Abstract
OBJECTIVES:
To assess hospital differences in empirical antibiotic use, bacterial epidemiology, and antimicrobial susceptibility for common antibiotic regimens among young infants with urinary tract infection (UTI), bacteremia, or bacterial meningitis.
METHODS:
We reviewed medical records from infants <90 days old presenting to 8 US children’s hospitals with UTI, bacteremia, or meningitis. We used the Pediatric Health Information System database to identify cases and empirical antibiotic use and medical record review to determine infection, pathogen, and antimicrobial susceptibility patterns. We compared hospital-level differences in antimicrobial use, pathogen, infection site, and antimicrobial susceptibility.
RESULTS:
We identified 470 infants with bacterial infections: 362 (77%) with UTI alone and 108 (23%) with meningitis or bacteremia. Infection type did not differ across hospitals (P = .85). Empirical antibiotic use varied across hospitals (P < .01), although antimicrobial susceptibility patterns for common empirical regimens were similar. A third-generation cephalosporin would have empirically treated 90% of all ages, 89% in 7- to 28-day-olds, and 91% in 29- to 89-day-olds. The addition of ampicillin would have improved coverage in only 4 cases of bacteremia and meningitis. Ampicillin plus gentamicin would have treated 95%, 89%, and 97% in these age groups, respectively.
CONCLUSIONS:
Empirical antibiotic use differed across regionally diverse US children’s hospitals in infants <90 days old with UTI, bacteremia, or meningitis. Antimicrobial susceptibility to common antibiotic regimens was similar across hospitals, and adding ampicillin to a third-generation cephalosporin minimally improves coverage. Our findings support incorporating empirical antibiotic recommendations into national guidelines for infants with suspected bacterial infection.
In infants <90 days of age, concern for bacterial meningitis, bacteremia, and urinary tract infection (UTI) is a common reason for emergency care and hospitalization, and a driver of empirical antibiotic use.1 These patients often present with fever alone, and because of the infants’ immature immune system and risk for serious complications, they need urgent evaluation.1 To prevent delays in appropriate treatment, patients with concern for bacterial infection often receive antibiotics before bacterial culture results are available.2 Although mounting evidence has informed diagnostic evaluation strategies for this population, large-scale, regionally diverse data are still needed to develop national recommendations for empirical antimicrobial use.3–6
Treatment with a third-generation cephalosporin targets common pathogens such as Escherichia coli, group B Streptococcus (GBS), and most other Gram-negative rods. Ampicillin plus gentamicin is also a common empirical regimen that has the potential stewardship benefits of avoiding a third-generation cephalosporin,7 with the caveat that gentamicin does not penetrate well into the cerebrospinal fluid (CSF).8 The addition of ampicillin to an empirical regimen increases coverage of rare or resistant organisms, such as Listeria monocytogenes and Enterococcus spp. Little is known about geographic differences in the epidemiology of these organisms in infants, although recent studies suggest that L monocytogenes infections are increasingly rare.9,10
Fostering judicious use of antibiotics has been identified as a priority in the United States as a means to limit the spread of antibiotic resistance, medical complications, caregiver stress, and costs.11,12 The main objective of this study was to describe empirical antibiotic use, bacterial epidemiology, and antibiotic susceptibility patterns for the most common pathogens in infants <90 days old with confirmed bacterial infection across 8 regionally diverse US children’s hospitals. Secondarily, we sought to assess how often common empirical antimicrobial regimens are concordant with the antimicrobial susceptibility for the isolated pathogens.
Methods
Study Design and Setting
We conducted a multicenter retrospective cohort study of infants <90 days old without substantial comorbidities who were evaluated in the emergency department (ED) of 8 US children’s hospitals between July 1, 2012 and June 30, 2014, and had culture confirmation of a bacterial infection of the urine, blood, or CSF. The study included freestanding pediatric academic medical centers that contribute data to the Pediatric Health Information System (PHIS); 2 are located in the South, 2 in the Midwest, 1 in the West, and 3 in the East.
The PHIS database contains administrative data from 44 participating hospitals and is managed by the Children’s Hospital Association and participating hospitals. It was used for initial patient identification, empirical antibiotic therapy, and patient demographics (age, sex, race, and insurance). Medical record review was conducted at each of the 8 study sites and was used for the verification of study eligibility and to obtain clinical data including presence of fever, medical comorbidities, bacterial pathogen, site, and pathogen susceptibility.
