INTRODUCTION
When I was a junior faculty member at the University of Pittsburgh in the 1980s, one of my first HIV-positive patients was a priest who worked in Chicago. He developed chronic weight loss, visual problems, and cognitive changes, and as a result came home to be with his family. When I saw him, he was wasted, demented, and essentially blind from cytomegalovirus retinitis. He died several months later and, as you can imagine, his family was devastated. This occurred before accurate testing for HIV and before any antiretroviral agents was available. I have cared for HIV patients ever since and have seen dramatic changes in the manifestations and prognosis of this viral illness. Now, rather than watching patients die as in the early years, I have had what seems like the almost miraculous experience of seeing my patients living normal lives on antiretroviral therapy.
As the years have passed, many of my patients are becoming older and now face the issues of aging. For a more complete discussion of HIV and aging, please see my recent article “HIV and Aging” published in the International Journal of Infectious Diseases (1).
EPIDEMIOLOGY
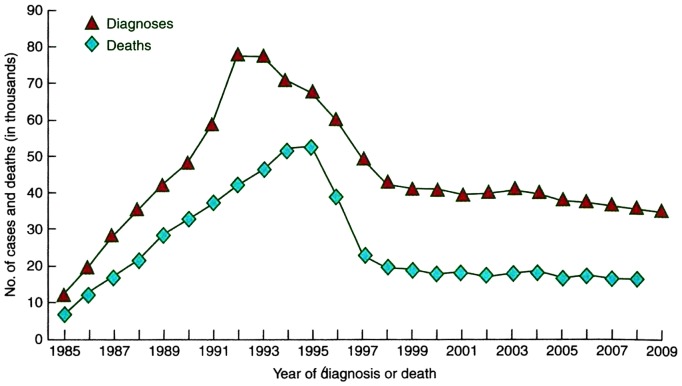
The first cases of HIV in the United States were recognized in 1981. The early years of the epidemic saw a steady increase in the number of diagnoses and deaths as shown Figure 1. In a report of the first 1,000 cases of HIV in the United States, the average age was 34 years and almost all patients could be classified in one of the following groups: men who have sex with men; intravenous drug abusers; and Haitian natives or patients with hemophilia (2). The cases were centered in New York and California with smaller numbers in New Jersey and Florida. The principal markers for the disease were Kaposi’s sarcoma and Pneumocystis carinii pneumonia. Other infections and tumors occurred as a result of the immunodeficiency associated with HIV including Toxoplasma gondii encephalitis, Cryptococcus neoformans meningitis, Cryptosporidiosis colitis, Candida albicans mucosal disease, mycobacterial disease, Herpes virus infections, non-Hodgkin’s B cell lymphoma, as well as wasting and dementia. The mortality rate at the time of the report in 1983 was 39%. We now know that in untreated patients the time to the development of full blown AIDS from initial infection is 7 years to 10 years and the time to death after that is 1 year to 2 years. The early history of the AIDS epidemic was graphically and tragically described in The Band Played On by Randy Shilts published in 1987.
Fig. 1.
The number of HIV cases and diagnoses in thousands from 1985 to 2009 in the United States (From Longo, Fauci, Kasper, Hauser, Jameson, Loscalzo, eds. Harrison’s Principles of Internal Medicine, 18th Edition, New York, McGraw Hill, 2012).
In the mid-1990s, combination, highly effective antiretroviral therapy (ART), usually consisting of a three-drug regimen, became widely available in the United States, and the death rate decreased dramatically as shown in Figure 1. The number of cases of AIDS decreased as well since treatment was started before the full manifestations of HIV occurred (3). The cause of death also changed as the rate of AIDS-defining infections and tumors decreased and other illnesses such as cardiovascular disease and liver disease became more prominent. As a consequence, the number of people living with HIV (PLWH) increased steadily.
