Abstract
Mast cells and immunoglobulin E (IgE) antibodies are thought to promote health by contributing to host responses to certain parasites, but other beneficial functions have remained obscure. Venoms provoke innate inflammatory responses and pathology reflecting the activities of the contained toxins. Venoms also can induce allergic sensitization and development of venom-specific IgE antibodies, which can predispose some subjects to exhibit anaphylaxis upon subsequent exposure to the relevant venom. We found that innate functions of mast cells, including degradation of venom toxins by mast cell–derived proteases, enhanced survival in mice injected with venoms from the honeybee, two species of scorpion, three species of poisonous snakes, or the Gila monster. We also found that mice injected with sub-lethal amounts of honeybee or Russell’s viper venom exhibited enhanced survival after subsequent challenge with potentially lethal amounts of that venom, and that IgE antibodies, FcεRI, and probably mast cells contributed to such acquired resistance.
INTRODUCTION
Allergies currently afflict 20% to 30% of people worldwide, and represent detrimental acquired immune responses against any of a large variety of environmental antigens (1). Such antigens (called allergens) can elicit acquired type 2 immune responses which are dependent on CD4+ T helper type (Th)2 cells and include the production of allergen-specific immunoglobulin E (IgE) antibodies (2–4). In such Th2 cell–associated type 2 immune responses, IgE enables antigen-specific function of effector cells by binding to high affinity receptors for IgE (FcεRI) on the cells’ surface (5,6). FcεRI are expressed on mast cells, that reside in most vascularized tissues in mammals and other vertebrates, and on basophilic granulocytes (“basophils”), that ordinarily circulate in very low numbers in the blood but which can be recruited to sites of inflammation (3, 5–10).
When mast cell– or basophil-bound IgE antibodies recognize antigens that are at least bivalent, rapid aggregation of the FcεRI initiates complex intra-cellular signaling pathways. This ultimately results in the release, by such activated effector cells, of a wide variety of mediators with diverse biological effects (5,6,8–11). Some of these mediators are stored in the cells’ cytoplasmic granules, ready for immediate release, including, in mast cells, histamine, heparin and other proteoglycans, proteases such as carboxypeptidase A3 (CPA3), tryptases and chymases, and some cytokines; in addition, products of arachidonic acid metabolism (via the cyclo-oxidase or lipoxygenase pathways; e.g., prostaglandins and cysteinyl leukotrienes) and a diverse group of cytokines, chemokines, and growth factors are secreted after upregulation of their transcription as a result of FcεRI–dependent cell activation (3,5–7,12,13). Basophils activated via FcεRI aggregation can release a panel of mediators partially overlapping with those of mast cells, but, as compared to mast cells, they contain much lower amounts of proteases and appear to produce fewer cytokines and chemokines (8–10).
In addition to IgE and specific antigen, many stimuli can activate at least some mast cell populations via innate mechanisms (i.e., independently of an antigen-specific acquired immune response), including products of complement activation (e.g., C3a, C5a), products of pathogens (e.g., lipopolysaccharide (LPS) and other pathogen-associated molecular patterns), certain cytokines or growth factors (including interleukin 33 [IL-33] and the Kit ligand, stem cell factor), products of other hematopoietic cells, certain endogenous peptides (including endothelin-1 [ET-1] and vasoactive intestinal polypeptide [VIP]), and components of the venoms of many different vertebrates and invertebrates (10,14–18). Within or among different mammalian species, individual mast cell subpopulations can vary in their susceptibility to activation via these innate mechanisms, likely reflecting such factors as microenvironmentally regulated differences in levels of expression of the cognate receptors (14,19). Also, various stimuli can differ in their ability to elicit the release of granule-stored, lipid, or cytokine mediators. For example, certain peptides such as substance P can activate some mast cell populations to undergo extensive release of the granule-stored mediators; however, compared to the same cells activated via the FcεRI, such stimuli may less potently elicit release of cytokines or lipid mediators (14,20,21). In contrast, for at least some mast cell populations, pathogen-associated molecular patterns are more effective in eliciting release of cytokines and chemokines than granule-stored mediators (16,17). Because mast cells or basophils participating in innate or adaptive immune responses may encounter simultaneously or sequentially several different stimuli of activation, it may be difficult to predict which mast cell– or basophil-derived mediators will be released and in what amounts in these settings, and even more challenging to guess what the net effects of all such mediators might be during that particular biological response.
It is now generally accepted that mast cells and basophils can contribute importantly to the pathology associated with allergic disorders, including potentially fatal anaphylaxis (3,22,23). Yet the evolutionary advantages which might by conferred by IgE, mast cells, and basophils have been more difficult to identify. A major hypothesis about the potential “beneficial functions” of such allergic effector mechanisms is that IgE-associated type 2 immune responses contribute to host defense against helminths and certain other parasites (4,24–26). It should be noted, however, that it has been challenging to prove that IgE, mast cells, or basophils dramatically influence the survival of the parasite-infected animals. Abnormalities in host responses to certain parasites have been observed in mice that genetically lack IgE (27,28), mast cells (29–33), or basophils (28,33), but such studies generally have not included an analysis of the effects of those deficiencies on the overall survival or reproductive success of the infected hosts. And some findings even suggest that, in certain settings, IgE or mast cells may have effects during host responses to parasites (e.g., effects which directly or indirectly result in increased parasite egg production) that may favor the parasite rather than the host (34–36).
The complexity of the relationships between parasites and their hosts is not surprising given that vertebrates have been co-evolving with such parasites for millions of years. Therefore, it also is not surprising that, depending on the parasites and the particular setting, immune effector mechanisms such as IgE, mast cells, and basophils might be exploited by the parasites to their own advantage. For example, one can speculate that by eliciting a type 2 immune response that results in IgE-dependent mast cell activation and release of vasoactive mediators in response to parasite antigens at sites of parasite infection, the parasite could influence local blood flow and vascular permeability in ways that enhance the parasite’s nutrition.
In contrast to parasites, most allergens do not represent a direct threat to the non-sensitized host. This is why such type 2 immune responses are widely considered to be “misdirected” or “maladaptive” immune responses (37,38). However, in 1991, Margie Profet proposed a radically different idea, based in part on the observation that a common feature of most allergens is their origin from sources such as seafood, nuts, or venoms which either might contain toxins (e.g., foods) or always do (e.g., venoms) (39). Profet proposed that acute allergic reactions, manifested as immediately occurring symptoms in response to allergen exposure, such as sneezing, coughing, vomiting, and diarrhea, evolved as defense mechanisms allowing the sensitized host to respond immediately to, and to expel, neutralize, and/or avoid noxious substances which might be indicative of potentially life-threatening situations (39). Even before Profet’s 1991 paper, James Stebbings, Jr. hypothesized that “a major function of the immediate hypersensitivity reactions has been the protection of terrestrial vertebrates from the bites of, or invasion by, arthropods” (40). However, until recently (41), Profet’s “toxin hypothesis” was largely ignored by the scientific community; Stebbings’ paper was even more neglected (42). Over the last few years, my colleagues and I have been able to provide experimental evidence from studies in mice which supports this “toxin hypothesis” of allergy.
MATERIALS AND METHODS
Mast Cell Knock-in Mice
Yukihiko Kitamura et al discovered that (WB/Re-W/+ X C57BL/6-Wv/+)F1-“W/Wv” mice (now known as WBB6F1-KitW/KitW-v mice, since “W” later was shown to encode c-kit) (43,44) not only had a moderate macrocytic anemia, a phenotype which had been reported decades earlier, but were profoundly deficient in tissue mast cells (45). They also showed that mast cells developed in WBB6F1-W/Wv mice which had been engrafted with bone marrow cells from the (wild-type) WT littermate WBB6F1-+/+ mice (45). However, the recipient W/Wv mice also were cured of their anemia (45), as was initially shown by Elizabeth Russell (46). Moreover, eventually, non-irradiated WBB6F1-W/Wv mice engrafted with sufficient (e.g., 1 x 107) WBB6F1-+/+ or other genetically compatible WT whole bone marrow cells also undergo virtually complete replacement of multiple other hematopoietic lineages (including granulocytes and lymphocytes) with cells of donor origin (47–49).
Because transfer of WT bone marrow cells into W/Wv mice did not result in the selective engraftment of mast cells because of the presence in bone marrow cells of hematopoietic stem and progenitor cell populations, an effort was undertaken to attempt to achieve a more selective “repair” of the mast cell deficiency of W/Wv mice by transferring in vitro–derived, “lineage-committed” mast cells to the mice instead of whole bone marrow cells. This approach appeared plausible because it was clear that large populations of cells with features of “immature” mast cells could be generated in vitro from mouse hematopoietic progenitor cells (50) and that such cells exhibited features of additional mast cell maturation when exposed to sodium butyrate in vitro (51). Nakano et al. showed that the transfer of WBB6F1-+/+ mouse bone marrow–derived cultured mast cells (BMCMCs) intravenously, intraperitoneally, or intradermally into WBB6F1-W/Wv mice had no effect on the anemia of the recipient mice but resulted in the appearance of mast cells in their tissues, and that, over time, these mast cell populations came to exhibit certain phenotypic features similar to those present in the corresponding anatomical sites in WT mice (52).
