Abstract
Mammalian cells sense changes in oxygen and transduce that information into adaptive changes in gene expression using a conserved pathway that converges on the heterodimeric transcription factor called hypoxia-inducible factor (HIF), which contains a labile alpha subunit and a stable beta subunit. In the presence of oxygen, the alpha subunit is hydroxylated on one (or both) of two highly conserved prolyl residues by an Egg-Laying Defective Nine (EglN) [also called Prolyl Hydroxylase Domain (PHD)] dioxygenase, which recruits an ubiquitin ligase complex containing the VHL tumor suppressor gene product. Germline VHL mutations cause von Hippel-Lindau (VHL) disease, which manifest as angiogenic tumors such as hemangioblastomas and kidney cancers. Somatic VHL inactivation and deregulation of HIF (especially HIF2α) drives sporadic kidney cancers and an HIF2α inhibitor is showing promise for this disease. VHL, EglN1, and HIF2α polymorphisms have been linked to familial polycythemia and adaptation to high altitude. Orally available EglN inhibitors are being developed for the treatment of anemia and ischemic diseases.
INTRODUCTION
In 1894, the British geneticist Treacher Collins reported the cases of a brother and a sister who both had retinal vascular proliferations that would now be called retinal hemangioblastomas (1). In hindsight, this is now believed to be the first description of individuals with what is now called von Hippel-Lindau (VHL) disease. The familial occurrence of retinal hemangioblastomas was again described in 1904 by the German ophthalmologist Eugen von Hippel (2). It was the Swedish neuropathologist Arvid Lindau who appreciated that these familial retinal lesions were a marker for a systemic disease that was associated with an increased risk of hemangioblastomas of the brain (especially the cerebellum) and spinal cord, as well as an increased risk of kidney cancers and paragangliomas (3).
Clinically, VHL disease appears to be transmitted in autosomal dominant fashion with high penetrance (4). The VHL gene was isolated in 1993 using a positional cloning strategy by a group at the National Cancer Institute led by Marston Linehan, Michael Lerman, and Bert Zbar in collaboration with Eamon Maher, who was then at the University of Birmingham in England, based upon earlier linkage studies that had correctly localized the VHL susceptibility locus to chromosome 3p25 (5). At the molecular level, patients with VHL disease have inherited a defective VHL allele from one of their parents (4). Pathology develops when the remaining wild-type VHL allele is mutated, silenced, or lost. Importantly, biallelic VHL inactivation due to somatic mutations or, less commonly, hypermethylation, is very common in nonhereditary (sporadic) kidney cancer and hemangioblastomas (6). In fact, VHL inactivation is typically the first, or “truncal,” mutation in the pathogenesis of clear cell renal carcinoma, which is the most common form of kidney cancer (7–9).
The VHL gene product, pVHL, is a multifunctional protein that shuttles between the nucleus and cytoplasm (10). Its best-documented function, and the one most firmly linked to the pathogenesis of VHL disease, relates to its ability to form an ubiquitin ligase complex that also contains Elongin B, Elongin C, Cullin 2 (Cul2), and Ring Box 1 (RBX1) (11). In this complex, pVHL serves as the substrate recognition unit. pVHL contains two mutational hotspots: the alpha domain and the beta domain (12). The alpha domain recruits the Elongins, Cul2, and RBX1, while the beta domain is a substrate-binding domain (11).
