poly(ADP-ribose) polymerase PARP-1 is required for oxidative stress-induced TRPM2 activation in lymphocytes. J Biol Chem. 2008;283:24571–83. doi: 10.1074/jbc.M802673200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ, et al. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem. 2003;278:11002–6. doi: 10.1074/jbc.M210810200. [DOI] [PubMed] [Google Scholar]
- 31.Du J, Xie J, Yue L, et al. Intracellular calcium activates TRPM2 and its alternative spliced isoforms. Proc Natl Acad Sci U S A. 2009;106:7239–44. doi: 10.1073/pnas.0811725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Togashi K, Hara Y, Tominaga T, et al. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. Embo J. 2006;25:1804–15. doi: 10.1038/sj.emboj.7601083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du J, Xie J, Yue L, et al. Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity. J Gen Physiol. 2009;134:471–88. doi: 10.1085/jgp.200910254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starkus JG, Fleig A, Penner R, et al. The calcium-permeable non-selective cation channel TRPM2 is modulated by cellular acidification. J Physiol. 2010;588:1227–40. doi: 10.1113/jphysiol.2010.187476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Csanady L, et al. Permeating proton found guilty in compromising TRPM2 channel activity. J Physiol. 2010;588:1661–2. doi: 10.1113/jphysiol.2010.190223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller BA, Hoffman NE, Merali S, et al. TRPM2 channels protect against cardiac ischemia-reperfusion injury: role of mitochondria. J Biol Chem. 2014;289:7615–29. doi: 10.1074/jbc.M113.533851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffman NE, Miller BA, Wang J, et al. Ca2+ entry via Trpm2 is essential for cardiac myocyte bioenergetics maintenance. Am J Physiol Heart Circ Physiol. 2015;308:H637–50. doi: 10.1152/ajpheart.00720.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsutsui H, Kinugawa S, Matsushima S, et al. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–90. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 39.Singal PK, Iliskovic N, et al. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–5. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 40.Kang YJ, Chen Y, Epstein PN, et al. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem. 1996;271:12610–6. doi: 10.1074/jbc.271.21.12610. [DOI] [PubMed] [Google Scholar]
- 41.Loor G, Kondapalli J, Iwase H, et al. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim Biophys Acta. 2011;1813:1382–94. doi: 10.1016/j.bbamcr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ, et al. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–14. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cross JV, Templeton DJ, et al. Regulation of signal transduction through protein cysteine oxidation. Antiox Redox Signal. 2006;8:1819–27. doi: 10.1089/ars.2006.8.1819. [DOI] [PubMed] [Google Scholar]
- 44.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS, et al. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 45.Murphy E, Steenbergen C, et al. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Widlansky ME, Gutterman DD, et al. Regulation of endothelial function by mitochondrial reactive oxygen species. Antiox Redox Signal. 2011;15:1517–30. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zinkevich NS, Gutterman DD, et al. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol. 2011;301:H647–53. doi: 10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaneko S, Kawakami S, Hara Y, et al. A critical role of TRPM2 in neuronal cell death by hydrogen peroxide. J Pharmacol Sci. 2006;101:66–76. doi: 10.1254/jphs.fp0060128. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, Hirschler-Laszkiewicz I, Tong Q, et al. TRPM2 is an ion channel that modulates hematopoietic cell death through activation of caspases and PARP cleavage. Am J Physiol Cell Physiol. 2006;290:C1146–59. doi: 10.1152/ajpcell.00205.2005. [DOI] [PubMed] [Google Scholar]
- 50.Knowles H, Heizer JW, Li Y, et al. Transient receptor potential melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proc Natl Acad Sci U S A. 2011;108:11578–83. doi: 10.1073/pnas.1010678108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knowles H, Li Y, Perraud AL, et al. The TRPM2 ion channel, an oxidative stress and metabolic sensor regulating innate immunity and inflammation. Immunol Res. 2013;55:241–8. doi: 10.1007/s12026-012-8373-8. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto S, Shimizu S, Kiyonaka S, et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–47. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gelderblom M, Melzer N, Schattling B, et al. Transient receptor potential melastatin subfamily member 2 cation channel regulates detrimental immune cell invasion in ischemic stroke. Stroke. 2014;45:3395–402. doi: 10.1161/STROKEAHA.114.005836. [DOI] [PubMed] [Google Scholar]