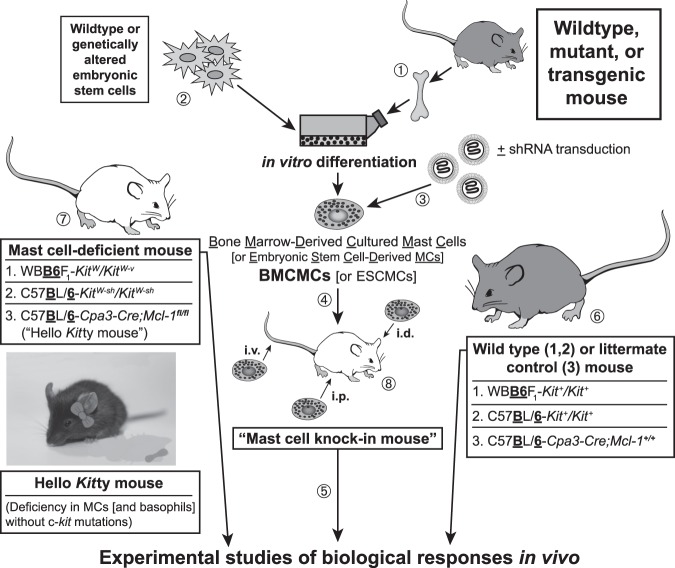
Since that first study, many groups have used such mast cell–engrafted or, as we refer to them in the Galli lab, “mast cell knock-in mice” (Figure 1), to analyze mast cell development, phenotype, heterogeneity, and function in vivo (14,60,61). An attractive aspect of this approach is that one can transfer into different genetically mast cell–deficient recipients either WT mast cells or mast cells that have been genetically manipulated or that are derived from various mutant or transgenic mice, so that one can compare the function in vivo of mast cells that are normal or that lack (or have altered function of) various receptors, signaling molecules or mediators. Moreover, Tsai et al. showed that one also can generate such mast cells from embryonic stem cells, permitting the analysis in vivo of mast cells which lack products which, if absent in the germ line, would result in embryonic or perinatal lethality (53).
Fig. 1.
Making “mast cell knock-in mice.” (1) Mast cells can be generated from bone marrow cells (or other hematopoietic cells, e.g., those in the fetal liver) from wild-type mice or from mutant or transgenic mice with specific genetic alterations of interest (50–52). Alternatively, (2) embryonic stem (ES) cell–derived cultured mast cells (ESCMCs) can be generated from wild-type or genetically altered ES cells (53,54), or (3) various mast cell populations can be transduced in vitro with shRNA to diminish expression of specific genes of interest (15,55). (4) Such bone marrow–, ES cell–, (or fetal liver–) derived cultured mast cells, or shRNA-transduced mast cells, can then be transplanted into mast cell–deficient c-kit mutant mice, such as WBB6F1-KitW/KitW-v mice (52,56) or C57BL/ 6-KitW-sh/KitW-sh mice (57,58), or into C57BL/6-Cpa3-Cre;Mcl-1fl/fl mice (59) (which we informally refer to as “Hello Kitty mice”, which have wild-type c-kit), to produce mast cell knock-in mice. Note: Mouse bone marrow–derived cultured mast cells (BMCMCs) can be injected into genetically mast cell-deficient mice intravenously (i.v.), intraperitoneally (i.p.), or intradermally (i.d.), or into the joints or meninges, etc., but there is a more limited experience with the engraftment of other types of MCs, such as EMCMCs, than with BMCMCs. (5) A suitable interval is then allowed for engraftment and phenotypic “maturation” of the adoptively transferred mast cells (the length of this interval can be varied based on the route of mast cell transfer, the anatomical site of interest, the particular biological response being analyzed, etc.). The importance of mast cell function(s) in biological responses can be analyzed by comparison of the responses in the appropriate wild-type or littermate control mice (6), the corresponding mutant mast cell-deficient mice (7), and selectively mast cell–engrafted mutant mice (mast cell knock-in mice) (8). The contributions of specific mast cell products (surface structures, signaling molecules, secreted products, and so on) to such biological responses can be analyzed by comparing the features of the responses of interest in mast cell knock-in mice engrafted with wild-type mast cells versus mast cells derived from mice or ES cells that lack or express genetically altered forms of such products or that have been transduced with shRNA to silence the specific genes that encode these products. An important part of the analysis of the mast cell knock-in mice used in particular experiments is to assess the numbers and anatomic distribution, and, for certain experiments, aspects of the phenotype, of the adoptively-transferred mast cells, as, depending on the type of in vitro–derived mast cells used, the route of administration, and other factors, these may differ from those of the corresponding native populations of mast cells in the corresponding wild type mice (14,60,61). [This is a modified version of Figure 2 from Metz M, Grimbaldeston MA, Nakae S, et al. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev 2007;217:304-28 (ref. 60), reprinted with the permission of the publisher, John Wiley and Sons.]
In addition to using the original mast cell–deficient WBB6F1-KitW/KitW-v mice to prepare mast cell knock-in mice, this approach also can be employed using C57BL/6-KitW-sh/KitW-sh mice, which have the advantage of being inbred, fertile, and not anemic (57,58,62). We recently showed that this approach also can be pursued using C57BL/6-Cpa3-Cre+-Mcl-1fl/fl mice, which are mast cell–deficient (and also have substantially diminished numbers and function of basophils) due to the lineage-restricted ablation of the anti-apoptotic factor, myeloid cell leukemia 1 (Mcl-1), in lineages with sufficiently high expression of the Cpa3 gene (59). Because the latter mice are mast cell–deficient but, unlike WBB6F1-KitW/KitW-v or C57BL/6-KitW-sh/KitW-sh mice, have normal c-kit, we informally call them “Hello Kitty” mice (59) (Figure 1).
NEW MODELS FOR ANALYZING THE FUNCTIONS OF MAST CELLS AND BASOPHILS
In addition to the models described above, those interested in the biology of mast cells, basophils, or their mediators are now fortunate to have a large number of additional models to choose from, including other lines of mice that exhibit constitutive or inducible deficiencies in populations of mast cells or in basophils, or which constitutively or inducibly lack various mast cell mediators or other molecules (61). As reviewed in detail elsewhere (61,63,64), each of the various models currently available has features that must be kept in mind when interpreting the results of studies using such mice, and the importance of particular mast cell (or basophil) roles in individual biological responses may vary both according to the details of the model used to study that biological response (e.g., whether one is studying a “weak” or “strong” model of that response) and based on the strain background of the mice. Accordingly, we have recommended that investigators consider using more than one type of genetic model to investigate the functions and importance of mast cells (or basophils) and/or their individual products in biological responses in vivo (61).
RESULTS
Identifying a Beneficial Role for Mast Cells in Enhancing Innate Resistance to Venoms
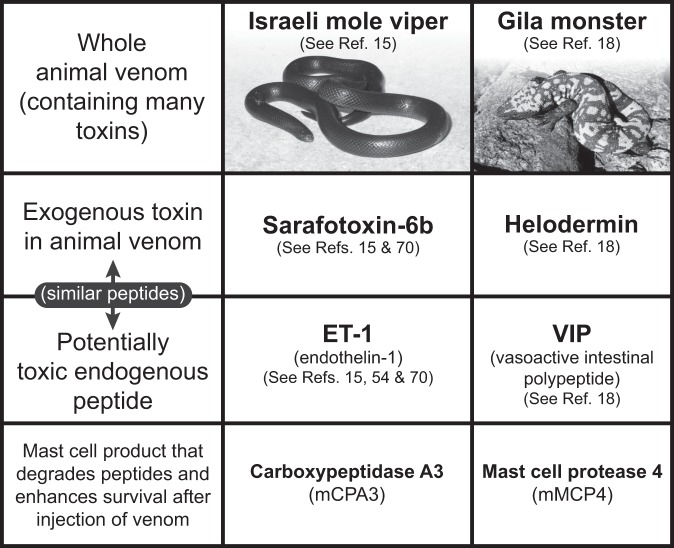
Early work by Higginbotham et al suggested that mast cells might be able to diminish the toxicity of certain venoms by degranulating and releasing heparin in response to venom exposure (65,66). However, this work was conducted before the description of mice deficient in mast cells or their individual mediators, so the importance of the roles of mast cells and their products in innate resistance to venoms could not be investigated more definitively in vivo. One step in that direction was the finding that ET-1 can initiate a homeostatic mechanism whereby proteases released by mast cells activated by ET-1 can degrade that vasoactive peptide and thereby diminish its potential toxicity in vivo (54). Using mast cell knock-in C57BL/6-KitW-sh/KitW-sh mice engrafted with ETA receptor-deficient or WT mast cells derived in vitro from ETA-/- or ETA-/+ embryonic stem cells, Maurer et al. found that mast cell activation by ET-1 via the ETA receptor contributed to this effect (54).
A homology search revealed that ET-1 was structurally similar to sarafotoxin 6b, one of the major toxins in the venom of the Israeli mole viper (Atractaspis engaddensis) (67). Sarafotoxin 6b can induce activation of cells in envenomated animals by binding to endothelin receptors (68). Metz et al. showed that mast cells not only diminished the toxicity of sarafotoxin 6b and enhanced the survival of mice injected with that peptide, but also did so in mice injected with the whole venom of A. engaddensis (15). Testing the ability of mast cells to influence responses to whole venoms is important, since snakes (and arthropods) do not envenomate their prey with a single toxin but with a complex mixture of toxins that can induce pathology by different mechanisms (69). Our initial pharmacological studies suggested that chymase was the critical mast cell protease in this setting (54). However, later work by our group, using both pharmacological approaches and shRNA knock down of CPA3 in adoptively transferred mast cells (15), as well as elegant studies by Schneider et al., who exploited a mouse they created which expressed only a catalytically inactive CPA3 (70), indicated that CPA3 is the key mast cell–derived protease that detoxifies both ET-1 and sarafotoxin 6b. Using mast cell knock-in mice, Metz et al. also provided evidence that mast cells were important in substantially enhancing the innate resistance of mice to honeybee (Apis mellifera) venom and to the venoms of two North American pit vipers, the western diamondback rattlesnake (Crotalus atrox) and the southern copperhead (Agkistrodon contortrix contortrix) (15).
