Abstract
Purpose:
To assess and compare preoperative refractive, aberrometric, topographic, and contrast sensitivity (CS) measurements with postoperative values after corneal collagen cross-linking (CXL) in patients with progressive keratoconus.
Methods:
Twenty-two eyes of 11 patients with keratoconus were enrolled in this prospective study. Uncorrected distance visual acuity (UDVA), best spectacle corrected visual acuity (BSCVA), CS, and higher order aberrations (HOAs) were evaluated at baseline and 1, 3, 6, and 8 months after surgery.
Results:
The mean total HOAs of the included patients were 2.24, 2.34, 2.28, 2.17, and 2.03 μm before and 1, 3, 6, and 8 months after CXL, respectively. A significant reduction in corneal HOAs including vertical coma, vertical and horizontal trefoil and spherical aberration was observed 6 and 8 months after CXL. UDVA and BSCVA improved significantly in all patients who completed the follow-up period (P = 0.001). Although mean CS declined significantly 1 month postoperatively, it improved significantly after 3, 6, and 8 months (P<0.001). Maximum keratometry was significantly lower 8 months postoperatively compared to the preoperative value. (P = 0.006).
Conclusions:
CXL seems to improve UCVA, BSCVA, and CS and reduce most corneal HOAs in progressive forms of keratoconus.
Keywords: Collagen Cross-linking, Contrast Sensitivity, Corneal Aberration, Keratoconus
INTRODUCTION
Keratoconus is a progressive, bilateral, asymmetric, non-inflammatory, degenerative disease that leads to central or inferotemporal thinning of the cornea. It may also occur in the superior region of the cornea. It is the most common cause of corneal ectasia. The conical shape of the cornea causes high myopia and irregular astigmatism and interferes with visual function. The disease usually appears in the second decade of life during puberty and usually progresses until the fourth decade of life.[1,2,3,4] The prevalence of keratoconus is 5 to 23 per 10,000, and the incidence is 4.5 per 10,000.[2,3,5] Different studies report different rates which is due to diversity in the descriptive or diagnostic criteria in various studies.[4,5] The disease affects all races; it is more common in women in some studies and in men in others.[5,6,7,8,9] Ocular signs and symptoms of keratoconus may vary according to the severity of the disease. Disease progression results in severe irreversible loss of visual acuity. The emergence of “scissors-like” reflex in retinoscopy is an indication of irregular astigmatism. In cases of moderate and advanced keratoconus, circular arc deposition of hemosiderin (Fleischer's ring) is often evident. Iron accumulation results from extreme change of corneal curvature.[10] Munson's sign and Rizzuti's sign are often observed in advanced stages, and Descemet's membrane rupture in severe keratoconus causes sudden loss of vision and significant pain.[11] Increased high order aberrations are another problem of keratoconus which can lead to impaired visual acuity.[12,13]
Keratoconus treatment depends on the severity of the disease. Patients in early stages can wear glasses. Patients with mild to moderate keratoconus can use hard contact lens, and patients with severe form of the disease may need corneal transplantation.
The CXL technique involves formation of covalent cross-links between collagen fibrils. This method uses a photosensitive material, riboflavin, and a specific wavelength of ultraviolet irradiation, to increase the biomechanical stability of the cornea.[14] The purpose of this study was to assess the effects of CXL on refractive and aberrometric measurements and contrast sensitivity in patients with progressive keratoconus.
METHODS
Study Subjects
This study enrolled 22 eyes of 11 patients with keratoconus. All patients provided informed consent and the study which followed the tenets of the Declaration of Helsinki was approved by the Ethics Committee of our university. The inclusion criteria were as follows: clinical or subclinical keratoconus (clinical signs and abnormal retinoscopy reflex), clear cornea without central opacity, thinnest corneal thickness >400 μm, and corneal mean keratometry of less than 58 diopters (D). Patients older than 30 years of age, patients who failed to attend the follow-up examinations until 6 months postoperatively or longer, and pregnant and nursing patients were excluded.