Study Population
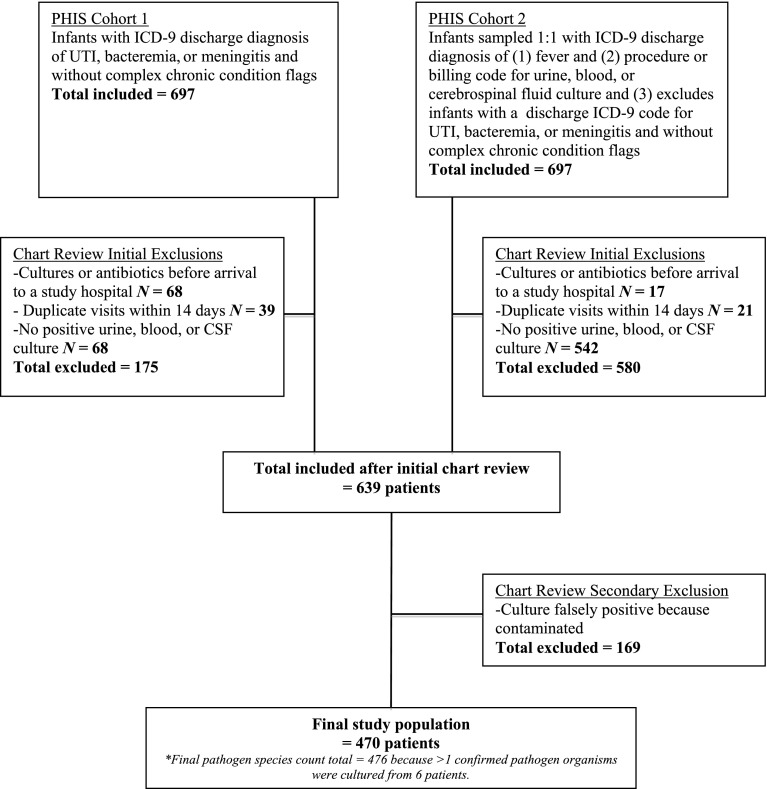
We used a previously published and validated method to identify the study cohort.13 We first identified potential cases by searching the PHIS database for all infants aged <90 days evaluated in the participating hospital ED and with an International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnosis indicative of UTI, bacteremia, or bacterial meningitis (Supplemental Information). Because some infants with UTI, bacteremia, or meningitis may have only a discharge diagnosis of fever, we also sampled an equal number of medical records of patients with an admission or discharge diagnosis ICD-9 code for fever and a billing code for culture of urine, blood, or CSF (Fig 1). We chose an equal number of medical records because it was not feasible to review all the medical records of all patients with an ICD-9 code for fever (n = 3076).
FIGURE 1.
Study population.
To ensure availability of microbiology data, we excluded patients transferred from another hospital as indicated by a PHIS transfer code or determined in narrative notes upon medical record review. Because the risk of bacterial infection and the management often differ in infants with many comorbid conditions, we excluded infants with ICD-9 diagnosis codes indicating a complex chronic condition by using Feudtner’s updated list of ICD-9 codes that indicate a disease that is likely to last >12 months or lead to death (eg, congenital heart disease, chronic lung disease, and prematurity).14 Empirical antibiotics were defined as antibiotics given in the ED or on the first day of hospitalization (PHIS flag “day 0 or 1”).15
Medical record review was performed at each site via a standardized data collection form on REDCap, which is a secure, Web-based electronic data capture tool.16 Among infants with a positive culture, we excluded patients with cultures determined to be contaminants. Because hospitals differed in the clinical determination of contaminated cultures and range of reporting for the quantification of colony-forming units, we determined definitions for contaminated culture a priori based on national recommendations and research team consensus, which included 2 board-certified pediatric infectious disease experts (R.J.M. and A.L.M.). In general, a contaminant was defined as a urine culture with <10 000 colony-forming units, a urine culture with 10 000 to 100 000 colony-forming units if the urinalysis was negative for nitrite or leukocyte esterase with the exception of GBS, or a single isolate of a bacteria generally regarded as a contaminant in the urine, blood, or CSF that was not found in other cultures from the same patient (eg, a single isolate of a coagulase-negative Staphylococcus species from a blood culture) (Supplemental Information).17 Patients who were infected with >1 true pathogen according to these rules were included in multiple categories.