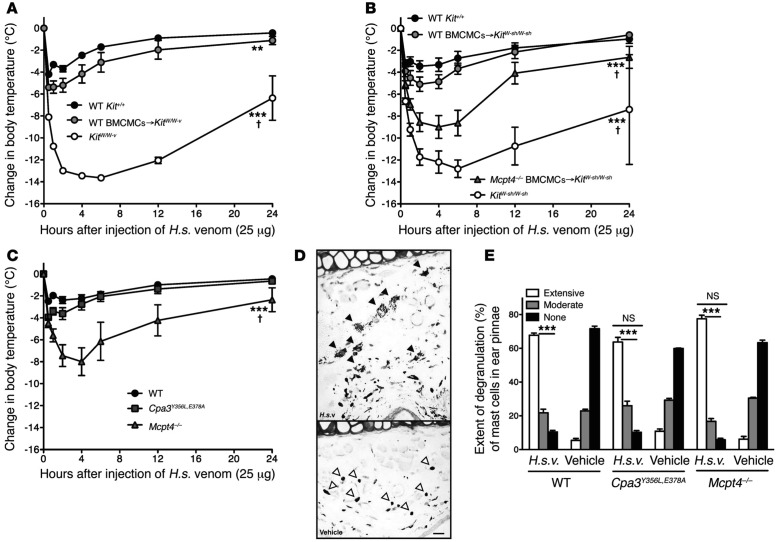
A project led by Mitsuteru Akahoshi and Chang Ho Song then analyzed whether mast cells might enhance innate resistance to another pair of biologically active peptides, the endogenous mammalian peptide VIP and the structurally similar peptide helodermin (also known as exendin-2), which is one of the toxins present in the venom of the Gila monster (Heloderma suspectum) (18). Testing of both mast cell knock-in mice (including C57BL/6-KitW-sh/KitW-sh mice engrafted with WT versus chymase [mMCP4]–deficient mast cells) (Figures 2A and 2B) and mice which had mast cells but were genetically deficient in mMCP4 (71) or CPA3 (70) or produced a catalytically inactive CPA3 (70) (Figure 2C), showed that mast cells could diminish the toxicity of VIP, helodermin, and the whole venom of H. suspectum, and that this was largely or wholly dependent on mast cell–derived mMCP4 rather than CPA3 (18). Similar approaches were used to provide evidence that mast cells and mMCP4 can contribute to enhanced innate resistance of mice to the venoms of two scorpions, one from the old world, the Deathstalker (Leiurus quiquestriatus hebraeus), and one from the new world, the Arizona bark scorpion (Centruroides exilicauda) (18).
Fig. 2.
Mast cells can diminish Heloderma suspectum venom (H.s.v.)–induced hypothermia and mortality through mast cell protease 4–dependent mechanisms. Changes in rectal temperatures after intradermal injection of H.s.v. (25 mg in 20 ml Dulbecco Modified Eagle Medium [DMEM] solution) into the ear pinnae (one ear pinna of each mouse) of: (A) wild-type (WT) WBB6F1-Kit+/+, mast cell-deficient WBB6F1-KitW/W-v, and WT BMCMCs→KitW/W-v mice (i.e., WBB6F1-KitW/W-v mice which had been engrafted, 6 to 8 weeks before venom challenge, in one ear pinna with 2 million bone marrow–derived cultured mast cells (BMCMCs) derived from WT WBB6F1-Kit+/+ mice) (the death rates of Kit+/+, WT BMCMCs→KitW /W-v, and KitW /W-v mice within 24 hours after H.s.v. injection were 0% [0/21], 7% [1/15, P = 0.42 vs. Kit+/+ mice], and 65% [13/20, P <0.0001 vs. Kit+/+ mice], respectively); (B) WT C57BL/6-Kit+/+, mast cell–deficient C57BL/6-KitW-sh/W-sh, WT BMCMCs→KitW-sh/W-sh, and Mcpt4-/- BMCMCs→KitW-sh/W-sh mice (the death rates of Kit+/+, WT BMCMCs→KitW-sh/W-sh, Mcpt4-/- BMCMCs→KitW-sh/W-sh, and KitW-sh/W-sh mice within 24 hours after H.s.v. injection were 5% [1/19], 11% [2/18, P = 0.48 vs. Kit+/+ mice], 43% [6/14, P = 0.01 vs. Kit+/+ mice], and 50% [10/20, P = 0.006 vs. Kit+/+ mice], respectively); or (C) WT C57BL/6-Kit+/+ mice, C57BL/6-Cpa3Y356L,E378A mice (which have a catalytically inactive CPA3) and C57BL/6-Mcpt4-/- mice (the death rates of Kit+/+, Cpa3Y356L,E378A, and Mcpt4-/- mice within 24 hours after H.s.v. injection were 7% [1/15], 0% [0/14, P = 0.52 vs. Kit+/+ mice], 40% [6/15, P = 0.007 vs. Kit+/+ mice], respectively). Each figure shows data pooled from at least three independent experiments with each group of mice (n = 2-5 mice per group per each individual experiment). **P <0.01, ***P <0.001 versus WT WBB6F1-Kit+/+ or WT C57BL/6-Kit+/+ mice; †P <0.01~0.001 versus each other group (A–C). (D) Extensive degranulation of mast cells (some indicated by closed arrowheads) 1 hour after intradermal injection of H.s.v. (25 mg in 20 ml DMEM), but not vehicle (DMEM) alone (mast cells without evidence of degranulation are indicated by open arrowheads) in WT C57BL/6 mice (Toluidine blue stain; scale bar: 50 µm). (E) Degranulation of mast cells 60 minutes after intradermal injection of H.s.v. (25 mg in 20 ml DMEM) or vehicle (DMEM) alone in WT C57BL/6, Mcpt4-/-, or Cpa3Y356L,E378A mice (injection was into one ear pinna of each mouse). ***P <0.001 versus corresponding vehicle-injected groups; NS = not significant (P >0.05) versus values for WT mice. [This is a reproduction of Figure 1 from Akahoshi M, Song CH, Piliponsky AM, et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J Clin Invest 2011;121:4180-91 (reference 18), reprinted with the permission of the publisher, the American Society for Clinical Investigation.]
It is possible that future work will reveal that mast cell activation can increase rather than decrease the toxicity of some types of venoms. However, our initial evidence indicated that mast cells can increase the innate resistance and enhance the survival of mice upon their first exposure to the venoms of 3 species of poisonous snakes, the Gila monster, the honeybee, or two especially dangerous scorpions. Moreover, mast cells contain at least two different proteases, CPA3 and chymase (mMCP4), which permit mast cells to respond, after their activation via cognate receptors that can bind either the endogenous or the structurally similar reptile-derived peptides, to high and potentially toxic levels of ET-1 and VIP, respectively, as well as to high levels of the similar peptides contained in the reptile venoms (Israeli mole viper sarafotoxin 6b and Gila monster helodermin, respectively) (Figure 3). By undergoing degranulation and releasing proteases that can inactivate potentially toxic endogenous peptides or peptides in venoms, mast cells can help to restore homeostasis, albeit while also enhancing features of the ensuing local and perhaps systemic inflammatory responses.
Fig. 3.
Mast cells can enhance innate resistance to high levels of endogenous peptides and structurally similar peptides in reptile venoms. Mast cell cytoplasmic granules contain proteases such as carboxypeptidase A3 (CPA3 [mCPA3 = mouse CPA3]) and mast cell protease 4 (MCP4 [mMCP4 = mouse MCP4]) that, upon secretion by activated mast cells, can degrade certain endogenous peptides, such as endothelin-1 (ET-1) and vasoactive intestinal polypeptide (VIP), respectively, as well as structurally similar peptides contained in the venoms of poisonous reptiles, such as sarafotoxin 6b in the venom of the Israeli mole viper (Atractaspis engaddensis) and helodermin in the venom of the Gila monster (Heloderma suspectum). The ability of mast cells to be activated to degranulate by components of venoms such as these, which can act at the same receptors which recognize the corresponding structurally similar endogenous peptides, permits mast cells to release proteases that can reduce the toxicity of these peptides and which help to enhance the survival of mice injected with the whole venoms of these reptiles, that contain many toxins in addition to sarafotoxin 6b and helodermin. This mechanism may also permit mast cells to restore homeostasis in settings associated with markedly increased levels of the endogenous peptides. [This is a reproduction, in modified form, of Figure 4 from Galli SJ. Rous-Whipple Award Lecture. The mast cell-IgE paradox: from homeostasis to anaphylaxis. Am J Pathol 2016;186:212-24 (reference 42), reprinted with the permission of the publisher, Elsevier, for the American Society for Investigative Pathology.]
Depending on the mammalian species, mast cells can contain several tryptases and chymases of distinct substrate specificity, as well as CPA3 (13,61,72). This raises the possibility that one of the reasons that the mast cells of various species contain several different proteases in their cytoplasmic granules is so that these cells, which are positioned in large numbers in the skin, the most common site of envenomation, are equipped to release a panel of proteases with the potential to degrade a variety of structurally distinct toxic compounds contained in animal venoms. Mast cells might also contribute to innate resistance to venoms in other ways, such as by increasing local vascular permeability and thereby favoring the interstitial access of circulating molecules that can antagonize the effects of venom proteases (73) and other toxins.
IgE Antibodies Can Contribute to Host Defense Against Arthropod and Reptile Venoms
Many animals and some humans experience multiple episodes of envenomation by arthropods such as bees, wasps, and scorpions, or by various reptiles. Such envenomation not only provokes an innate inflammatory response and pathology related to the biological activities of the venoms’ toxins (74–76), but also can induce allergic sensitization associated with the development of specific IgE antibodies that recognize components of the venoms (77–82). In some unfortunate people, such IgE responses to venoms put these individuals at risk to develop potentially fatal episodes of anaphylaxis (3,7,23,82). But recent findings suggest, in accord with Profet’s “toxin hypothesis of allergy,” that this same “allergic” mechanism — involving IgE and mast cells — also can enhance host resistance to venoms.