- 54.Shirakawa H, Sakimoto S, Nakagawa T, Kaneko S, et al. [Pathophysiology of immune cells during the progression of cerebral ischemic injury-involvement of TRPM2-mediated induction of iNOS in microglia/macrophage] Nihon Yakurigaku Zasshi. 2014;144:104–9. doi: 10.1254/fpj.144.104. [DOI] [PubMed] [Google Scholar]
- 55.Xu C, Macciardi F, Li PP, et al. Association of the putative susceptibility gene, transient receptor potential protein melastatin type 2, with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:36–43. doi: 10.1002/ajmg.b.30239. [DOI] [PubMed] [Google Scholar]
- 56.Xu C, Li PP, Cooke RG, et al. TRPM2 variants and bipolar disorder risk: confirmation in a family-based association study. Bipolar Disord. 2009;11:1–10. doi: 10.1111/j.1399-5618.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 57.Herson PS, Ashford ML, et al. Activation of a novel non-selective cation channel by alloxan and H2O2 in the rat insulin-secreting cell line CRI-G1. J Physiol. 1997;501:59–66. doi: 10.1111/j.1469-7793.1997.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uchida K, Dezaki K, Damdindorj B, et al. Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes. 2011;60:119–26. doi: 10.2337/db10-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao G, Wang W, Tadagavadi RK, et al. TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1. J Clin Invest. 2014;124:4989–5001. doi: 10.1172/JCI76042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller BA, Wang J, Hirschler-Laszkiewicz I, et al. The second member of transient receptor potential-melastatin channel family protects hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2013;304:H1010–22. doi: 10.1152/ajpheart.00906.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang KT, Chang WL, Yang PC, et al. Activation of the transient receptor potential M2 channel and poly(ADP-ribose) polymerase is involved in oxidative stress-induced cardiomyocyte death. Cell Death Differ. 2006;13:1815–26. doi: 10.1038/sj.cdd.4401813. [DOI] [PubMed] [Google Scholar]
- 62.Di A, Gao XP, Qian F, et al. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nature Immunol. 2012;13:29–34. doi: 10.1038/ni.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hermosura MC, Cui AM, Go RC, et al. Altered functional properties of a TRPM2 variant in Guamanian ALS and PD. Proc Natl Acad Sci U S A. 2008;105:18029–34. doi: 10.1073/pnas.0808218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia R, Mei ZZ, Mao HJ, et al. Identification of pore residues engaged in determining divalent cationic permeation in transient receptor potential melastatin subtype channel 2. J Biol Chem. 2008;283:27426–32. doi: 10.1074/jbc.M801049200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bai JZ, Lipski J, et al. Differential expression of TRPM2 and TRPV4 channels and their potential role in oxidative stress-induced cell death in organotypic hippocampal culture. Neurotoxicol. 2010;31:204–14. doi: 10.1016/j.neuro.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Chen SJ, Zhang W, Tong Q, et al. Role of TRPM2 in cell proliferation and susceptibility to oxidative stress. Am J Physiol Cell Physiol. 2013;304:C548–60. doi: 10.1152/ajpcell.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanley BA, Sivakumaran V, Shi S, et al. Thioredoxin reductase-2 is essential for keeping low levels of H2O2 emission from isolated heart mitochondria. J Biol Chem. 2011;286:33669–77. doi: 10.1074/jbc.M111.284612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberge S, Roussel J, Andersson DC, et al. TNF-α-mediated caspase-8 activation induces ROS production and TRPM2 activation in adult ventricular myocytes. Cardiovasc Res. 2014;103:90–9. doi: 10.1093/cvr/cvu112. [DOI] [PubMed] [Google Scholar]
- 69.Hiroi T, Wajima T, Negoro T, et al. Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischemia/reperfusion injury. Cardiovasc Res. 2012;97:271–81. doi: 10.1093/cvr/cvs332. [DOI] [PubMed] [Google Scholar]
- 70.Baxter GF, et al. The neutrophil as a mediator of myocardial ischemia-reperfusion injury: time to move on. Basic Res Cardiol. 2002;97:268–75. doi: 10.1007/s00395-002-0366-7. [DOI] [PubMed] [Google Scholar]