Analysis
Patient and pathogen characteristics were summarized for the entire sample and for each hospital. Pathogens isolated from multiple infection sites (eg, blood and urine) in the same patient were counted only once per patient. Infection sites were categorized by single site and by a combination of sites when present: UTI alone, UTI with bacteremia, bacteremia alone, bacteremia with meningitis, meningitis alone, and as a binary variable with categories UTI alone versus bacteremia or meningitis (with or without UTI). Gram-negative organisms with inducible B-lactamases (eg, Enterobacter, Citrobacter, and Serratia species) were grouped together for analysis. Patient age in days at ED presentation was categorized as ≤6 days, 7 to 28 days, 29 to 60 days, and 61 to 89 days.18 Results of in vitro antimicrobial resistance testing were categorized as susceptible, resistant, or intermediately susceptible, based on laboratory reports. Susceptibility to common empirical antibiotic regimens (third-generation cephalosporin with or without ampicillin, and gentamicin with ampicillin) was determined for each pathogen according to predefined criteria and in vitro susceptibility data (Supplemental Table 4).19 Gentamicin monotherapy was not considered effective empirical therapy for meningitis because of its poor penetration into CSF, whereas ampicillin plus gentamicin was considered to have activity for meningitis.20
Categorical variables were reported as frequencies with percentages. Comparisons across hospitals or age groups used Pearson’s χ2 test or Fisher’s exact test as appropriate for small counts. We compared frequencies of demographic variables, microbiological testing, infection source, individual pathogens, and use of common empirical parenteral antimicrobial regimens across hospitals. For each empirical regimen, we then compared antimicrobial susceptibility across hospitals for all tested isolates, followed by post hoc individual tests of susceptibility by hospital for categories of infection source; the post hoc tests were not adjusted for multiple comparisons. Because not all patients had isolates tested for susceptibility to every empirical regimen, we limited our susceptibility analyses to within-regimen tests where data were independent, because each patient was represented only once. A P < .05 was considered statistically significant. The study was approved by the institutional review board at each of the participating sites.
Results
The final study cohort, excluding those with contaminated cultures, included 470 infants with 476 identified pathogens. Patient characteristics and microbiological evaluations are summarized overall and across hospitals in Table 1. UTI alone was the most common infection identified (77%), followed by bacteremia alone (11%) and bacteremia with UTI (10%). There were 13 cases of meningitis (3%). Patient characteristics and diagnostic test use significantly varied across hospitals. However, the proportion of patients with a UTI alone versus more invasive infections (eg, bacteremia and meningitis with or without UTI) did not vary across hospitals (P = .85) (Table 1). Given the small number of children with bacterial meningitis, bacteremia and meningitis were grouped together as severe invasive bacterial infections for the purposes of some of our analysis.
TABLE 1.
Patient Characteristics, Testing, and Management
| All Patients | By Hospital | |||
|---|---|---|---|---|
| N = 470 | N = 8 | |||
| N | % (95% Confidence interval) | Minimum– Maximum, % | Pa | |
| Sex | <.01 | |||
| Male | 223 | 47 (43–52) | 31–67 | |
| Female | 247 | 53 (48–57) | 33–69 | |
| Age, d | .07b | |||
| 0–6 | 16 | 3 (2–5) | 0–7 | |
| 7–28 | 120 | 26 (22–30) | 18–35 | |
| 29–60 | 208 | 44 (40–49) | 31–62 | |
| 61–90 | 126 | 27 (23–31) | 11–40 | |
| Race | <.01 | |||
| Non-Hispanic white | 199 | 42 (38–47) | 5–55 | |
| Non-Hispanic black | 75 | 16 (13–20) | 0–30 | |
| Hispanic | 99 | 21 (17–25) | 0–51 | |
| Asian | 27 | 6 (4–8) | 0–21 | |
| Other | 59 | 13 (10–16) | 2–39 | |
| Unknown | 11 | 2 (1–4) | 0–15 | |
| Payer | <.01 | |||
| Private | 213 | 45 (41–50) | 29–67 | |
| Government | 224 | 48 (43–52) | 33–62 | |
| Other | 16 | 3 (2–5) | 0–9 | |
| Unknown | 17 | 4 (2–6) | 0–27 | |
| Microbiological testing obtainedc | .04 | |||
| Urine, blood, and CSF | 322 | 69 (64–73) | 56–85 | |
| Urine and blood | 125 | 27 (23–31) | 15–37 | |
| Urine only | 22 | 5 (3–7) | 0–11 | |
| Infection source | .69 | |||
| UTI | 362 | 77 (73–81) | 71–82 | |
| Bacteremia | 50 | 11 (8–14) | 7–17 | |
| Bacteremia and UTI | 45 | 10 (7–13) | 2–13 | |
| Meningitis and bacteremia | 7 | 1 (1–3) | 0–6 | |
| Meningitis | 5 | 1 (0–2) | 0–2 | |
| Meningitis, bacteremia, and UTI | 1 | 0 (0–1) | 0–2 | |
χ2 test of variable by hospital.
Age group 0–28 d by hospital.
One patient had blood culture testing without urine or CSF testing.