Honeybee (Apis mellifera) venom consists of a mixture of cytolytic peptides (e.g., melittin), enzymes (e.g., phospholipase A2 [PLA2; considered the main allergen in bee venom]), hyaluronidase, neurotoxins, and bioactive amines (74), and accounts for a large fraction of venom allergies in humans (82). The venom of the Russell’s viper (Daboia russelii), one of the most dangerous snakes in the Indian subcontinent (83), is a complex mixture of growth factors and enzymes with pro-coagulant and neurotoxic activities (76). We found that, in mice, IgE-associated type 2 immune responses against honeybee venom or Russell’s viper venom (RVV) were able to increase significantly host resistance to challenge with potentially lethal doses of those venoms (84).
This was unexpected because both IgE and immunoglobulin G1 (IgG1) antibodies produced during type 2 immune responses can orchestrate anaphylaxis and other allergic reactions in mice (7,23,85,86) and because type 2 immune responses against venoms (that include the development of anti-venom IgG1 [in mice] and IgE antibodies) are classically thought to exacerbate the outcome of subsequent venom exposure (77–82,87). By contrast, immunoglobulin G (IgG) class antibodies raised against animal venoms (or the Fab fragments of such anti-venom antibodies) are used to treat envenomated humans or animals (88).
So, it was important to identify which antibodies contributed to the enhanced resistance to honeybee venom observed in mice with type 2 immune responses to that venom. Our evidence showed that IgE antibodies were the critical elements of the acquired host resistance to honeybee or RVV. We found that 1) most or all of the acquired resistance induced in mice by a single exposure to honeybee venom was transferable to naïve mice with only 250 µl of serum from honeybee venom–immunized mice; 2) when such “immune serum” was depleted of functionally active IgE either by adding a neutralizing antibody to IgE (35,89) or by heating (56o C, 1 hour, which eliminates the ability of IgE to bind to FcεRI and induce passive cutaneous anaphylaxis [90] while the function of other antibody classes, including IgG1, is not affected) (91), the immune serum’s ability to transfer enhanced resistance to naïve mice was essentially lost; and 3) such immune serum failed to transfer enhanced venom resistance to mice lacking either the IgE antibody-binding α chain of the FcεRI or the γ chain of FcεRI that is necessary for signaling initiated by aggregation of the receptor (5,6).
We also found that 1) genetically IgE-deficient mice (85) could not develop acquired immunity to honeybee venom, even though they developed a robust IgG1 antibody response to the venom; 2) immune serum from WT mice could passively transfer enhanced resistance to honeybee venom to naïve IgE-deficient mice, unless such immune serum was first treated to neutralize IgE or impair its ability to bind to FcεRI; and 3) naïve genetically mast cell–deficient C57BL/6-KitW-sh/KitW-sh or C57BL/6-Cpa3-Cre+-Mcl-1fl/fl mice which received immune serum from honeybee venom–immunized C57BL/6 WT mice actually exhibited worse survival after challenge with a high dose of honeybee venom than did mast cell–deficient mice which had received serum from phosphate-buffered saline (PBS) mock-immunized C57BL/6 WT mice (84). The latter finding suggested that mast cells can contribute to IgE-mediated acquired resistance to honeybee venom, as well as enhance the innate resistance of mice to a first exposure to that venom (15). Independently of our work, Palm et al. showed that mice immunized with the major allergen contained in honeybee venom, bee venom phospholipase A2 (bvPLA2), exhibited enhanced resistance to the ability of bvPLA2 to induce hypothermia upon its injection into mice, and provided evidence that this enhanced immunity required B cells and was diminished significantly in mice which lacked the IgE-binding α chain of the FcεRI (92). Taken together, these two initial studies (85,92) support the hypothesis that one physiological function of IgE is to protect the host against noxious substances.
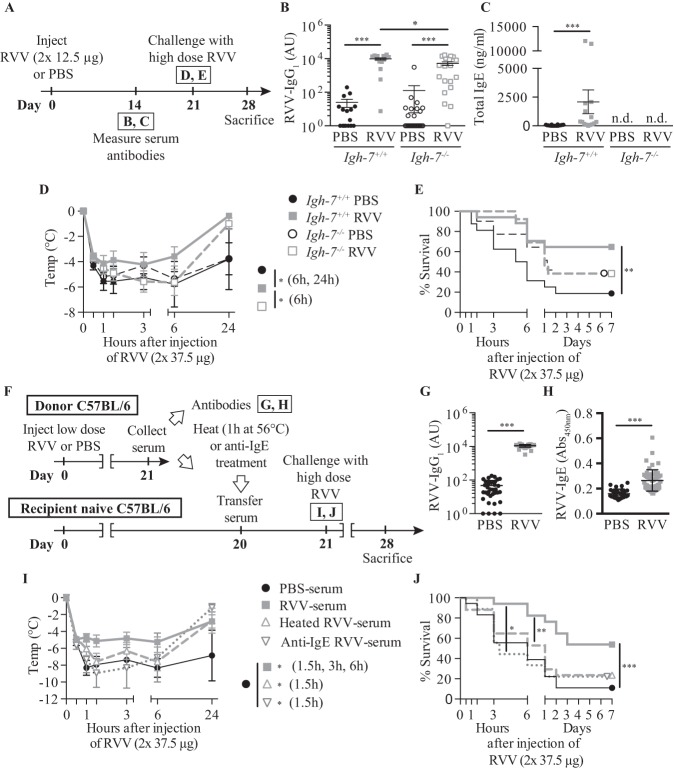
Subsequently, we found that the acquired enhanced resistance to RVV which we observed in mice that had developed type 2 immune responses to that venom (84) also was highly dependent on IgE (Figure 4) and FcεRI (see Figures 4A-4E in ref. 93), and could be effectively transferred by immune serum into normal mice (see Figures 3F-3J in ref. 93) but not into C57BL/6-Cpa3-Cre;Mcl-1fl/fl mice which were genetically markedly deficient in mast cells and which also had diminished numbers of basophils (see Figures 4G and 4H in Starkl et al.) (93). Notably, two different types of genetically mast cell–deficient mice also exhibited significantly diminished innate resistance to the toxicity and lethality of RVV (see Figures 6A-6C and 6E and 6F in Starkl et al.) (93), supporting Higginbotham’s hypothesis that mast cells can contribute to enhanced innate resistance to this venom (65). Compared to the corresponding mast cell–sufficient mice, such naïve mast cell–deficient mice also exhibited many fewer attempts to scratch sites of RVV injection (see Figures 6D and 6G in Starkl) (93). The latter finding supports the idea proposed both by Stebbings (40) and Profet (39) that elements of allergic responses, in this case, mast cells, can confer benefit to hosts experiencing attacks by arthropods (40) or other sources of toxins (39) by having effects which permit the host to become aware of the threat and which also prompt behaviors that would help to eliminate it.
Fig. 4.
Evidence that immunoglobulin E (IgE) antibodies contribute to acquired enhanced resistance to the toxicity and lethality of Russell’s viper venom (RVV). (A) Outline of experiments with IgE-deficient (Igh-7-/-) and control (Igh-7+/+) C57BL/6 mice (B-E). (B,C) Serum RVV-specific immunoglobulin G1 (IgG1) (B) and total IgE (C). (D) Body temperature. (E) Survival. (F) Outline of serum transfer experiments in C57BL/6 mice (G–J). (G) Serum RVV-specific IgG1. (H) Serum total IgE. (I) Body temperature. (J) Survival. Data were pooled from three to four experiments (n = 9-25/group). P values: Mann-Whitney test (B, C, G, H), Student t test (D, I) and Mantel-Cox test (E, J). Abbreviation: PBS, phosphate-buffered saline. [This is a reproduction of Figure 3 from Starkl P, Marichal T, Gaudenzio et al. IgE antibodies, FcεRIα and IgE-mediated local anaphylaxis can limit snake venom toxicity. J Allergy Clin Immunol 2016;137:246-57.e11. (reference 93), reprinted with the permission of the publisher, Elsevier.]
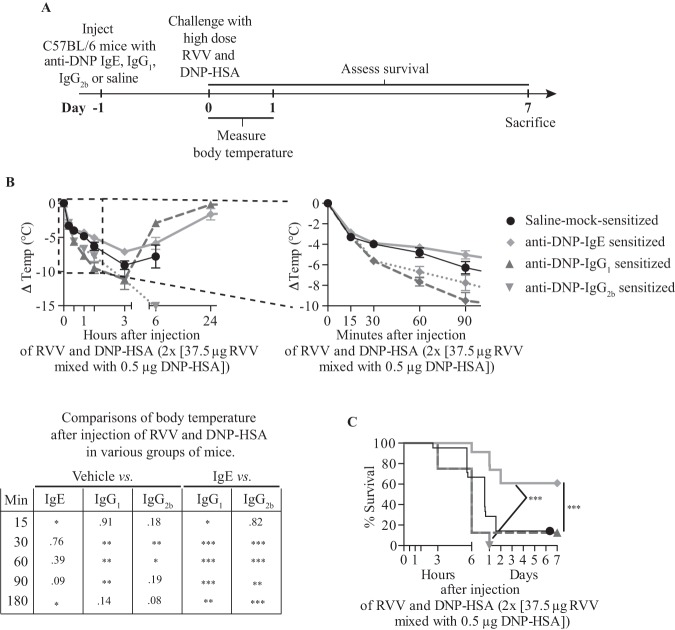
As noted above, Palm et al. reported that immunization of mice with honeybee venom–derived bvPLA2, which represents approximately 10% of the dry weight of whole BV (74), can reduce the toxicity-related hypothermia induced by subsequent challenge with a high dose of the same allergen in an antibody- and FcεRIα-dependent manner (92). However, it was not clear whether an IgE response to a single constituent of an animal venom would be able to enhance resistance to the entire group of toxins contained in that venom. To investigate this, we passively sensitized WT mice locally against dinitrophenylated human serum albumin (DNP-HSA) by subcutaneous injections of anti-DNP IgE (94) (or with anti-DNP IgG1 or IgG2b as controls), or mock-sensitized the mice with saline, then challenged the animals subcutaneously at the same site 24 hours later by injecting a mixture of RVV and DNP-HSA (Figure 5A). We used amounts of anti-DNP IgE and DNP-HSA which were able to induce a local increase in vascular permeability at the DNP-HSA injection site without resulting in systemic hypothermia, and showed that the amount of DNP-HSA used did not by itself influence the toxicity of RVV (see Figure E5 in the Online Repository of Starkl et al.) (93).