- 71.Mittal M, Urao N, Hecquet CM, et al. Novel role of reactive oxygen species-activated Trp melastatin channel-2 in mediating angiogenesis and postischemic neovascularization. Arterioscler Thromb Vasc Biol. 2015;35:877–87. doi: 10.1161/ATVBAHA.114.304802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lucchesi PA, Sweadner KJ, et al. Postnatal changes in Na,K-ATPase isoform expression in rat cardiac ventricle. Conservation of biphasic ouabain affinity. J Biol Chem. 1991;266:9327–31. [PubMed] [Google Scholar]
- 73.Berry RG, Despa S, Fuller W, Bers DM, Shattock MJ, et al. Differential distribution and regulation of mouse cardiac Na+/K+-ATPase α1 and α2 subunits in T-tubule and surface sarcolemmal membranes. Cardiovasc Res. 2007;73:92–100. doi: 10.1016/j.cardiores.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Tello D, Balsa E, Acosta-Iborra B, et al. Induction of the mitochondrial NDUFA4L2 protein by HIF-1α decreases oxygen consumption by inhibiting Complex I activity. Cell Metab. 2011;14:768–79. doi: 10.1016/j.cmet.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Zhang H, Bosch-Marce M, Shimoda LA, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Semenza GL, et al. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophy Acta. 2011;1813:1263–8. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cardenas C, Miller RA, Smith I, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–83. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murphy E, Steenbergen C, Levy LA, Gabel S, London RE, et al. Measurement of cytosolic free calcium in perfused rat heart using TF-BAPTA. Am J Physiol. 1994;266:C1323–9. doi: 10.1152/ajpcell.1994.266.5.C1323. [DOI] [PubMed] [Google Scholar]
- 79.Cheung JY, Bonventre JV, Malis CD, Leaf A, et al. Calcium and ischemic injury. N Engl J Med. 1986;314:1670–6. doi: 10.1056/NEJM198606263142604. [DOI] [PubMed] [Google Scholar]
- 80.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL, et al. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davidson SM, Yellon DM, Murphy MP, Duchen MR, et al. Slow calcium waves and redox changes precede mitochondrial permeability transition pore opening in the intact heart during hypoxia and reoxygenation. Cardiovasc Res. 2012;93:445–53. doi: 10.1093/cvr/cvr349. [DOI] [PubMed] [Google Scholar]
- 82.Tian D, Litvak V, Lev S, et al. Cerebral ischemia and seizures induce tyrosine phosphorylation of Pyk2 in neurons and microglial cells. J Neurosci. 2000;20:6478–87. doi: 10.1523/JNEUROSCI.20-17-06478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsui A, Okigaki M, Amano K, et al. Central role of calcium-dependent tyrosine kinase Pyk2 in endothelial nitric oxide synthase-mediated angiogenic response and vascular function. Circulation. 2007;116:1041–51. doi: 10.1161/CIRCULATIONAHA.106.645416. [DOI] [PubMed] [Google Scholar]
- 84.Lang D, Glukhov AV, Efimova T, Efimov IR, et al. Role of Pyk2 in cardiac arrhythmogenesis. Am J Physiol Heart Circ Physiol. 2011;301:H975–83. doi: 10.1152/ajpheart.00241.2011. [DOI] [PubMed] [Google Scholar]
- 85.O-Uchi J, Jhun BS, Xu S, et al. Adrenergic signaling regulates mitochondrial Ca2+ uptake through Pyk2-dependent tyrosine phosphorylation of the mitochondrial Ca2+ uniporter. Antiox Redox Signal. 2014;21:863–79. doi: 10.1089/ars.2013.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu YV, Hubbi ME, Pan F, et al. Calcineurin promotes hypoxia-inducible factor 1α expression by dephosphorylating RACK1 and blocking RACK1 dimerization. J Biol Chem. 2007;282:37064–73. doi: 10.1074/jbc.M705015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bakker WJ, Harris IS, Mak TW, et al. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007;28:941–53. doi: 10.1016/j.molcel.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 88.Kops GJ, Dansen TB, Polderman PE, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–21. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 89.Mohlin S, Hamidian A, Pahlman S, et al. HIF2α and IGF2 expression correlates in human neuroblastoma cells and normal immature sympathetic neuroblasts. Neoplasia. 2013;15:328–34. doi: 10.1593/neo.121706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klumpp D, Misovic M, Szteyn K, Shumilina E, Rudner J, Huber SM, et al. Targeting TRPM2 channels impairs radiation-induced cell cycle arrest and fosters cell death of T cell leukemia cells in a Bcl-2-dependent manner. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/8026702. 8026702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller BA, et al. TRPM2 function and potential as a drug target. Methods Pharmacol Toxicol. 2012;I:89–102. [Google Scholar]