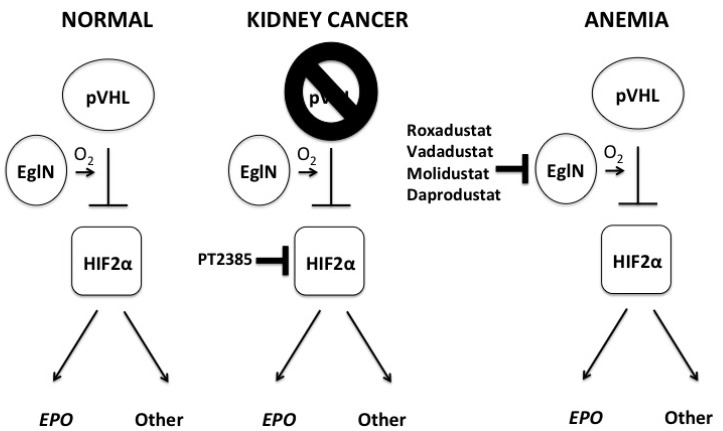
The search for pVHL’s substrates was aided tremendously by the appreciation that the neoplasms caused by VHL inactivation are highly vascular due to overproduction of vascular endothelial growth factor (VEGF) and sometimes cause erythrocytosis by elaborating erythropoietin (EPO) (13–17). VEGF and EPO are the products of hypoxia (low oxygen) –inducible mRNAs and are controlled by the hypoxia-inducible factor (HIF) transcription factor (18). HIF contains a labile alpha subunit (such as HIF1α or HIF2α) that is normally degraded if oxygen is present (hence is hypoxia-inducible) and a stable beta subunit [HIF1β or Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT)]. In the presence of oxygen, HIFα becomes hydroxylated on one (or both) of two prolyl residues by members of the Egg-Laying Defective Nine (EglN) [also called Prolyl Hydroxylase Domain (PHD)] 2-oxoglutarate-dependent dioxygenase family (19–24). Once prolyl hydroxylated, HIFα is recognized by pVHL, polyubiquitylated, and destroyed by the proteasome (Figure 1). Under low oxygen conditions, or in cells functional pVHL, HIFα is stabilized, dimerizes with ARNT, and activates hundreds of genes, many of which (such as the above mentioned VEGF and EPO) normally serve to promote acute or chronic adaptation to hypoxia (25). In pVHL-defective renal cancers the HIF program is co-opted to promote tumorigenesis.
Fig. 1.
Pharmacological manipulation of the oxygen-sensing pathway. When oxygen is available an EglN (also called PHD) prolyl hydroxylase, such as EglN1 (also called PHD2), hydroxylates HIFα subunits on one of two prolyl residues, which then generates a binding site for an ubiquitin ligase containing the VHL gene product, pVHL. Once bound, pVHL earmarks the alpha subunit for proteasomal degradation. When oxygen levels are low, or pVHL is defective, HIFα becomes stable, dimerizes with HIFβ, and transcriptionally activates HIF-responsive genes such as EPO and VEGF. EPO is an example of a HIF-responsive gene that is exclusively regulated by HIF2 and HIF2 also appears to be the primary driver of pVHL-defective kidney cancers. Note that pVHL also has HIF-independent functions (not shown for simplicity) and that pVHL loss is not sufficient to cause kidney cancer (this requires additional cooperating genetic events). The small molecule PT-2385 (and related tool compound PT-2399) is being developed for the treatment of kidney cancer, while multiple EglN inhibitors, such as Roxadustat, Vadadustat, Molidustat, and Daprodustat, are undergoing clinical trials for anemia linked to chronic renal failure. Abbreviations: Eg1N, Egg-Laying Defective Nine; PHD, Prolyl Hydroxylase Domain; HIF, hypoxia-induced factor; VHL, von Hippel Lindau; EPO, erthropoeitin; VEGF, vascular endothelial growth factor.
In preclinical models, downregulation of HIF, and particularly HIF2, appears to be both necessary and sufficient for pVHL to suppress ectopic blood vessel formation and clear cell renal carcinoma (26–33). Amongst solid tumors, clear cell renal carcinomas have the highest VEGF levels, presumably because of loss of pVHL and deregulation of HIF2, and are the most sensitive to single agents directed against VEGF, such as bevacizumab, or its receptor Kinase Insert Domain Receptor (KDR), such as sunitinib, sorafenib, axitininb, pazopabib, and cabozantinib (34). HIF2α itself would classically have been viewed as “undruggable.” However, Rick Bruick and Kevin Gardner identified a potentially druggable pocket in the HIF2α Per-Arnt-Sim B (PAS B) domain and identified chemicals that can bind to this pocket and prevent HIF2α from dimerizing with ARNT (35–37). These initial chemical scaffolds formed the basis for subsequent medicinal chemistry efforts at Peloton Therapeutics, resulting in the first-in-class HIF2α inhibitor PT-2385 and the closely related tool compound PT-2399 (Figure 1) (38–40). PT-2399 displays on-target antitumor activity against a significant percentage of pVHL-defective clear cell renal carcinoma lines and patient-derived xenografts in preclinical models and early clinical data for PT-2385 in heavily pretreated patients with metastatic clear cell renal carcinoma are encouraging (38–40).