Despite this dramatic change, the mortality rates and life expectancy of PLWH never returned to that of the general population. Mortality rates plateaued at 2- to 7-times that of the general population and the calculated life expectancy ranged from 10 years to 20 years less than the uninfected population (4–9). Of interest for this discussion, in subpopulation analyses, those with a CD4 count >500 cells/µL and a suppressed viral load on ART had a life expectancy that approached the uninfected population (9).
The demographics of the HIV epidemic have steadily changed over the past 20 years (10). In addition to the original risk groups, the number of women and children with HIV has increased. Furthermore, of the world’s estimated 38.8 million PLWH, 10% are now older than 50 years of age. Of the US’s 1.4 million PLWH, 50% are now older than 50 years of age (11), and by the year 2030, in 14 years, it is estimated that 70% of the PLWH in the United States will be older than 50 years of age. The primary reasons for this demographic shift are the effectiveness of antiretroviral drugs as well as accurate testing and early diagnosis. In addition, the complications of HIV such as cardiovascular disease are being recognized and treated earlier. Adding to the elderly population are the 18% of newly diagnosed patients older than 50 years of age (12).
COMORBIDITIES
Oftentimes now, the HIV-positive patient faces the challenges of aging. These include chronic comorbidities such as cardiovascular disease, osteoporosis and fractures, renal disease, chronic neurological disease, malignancies, liver disease, and metabolic syndrome as well as geriatric syndromes and frailty. Of great interest to patients and physicians caring for them is the very strong evidence that these comorbidities in HIV patients occur at increased rates at all ages compared to the uninfected population.
For example, a large cohort, cross-sectional study at VA hospitals showed a 50% increase rate of myocardial infarction in HIV-infected veterans compared to uninfected veterans (13). Age and risk factors such as smoking and hypertension were controlled for and all HIV-positive patients were on ART. Interestingly, the risk was increased 50% at all ages and did not seem to increase with time of infection. Whereas some of the older antiretroviral drugs were associated with cardiovascular risk, such as protease inhibitors and abacavir, newer drugs including the integrase inhibitors have much less if any risk. Similar risks for cardiovascular disease have been noted in other large studies, although in one recent longitudinal study, the risk seems to have decreased in recent years, perhaps as a result of more aggressive preventative therapy such as the use of statins (14). The risk for stroke is similarly increased (15).
Patients with HIV are at risk for osteoporosis and fractures (16,17). Cross-sectional studies from Denmark showed a 3-fold increase in the rate of fractures in HIV patients compared to uninfected patients (18).
Chronic renal disease is common in patients with HIV and is often multifactorial (19,20). Risk factors for renal disease in HIV patients include hypertension, diabetes mellitus, black race (particularly HIV nephropathy), low CD4 count, high viral load, and use of tenofovir. A recent reformulation of tenofovir, tenofovir alafenamide, has less renal and bone toxicity and is available in newer combinations of antiretroviral drugs.
Dementia, which was a dreaded complication of HIV in the pre-ART era, is now far less common. HIV-associated neurocognitive disease (HAND) refers to both symptomatic and asymptomatic neurocognitive changes found in HIV patients in the ART era. A recent study from the MAC cohort found an overall prevalence of HAND of 33% with asymptomatic, minor, and dementia forms of HAND of 14%, 14%, and 5%, respectively (21). As long as patients were on ART, there did not seem to be consistent progression of disease.
Malignancies as a whole have decreased in incidence in the ART era (22–24). As would be expected, Kaposi’s sarcoma and non-Hodgkin’s B cell lymphoma have become much less frequent although they are still far more common in the HIV population than the uninfected population. Rates of lung cancer and virally related cancers now account for an increased percentage of all cancers. Lung cancer has increased absolutely, probably as a result of the increased smoking rates in PLWH. Viral-related tumors such as hepatocellular carcinoma (hepatitis C and B) have increased and are a major risk for HIV patients (25). Similarly, human papillomavirus – related cancers including cervical, anal, and oral cancer are increased in the HIV population.