Study Measurements
Complete preoperative ophthalmic evaluations were conducted for all patients. Uncorrected distance visual acuity (UDVA) and best spectacle corrected visual acuity (BSCVA) were measured using logarithmic scale charts (Lighthouse International, New York, NY, USA). BSCVA (CDVA) was measured using trial frames at a distance of 6 m from the chart box. Auto Kerato-Refractometer KR-1/RM-1(Topcon Medical Systems, Inc., Oakland, CA, USA) was used to measure manifest and cycloplegic refraction and keratometry. A CSV-1000 contrast (CS) sensitivity test was conducted. Total wavefront analysis was performed with a Pentacam (Pentacam® HR, Type 70900, OCULUS, Wetzelar, Germany). The device measures mean total corneal higher order aberrations (HOAs), mean vertical and horizontal coma, and trefoil and mean corneal spherical aberration. All parameters were evaluated before and 1, 3, 6, and 8 months after surgery.
Cross-linking Surgery
All patients underwent standard CXL under sterile conditions in the operating room. Topical anesthesia of 0.05% tetracain eye drops was administered. Epithelium removal of an area 7-9 mm in diameter was done using a no. 15 surgical knife (Swann- Morton, Sheffield, England). In addition, a solution of 0.1% riboflavin (Biotech Visioncare) in 20% dextran (T500) (10 mg riboflavin in 0.1 mL dextran) was applied every 2-3 min. After 30 min, the patient was examined using a slit lamp with a cobalt blue filter for the presence of fluorescein in the anterior chamber. The cornea was then irradiated with calibrated UV-A light with a wavelength of 370 nm and an irradiance of 3 mW/cm2 for 30 minutes. During irradiation, riboflavin drops were applied to the cornea every 2 minutes to sustain the required concentration of riboflavin and to prevent desiccation of the cornea. Postoperatively, the patient received topical chloramphenicol (Clobiotic 0.5% ophthalmic drops, Sina Darou, Tehran, Iran) and betamethasone (Betasonate 0.1% ophthalmic drops, Sina Darou, Tehran, Iran). A bandage contact lens (CIBA Vision, Duluth, GA, USA) was fitted until reepithelialization was complete (usually after 3 days). The patient was followed up on consecutive days after surgery until contact lens removal. Chloramphenicol and betamethasone 0.1% eye drops were administered 4 times per day during the first week. Betamethasone was replaced in the second week with fluorometholone 4 times per day. After 7 days, the dose was tapered over 4-6 weeks. Follow-up visits were scheduled at 1, 3, 6, and 8 months after surgery.
Statistical Analysis
Data were analyzed using the statistical software PASS 11 (NCSS, LLC., Kaysville, UT, USA). P < 0.05 was considered significant.
RESULTS
Twenty-two eyes of 11 patients were included in the study and followed up for 8 months postoperatively. No postoperative complications were observed.
Visual Acuity Changes
Table 1 shows the postoperative effects of CXL on UCVA and BSCVA. After 8 months, the mean UCVA improved significantly compared to preoperative measurements, from 0.72 ± 0.027 to 0.53 ± 0.023 logMAR (P = 0.001). Mean preoperative BSCVA was 0.26 ± 0.029 logMAR. One month after CXL, it was 0.23 ± 0.015 logMAR. At 3, 6, and 8 months, it was 0.18 ± 0.013, 0.17 ± 0.015, and 0.17 ± 0.014 logMAR, respectively. The changes were statistically significant over 8 months (P = 0.001).
Table 1.
Visual acuity changes over time in eyes treated with cross-linking

Corneal Wavefront
The mean preoperative total HOA changed significantly, from 2.24 ± 0.57 μm to 2.33 ± 0.53 μm, 2.17 ± 0.53 μm, and 2.02 ± 0.54 μm, at 1, 6, and 8 months after CXL, respectively (P = 0.0078, P = 0.012, P = 0.001 respectively), but the difference at 3 months was not significant (P = 0.29). Analysis of the vertical coma revealed significant changes at all follow-up visits (P = 0.001), but horizontal coma changes were not significant. Significant changes were also observed for vertical and horizontal trefoils after 8 months (P = 0.001, P < 0.001) [Table 2].
Table 2.