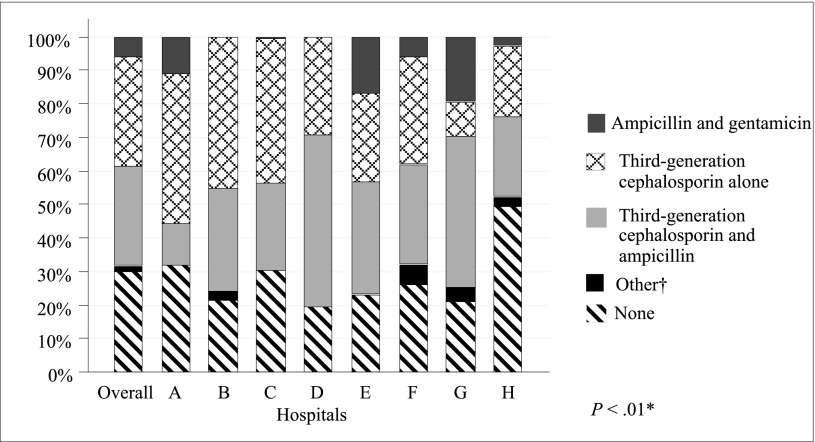
The most common empirical regimen used was a parenteral third-generation cephalosporin alone (43%; hospital range 39%–48%), followed by ampicillin plus a third-generation cephalosporin (39%; 35%–44%), and ampicillin plus gentamicin (8%; 6%–11%). Empirical antimicrobial treatment varied across hospitals (P < .01) (Fig 2).
FIGURE 2.
Empirical antibiotics by hospital. *Pearson’s χ2 test. †Includes other cephalosporin, amikacin, oxacillin, clindamicin, or ciproflaxin.
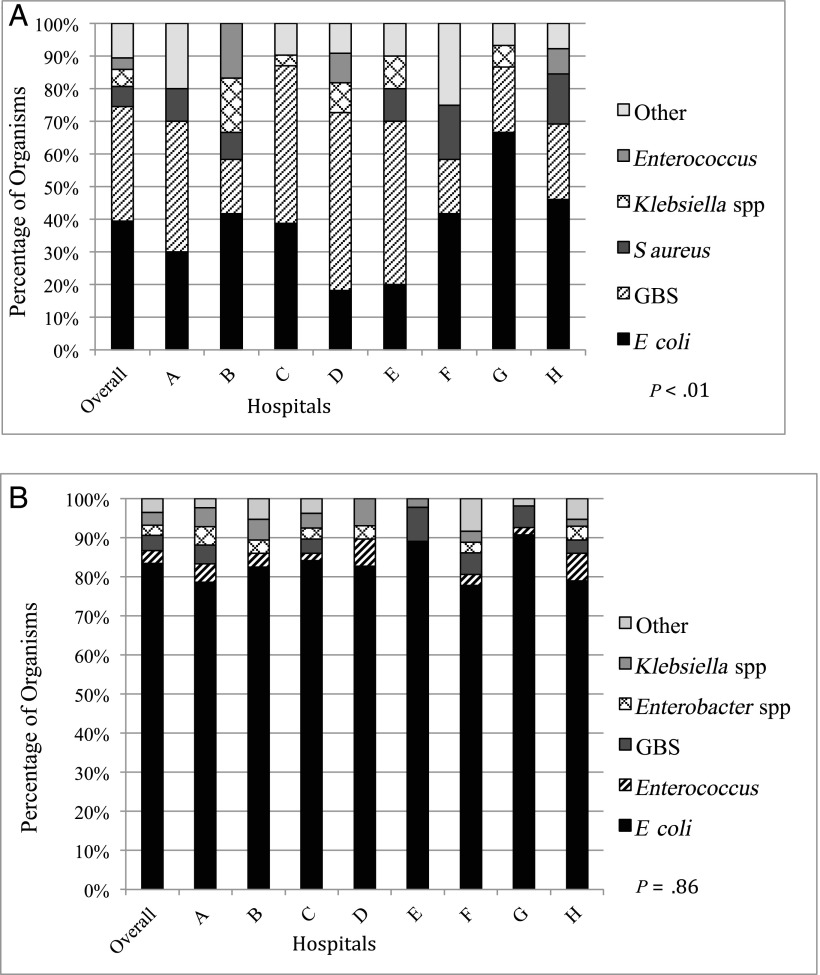
Of the 476 isolated organisms, the majority were E coli (74%; hospital range 66%–84%), Streptococcus agalactiae, also known as GBS (11%; 3%–16%), Klebsiella spp (4%; 2%–6%), Enterococcus spp (3%; 2%–8%), Enterobacter spp (2%; 0%–4%), Staphylococcus aureus (2%; 0%–5%), Citrobacter spp (1%; 0%–2%), and Streptococcus pneumoniae (1%; 0%–3%). Ten other organisms accounted for the remaining 2% of infections, and most of these organisms were Gram-negative bacteria. There were no cases of L monocytogenes identified in the study cohort. Across the 8 study sites, no significant difference was observed among UTI pathogens (P = .86) (Fig 3A). Differences existed in the specific organisms causing bacteremia and meningitis (P < .01) (Fig 3B). The 13 cases of meningitis were caused by GBS (N = 5), S pneumoniae (N = 2), E coli (N = 2), Klebsiella oxytoca (N = 1), Salmonella enteritidis (N = 1), Paenibacillus spp (N = 1), and S aureus (N = 1). Paenibacillus spp was identified as a pathogen because it was cultured from 2 separate sources from the same patient (blood and CSF).