Hepatitis C in combination with HIV confers an increased risk for PLWH for cirrhosis and hepatocellular carcinoma. Curative oral treatment for hepatitis C has revolutionized the outlook for patients dually infected with hepatitis C and HIV and is now in widespread use (26). PLWH also appear to be at increased risk for cirrhosis and hepatocellular carcinoma from fatty liver and nonalcoholic steatohepatitis. Alcohol abuse, which occurs at significant rates in HIV-positive populations, adds to the risk for liver disease (27).
The rates of metabolic syndrome and diabetes mellitus were increased in the pre-ART era and are increased in the obese HIV-positive population (28,29). Complicating previous studies has been the tendency of older antiretroviral drugs to cause dyslipidemia, insulin resistance, and lipoatrophy. Perhaps the biggest risk now for metabolic syndrome and diabetes in the HIV population is obesity. Obesity rates in PLWH approach the rates in the US population with more than 25% of the population having a body mass index (BMI) >30 kg/m2 or greater. Obesity itself confers independent risks for hypertension, cardiovascular disease, and cancer as well as for insulin resistance, metabolic syndrome, and diabetes mellitus. Optimizing approaches to the treatment of obesity in the HIV population is an important research priority.
Finally, PLWH are more at risk for geriatric syndromes and frailty than the non-HIV population (30,31). Geriatric syndromes include falls, urinary incontinence, difficulties with activities of daily living, sensory deficiencies including eyesight and hearing, and frailty. Frailty, which can be defined by weight loss, exhaustion, decreased strength, decreased walking speed, and decreased activities, confers risk for morbidity, hospitalization, and death. Whether frailty can be stabilized or reversed by interventions such as exercise is being actively explored.
HIV providers, including myself, are increasingly facing the issues of aging when caring for PLWH. The principles developed in Geriatric Medicine are now applying to a large portion of HIV patients. Guidelines and recommendations for the care of aging patients with HIV have been published (32,33). These include recommendations for treatment of HIV as well as for prevention and treatment of comorbidities.
DOES HIV ACCELERATE AGING?
“Does HIV make me age faster?” or “Am I going to die earlier because of HIV?” are questions I often hear from my patients. This question of accelerated aging due to HIV is controversial and there is evidence on both sides of the issue (Table 1) (34). Evidence against accelerated aging includes the subgroup analysis referred to previously indicating that PLWH on ART who have a CD4 T cell count of >500/µL and an undetectable viral load have a mortality rate and calculated life expectancy that approach those of the uninfected population. If HIV alone accelerated aging, all infected patients would be expected to have a shortened life expectancy. In addition, risks for comorbidities are similar (for example, 50% increase in risk for cardiovascular disease) at any age. If the risk were related to an accelerated aging, then it would increase over time (for example, from 50% increase to higher rates of increase). Finally, illnesses or defects that increase with age, such as prostate and breast cancer or hearing loss, would be expected to be increased in HIV patients. They are not.
TABLE 1.
Does HIV Accelerate Aging?
| Evidence against | Mortality rates and calculated life expectancy approach normal if CD4 >500 and VL undetectable on ART |
| Comorbidity risk similar at all ages | |
| Age-related processes including prostate and breast cancer and hearing loss not increased | |
| Evidence for | Mortality rates increased and calculated life expectancy shortened on cross-sectional population-based studies |
| Increased rates of comorbidities, geriatric syndromes, and frailty | |
| Evidence for accelerated aging as determined by abnormalities in certain biological mechanisms of aging |
Abbreviations: VL, viral load; ART, antiretroviral therapy.
Arguments for accelerated aging caused by HIV include the increased mortality and shortened calculated life expectancy noted in numerous large cross-sectional controlled studies. The post-hoc subgroup analysis referred to in the previous paragraph may be skewed in favor of unknown factors favoring the subgroup over the general population. Similarly, the rates of comorbidities are increased in general in PLWH, in particular geriatric syndromes and frailty — conditions strongly related to aging, risks for morbidity, and mortality. Finally, PLWH have been shown to have increased biomarkers of aging compared to uninfected patients.