Higher-order corneal aberrations compared before and after surgery
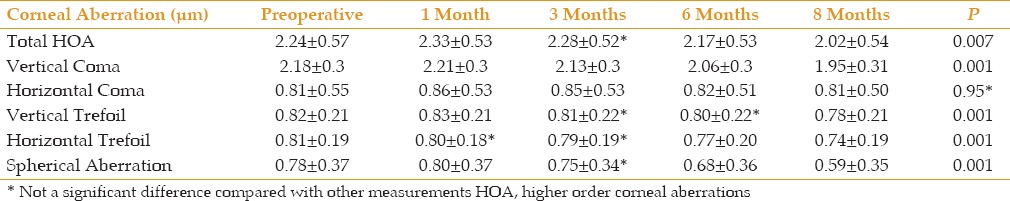
Mean corneal spherical aberrations decreased significantly, from 0.78 ± 0.37 μm preoperatively to 0.59 ± 0.35 μm 8 months after surgery (P = 0.001). The differences in mean values at all except the 3-month post-surgical follow-up were statistically significant [Table 2].
Contrast Sensitivity
The average preoperative CS for all patients was 1.46 ± 0.06 log CS, which decreased to 1.41 ± 0.07 log one month postoperatively and then increased to 1.62 ± 0.05 log at the final follow up examination. Table 3 summarizes the CS data and demonstrates that there was no significant difference throughout the study period.
Table 3.
Contrast sensitivity before and after cross-linking in 22 eyes
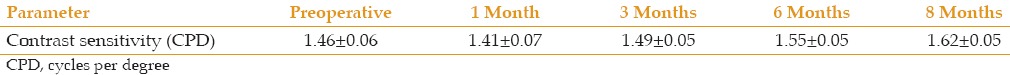
Maximum Simulated Keratometry Value
The mean maximum simulated keratometry value (Kmax) before CXL was 52.5 D (47.25-54.5 D); it was 51.1 D (45.2-54 D) after 3 months and 49.5 D (47.2-51.15 D) after 6 months. Eight months after surgery, Kmax was 51.5 D (47.1-54.25 D) which was significantly lower than preoperative measurements (P = 0.006).
DISCUSSION
The primary goal of CXL is the stabilization of keratoconus. Astigmatism treatment, increase of visual acuity, and better vision quality are also the secondary goals.[15,16] Our study identified statistically significant differences between the primary parameters obtained before and after CXL.
According to the UCVAs of the patients in this study, the accelerated downward trend decreased over time and the differences compared with previous months were statistically significant (P<0.001). UCVA changed from 0.72 preoperatively to 0.64, 0.55, 0.54, and 0.54 after 1, 3, 6, and 8 months after surgery, respectively. In this study, we found that BSCVA followed a pattern similar to that of UCVA. A statistically significant improvement was seen in this variable (P = 0.003) in the first month after cross-linking that was slower in subsequent months (0.24, 0.18, 0.17, and 0.17 in 1, 3, 6, and 8 months after surgery). These results are consistent with the results of previous similar studies.[15,17,18,19,20]
The effective factor to improve visual acuity after crosslinking is not clear. We don’t know even if topographic examinations,[21] pachymetry,[22] corneal haziness,[23] or cornea biomechanics[24] after CXL are the clinical characteristics associated with visual acuity after treatment.
Some studies have shown a significant decrease in Kmax after CXL,[25,26,27,28,29,30] and others reported stability in corneal keratometry.[31,32] The patients in our study showed a significant decrease in Kmax after CXL, which reveals a flattening effect of this procedure.
Increased anterior corneal and posterior corneal HOAs are the factors responsible for decreased visual acuity in keratoconic eyes.[21,33,34,35] Cross-linking was first used to prevent progression of corneal ectasia, but was later found to be useful for visual acuity and improvement in corneal topographic characteristic in some patients.[16,21,35,36,37] In the present study, the total corneal HOA was observed to increase initially after surgery, but decreased at 6 and 8 months postoperatively. after surgery. A similar pattern of a significant increase in the first month followed by a significant decrease in the following months, especially in the sixth and eight months, was observed in other HOAs, including vertical coma (the most important Zernike coefficient affecting visual acuity), horizontal coma, vertical trefoil, horizontal trefoil, and spherical aberration. Among these measures, the horizontal coma followed this pattern, but none of the changes were statistically significant. Generally, results associated with HOA corresponded to the results of similar previous studies.[18,38,39] It indicated that after CXL, the corneal shape undergoes a process of regularization.