FIGURE 3.
Bacterial epidemiology across hospitals by source. A, Blood and CSF pathogens, with and without UTI (n = 114). “Other” category includes S pneumoniae, Moraxella spp, Proteus mirabilis, S enteritidis, Paenibacillus, Citrobacter koseri, Staphylococcus lugdunensis, Haemophilus parainfluenzae, and Serratia marcescens. B, UTI pathogens (n = 428). Includes patients with cultures that were positive for >1 organism. “Other” category includes S aureus, S marcescens, Citrobacter, P mirabilis, Streptococcus sanguinis, and Neisseria gonorrhoeae.
When we tested for across-hospital variability in susceptibility to each of 3 common antimicrobial regimens, we found no statistically significant differences in any regimen for the overall sample or when testing separately for infection sources UTI alone or bacteremia and meningitis (Table 2). When testing was conducted separately by pathogen, we found statistically significant across-hospital differences for third-generation cephalosporin alone and third-generation cephalosporin plus ampicillin, but not for ampicillin plus gentamicin (Table 3). According to the in vitro susceptibilities, the use of gentamicin plus ampicillin would have empirically covered 95% of all isolates, including 96% of UTI and 92% of bacteremia or meningitis (with or without UTI). When patients were evaluated by age, the use of ampicillin and gentamicin or ampicillin and a third-generation cephalosporin appeared to improve antimicrobial coverage for the small number of 0- to 6-day-olds compared with a third-generation cephalosporin alone. However, the small sample size prevents a meaningful comparison for this age group. Only modest improvement in coverage was seen for 7- to 28-day-olds and even less for 29- to 89-day-olds.
TABLE 2.
Antimicrobial Susceptibility to Common Empirical Antibiotic Regimens by Infection Source
| Third-Generation Cephalosporin | Third-Generation Cephalosporin + Ampicillina | Gentamicin + Ampicillinb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients Susceptible Isolates | By Hospital | All Patients Susceptible Isolates | By Hospital | All Patients Susceptible Isolates | By Hospital | |||||||
| Nc | n (%) | Minimum–Maximum, % | Pb | Nc | n (%) | Minimum–Maximum, % | Pb | Nc | n (%) | Minimum–Maximum, % | Pb | |
| Overall, d | 467 | 418 (90) | 82–98 | .16 | 454 | 421 (93) | 87–98 | .35 | 455 | 433 (95) | 91–98 | .62 |
| 0–6 | 12 | 7 (58) | 0–100 | 9 | 8 (89) | 50–100 | 9 | 9 (100) | — | |||
| 7–28 | 117 | 104 (89) | 77–100 | 113 | 106 (94) | 80–100 | 114 | 102 (89) | 79–100 | |||
| 29–89 | 338 | 307 (91) | 84–100 | 332 | 307 (92) | 88–100 | 332 | 322 (97) | 93–100 | |||
| By infection sourcea | ||||||||||||
| UTI alone, d | ||||||||||||
| 0–89 | 365 | 330 (90) | 84–100 | .14 | 357 | 334 (94) | 86–100 | .23 | 358 | 344 (96) | 91–100 | .56 |
| 0–6 | 8 | 5 (63) | 0–100 | 6 | 6 (100) | — | 6 | 6 (100) | — | |||
| 7–28 | 74 | 67 (91) | 75–100 | 71 | 69 (97) | 80–100 | 72 | 65 (90) | 83–100 | |||
| 29–89 | 283 | 258 (91) | 84–100 | 280 | 259 (93) | 86–100 | 280 | 273 (98) | 91–100 | |||
| Bacteremia or meningitis, with or without UTI, d | ||||||||||||
| 0–89 | 102 | 88 (86) | 75–100 | .14 | 97 | 87 (90) | 79–100 | .22 | 97 | 89 (92) | 80–100 | .31 |
| 0–6 | 4 | 2 (50) | 0–100 | 3 | 2 (67) | 0–100 | 3 | 3 (100) | — | |||
| 7–28 | 43 | 37 (86) | 67–100 | 42 | 37 (88) | 67–100 | 42 | 37 (88) | 67–100 | |||
| 29–89 | 55 | 49 (89) | 50–100 | 52 | 48 (92) | 50–100 | 52 | 49 (94) | 50–100 | |||
Each pathogen counted only once per patient if >1 infection source.
P value from Pearson’s χ2 test of susceptibility × hospital for age <90 d.
Total number of cases with nonmissing data varies by empirical regimen.
—, no range given as all sites have equal percentages.
TABLE 3.