MECHANISMS OF AGING
Table 2 shows some of the major biological mechanisms of aging outlined in a classic article “Hallmarks of Aging” by Lopez-Otin et al (35). Aging is one of the most intensely investigated and rapidly evolving areas of biology research today. Many mechanisms have been investigated in organisms ranging from yeast to worms, flies, vertebrates, and mammals. Although many processes have been elucidated, there is no integrated, overarching theory of aging to date. Mechanisms in Table 2 labeled with an asterisk have been shown to be altered in PLWH.
TABLE 2.
Mechanisms of Aging
| Altered intercellular communication e.g., inflammation and lack of immune regulation* |
| Genomic damage |
| Telomere shortening* |
| Epigenetic changes* |
| Abnormal proteostasis |
| Altered nutrient sensing, GH/IGF-1/Insulin pathway* |
| Mitochondrial dysfunction* |
| Cellular senescence* |
| Stem cell exhaustion |
Aging process known to be affected adversely in PLWH.
Abbreviations: GH, growth hormone; IGF-1, insulin-like growth factor 1; PLWH, people living with HIV.
The most intensely investigated is the altered intracellular communications exemplified by increased inflammation and decreased immune regulation. Patients with HIV have been shown to have low-level chronic inflammation even when the CD4 count is >500 and the viral load is suppressed. Biomarkers such as interleukin 6, D-dimer, C-reactive protein, and sCD14 are elevated (36–40). The causes of increased inflammation include low-level viral replication (including HIV itself and herpes viruses such as cytomegalovirus) (41,42) loss of immune regulation, immunosenescence, and microbial translocation in the gut (43). Low-level inflammation can also be shown in aging uninfected patients but at older ages than HIV patients. Clinical trials using the anti-inflammatory properties of statins and methotrexate are underway in an attempt to counteract the aging process in HIV patients. Decreased immune regulation results from the decreased number of CD4 T cells and the increased percentage of immunosenescent CD8 T cells (see below).
Telomeres are terminal DNA-protein complexes that protect chromosomes but shorten with aging with each chromosomal replication and are thus a marker for biological age. Telomere length has been shown to be shortened in HIV patients compared to uninfected people (44). Epigenetic alterations in DNA and histone methylation patterns are an additional biomarker for aging. Such alterations potentially affect such basic biological functions such as the transcription processes. Patients with HIV have been shown to have an increase in epigenetic methylation patterns in both blood and brain corresponding to aging accelerated by at least 5 years (45).
Patients with HIV appear to have abnormalities in the growth hormone/insulin-like growth factor 1 (IGF-1)/Insulin (GH/IGF-1) pathway. This pathway is one of the most conserved aging pathways, and nutrient deprivation has been shown to lengthen lifespan in representative organisms from yeasts to mammals. Patients with HIV have been shown to have increased insulin resistance and in some studies increased rates of the metabolic syndrome and diabetes mellitus. Manipulation of the pathway to stabilize the aging process is under intense investigation and includes drugs such as rapamycin. Rapamycin affects several aging pathways, but can directly affect the GH/IGF-1 pathway and extend life in animals.
Aging results in progressive mitochondrial dysfunction (secondary to mitochondrial DNA mutations and increased free radical production) bringing about decreased respiratory efficiency (46). Certain antiretroviral drugs appear to contribute to increased aging by increased clonal expansion of mitochondrial DNA mutations thus affecting mitochondrial function. Drugs such as metformin may counteract decreased mitochondrial function, and thus counteract the accelerated aging process.