HIF deregulation plays an important role in pVHL-defective renal carcinogenesis. Nonetheless, there is no evidence that HIF deregulation is sufficient to cause cancer and some evidence that it is not sufficient. For example, even ignoring pVHL’s HIF-independent functions, it is notable that biallelic VHL inactivation in mice and man causes preneoplastic renal cysts and HIF deregulation, but not cancer (41,42). Additional cooperating genetic events, such as loss of PBRM1 or BAP1, gain of chromosome 5q, and loss of chromosome 14q, are required to cause human clear cell renal carcinomas (43). Moreover, there are many conditions that are associated with adaptive increases in erythrocytosis, such as cardiac and lung diseases that impair oxygen delivery, where the erythrocytosis is presumably caused by chronic HIF activation and yet not linked to the usual stigmata of VHL disease (44). Rare cases of carotid body paragangliomas have been reported in individuals living at extremely high altitude (Andeas mountain dwellers) (45), but there is some evidence that the pathogenesis of paragangliomas is linked to abnormal sculpting of the sympathetic nervous system during embryogenesis and so might not translate into a risk associated with increased HIF activity in adults (46).
Also notable in this regard is the fact some cases of familial polythemia in man are caused by homozygous (or compound heterozygous) hypomorphic VHL alleles, hypermorphic HIF2α alleles, or hypomorphic EglN1 alleles (47). This suggests that subtle, quantitative defects in the pVHL-EglN1-HIF2α can cause polycythemia without dramatically increasing the risk of neoplasia. Similarly, EglN1 inactivation in mice causes polycythemia (48,49). Such mice die of cardiac failure before developing any signs of cancer (48,50).
Although the kidney is the major source of EPO in adult mammals, the liver is the primary source during fetal life. Genetic inactivation of EglN1 causes a transient spike in hepatic EPO production that is then dampened, presumably by HIF-dependent induction of EglN3 (51,52). In contrast, inactivation of all three EglN paralogs causes sustained, high-level, hepatic EPO production and can correct anemia in anephric rodents (51,53,54). The EglNs can be inhibited with drug-like molecules in vitro and in vivo (24,54–59). First-generation, orally available EglN inhibitors have now advanced to Phase 3 clinical testing in man for anemia caused by chronic renal disease based on promising Phase 2 data (Figure 1) (59–61). Inhibition of EglN, using genetic and pharmacological tools, is also beneficial in rodent models of myocardial infarction and stroke (48,62–69). It remains to be seen, however, if these benefits will also be observed in large animal models.
In summary, clinical clues stemming from studies of VHL disease, coupled with fundamental studies related to the HIF transcription factor, led to our current understanding of the pathway used by metazoans to sense and transcriptionally adapt to hypoxia. This pathway is coopted by certain cancers, notably kidney cancer, and also plays an important role in many diseases linked to abnormal oxygen delivery. This knowledge has led to first-in-class molecules being tested for the treatment of kidney cancer, anemia, and ischemic diseases.
Footnotes
Potential Conflicts of Interest: Dr Kaelin has a financial interest in Fibrogen, Inc., which is developing EglN inhibitors, and Peloton Therapeutics, Inc., which is developing HIF2α inhibitors. Supported by grants from the NIH and by HHMI.
DISCUSSION
Rubin, Chapel Hill: There’s a young researcher at UNC named Qing Zhang who works on EglN1. He has shown that EglN1 is regulated by estrogen, at least in breast tumors, and I wonder if any of this has gender specificity.
Kaelin, Boston: That’s a great question. Qing Zhang actually trained in my lab as a post doc and you are correctly pointing out that there are actually three members of this prolyl hydroxylase family — the work horse member of which I refer to as EglN1 because it does most of the work on a day-to-day basis. The other two family members are EglN2 and EglN3, which we think of as sort of the spare tires. But it’s becoming clear that they have some other jobs unrelated to HIF. It is actually EglN2 that is induced by estrogen and Qing’s work strongly suggests that EglN2 plays a role in estrogen-dependent proliferation of breast cancer cells. We’re still frankly trying to understand the mechanism underlying that, but I think that it is sort of a moonlighting job reflecting a HIF-independent function of EglN2.
Tweardy, Houston: Very exciting talk. I was wondering if you could comment just in terms of the potential utility to prolyl hydroxylase inhibitors and ischemic injuries. When can they be given in terms of the timing of the insult?