Antimicrobial Susceptibility to Common Empirical Antibiotic Regimens by Pathogen
| Third-Generation Cephalosporin | Third-Generation Cephalosporin + Ampicillina | Gentamicin + Ampicillinb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients Susceptible Isolates | By Hospital | All Patients Susceptible Isolates | By Hospital | All Patients Susceptible Isolates | By Hospital | |||||||
| Nc | n (%) | Minimum–Maximum, % | Pb | Nc | n (%) | Minimum–Maximum, % | Pb | Nc | n (%) | Minimum–Maximum, % | Pb | |
| Pathogena | ||||||||||||
| E coli | 352 | 338 (96) | 90–100 | .03 | 352 | 338 (96) | 90–100 | .03 | 352 | 339 (96) | 90–100 | .44 |
| GBS | 51 | 51 (100) | — | 38 | 38 (100) | — | 38 | 38 (100) | — | |||
| Klebsiella spp | 18 | 17 (94) | 50–100 | 18 | 17 (94) | 50–100 | 18 | 18 (100) | — | |||
| SCEd | 18 | 8 (44) | 0–67 | 18 | 8 (44) | 0–67 | 18 | 18 (100) | — | |||
| Enterococcus | 16 | 0 (0) | — | 16 | 16 (100) | — | 16 | 16 (100) | — | |||
| S aureus | 8 | 0 (0) | — | 8 | 0 (0) | — | 8 | 0 (0) | — | |||
| S pneumoniae | 3 | 3 (100) | — | 3 | 3 (100) | — | 3 | 3 (100) | — | |||
Each pathogen counted only once per patient if >1 infection source.
P value from Pearson’s χ2 test of susceptibility × hospital for age <90 d.
Total number of cases with nonmissing data varies by empirical regimen.
Gram-negative bacteria with inducible β-lactamase genes Serratia, Citrobacter, and Enterobacter.
—, no range given as all sites have equal percentages.
E coli was the most common organism, and 96% of the isolates were susceptible to 1 of the empirical regimens. There were 14 cases of cephalosporin-resistant bacteremia and meningitis, including S aureus (n = 7), Enterococcus spp (n = 4), and E coli (n = 3). The addition of ampicillin to a third-generation cephalosporin would have improved coverage of only the 4 cases of Enterococcus spp bacteremia and meningitis.
Discussion
We identified significant variation in empirical antibiotic regimens in this 2-year review of infants <90 days old with culture-confirmed UTI, bacteremia, and meningitis across 8 regionally diverse children’s hospitals. There was no significant difference in the antimicrobial susceptibility for common empirical regimens, age, and proportion of invasive infections across these same hospitals, although there was a difference in the pathogens causing bacteremia and meningitis. The addition of ampicillin to a third-generation cephalosporin would have improved coverage in only 4 out of 476 isolates causing bacteremia or meningitis. These findings suggest that the observed variability in antibiotic use, particularly for ampicillin, may result from perceived rather than actual differences in pathogen prevalence and thus antimicrobial susceptibility.
Our findings in this study are consistent with previous research from predominantly regional studies. First, other multisite studies evaluating bacteremia found that bacterial epidemiology was similar across hospitals.21,22 Second, no cases of L monocytogenes were found in this study, which has been previously reported and has historically been a driver of ampicillin use.23–25 Third, studies that used data from multisite health systems in northern California and Utah and assessed the severity of bacterial infections in infants also found that UTI accounted for most bacterial infections in infants <90 days old and that bacteremia and meningitis accounted for <2% of infections. Finally, using administrative data, our group previously reported significant variation across hospitals in the use of diagnostic tests, rates of hospital admission from the ED, and overall antibiotic use in infants with suspected UTI, bacteremia, and meningitis.8,26 Our study builds on this previous work by providing epidemiologic and susceptibility data on meningitis, bacteremia, and UTI in young infants representing a large, regionally diverse population.
Although we found across-hospital variability in the types of pathogens, most infections regardless of whether they were isolated in the urine, blood, or CSF would have still been empirically covered by a third-generation cephalosporin alone. The most common pathogen, E coli, was susceptible to a third-generation cephalosporin in 96% of the isolates, which was equal to its susceptibility to ampicillin and gentamicin. The high percentage of isolates susceptible to a third-generation cephalosporin suggests that extended-spectrum β-lactamase producing E coli is not a common cause of infection in infants <90 days old with suspected bacterial infection. Similar patterns of susceptibility to common empirical antimicrobial regimens across 8 children’s hospitals indicate that there is an opportunity for national antibiotic stewardship, as suggested by previous, smaller-scale studies reporting on the adequacy of currently used empirical antibiotic regimens.27
Empirical therapy failed to provide adequate coverage in 10% of our cohort. Most of this discordance in therapy occurred in patients with UTI. Fourteen cases of bacteremia or meningitis also received discordant empirical therapy. Given the small sample size of discordant therapy, the clinical impact on patient outcomes is uncertain. Clinicians should consider broadening empirical coverage for patients who are clinically unstable or who do not improve with empirical therapy.