Cellular senescence occurs through telomere shortening or other mechanisms resulting in an inability of cells to regenerate. In PLWH there is decreased telomere length (44) and an increased percentage of senescent CD8 T cells (CD57+CD28−) (47). These cells are unable to react to new antigens and are themselves proinflammatory contributing to the aging process.
Each of these mechanisms may contribute to accelerated aging in PLWH. As mentioned, there is intense interest in pharmacological approaches to stabilizing the aging process in HIV patients. Drugs such as statins, methotrexate, rapamycin, and metformin have all been used or proposed to be used in clinical trials.
A PRESCRIPTION FOR YOUTH
Out of interest, I have included a picture of one of the longest lived individuals in history, Jean Calment, who lived to 122 years, passing away in 1997 (Figure 2). Shown is Ms. Calment on her 121st birthday. She ascribed her long life to olive oil, port wine, and 2.5 pounds of chocolate/week, an equivalent of 25 Hershey bars/week. We don’t know if these shortened or lengthened her life.
Fig. 2.
Jean Calment.
In Summary:
The mortality of patients living with HIV decreased dramatically in the mid-1990s due to ART. Correspondingly, calculated life expectancy increased.
Fifty percent of PLWH in the United States are now older than 50 years of age, and by 2030 70% will be older than 50 years of age.
The rate of comorbidities is greater in PLWH compared to uninfected people.
Low-level inflammation is increased in PLWH.
Whether HIV accelerates the underlying mechanisms of aging is controversial and an area of active research.
Footnotes
Potential Conflicts of Interest: None to disclose.
DISCUSSION
Zeidel, Boston: Wonderful talk and you point out a population that we are all taking care of and maybe not paying attention to. I guess the question that comes up is: Is it the HIV, is it the drugs they are on, or the other five things that these folks have which related to how they got the HIV that relate to some of these changes? Are studies underway where we can try to tease those out so we understand more about the population you describe?
Wing, Providence: Yes, so Mark, that’s a very good question. There are articles written on trying to develop control groups for HIV studies. And it’s difficult. There are many, many factors which potentially distinguish the HIV population. The best cross-sectional studies take into account as many things as they can, but there’s always the question of what’s the difference.
Michael Gershon, New York City: Are there any studies, perhaps with animals, that can distinguish whether the problem is long-term living with HIV infection, or long-term living with the drugs that are used to treat HIV infection?
Wing, Providence: So, the initial drugs were very toxic. They had many toxicities associated with them and some of them like lipodystrophy change the risk for cardiovascular disease seen in early studies. The newer studies, which include drug classes like the integrase inhibitors, and should include formulations of drugs like tenofovir, which don’t have as many metabolic effects. The Drug therapies are always in the background as a potential problem in terms of analyzing the data.
Michael Gershon, New York City: But those studies could be mimicked in animals. In other words, without the HIV virus you could give the drugs that are used to treat people with HIV to animals who have much shorter live spans than humans do, and perhaps get an idea as to whether they accelerate problems of aging such as mitochondrion damage.
Wing, Providence: That’s a good point and there are also models of animals infected with HIV. They’re not perfect but they could be studied.
Calkins, Baltimore: I was fascinated by your talk. Can you clarify the link between HIV and coronary disease, atrial fibrillations, and other common cardiac conditions?
Wing, Providence: So, there’s a clear association with increased risk for a variety of cardiac conditions that include vascular disease, coronary artery disease, cerebrovascular disease. There’s an association with the myocardial stiffness. And the myocardium itself can be infected by the virus as can vasculature. There’s a lot of evidence that there are direct connections between the HIV virus and the myocardium and vascular structures.
Olds, Granada: Wonderful talk Ed....I was going to ask you maybe a more political question about HIV. We have a history when we do a good job with an illness to kind of let up on the efforts surround that. We did that in tuberculosis back in the 50s and it came back to become a problem. I’m a bit concerned that now that HIV is much better managed, that a lot of the political commitment may be reduced. HIV is going to continue to be a major health problem in our country, but perhaps the Ryan White Funds, the special funds that were partially responsible for our success, could go on to support the latest disease.