Kaelin, Boston: As you might imagine, it works better if you give the inhibitor prior to the insult. Of course, at first, you would say that’s cheating, Bill, because most people don’t walk around saying I think I’ll have a heart attack in another 4 hours. Having said that, there are certainly situations where we know people are at significant risk of having an ischemic attack, such as people with unstable angina or undergoing an elective cardiac surgery procedure. Here you can imagine prophylaxis or prevention would work. But, fortunately, there is a benefit even if these inhibitors are given a few hours after the insult. It’s just simply not as great.
Ziegelstein, Baltimore: Thanks for your great talk, Bill. My question relates to high altitudes. Can you talk a little bit about HIF signaling and chronic adaptation to high altitudes? I know, for example, that Tibetan people don’t get erythrocytosis at high altitudes.
Kaelin, Boston: Terrific question. I am often asked, “aren’t these HIF agonists going to cause VHL disease or kidney cancer?” So, the first answer to that is that we know that in VHL-associated cancers such as kidney cancers, VHL is lost, but many other mutations had to occur as well, sort of like tumblers on a lock falling in place. There is no evidence that VHL loss or HIF activation is sufficient to cause cancer. And then there is lots of indirect evidence that it’s probably going to be okay, meaning possibly safe, to pharmacologically activate HIF. People living at high altitudes have been very well studied. Many of them do have slightly increased red blood cell production. However epidemiologically, if anything, they have better survival than people living at sea level. I understand there can be many confounders there but they certainly don’t get VHL disease and with a few exceptions they are not at increased risk of cancer. Now, you bring up an interesting point, which is at extremely high altitude — if anything the erythrocytic response is blunted in some populations such as the Tibetans. We now know that some of these populations have polymorphisms in this pathway that further fine tune the response. Because you can imagine you don’t want to overshoot and have so many red blood cells that you’re getting into hyperviscosity problems. So, it’s becoming clear that some populations, as a form of adaptation, have modified this pathway to optimize their response to high altitude.
Spivak, Baltimore: Thank you for a wonderful talk. You mentioned in addition to 2-oxoglutarate and oxygen, iron as a regulator of HIF in anemia of cancer. I think part of it is you accumulate iron in cells and suppress erythropoietin production. So, in terms of treating anemia with PHD inhibitors, what do you think about the importance of iron?
Kaelin, Boston: That’s a great question, and I alluded to this in passing. I think there are a lot of advantages of turning on the entire erythrocytic response and not just giving EPO by itself. As part of that erythrocytic response, you do, as you know better than I do, change the way you absorb and utilize iron. And so, for example, these PHD inhibitors work in the anemia of chronic disease and it’s at least associated with changes in hepcidin and other genes linked to iron utilization. And so, you’re effectively correcting part of the pathogenesis, at least I understand it, of the anemia of chronic disease.
Crowley, Boston: Just a quick comment which is the throw away. A friend of mine in the School of Public Health has been interested in the Sherpas in the Himalayas because of their physiology. He had a post-doc fellow who went up there who lived with these people for some time. And it’s an interesting thing that the way they heat their tents involves the fire going through and underneath the beds before the smoke gets out. These people live with carbon monoxide levels that are out of sight! Everybody has high carbon monoxide levels without any consequence. So, this is an interesting observation. It may very well have an activation to the system and would merit some studying because the genetic polymorphisms you are talking about may very well be a clue to how they adapt to that.
Kaelin, Boston: Yes, there’s no question, and I think that carbon monoxide can potentially affect these enzymes, so it’s very, very interesting.
Feldman, Philadelphia: Bill, one last quick question: there’ve been some papers that have shown an association between HIF-1 expression and BAG3 expression in some cancers. Have you looked at that?
Kaelin, Boston: I haven’t looked at that one. So, I’ll do a medical student trick and answer a related question: There is a lot of literature pointing to the role of HIF-1, and HIF-1 target genes, in cancer, whether it’s BAG3 or whatever. But you know, part of this is guilt by association, because aggressive tumors outgrow their blood supplies, become hypoxic and upregulate HIF. So, there may be cases where HIF is driving the bad behavior. But I am sure in many cases HIF is simply a marker for the bad behavior. So, I think we have to be very careful of thinking about the actual causal role of HIF in cancer progression.