When comparing our observed susceptibility results to the 3 most commonly used empirical antimicrobial regimens, we found that they provided equivalent coverage in infants ≥7 days old but not in infants <7 days old. For infants ≥7 days old, a third-generation cephalosporin performed as well as ampicillin plus gentamicin, although it is important to note that in studies performed with older children and adolescents gentamicin does not cross the blood-brain barrier sufficiently to treat meningitis.8 Data are limited regarding differences in penetration of antibiotics into the CSF of neonates and young infants with meningitis as compared with older children. We could not draw conclusions about infants 0 to 6 days because of small sample size.
Ampicillin was commonly used in conjunction with a third-generation cephalosporin, but its addition only minimally improved antimicrobial coverage (86%–90%). The gain in spectrum of activity resulted primarily from coverage of Enterococcus spp, bacteria that are less virulent and rarely responsible for infection beyond UTI in young infants. The addition of ampicillin to a third-generation cephalosporin would have improved coverage for 13 cases of UTI and 4 cases of bacteremia and meningitis.
Our study has limitations. First, it is possible that we underclassified cases because of administrative coding differences. However, we used a previously validated method to identify similar cases and assessed for missing infections among a matching cohort of infants with fever codes.8 Second, our findings do not apply to patients with many comorbidities. Such patients were excluded from this study because they are at greater risk of infection from multi-drug resistant bacteria and have greater risk of more severe illness and therefore should not be treated similarly to otherwise healthy febrile infants. Third, infants classified with contaminated microbiological cultures could have been misclassified. By comparison, previous studies have used provider interpretation to identify true pathogens.18 Our method of using available evidence, national standards, and consensus opinion to adjudicate culture results had the benefit of applying uniform criteria to all culture results and reducing interinstitution variability in pathogen classification. Fourth, we identified only 13 patients with meningitis (7 also of whom had bacteremia), limiting our ability to assess susceptibility patterns for meningitis alone across the 8 hospitals. Grouping these patients with those with only bacteremia allowed us to detect a difference for the most severe infections. However, it is possible that a larger sample size might yield a different finding. Fifth, the study is unable to determine the driver for variability in empirical antibiotic selection. Local efforts will be needed to identify and mitigate any addressable causes. Sixth, the associated clinical outcomes of children receiving discordant empirical antibiotic therapy with final culture results were not assessed; future studies should focus on the potential impact of discordant antibiotic therapy on management or clinical outcomes. Finally, this is a cohort of only 8 tertiary pediatric hospitals out of the 44 hospitals that participate in PHIS, and these results may not be generalizable to other pediatric settings.
Conclusions
This study demonstrates that common empirical antimicrobial regimens provide similar coverage in infants <90 days old with UTI, bacteremia, and bacterial meningitis across 8 geographically diverse US children’s hospitals. A third-generation cephalosporin without the addition of ampicillin provides adequate empirical coverage in the majority of cases, particularly for those with UTI or bacteremia. Ampicillin with gentamicin is a reasonable alternative in neonates without Gram-negative meningitis.
These findings, along with careful consideration of caregiver preferences and the perceived risks and benefits of overempirical and underempirical antimicrobial treatment,28 could be used to support the development of a national guideline for the treatment of infants with suspected bacterial infection. To better align antibiotic stewardship efforts with optimal clinical outcomes, more study is needed to elucidate the clinical impact of discordant empirical antibiotics.
Acknowledgments
The authors acknowledge Assaf Oron, PhD at Seattle Children’s Hospital for his work on data analysis.
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded in part by The Gerber Foundation Novice Researcher Award, ref 1827-3835, and the National Center for Advancing Translational Sciences of the National Institutes of Health under awards UL1TR000423 and TL1TR000422. Dr Balamuth receives career development support from NICHD K23 HD082368. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Dr Feldman conceptualized and designed the study and data collection instrument, acquired data for her site, interpreted the data, and drafted the initial manuscript; Dr McCulloh made a substantial contribution to the conception and design of the study and data collection instrument, contributed expertise in the field of infectious disease, and revised the manuscript for intellectual content; Dr Myers made a substantial contribution to the conception and design of the study, contributed expertise in the field of infectious disease, and revised the manuscript for intellectual content; Dr Aronson made a substantial contribution to the conception and design of the study including cohort selection, acquired data for his site, and revised the manuscript for intellectual content; Dr Neuman made a substantial contribution to the conception and design of the study, revised the manuscript for intellectual content, and acquired data for his site; Ms Bradford contributed the statistical analysis of data and drafted the figures and tables; Drs Alpern, Balamuth, Blackstone, Browning, Marble, Roben, and Williams made a substantial contribution to the conception and design of the study and revised the manuscript for intellectual content; Ms Hayes and Drs Korman, Leazer, and Nigrovic made a substantial contribution to the conception and design of the study, acquired data for their sites, and revised the manuscript for intellectual content; Dr Tieder made a substantial contribution to the conception and design of the study and data collection instrument and revised the manuscript for intellectual content; and all authors approved the final manuscript as submitted.