Wing, Providence: I think that’s accurate.... we still have 50,000 new cases of HIV in the country every year. We have an aging population with the issues that I mentioned are becoming very important. Eighty percent of the diagnoses of HIV are in sub-Saharan Africa where there is much less of a commitment and much fewer resources. And you’re right about the politics. And one of the issues that the NIH has tried to deal with is if we are spending too much money on HIV research and HIV programs. There’s a real push away from HIV at the moment....so, good point.
Barondess, New York City: I have what’s almost a philosophic point I suppose. But it does have some practical implications. We tend to use the terms disease and illness interchangeably but they actually refer to two related but quite distinct kinds of phenomena I would suggest. You can see a disease as a biological phenomenon and illness as a subjective experience frequently caused by disease but not always. And if you think further, then it’s possible to have a disease without being ill which is the objective of therapy for much of how we manage the common, the leading causes of death of chronic diseases that are hyperendemic now. It does make a difference in terms of therapeutic clarity if not fundamental research objectives. We should consider this as we manage and perform research on many chronic, hyperendemic conditions.
Wing, Providence: Very interesting point. You know, one thing to keep in mind about HIV is the typical cascade as we diagnose maybe 80% of all patients with HIV. Which means we don’t diagnose 20%. We bring into therapy maybe another 20% or 30% and then only about 30% or so are actually have suppressed viral loads. So we don’t do a good job of managing the illness and the disease. So again, it’s a national problem.
Merajver, Ann Arbor: I’m curious about your opinion as a practicing physician in the HIV population. The impact of treatment and prevention with HIV in international circles is considered a landmark condition for prevention. With discordant couples, we treat the uninfected partner; what has been your experience with “treatment for prevention”?
Wing, Providence: Right....so, I’ve had experience in Africa — we have a program in Kenya where it was almost like going back in time to treat people with HIV. When I first went there, there was no therapy at all. No therapy for infected people, no therapy for people with AIDS, no pre-exposure prophylaxis, no treatment of discordant couples. And that’s changed with the PEPFAR (The President’s Emergency Plan for AIDS Relief ) money and with international money. That money has plateaued by the way and there is still a tremendous need. But the treatment of individuals where the viral load comes down to almost undetectable or is undetectable basically prevents the spread of the illness. It’s critical, it’s part of the epidemiological efforts to control HIV and that’s particularly true in Africa so prevention efforts have had a huge impact.
REFERENCES
- 1.Wing EJ. HIV and aging. Int J Infect Dis. 2016;53:61–8. doi: 10.1016/j.ijid.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe HW, Bregman DJ, Selik RM. Acquired immune deficiency syndrome in the United States: the first 1,000 cases. J Infect Dis. 1983;148((2)):339–45. doi: 10.1093/infdis/148.2.339. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Ruppik M, Rickenbach M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013;14((4)):195–207. doi: 10.1111/j.1468-1293.2012.01051.x. [DOI] [PubMed] [Google Scholar]
- 4.Antiretroviral Therapy Cohort C. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372((9635)):293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collaboration of Observational HIVEREiE. Lewden C, Bouteloup V, et al. All-cause mortality in treated HIV-infected adults with CD4 ≥500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol. 2012;41((2)):433–45. doi: 10.1093/ije/dyr164. [DOI] [PubMed] [Google Scholar]
- 6.Legarth RA, Ahlstrom MG, Kronborg G, et al. Long-term mortality in HIV-infected individuals 50 years or older: a nationwide, population-based cohort study. J Acquir Immune Defic Syndr. 2016;71((2)):213–8. doi: 10.1097/QAI.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 7.Lewden C, Chene G, Morlat P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr. 2007;46((1)):72–7. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]
- 8.Marcus JL, Chao CR, Leyden WA, et al. J Acquir Immune Defic Syndr. 2016. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodger AJ, Lodwick R, Schechter M, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS. 2013;27((6)):973–9. doi: 10.1097/QAD.0b013e32835cae9c. [DOI] [PubMed] [Google Scholar]
- 10.HIV and Aging. A special supplement to the UNAIDS Report on the Global AIDS Epidemic 2013. 2013. 978-92-9253-004-0. [Google Scholar]
- 11.O’Keefe KJ, Scheer S, Chen MJ, Hughes AJ, Pipkin S. People fifty years or older now account for the majority of AIDS cases in San Francisco, California, 2010. AIDS care. 2013;25((9)):1145–8. doi: 10.1080/09540121.2012.752565. [DOI] [PubMed] [Google Scholar]
- 12.HIV Surveillance Report. 2013. In: Prevention CfDCa 2015 Available at: http://www.cdc.gov/hiv/library/reports/surveillance/2013/surveillance_report_vol_25.html .