REFERENCES
- 1.Collins ET, et al. Intra-ocular growths (two cases, brother and sister, with peculiar vascular new growth, probably retinal, affecting both eyes) Trans Ophthal Soc UK. 1894;14:141–9. [Google Scholar]
- 2.von Hippel E, et al. Ueber eine sehr seltene Erkrankung der Nethaut. Graefe Arch Ophthal. 1904;59:83–106. [Google Scholar]
- 3.Lindau A, et al. Zur Frage der Angiomatosis Retinae und Ihrer Hirncomplikation. Acta Opthal. 1927;4:193–226. [Google Scholar]
- 4.Maher E, Kaelin WG, et al. von Hippel-Lindau Disease. Medicine. 1997;76:381–91. doi: 10.1097/00005792-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–20. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 6.Kim WY, Kaelin WG, et al. The role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22(24):4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 7.Gerlinger M, Horswell S, Larkin J, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014;46:225–33. doi: 10.1038/ng.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Hou Y, Yin X, et al. Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell. 2012;148:886–95. doi: 10.1016/j.cell.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Neumann M, Stearman R, et al. Transcription-dependent nuclear-cytoplasmic trafficking is required for the function of the von Hippel-Lindau tumor suppressor protein. Mol Cell Biol. 1999;19:1486–97. doi: 10.1128/mcb.19.2.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaelin WG, et al. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2:673–82. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- 12.Stebbins CE, Kaelin WG, Pavletich NP, et al. Structure of the VHL-ElonginC-elonginB complex: implications for VHL tumor suppressor function. Science. 1999;284:455–61. doi: 10.1126/science.284.5413.455. [DOI] [PubMed] [Google Scholar]
- 13.Wizigmann-Voos S, Breier G, Risau W, Plate K, et al. Up-regulation of vascular endothelial growth factor and its receptors in von Hippel-Lindau disease-associated and sporadic hemangioblastomas. Cancer Res. 1995;55:1358–64. [PubMed] [Google Scholar]
- 14.Siemeister G, Weindel K, Mohrs K, Barleon B, et al. Reversion of deregulated expression of vascular endothelial growth factor in human renal carcinoma cells by von Hippel-Lindau tumor suppressor protein. Cancer Res. 1996;56:2299–301. [PubMed] [Google Scholar]
- 15.Iliopoulos O, Jiang C, Levy AP, Kaelin WG, Goldberg MA, et al. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci U S A. 1996;93:10595–9. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gnarra JR, Zhou S, Merrill MJ, et al. Post-transcriptional regulation of vascular endothelial growth factor mRNA by the VHL tumor suppressor gene product. Proc Natl Acad Sci U S A. 1996;93:10589–94. doi: 10.1073/pnas.93.20.10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieg M, Marti H, KH P, et al. Coexpression of erythropoietin and vascular endothelial growth factor in nervous system tumors associated with von hippel-lindau tumor suppressor gene loss of function. Blood. 1998;92:3388–93. [PubMed] [Google Scholar]
- 18.Semenza GL, et al. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 20.Jaakkola P, Mole D, Tian Y, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 21.Yu F, White S, Zhao Q, Lee F, et al. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A. 2001;98:9630–5. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein A, Gleadle J, McNeill L, et al. C. elegans EGL-9 and Mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 23.Bruick R, McKnight S, et al. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–40. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 24.Ivan M, Haberberger T, Gervasi DC, et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci U S A. 2002;99:13459–64. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaelin WG Jr, Ratcliffe PJ, et al. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG, et al. Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–46. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 27.Kondo K, Kim WY, Lechpammer M, Kaelin WG Jr. Inhibition of HIF2alpha Is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1:439–44. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmer M, Doucette D, Siddiqui N, Iliopoulos O, et al. Inhibition of hypoxia-inducible factor is sufficient for growth suppression of VHL−/− tumors. Mol Cancer Res. 2004;2:89–95. [PubMed] [Google Scholar]