Fig. 5.
Immunoglobulin E (IgE)–dependent local mast cell activation induced by activation with a single antigen can enhance resistance to the lethality of Russell’s viper venom (RVV). (A) Experimental outline. (B) Body temperature and (C) Survival of C57BL/6 mice treated with 3 subcutaneous injections of saline alone or containing 50 ng anti-dinitrophenyl (anti-DNP) IgE, IgG1 or IgG2b antibody and challenged 18 hours later with 2 subcutaneous injections, each containing 37.5 µg RVV and 0.5 µg dinitrophenylated human serum albumin (DNP-HSA). Data were pooled from two to five independent experiments (n = 10 to 25/group). P values: Student t test (B); Mantel-Cox test (C). Abbreviation: PBS, phosphate-buffered saline.[This is a reproduction of Figure 5 from Starkl P, Marichal T, Gaudenzio et al. IgE antibodies, FcεRIα and IgE-mediated local anaphylaxis can limit snake venom toxicity. J Allergy Clin Immunol 2016;137:246-57.e11. (reference 93), reprinted with the permission of the publisher, Elsevier.]
We found that pre-sensitization with anti-DNP IgE significantly increased the resistance of C57BL/6 (Figures 5B and 5C) or BALB/c (see Figures E5 and 5H-5I in the online repository of Starkl et al.) (93) mice to challenge with a potentially lethal amount of RVV admixed with DNP-HSA (93). However, pre-sensitization of C57BL/6 mice with anti-DNP IgG1 or IgG2b, DNP-specific IgG isotypes with the capacity to activate effector cells via Fcγ receptors (95), not only failed to increase protection but also resulted in increased hypothermia at early time points compared to vehicle-treated or IgE-sensitized mice (Figure 5B) (93). These findings show that local tissue responses mediated by IgE and antigen can enhance host resistance against RVV even when that antigen is not a native constituent of the venom, and are consistent with the general idea that the host needs only to generate an IgE response against a limited number of the components of a complex venom (perhaps as few as one component) to manifest enhanced acquired resistance to that venom.
CONCLUSIONS
Tissue resident cells with morphological, biochemical, and functional properties of mammalian mast cells, and which can produce histamine, heparin, and serine proteases, are present in tunicates, whose ancestors appeared in evolution before the development of adaptive immunity (96,97). Such tunicates also have been reported to have cells resembling basophils (98). After the appearance of acquired immunity and the development of antibodies, these ancient hematopoietic lineages acquired the ability to bind immunoglobulins such as IgE (in mammals) to their surface. This allowed such tissue-resident cells to become “immunologically primed” or “sensitized” to undergo activation for mediator release upon encountering relatively small amounts of the antigen identified by their surface-bound IgE antibodies. The most extreme example of an IgE-associated immune response resulting in the activation of mast cells (and basophils) is fatal anaphylaxis, in which the rapid, systemic, and extensive release of mediators stored in these FcεRI-bearing effector cells results in a catastrophic and quickly lethal outcome.
Observational and epidemiological studies in humans, as well as studies in experimental animals (including those using mice genetically deficient in mast cells, basophils, or IgE), strongly suggest that one beneficial role of IgE, mast cells, and basophils is to help to defend the host against ectoparasites such as ticks, and to diminish the numbers of parasites and burden of disease in mammals infected with certain helminths [reviewed in Mukai et al (99)]. However, in addition to being infected with parasites, vertebrates also have been subjected to evolutionary pressure through millions of years of co-evolution with venomous arthropods, reptiles, and other species. Evidence from studies in mice indicates that mast cells can enhance innate resistance of mice to four species of poisonous snakes, the Gila monster, 2 species of scorpions, and the honeybee; and that mast cell proteases (specifically, CPA3 and the chymase MCP4) can contribute to such mast cell–dependent innate defenses by degrading toxins present in some of these venoms. Moreover, type 2 immune responses induced by a single exposure to honeybee venom or RVV, which “arm” mast cells with IgE antibodies that bear specificity for components of those venoms, can significantly increase the survival of such mice to challenge with doses of the venoms which would be lethal in naïve mice (84).
This evidence supports the notion (39,40,41) that key elements of allergic reactivity, including mast cells and IgE, indeed can importantly enhance innate and acquired host resistance to venoms. Yet much work remains to be done to answer several related, but unresolved, questions. These include: 1) In addition to releasing proteases, are there other mechanisms by which mast cells can contribute to enhanced resistance to venoms during innate or acquired immune responses [e.g., ex vivo studies indicate that mast cell-derived heparin, that is highly anionic, can bind and thereby reduce the toxicity of cationic toxins in RVV (65)]; 2) In what ways do venoms induce Th2 cell and IgE responses (for honeybee venom, this appears to involve a pathway by which products of bvPLA2 acting on host lipid membranes induce IL-33 production, which in turn can activate ILC2 cells to release cytokines that drive IgE production (92); 3) During vertebrate evolution, what has been the relative importance of exposure to ectoparasites (and the pathogens for which they serve as vectors), infection with helminths and other parasites, and interactions with venomous animals in shaping the features, function and immunological roles of mast cells, basophils, and IgE? (99); 4) Given that mast cells and basophils cooperate to enhance acquired resistance to the feeding of certain ticks, and that the hematophagous fluids produced by tick salivary glands can contain peptides similar to those in venoms (100), is there a role for basophils in enhancing resistance to some venoms?; and 5) Why do some subjects develop such severe IgE-dependent reactivity to venom that they are at risk for fatal anaphylaxis (an outcome far from a protective immune response)?
Our initial findings indicate that, in mice, the propensity to develop protective versus potentially harmful type 2 immune responses to venoms can depend on the genetic background of the animal, the type and amount of venom to which the animal is exposed, and/or the frequency of such venom exposures (93). But this is only the beginning of addressing this important issue. In considering this question, it should be noted that many people who develop type 2 immune responses to honeybee venom do not exhibit anaphylactic reactivity despite having venom-specific IgE antibodies (101). Also, there is abundant evidence that Th2 cell–mediated responses are subject to immune regulation which can diminish pathology related to IgE-dependent reactivity to the inducing antigen, including honeybee venom (102–104). One might speculate that such immune regulation of type 2 immune responses ideally would reduce the pathology associated with these responses while preserving their ability to confer enhanced protection when the elicited antigen is a toxin.
ACKNOWLEDGMENTS
We thank the past and current members of the Galli lab and the many collaborators who have made important contributions to the work reviewed herein.
Footnotes
Potential Conflicts of Interest: The work reviewed herein was supported by grants to Dr. Galli from the National Institutes of Health (e.g., R37 AI23990, R01 CA072074, R01 AR067145, and U19 AI104209) and the National Science Foundation, and from several other funding sources, including the Department of Pathology at Stanford University. Dr. Starkl was supported by a Max Kade Fellowship of the Max Kade Foundation and the Austrian Academy of Sciences, a Schroedinger Fellowship of the Austrian Science Fund (FWF): J3399-B21, and a Marie Curie fellowship of the European Commission (H2020-MSCA-IF-2014), 655153. Dr. Marichal was supported by a Marie Curie International Outgoing Fellowship for Career Development: European Union’s Seventh Framework Programme (FP7-PEOPLE-2011-IOF), 299954, and a “Charge de recherches” fellowship of the Belgian National Fund for Scientific Research (F.R.S-FNRS).
DISCUSSION
Billings, Baton Rouge: As you probably are aware, one of our eminent members, who is not here, is Craig Kitchens from Gainesville and he is a highly trained clotting doctor and does a lot of his work with venom. And he is somewhat the Jay Leno of our association. So as I was listening to your paper I was thinking my wife Susan and I have a camp which is 50 miles away from Baton Rouge in the southernmost part of Mississippi where pit vipers are not uncommon. I have 8 grandchildren between the ages of 7 months and 10 years and there are large timber rattlers there and I’m curious to know whether I should keep available CroFab® as an agent to use should one of my short people get bitten. The nearest emergency room, which is a small town emergency room, is about 15 minutes away and they do have CroFab® there.