- 13.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173((8)):614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein DB, Leyden WA, Xu L, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis. 2015;60((8)):1278–80. doi: 10.1093/cid/civ014. [DOI] [PubMed] [Google Scholar]
- 15.Sico JJ, Chang CC, So-Armah K, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84((19)):1933–40. doi: 10.1212/WNL.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kooij KW, Wit FW, Bisschop PH, et al. Low bone mineral density in patients with well-suppressed HIV infection: association with body weight, smoking, and prior advanced HIV disease. J Infect Dis. 2015;211((4)):539–48. doi: 10.1093/infdis/jiu499. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen LD, May MT, Kronborg G, et al. Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: a nationwide population-based cohort study. Lancet HIV. 2015;2((7)):e288–98. doi: 10.1016/S2352-3018(15)00077-6. [DOI] [PubMed] [Google Scholar]
- 18.Prieto-Alhambra D, Guerri-Fernandez R, De Vries F, et al. HIV infection and its association with an excess risk of clinical fractures: a nationwide case-control study. J Acquir Immune Defic Syndr. 2014;66((1)):90–5. doi: 10.1097/QAI.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 19.Abraham AG, Althoff KN, Jing Y, et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis. 2015;60((6)):941–9. doi: 10.1093/cid/ciu919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ando M, Tsuchiya K, Nitta K. How to manage HIV-infected patients with chronic kidney disease in the HAART era. Clin Exp Nephrol. 2012;16((3)):363–72. doi: 10.1007/s10157-012-0585-7. [DOI] [PubMed] [Google Scholar]
- 21.Sacktor N, Skolasky RL, Seaberg E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. 2016;86((4)):334–40. doi: 10.1212/WNL.0000000000002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franceschi S, Lise M, Clifford GM, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer. 2010;103((3)):416–22. doi: 10.1038/sj.bjc.6605756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park LS, Tate JP, Sigel K, et al. AIDS. 2016. Time trends in cancer incidence in persons living with HIV/AIDS in the antiretroviral therapy era: 1997-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simard EP, Pfeiffer RM, Engels EA. Cumulative incidence of cancer among individuals with acquired immunodeficiency syndrome in the United States. Cancer. 2011;117((5)):1089–96. doi: 10.1002/cncr.25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taddei TH, Lo Re V, 3rd, Justice AC. HIV, aging, and viral coinfections: taking the long view. Curr HIV/AIDS Rep. 2016;13((5)):269–78. doi: 10.1007/s11904-016-0327-7. [DOI] [PubMed] [Google Scholar]
- 26.Feeney ER, Chung RT, Yazdanpanah Y. Current guidelines and prioritizing treatment of hepatitis C virus in HIV-infected patients. Curr Opin HIV AIDS. 2015;10((5)):323–9. doi: 10.1097/COH.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 27.Edelman EJ, Tetrault JM, Fiellin DA. Substance use in older HIV-infected patients. Curr Opin HIV AIDS. 2014;9((4)):317–24. doi: 10.1097/COH.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monroe AK, Glesby MJ, Brown TT. Diagnosing and managing diabetes in HIV-infected patients: current concepts. Clin Infect Dis. 2015;60((3)):453–62. doi: 10.1093/cid/ciu779. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen LD, Mathiesen ER, Kronborg G, Pedersen C, Gerstoft J, Obel N. Risk of diabetes mellitus in persons with and without HIV: a Danish nationwide population-based cohort study. PloS ONE. 2012;7((9)):e44575. doi: 10.1371/journal.pone.0044575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brothers TD, Kirkland S, Guaraldi G, et al. Frailty in people aging with human immunodeficiency virus (HIV) infection. J Infect Dis. 2014;210((8)):1170–9. doi: 10.1093/infdis/jiu258. [DOI] [PubMed] [Google Scholar]