- 29.Raval RR, Lau KW, Tran MG, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–86. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC, et al. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–47. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordan JD, Lal P, Dondeti VR, et al. HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell. 2008;14:435–46. doi: 10.1016/j.ccr.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim WY, Safran M, Buckley MR, et al. Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. Embo J. 2006;25:4650–62. doi: 10.1038/sj.emboj.7601300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rankin EB, Rha J, Unger TL, et al. Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene. 2008;27:5354–8. doi: 10.1038/onc.2008.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choueiri TK, Motzer RJ, et al. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376:354–66. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 35.Scheuermann TH, Tomchick DR, Machius M, Guo Y, Bruick RK, Gardner KH, et al. Artificial ligand binding within the HIF2alpha PAS-B domain of the HIF2 transcription factor. Proc Natl Acad Sci U S A. 2009;106:450–5. doi: 10.1073/pnas.0808092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers JL, Bayeh L, Scheuermann TH, et al. Development of inhibitors of the PAS-B domain of the HIF-2alpha transcription factor. J Med Chem. 2013;56:1739–47. doi: 10.1021/jm301847z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheuermann TH, Li Q, Ma HW, et al. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat Chem Biol. 2013;9:271–6. doi: 10.1038/nchembio.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen W, Hill H, Christie A, et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016;539(7527):112–7. doi: 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho H, Du X, Rizzi JP, et al. On-Target Efficacy of a HIF2alpha antagonist in preclinical kidney cancer models. Nature. 2016;539:107–11. doi: 10.1038/nature19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace EM, Rizzi JP, Han G, et al. A small-molecule antagonist of HIF2alpha is efficacious in preclinical models of renal cell carcinoma. Cancer Res. 2016;76:5491–500. doi: 10.1158/0008-5472.CAN-16-0473. [DOI] [PubMed] [Google Scholar]
- 41.Rankin EB, Tomaszewski JE, Haase VH, et al. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res. 2006;66:2576–83. doi: 10.1158/0008-5472.CAN-05-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montani M, Heinimann K, von Teichman A, Rudolph T, Perren A, Moch H, et al. VHL-gene deletion in single renal tubular epithelial cells and renal tubular cysts: further evidence for a cyst-dependent progression pathway of clear cell renal carcinoma in von Hippel-Lindau disease. Am J Surg Pathol. 2010;34:806–15. doi: 10.1097/PAS.0b013e3181ddf54d. [DOI] [PubMed] [Google Scholar]
- 43.Kaelin W, et al. Molecular Biology of Kidney Cancer. Kidney Cancer: Principles and Practice. 2nd ed. New York, NY: Springer International Publishing; 2015. pp. 31–57. [Google Scholar]
- 44.Golde DW, Hocking WG, et al. Polycythemia: mechanisms and management. Ann Intern Med. 1981;95:71–87. doi: 10.7326/0003-4819-95-1-71. [DOI] [PubMed] [Google Scholar]
- 45.Jech M, Alvarado-Cabrero I, Albores-Saavedra J, Dahia PL, Tischler AS, et al. Genetic analysis of high altitude paragangliomas. Endocr Pathol. 2006;17:201–2. doi: 10.1385/ep:17:2:201. [DOI] [PubMed] [Google Scholar]
- 46.Lee S, Nakamura E, Yang H, et al. Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell. 2005;8:155–67. doi: 10.1016/j.ccr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Lee FS, Percy MJ, et al. The HIF pathway and erythrocytosis. Annu Rev Pathol. 2011;6:165–92. doi: 10.1146/annurev-pathol-011110-130321. [DOI] [PubMed] [Google Scholar]
- 48.Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG., Jr Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood. 2008;111:3236–44. doi: 10.1182/blood-2007-10-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeda K, Aguila HL, Parikh NS, et al. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood. 2008;111:3229–35. doi: 10.1182/blood-2007-09-114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moslehi J, Minamishima YA, Shi J, et al. Loss of hypoxia-inducible factor prolyl hydroxylase activity in cardiomyocytes phenocopies ischemic cardiomyopathy. Circulation. 2010;122:1004–16. doi: 10.1161/CIRCULATIONAHA.109.922427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Querbes W, Bogorad RL, Moslehi J, et al. Treatment of erythropoietin deficiency in mice with systemically administered siRNA. Blood. 