Galli, Stanford: Okay, that’s a very good question....in fact, I just saw a recent report that the incidence of pit viper bites among children is increasing in the United States. Having said that, number 1, over the United States in any given year, there are usually less than 10 fatalities related to snake bites. Number 2, one has to determine which snake bite victims need to be treated with CroFab®. CroFab® consists of the Fab fragments derived from a mixture of 4 separate monospecific IgG antibodies. However, the company states that that these antibody Fab fragments have substantial clinical effectiveness when used to treat envenomation by many types of rattlesnakes, cottonmouths/water moccasins, and copperheads. While CroFab® thus can be effective in those envenomated by a variety of different pit vipers, a consensus document recommends that it be withheld unless the bite has induced swelling that is “more than minimal” and is progressing, or there is an elevated prothrombin time, decreased fibrinogen and decreased platelets, or there are any systemic signs [Lavonas EJ, Ruha A-M, Banner W, et al. Unified treatment algorithm for the management of crotaline snakebite in the United States: results of an evidence-informed consensus workshop. BMC Emergency Medicine 2011;11:2 http://www.biomedcentral.com/1471-227X/11/2.] This is because, as I mentioned, sometimes when venomous snakes bite, they actually deliver little or no venom. We don’t know where in its tiny brain this information has been processed, but it is thought that the snake in some way knows it can’t swallow and consume a human, even a child, and so they may “decide not to waste” their venom in fending off such a perceived (inedible) threat. So usually, in the emergency room, bitten subjects with minimal or no signs and symptoms related to the bite will be carefully observed over several hours and if the bite is considered to have been “dry” (i.e., no venom was injected) or only mild signs and symptoms have developed, the patient won’t receive CroFab®. One reason not to use it in subjects with relatively mild envenomation is that a small proportion of subjects develop anaphylactoid reactions upon the first administration of the agent, and some subjects, after receiving CroFab®, will develop IgE antibodies to the foreign Fab fragments. This of course would put such “sensitized” individuals at risk to develop a serious anaphylactic reaction if they needed to receive the agent again. In a worst case, such subjects might even succumb to anaphylaxis induced by the treatment rather than to the toxic effects of the venom alone. Also, CroFab® is rather expensive. So, for these reasons, the consensus document recommends that CroFab® be administered only in cases of substantial envenomation. In addition, should an anaphylactoid or anaphylactic reaction develop upon injection of CroFab®, that complication would be managed more effectively in the ER than in the field. I am a pathologist and have not directly cared for people bitten by venomous snakes. However, because of the considerations noted, including the possibility of needing to treat an anaphylactoid or anaphylactic reaction to CroFab®, I think that transporting a snake bite victim to the emergency room generally is the better approach rather than attempting to treat the person in the field; and in any event, before administering CroFab®, one first should determine whether the bite has resulted in clinical evidence of substantial envenomation.
Billings, Baton Rouge: It’s my understanding that CroFab® is a sheep-based antibody whereas previously antibodies were from horse serum.... right down the road about 2 weeks ago a rattle snake bit a neighbor’s dog and the neighbor had CroFab® right there and gave it to his dog within 5 minutes and the dog died in 20 minutes.... the dog is the size of a grandchild.
Galli, Stanford: You are correct that CroFab® is a mixture of Fab fragments derived from sheep IgG antibodies. If the dog had previously been treated with CroFab®, it is possible that CroFab®–induced anaphylaxis contributed to its death. Was that the first time the dog got the anti-venom? [Billings, Baton Rouge: It was...yes.]
Galli, Stanford: Your questions prompt me to make another point related to the role of mast cells and IgE in enhancing innate and acquired resistance to venom. These are not the only mechanisms that can increase host resistance to venoms — because of the long period of coevolution of venomous reptiles with their prey (and also with predators that eat poisonous snakes), many mechanisms of enhanced resistance to venom components have developed. For example, the Virginia opossum is a predator of copperheads; they seek out, attack, and eat them. Opossums apparently show no behavioral adaptations to avoid being bitten, and they are often bitten. However, work by Sharon Jansa and Robert Voss provided evidence of rapid evolution of von Willebrand factor (vWF) in opossums that prey on pit vipers [Jansa SA, Voss RS. Adaptive evolution of the venom-targeted vWF protein in opossums that eat pitvipers. PLoS ONE 2011;6: e20997. doi:10.1371/journal.pone.0020997]. Specifically, such opossums have mutations in vWF that make it less able to bind botrocetin, a C-type lectin-like protein found in the venom of the South American pit viper, Bothrops jararaca. This reduces the toxicity of botrocetin, which contributes to bleeding by binding with the A1 domain of vWF and enhancing its affinity for platelet glycoprotein Ibα, thereby inducing platelet aggregation and thrombocytopenia. Such C-type lectin-like proteins are found in the venoms of many pit vipers, including the copperhead. This is just one example of many different mechanisms which have been identified that can enhance host resistance to the toxicity of components of various venoms.
Metcalfe, Bethesda: It’s good to see another mast cell lecture after 3 years here. So there aren’t mast cell–deficient humans [Galli, Stanford: There may have been one but it’s very rare.] So, is there any clinical evidence to suggest mast cell activation after a venom bite? In other words, elevated typtase or chymase or any of these things that may or may not have a role in the defense against a particular venom?
Galli, Stanford: You mean is there evidence of mast cell activation after envenomation? [Metcalf, Bethesda: Correct.] Well I am not aware that it’s been proven through laboratory tests in envenomated people, such as the detection of elevated levels of tryptase in the person’s blood. However, we and others have documented that many venoms can induce extensive mast cell degranulation in vivo in mice and other mammals, especially at the site of envenomation. Many venoms also can induce mast cell degranulation in vitro. So, I think that it is very likely that many venoms can induce mast cell degranulation in humans in vivo, either directly or indirectly, for example. via the activation of complement and the generation of anaphylatoxins such as C3a and C5a. [Note added in proof by Galli, Stanford: Statistically significant elevations in plasma levels of mast cell–derived tryptase have been reported in patients who had been envenomated by the Russell’s viper (vs. levels in healthy control subjects) and who were tested before administration of a horse-serum derived anti-venom, but the levels remained in the high end of the normal range; these envenomated subjects also exhibited marked elevation in blood levels of C3a and C5a, which can induce degranulation of human mast cells. However, mast cell tryptase levels increased further after anti-venom treatment, especially in those whose post-anti-venom reactions met the criteria of anaphylaxis [Stone SF, Isbister GK, Shahmy S, et al. Immune response to snake envenoming and treatment with antivenom; complement activation, cytokine production and mast cell degranulation. PLoS Negl Trop Dis. 2013;7:e2326. doi: 10.1371/journal.pntd.0002326]. In this context, one might ask: if mast cells already have the innate ability to release proteases in response to venom, why go through the trouble of generating an IgE response to the venom? We showed that mouse mast cells that bear on their surface IgE which recognizes bee venom components degranulated in vitro in response to a 100-fold lower concentration of bee venom than the amount needed to induce mast cell degranulation by innate, IgE-independent, mechanisms. So, we think that one of the reasons mammals have evolved the ability to produce an IgE response to venoms is that this permits mast cells to undergo activation, including the release of proteases which can reduce the toxicity of venom components, when they are exposed to relatively low concentrations of the venom. Such low concentrations of venom would occur, for example, at sites distant from the site of envenomation, as a result of the distribution of venom components via the circulation. So, if one of the main jobs of mast cells in the host response to venoms is rapidly to release proteases which degrade the venom components, then this could happen more quickly and would more likely become a systemic response if the envenomated animal has, bound to the surface of its mast cells, IgE that recognizes components of the venom.
Sacher, Cincinnati: I was originally born and raised in South Africa; of course, we have a lot of snakes there and we were always told that the clinical features of the snake envenomations were either cytotoxic, in other words locally hematotoxic, hemolytic, or neuro-toxic. Now I realize of course you’ve shown that there are different distributional functions of the mast cells but is there any correlation between those clinical spectra and perhaps mast cell activation?
Galli, Stanford: I am not sure about the relationship of mast cell activation to the clinical spectra observed in those envenomated by snakes with mainly hemolytic vs. neurotoxic venoms, but your question raises the general point of the potential role of mast cells in host defense against neurotoxic as opposed to hemolytic venoms. In this context, one of the mast cell–independent mechanisms that evolved as a defense against neurotoxic venoms (and this has been reported in the mongoose, the honey badger, and the hedgehog, and even in the domestic pig) is to have mutations in the genes encoding receptors that are targeted by constituents of neurotoxic venoms — the effect of such mutations being to reduce the ability of such venom components to bind to the receptors. For example, snake venom alpha-neurotoxins (like alpha-bungarotoxin, a component of the venom of the Taiwanese banded krait (Bungarus multicinctus)), competitively bind to nicotinic acetylcholine receptors at neuromuscular junctions, which can result in paralysis, respiratory failure, and death. The animals that I just mentioned, each of which can prey on and survive the bites of poisonous snakes with neurotoxic venoms, have independently evolved mutations in the toxin-binding sites of those receptors which diminish their ability to bind such toxins [Drabeck DH, Dean AM, Jansa SA. Why the honey badger doesn’t care: Convergent evolution of venom-targeted nicotinic acetylcholine receptors in mammals that survive venomous snake bites. Toxicon 2015;99:68–72]. So, returning to the mast cell, it is possible that mast cells may be more or less important in enhancing resistance to particular venoms depending on whether the snake has predominantly hemotoxic versus neurotoxic venoms. Indeed, when we challenged normal and mast cell–deficient mice with the venom of Naja naja (the Indian cobra often used by snake charmers, whose venom contains neurotoxic components), the results of our pilot experiments indicated that mast cells had neither a protective nor a detrimental effect on the mortality induced by that venom.
Michael Gershon, New York City: I wonder why mast cells, when they put out all those things they put out including much protease — they are not just degrading the venom but they are doing other things at the same time such as anaphylaxis which is counterproductive. So, I’m curious as to why you think that happens? I’ve thought evolution might have adapted a simpler way of just getting out one of the agents that it needed for this particular stimulus.