- 31.Greene M, Covinsky KE, Valcour V, et al. Geriatric syndromes in older HIV-infected adults. J Acquir Immune Defic Syndr. 2015;69((2)):161–7. doi: 10.1097/QAI.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58((1)):e1–34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 33.Work Group for HIV, Aging Consensus P. Summary report from the Human Immunodeficiency Virus and Aging Consensus Project: treatment strategies for clinicians managing older individuals with the human immunodeficiency virus. J Am Geriatr Soc. 2012;60((5)):974–9. doi: 10.1111/j.1532-5415.2012.03948.x. [DOI] [PubMed] [Google Scholar]
- 34.Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69((7)):833–42. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153((6)):1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borges AH, O’Connor JL, Phillips AN, et al. Factors associated with plasma IL-6 levels during HIV infection. J Infect Dis. 2015;212((4)):585–95. doi: 10.1093/infdis/jiv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freiberg MS, Bebu I, Tracy R, et al. D-dimer levels before HIV seroconversion remain elevated even after viral suppression and are associated with an increased risk of non-AIDS events. PloS ONE. 2016;11((4)):e0152588. doi: 10.1371/journal.pone.0152588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamat A, Misra V, Cassol E, et al. A plasma biomarker signature of immune activation in HIV patients on antiretroviral therapy. PloS ONE. 2012;7((2)):e30881. doi: 10.1371/journal.pone.0030881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV. Curr Opin HIV AIDS. 2010;5((6)):498–503. doi: 10.1097/COH.0b013e32833ed6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.So-Armah KA, Tate JP, Chang CC, et al. Do biomarkers of inflammation, monocyte activation, and altered coagulation explain excess mortality between HIV infected and uninfected people? J Acquir Immune Defic Syndr. 2016;72((2)):206–13. doi: 10.1097/QAI.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez-Picado J, Deeks SG. Persistent HIV-1 replication during antiretroviral therapy. Curr Opin HIV AIDS. 2016;11((4)):417–23. doi: 10.1097/COH.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naeger DM, Martin JN, Sinclair E, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PloS ONE. 2010;5((1)):e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinh DM, Volpe GE, Duffalo C, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2015;211((1)):19–27. doi: 10.1093/infdis/jiu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanet DL, Thorne A, Singer J, et al. Association between short leukocyte telomere length and HIV infection in a cohort study: no evidence of a relationship with antiretroviral therapy. Clin Infect Dis. 2014;58((9)):1322–32. doi: 10.1093/cid/ciu051. [DOI] [PubMed] [Google Scholar]
- 45.Horvath S, Levine AJ. HIV-1 Infection accelerates age according to the epigenetic clock. J Infect Dis. 2015;212((10)):1563–73. doi: 10.1093/infdis/jiv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Payne BA, Wilson IJ, Hateley CA, et al. Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat Genetics. 2011;43((8)):806–10. doi: 10.1038/ng.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol. 2012;24((4)):501–6. doi: 10.1016/j.coi.2012.05.004. [DOI] [PubMed] [Google Scholar]