2012;120:1916–22. doi: 10.1182/blood-2012-04-423715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minamishima YA, Moslehi J, Padera RF, Bronson RT, Liao R, Kaelin WG Jr. A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Mol Cell Biol. 2009;29:5729–41. doi: 10.1128/MCB.00331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minamishima YA, Kaelin WG, Jr. Reactivation of hepatic EPO synthesis in mice after PHD loss. Science. 2010;329:407. doi: 10.1126/science.1192811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Safran M, Kim WY, O’Connell F, et al. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci U S A. 2006;103:105–10. doi: 10.1073/pnas.0509459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mole DR, Schlemminger I, McNeill LA, et al. 2-oxoglutarate analogue inhibitors of HIF prolyl hydroxylase. Bioorg Med Chem Lett. 2003;13:2677–80. doi: 10.1016/s0960-894x(03)00539-0. [DOI] [PubMed] [Google Scholar]
- 56.Hsieh MM, Linde NS, Wynter A, et al. HIF-prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and fetal hemoglobin expression in rhesus macaques. Blood. 2007;110:2140–7. doi: 10.1182/blood-2007-02-073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernhardt WM, Wiesener MS, Scigalla P, et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol. 2010;21:2151–6. doi: 10.1681/ASN.2010010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDonough MA, Li V, Flashman E, et al. Cellular oxygen sensing: crystal structure of hypoxia-inducible factor prolyl hydroxylase (PHD2) Proc Natl Acad Sci U S A. 2006;103:9814–9. doi: 10.1073/pnas.0601283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SY, Yang EG, et al. Recent advances in developing inhibitors for hypoxia-inducible factor prolyl hydroxylases and their therapeutic implications. Molecules. 2015;20:20551–68. doi: 10.3390/molecules201119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta N, Wish JB, et al. Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am J Kidney Dis. 2017;pii(S0272-6386(17)30110-5) doi: 10.1053/j.ajkd.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 61.Becker K, Saad M, et al. A new approach to the management of anemia in CKD patients: a review on roxadustat. Adv Ther. 2017;34(4):848–53. doi: 10.1007/s12325-017-0508-9. [DOI] [PubMed] [Google Scholar]
- 62.Adluri RS, Thirunavukkarasu M, Dunna NR, et al. Disruption of hypoxia-inducible transcription factor-prolyl hydroxylase domain-1 (PHD-1-/-) attenuates ex vivo myocardial ischemia/reperfusion injury through hypoxia-inducible factor-1alpha transcription factor and its target genes in mice. Antioxid Redox Signal. 2011;15:1789–97. doi: 10.1089/ars.2010.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ratan RR, Siddiq A, Smirnova N, et al. Harnessing hypoxic adaptation to prevent, treat, and repair stroke. J Mol Med. 2007;85:1331–8. doi: 10.1007/s00109-007-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ratan RR, Siddiq A, Aminova L, et al. Translation of ischemic preconditioning to the patient: prolyl hydroxylase inhibition and hypoxia inducible factor-1 as novel targets for stroke therapy. Stroke. 2004;35:2687–9. doi: 10.1161/01.STR.0000143216.85349.9e. [DOI] [PubMed] [Google Scholar]
- 65.Siddiq A, Ayoub IA, Chavez JC, et al. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem. 2005;280:41732–43. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bao W, Qin P, Needle S, et al. Chronic inhibition of hypoxia-inducible factor prolyl 4-hydroxylase improves ventricular performance, remodeling, and vascularity after myocardial infarction in the rat. J Cardiovasc Pharmacol. 2010;56:147–55. doi: 10.1097/FJC.0b013e3181e2bfef. [DOI] [PubMed] [Google Scholar]
- 67.Hoelscher M, Silter M, Krull S, et al. Cardiomyocyte-specific prolyl-4-hydroxylase domain 2 knock out protects from acute myocardial ischaemic injury. J Biol Chem. 2011;286:11185–94. doi: 10.1074/jbc.M110.186809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Natarajan R, Salloum FN, Fisher BJ, Ownby ED, Kukreja RC, Fowler AA, 3rd. Activation of hypoxia-inducible factor-1 via prolyl-4 hydoxylase-2 gene silencing attenuates acute inflammatory responses in postischemic myocardium. Am J Physiol Heart Circ Physiol. 2007;293:H1571–80. doi: 10.1152/ajpheart.00291.2007. [DOI] [PubMed] [Google Scholar]
- 69.Olenchock BA, Moslehi J, Baik AH, et al. EGLN1 Inhibition and rerouting of alpha-ketoglutarate suffice for remote ischemic protection. Cell. 2016;164:884–95. doi: 10.1016/j.cell.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]