References
- 1.Hui C, Neto G, Tsertsvadze A, et al. Diagnosis and management of febrile infants (0–3 months). Evid Rep Technol Assess (Full Rep). 2012;(205):1–297 [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014;42(11):2409–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1). Available at: www.pediatrics.org/cgi/content/full/130/1/e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byington CL, Enriquez FR, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662–1666 [DOI] [PubMed] [Google Scholar]
- 5.Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329(20):1437–1441 [DOI] [PubMed] [Google Scholar]
- 6.Dagan R, Hall CB, Powell KR, Menegus MA. Epidemiology and laboratory diagnosis of infection with viral and bacterial pathogens in infants hospitalized for suspected sepsis. J Pediatr. 1989;115(3):351–356 [DOI] [PubMed] [Google Scholar]
- 7.Cantey JB. Optimizing the use of antibacterial agents in the neonatal period. Paediatr Drugs. 2016;18(2):109–122 [DOI] [PubMed] [Google Scholar]
- 8.Sullins AK, Abdel-Rahman SM. Pharmacokinetics of antibacterial agents in the CSF of children and adolescents. Paediatr Drugs. 2013;15(2):93–117 [DOI] [PubMed] [Google Scholar]
- 9.Leazer R, Perkins AM, Shomaker K, Fine B. A meta-analysis of the rates of Listeria monocytogenes and Enterococcus in febrile infants. Hosp Pediatr. 2016;6(4):187–195 [DOI] [PubMed] [Google Scholar]
- 10.Mischler M, Ryan MS, Leyenaar JK, et al. Epidemiology of bacteremia in previously healthy febrile infants: a follow-up study. Hosp Pediatr. 2015;5(6):293–300 [DOI] [PubMed] [Google Scholar]
- 11.The White House. National Action Plan for Combating Antibiotic-Resistant Bacteria. Published March 2015 Available at: https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf Accessed June 1, 2017
- 12.Aronson PL, Thurm C, Williams DJ, et al. ; Febrile Young Infant Research Collaborative. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aronson PL, Williams DJ, Thurm C, et al. ; Febrile Young Infant Research Collaborative. Accuracy of diagnosis codes to identify febrile young infants using administrative data. J Hosp Med. 2015;10(12):787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237–244 [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts KB; Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595–610 [DOI] [PubMed] [Google Scholar]
- 18.Tosif S, Baker A, Oakley E, Donath S, Babl FE. Contamination rates of different urine collection methods for the diagnosis of urinary tract infections in young children: an observational cohort study. J Paediatr Child Health. 2012;48(8):659–664 [DOI] [PubMed] [Google Scholar]
- 19.CLSI. Performance Standards for Antimicrobial Susceptibility Testing. CLSI supplement M100S. 26th ed Wayne, PA: Clinical and Laboratory Standards Institute; 2016 [Google Scholar]
- 20.Kim KS. Comparison of cefotaxime, imipenem–cilastatin, ampicillin–gentamicin, and ampicillin–chloramphenicol in the treatment of experimental Escherichia coli bacteremia and meningitis. Antimicrob Agents Chemother. 1985;28(3):433–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biondi E, Evans R, Mischler M, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132(6):990–996 [DOI] [PubMed] [Google Scholar]
- 22.Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595–599 [DOI] [PubMed] [Google Scholar]
- 23.Watt K, Waddle E, Jhaveri R. Changing epidemiology of serious bacterial infections in febrile infants without localizing signs. PLoS One. 2010;5(8):e12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee B, Newland JG, Jhaveri R. Reductions in neonatal listeriosis: “collateral benefit” of group B streptococcal prophylaxis? J Infect. 2016;72(3):317–323 [DOI] [PubMed] [Google Scholar]
- 25.Biondi EA, Mischler M, Jerardi KE, et al. ; Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844–849 [DOI] [PubMed] [Google Scholar]
- 26.Aronson PL, Thurm C, Alpern ER, et al. ; Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667–677 [DOI] [PubMed] [Google Scholar]
- 27.Cantey JB, Lopez-Medina E, Nguyen S, Doern C, Garcia C. Empiric antibiotics for serious bacterial infection in young infants: opportunities for stewardship. Pediatr Emerg Care. 2015;31(8):568–571 [DOI] [PubMed] [Google Scholar]
- 28.Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71–77 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.