Galli, Stanford: So, Mike, one can break your comment into two different questions: Can mast cells selectively release different mediators? The general answer to that is a qualified “probably yes.” but that’s a whole other story. However, cytoplasmic granules that store proteases also store other mediators, such as histamine, that can contribute to the signs and symptoms of anaphylaxis. So, if the release of stored proteases is needed to combat venom toxins, then there also is the risk, with extensive mast cell degranulation, of inducing anaphylaxis. But another question is, or I would interpret your comment to raise this question: Is anaphylaxis always bad for you? First, let’s consider the proposition that you will die if you’ve been envenomated with a certain amount of venom and you don’t detoxify the venom. If that is true, then the next question is: What risk would the envenomated animal be willing to take to make sure that sufficient venom is degraded to prevent death due to envenomation? If the venom is being distributed systemically, then one could argue that you would also want the mast cell proteases to be distributed systemically, i.e., the mast cell degranulation should be extensive and systemic: this typically will result in the signs and symptoms of anaphylaxis. So our speculation is that, across the entire population of animals at risk of envenomation, anaphylaxis may actually be beneficial in cases of extensive envenomation; of course as long as you survive it....
Billings, Baton Rouge: (after showing slide of snakes) So you can see my concern for my grandchildren.
Galli, Stanford: Do you know the “5 Ts” which are said to characterize the typical snake bite victim?: Testosterone — it’s usually a man; Tequila — there often is alcohol involved [Billings, Baton Rouge: I have six grandsons — that’s probably a problem] ....T-shirts, Trucks, and Teeth — lack thereof. [Note added in proof by Galli, Stanford (who looked it up in Google): sometimes two more “Ts” are added to the list: Tattoos and Teasing the snake.]
REFERENCES
- 1.Pawankar R, Canonica GW, Holgate ST, et al. Allergic diseases and asthma: a major global health concern. Curr Opin Allergy Clin Immunol. 2012;12:39–41. doi: 10.1097/ACI.0b013e32834ec13b. [DOI] [PubMed] [Google Scholar]
- 2.Paul WE, Zhu J, et al. How are TH2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–35. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galli SJ, Tsai M, et al. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulendran B, Artis D, et al. New paradigms in type 2 immunity. Science. 2012;337:431–5. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinet JP, et al. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–72. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 6.Rivera J, Fierro NA, Olivera A, et al. New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oettgen HC, Geha RS, et al. IgE in asthma and atopy: cellular and molecular connections. J Clin Invest. 1999;104:829–35. doi: 10.1172/JCI8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karasuyama H, Mukai K, Obata K, et al. Nonredundant roles of basophils in immunity. Annu Rev Immunol. 2011;29:45–69. doi: 10.1146/annurev-immunol-031210-101257. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan BM, Liang HE, Bando JK, et al. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–35. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voehringer D, et al. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. 2013;13:362–75. doi: 10.1038/nri3427. [DOI] [PubMed] [Google Scholar]
- 11.Kawakami T, Kitaura J, et al. Mast cell survival and activation by IgE in the absence of antigen: a consideration of the biologic mechanisms and relevance. J Immunol. 2005;175:4167–73. doi: 10.4049/jimmunol.175.7.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyce JA, et al. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev. 2007;217:168–85. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 13.Douaiher J, Succar J, Lancerotto L, et al. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv Immunol. 2014;122:211–52. doi: 10.1016/B978-0-12-800267-4.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galli SJ, Kalesnikoff J, Grimbaldeston MA, et al. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–86. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 15.Metz M, Piliponsky AM, Chen CC, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–30. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 16.Dawicki W, Marshall JS, et al. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007;19:31–8. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Abraham SN, St. John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–52. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akahoshi M, Song CH, Piliponsky AM, et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J Clin Invest. 2011;121:4180–91. doi: 10.1172/JCI46139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galli SJ, Borregaard N, Wynn TA, et al. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–44. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen BM, Frandsen PM, Raaby EM, et al. Molecular and stimulus-response profiles illustrate heterogeneity between peripheral and cord blood-derived human mast cells. J Leukoc Biol. 2014;95:893–901. doi: 10.1189/jlb.0712354. [DOI] [PubMed] [Google Scholar]
- 21.McNeil BD, Pundir P, Meeker S, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519:237–41. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portier MM, Richet C, et al. De l’action anaphylactique de certains venims. C R Soc Biol. 1902;54:170–2. [Google Scholar]
- 23.Finkelman FD, et al. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–15. doi: 10.1016/j.jaci.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 24.Stetson DB, Voehringer D, Grogan JL, et al. Th2 cells: orchestrating barrier immunity. Adv Immunol. 2004;83:163–89. doi: 10.1016/S0065-2776(04)83005-0. [DOI] [PubMed] [Google Scholar]
- 25.Finkelman FD, Shea-Donohue T, Morris SC, et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–55. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 26.Fitzsimmons CM, Dunne DW, et al. Survival of the fittest: allergology or parasitology? Trends Parasitol. 2009;25:447–51. doi: 10.1016/j.pt.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Spencer LA, Porte P, Zetoff C, et al. Mice genetically deficient in immunoglobulin E are more permissive hosts than wild-type mice to a primary, but not secondary, infection with the filarial nematode. Brugia malayi Infec Immun. 2003;71:2462–7. doi: 10.1128/IAI.71.5.2462-2467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz C, Turqueti-Neves A, Hartmann S, et al. Basophil-mediated protection against gastrointestinal helminths requires IgE-induced cytokine secretion. Proc Natl Acad Sci U S A. 2014;111:E5169–77. doi: 10.1073/pnas.1412663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nawa Y, Kiyota M, Korenaga M, et al. Defective protective capacity of W/Wv mice against Strongyloides ratti infection and its reconstitution with bone marrow cells. Parasite Immunol. 1985;7:429–38. doi: 10.1111/j.1365-3024.1985.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 30.Knight PA, Wright SH, Lawrence CE, et al. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–56. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuta T, Kikuchi T, Iwakura Y, et al. Protective roles of mast cells and mast cell-derived TNF in murine malaria. J Immunol. 2006;177:3294–302. doi: 10.4049/jimmunol.177.5.3294. [DOI] [PubMed] [Google Scholar]
- 32.Maurer M, Lopez Kostka S, Siebenhaar F, et al. Skin mast cells control T cell-dependent host defense in Leishmania major infections. FASEB J. 2006;20:2460–7. doi: 10.1096/fj.06-5860com. [DOI] [PubMed] [Google Scholar]
- 33.Ohnmacht C, Voehringer D, et al. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010;184:344–50. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 34.Arizono N, Kasugai T, Yamada M, et al. Infection of Nippostrongylus brasiliensis induces development of mucosal-type but not connective tissue-type mast cells in genetically mast cell-deficient Ws/Ws rats. Blood. 1993;81:2572–8. [PubMed] [Google Scholar]
- 35.Amiri P, Haak-Frendscho M, Robbins K, et al. Anti-immunoglobulin E treatment decreases worm burden and egg production in Schistosoma mansoni-infected normal and interferon gamma knockout mice. J Exp Med. 1994;180:43–51. doi: 10.1084/jem.180.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newlands GF, Miller HR, MacKellar A, et al. Stem cell factor contributes to intestinal mucosal mast cell hyperplasia in rats infected with Nippostrongylus brasiliensis or Trichinella spiralis, but anti-stem cell factor treatment decreases parasite egg production during N brasiliensis infection. Blood. 1995;86:1968–76. [PubMed] [Google Scholar]
- 37.Holgate ST, Polosa R, et al. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–30. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 38.Artis D, Maizels RM, Finkelman FD, et al. Forum: Immunology: allergy challenged. Nature. 2012;484:458–9. doi: 10.1038/484458a. [DOI] [PubMed] [Google Scholar]
- 39.Profet M, et al. The function of allergy: immunological defense against toxins. Q Rev Biol. 1991;66:23–62. doi: 10.1086/417049. [DOI] [PubMed] [Google Scholar]
- 40.Stebbings JH. Immediate hypersensitivity: a defense against arthropods? Perspect Biol Med. 1974;17:233–9. doi: 10.1353/pbm.1974.0027. [DOI] [PubMed] [Google Scholar]
- 41.Palm NW, Rosenstein RK, Medzhitov R, et al. Allergic host defences. Nature. 2012;484:465–72. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galli SJ, et al. Rous-Whipple Award Lecture. The mast cell-IgE paradox: from homeostasis to anaphylaxis. Am J Pathol. 2016;186:212–24. doi: 10.1016/j.ajpath.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chabot B, Stephenson DA, Chapman VM, et al. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–9. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 44.Geissler EN, Ryan MA, Housman DE, et al. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–92. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 45.Kitamura Y, Go S, Hatanaka K, et al. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–52. [PubMed] [Google Scholar]
- 46.Russell ES, et al. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- 47.Harrison DE, Astle CM, et al. Population of lymphoid tissues in cured W-anemic mice by donor cells. Transplantation. 1976;22:42–6. doi: 10.1097/00007890-197607000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Nakano T, Waki N, Asai H, et al. Lymphoid differentiation of the hematopoietic stem cell that reconstitutes total erythropoiesis of a genetically anemic W/Wv mouse. Blood. 1989;73:1175–9. [PubMed] [Google Scholar]
- 49.Nakano T, Waki N, Asai H, et al. Different repopulation profile between erythroid and nonerythroid progenitor cells in genetically anemic W/Wv mice after bone marrow transplantation. Blood. 1989;74:1552–6. [PubMed] [Google Scholar]
- 50.Nabel G, Galli SJ, Dvorak AM, et al. Inducer T lymphocytes synthesize a factor that stimulates proliferation of cloned mast cells. Nature. 1981;291:332–4. doi: 10.1038/291332a0. [DOI] [PubMed] [Google Scholar]
- 51.Galli SJ, Dvorak AM, Marcum JA, et al. Mast cell clones: a model for the analysis of cellular maturation. J Cell Biol. 1982;95:435–44. doi: 10.1083/jcb.95.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakano T, Sonoda T, Hayashi C, et al. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985;162:1025–43. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai M, Wedemeyer J, Ganiatsas S, et al. In vivo immunological function of mast cells derived from embryonic stem cells: an approach for the rapid analysis of even embryonic lethal mutations in adult mice in vivo. Proc Natl Acad Sci U S A. 2000;97:9186–90. doi: 10.1073/pnas.160254997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maurer M, Wedemeyer J, Metz M, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–6. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 55.Piliponsky AM, Chen CC, Nishimura T, et al. Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis. Nat Med. 2008;14:392–8. [Google Scholar]
- 56.Galli SJ, Kitamura Y, et al. Genetically mast-cell-deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo. Am J Pathol. 1987;127:191–8. [PMC free article] [PubMed] [Google Scholar]
- 57.Grimbaldeston MA, Chen CC, Piliponsky AM, et al. Mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolters PJ, Mallen-St Clair J, Lewis CC, et al. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient KitW-sh/KitW-sh sash mice. Clin Exp Alergy. 2005;35:82–8. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lilla JN, Chen CC, Mukai K, et al. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–8. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Metz M, Grimbaldeston MA, Nakae S, et al. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304–28. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 61.Galli SJ, Tsai M, Marichal T, et al. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv Immunol. 2015;126:45–127. doi: 10.1016/bs.ai.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nigrovic PA, Gray DH, Jones T, et al. Genetic inversion in mast cell-deficient Wsh mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173:1693–701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown MA, Hatfield JK, et al. Mast cells are important modifiers of autoimmune disease: with so much evidence, why is there still controversy? Front Immunol. 2012;3:147. doi: 10.3389/fimmu.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodewald HR, Feyerabend TB, et al. Widespread immunological functions of mast cells: fact or fiction? Immunity. 2012;37:13–24. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Higginbotham RD, et al. Mast cells and local resistance to Russell’s viper venom. J Immunol. 1965;95:867–75. [PubMed] [Google Scholar]
- 66.Higginbotham RD, Karnella S, et al. The significance of the mast cell response to bee venom. J Immunol. 1971;106:233–40. [PubMed] [Google Scholar]
- 67.Kloog Y, Ambar I, Sokolovsky M, et al. Sarafotoxin, a novel vasoconstrictor peptide: phosphoinositide hydrolysis in rat heart and brain. Science. 1988;242:268–70. doi: 10.1126/science.2845579. [DOI] [PubMed] [Google Scholar]
- 68.Kochva E, Bdolah A, Wollberg Z, et al. Sarafotoxins and endothelins: evolution, structure and function. Toxicon. 1993;31:541–68. doi: 10.1016/0041-0101(93)90111-u. [DOI] [PubMed] [Google Scholar]
- 69.Fry BG, et al. From genome to “venome”: molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res. 2005;15:403–20. doi: 10.1101/gr.3228405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schneider LA, Schlenner SM, Feyerabend TB, et al. Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J Exp Med. 2007;204:2629–39. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tchougounova E, Pejler G, Abrink M, et al. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J Exp Med. 2003;198:423–31. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wernersson S, Pejler G, et al. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014;14:478–94. doi: 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- 73.Neves-Ferreira AG, Perales J, Fox JW, et al. Structural and functional analyses of DM43, a snake venom metalloproteinase inhibitor from Didelphis marsupialis serum. J Biol Chem. 2002;277:13129–37. doi: 10.1074/jbc.M200589200. [DOI] [PubMed] [Google Scholar]
- 74.Habermann E, et al. Bee and wasp venoms. Science. 1972;177:314–22. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 75.Mukherjee AK, Ghosal SK, Maity CR, et al. Some biochemical properties of Russell’s viper (Daboia russelli) venom from Eastern India: correlation with clinico-pathological manifestation in Russell’s viper bite. Toxicon. 2000;38:163–75. doi: 10.1016/s0041-0101(99)00125-7. [DOI] [PubMed] [Google Scholar]
- 76.Risch M, Georgieva D, von Bergen M, et al. Snake venomics of the Siamese Russell’s viper (Daboia russelli siamensis) — relation to pharmacological activities. J Proteomics. 2009;72:256–69. doi: 10.1016/j.jprot.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 77.Saelinger CB, Higginbotham RD, et al. Hypersensitivity responses to bee venom and the mellitin. Int Arch Allergy Appl Immunol. 1974;46:28–37. doi: 10.1159/000231110. [DOI] [PubMed] [Google Scholar]
- 78.Charavejasarn CC, Reisman RE, Arbesman CE, et al. Reactions of anti-bee venom mouse reagins and other antibodies with related antigens. Int Arch Allergy Appl Immunol. 1975;48:691–7. doi: 10.1159/000231356. [DOI] [PubMed] [Google Scholar]
- 79.Jarisch R, Yman L, Boltz A, et al. IgE antibodies to bee venom, phospholipase A, melittin and wasp venom. Clin Allergy. 1979;9:535–41. doi: 10.1111/j.1365-2222.1979.tb02518.x. [DOI] [PubMed] [Google Scholar]
- 80.Wadee AA, Rabson AR, et al. Development of specific IgE antibodies after repeated exposure to snake venom. J Allergy Clin Immunol. 1987;80:695–8. doi: 10.1016/0091-6749(87)90289-2. [DOI] [PubMed] [Google Scholar]
- 81.Annila I, et al. Bee venom allergy. Clin Exp Allergy. 2000;30:1682–7. doi: 10.1046/j.1365-2222.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- 82.Bilo BM, Rueff F, Mosbech H, et al. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339–49. doi: 10.1111/j.1398-9995.2005.00963.x. [DOI] [PubMed] [Google Scholar]
- 83.Simpson ID, Norris RL, et al. Snakes of medical importance in India: is the concept of the “big 4” still relevant and useful? Wilderness Environ Med. 2007;18:2–9. doi: 10.1580/06-weme-co-023r1.1. [DOI] [PubMed] [Google Scholar]
- 84.Marichal T, Starkl P, Reber LL, et al. A beneficial role for immunoglobulin E in host defense against honeybee venom. Immunity. 2013;39:963–75. doi: 10.1016/j.immuni.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oettgen HC, Martin TR, Wynshaw-Boris A, et al. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–70. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 86.Miyajima I, Dombrowicz D, Martin TR, et al. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and FcγRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–14. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reimers AR, Weber M, Muller UR, et al. Are anaphylactic reactions to snake bites immunoglobulin E-mediated? Clin Exp Allergy. 2000;30:276–82. doi: 10.1046/j.1365-2222.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- 88.Meier J, et al. Commercially available antivenoms (“hyperimmune sera,” “antivenins,” “antisera”) for antivenom therapy. In: Meier, editor. Handbook of Clinical Toxicology of Animal Venoms and Poisons. Boca Raton, Florida: CRC Press; 1995. pp. 689–721. [Google Scholar]
- 89.Haak-Frendscho M, Saban R, Shields RL, et al. Anti-immunoglobulin E antibody treatment blocks histamine release and tissue contraction in sensitized mice. Immunology. 1998;94:115–21. doi: 10.1046/j.1365-2567.1998.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prouvost-Danon A, Binaghi RA, Abadie A, et al. Effect of heating at 56 degrees C on mouse IgE antibodies. Immunochemistry. 1977;14:81–4. doi: 10.1016/0019-2791(77)90284-1. [DOI] [PubMed] [Google Scholar]
- 91.Strait RT, Morris SC, Finkelman FD, et al. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and FcγRIIb cross-linking. J Clin Invest. 2006;116:833–41. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palm NW, Rosenstein RK, Yu S, et al. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39:976–85. doi: 10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Starkl P, Marichal T, Gaudenzio N, et al. IgE antibodies, FcεRIα and IgE-mediated local anaphylaxis can limit snake venom toxicity. J Allergy Clin Immunol. 2016;137:246–57. doi: 10.1016/j.jaci.2015.08.005. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu FT, Bohn JW, Ferry EL, et al. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980;124:2728–37. [PubMed] [Google Scholar]
- 95.Bruhns P, et al. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–9. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 96.Cavalcante MC, de Andrade LR, Du Bocage Santos-Pinto C, et al. Colocalization of heparin and histamine in the intracellular granules of test cells from the invertebrate Styela plicata (Chordata-Tunicata) J Struct Biol. 2002;137:313–21. doi: 10.1016/s1047-8477(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 97.Wong GW, Zhuo L, Kimata K, et al. Ancient origin of mast cells. Biochem Biophys Res Commun. 2014;451:314–8. doi: 10.1016/j.bbrc.2014.07.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Barros CM, Andrade LR, Allodi S, et al. The Hemolymph of the ascidian Styela plicata (Chordata-Tunicata) contains heparin inside basophil-like cells and a unique sulfated galactoglucan in the plasma. J Biol Chem. 2007;282:1615–26. doi: 10.1074/jbc.M604056200. [DOI] [PubMed] [Google Scholar]
- 99.Mukai K, Tsai M, Starkl P, et al. IgE and mast cells in host defense against parasites and venoms. Semin Immunopathol. 2016;38:581–603. doi: 10.1007/s00281-016-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fry BG, Roelants K, Champagne DE, et al. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annu Rev Genomics Hum Genetics. 2009;10:483–511. doi: 10.1146/annurev.genom.9.081307.164356. [DOI] [PubMed] [Google Scholar]
- 101.Antonicelli L, Bilo MB, Bonifazi F, et al. Epidemiology of hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341–6. doi: 10.1097/00130832-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 102.Muller UR, et al. Bee venom allergy in beekeepers and their family memberws. Curr Opin Allergy Clin Immunol. 2005;5:343–7. doi: 10.1097/01.all.0000173783.42906.95. [DOI] [PubMed] [Google Scholar]
- 103.Meiler F, Zumkehr J, Klunker S, et al. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205:2887–98. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ozdemir C, Kucuksezer UC, Akdis M, et al. Mechanisms of immunotherapy to wasp and bee venom. Clin Expl Allergy. 2011;41:1226–34. doi: 10.1111/j.1365-2222.2011.03812.x. [DOI] [PubMed] [Google Scholar]