Contrast sensitivity is a valuable index in visual function.[40,41] CS changes in cases of keratoconus after CXL have not been well studied. Even if the corrected visual acuity is optimal, contrast sensitivity may be reduced due to an increase in HOA in patients with keratoconus.[42,43] In this study, the findings associated with contrast sensitivity also follow a pattern similar to that of HOA. Compared to preoperative value, CS was significantly decrease at postoperative month 1. However, it increased significantly at postoperative months 3, 6, and 8. Lamy et al[44] found in their study of 68 keratoconic eyes that CS improved over 2 years of follow-up (P < 0.001) in contrast with control eyes, in which changes were not significant over time (P = 0.228). The Pelli-Robson test was used for CS measurements and compared to our study, the final CS after 2 years was higher (1.69 compared with 1.62 after 8 months in our study).
CXL can increase corneal rigidity up to 328.9%.[45] The improvement in visual acuity and contrast sensitivity after CXL is the result of decreasing corneal curvature, astigmatism, and biomechanical stability.
The major limitations of this study were the small sample size and the recruitment of both eyes of a single patient and short follow-up duration. Larger studies with longer follow-up periods are required to better define the outcomes of CXL.
In conclusion, CXL is considered one of the most promising developments of recent decades for the treatment of keratoconus as it improves corneal HOAs, in particular coma, and contrast sensitivity. According to studies conducted to date, it seems that this method could potentially reduce morbidity from progressive forms of the disease and may ultimately reduce the need for corneal transplantation.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Nottingham J. Practical observations on conical cornea. London: Churchill, London; 1984. pp. 1–19. [Google Scholar]
- 2.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101:267–273. doi: 10.1016/0002-9394(86)90817-2. [DOI] [PubMed] [Google Scholar]
- 3.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Rabinowitz YS, Rasheed K, Yang H. Longitudinal study of the normal eyes in unilateral keratoconus patients. Ophthalmology. 2004;111:440–446. doi: 10.1016/j.ophtha.2003.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Krachmer JH, Feder RS, Belin MW. Keratoconus and related non-inflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 6.Stein HA, Stein RM, Freeman MI. The ophthalmic assistant: A text for allied and associated ophthalmic personnel. Canada: Elsevier Mosby; 2006. p. 396. [Google Scholar]
- 7.Owens H, Gamble G. A profile of keratoconus in New Zealand. Cornea. 2003;22:122–5. doi: 10.1097/00003226-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Wagner H, Barr JT, Zadnik K. Collaborative longitudinal evaluation of keratoconus (CLEK) study: Methods and findings to date. Contact Lens Anterior Eye. 2007;30:223–232. doi: 10.1016/j.clae.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgiou T, Funnell CL, Cassels-Brown A, O’Conor R. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asians and white patients. Eye (Lond) 2004;18:379–383. doi: 10.1038/sj.eye.6700652. [DOI] [PubMed] [Google Scholar]
- 10.Weed KH, MaCEwen CJ, Giles T, Low J, McGhee CN. The Dundee University Scottish Keratoconus study: Demographics, corneal signs, associated diseases, and eye rubbing. Eye (Lond) 2008;22:534–541. doi: 10.1038/sj.eye.6702692. [DOI] [PubMed] [Google Scholar]
- 11.Ziaei H, Jafarinasab MR, Javadi MA, Karimian F, Poorsalman H, Mahdavi M, Shoja MR, Katibeh M. Epidemiology of keratoconus in an Iranian population. Cornea. 2012;31(9):1044–1047. doi: 10.1097/ICO.0b013e31823f8d3c. [DOI] [PubMed] [Google Scholar]
- 12.Maeda N, Fujikado T, Kuroda T, Mihashi T, Hirohara Y, Nishida K, et al. Wavefront aberrations measured with Hartmann-Shack sensor in patients with keratoconus. Ophthalmology. 2002;109:1996–2003. doi: 10.1016/s0161-6420(02)01279-4. [DOI] [PubMed] [Google Scholar]
- 13.Applegate RA, Hilmantel G, Howland HC, Tu EY, Starck T, Zayac EJ, et al. Corneal first surface optical aberrations and visual performance. J Refract Surg. 2000;16:507–514. doi: 10.3928/1081-597X-20000901-04. [DOI] [PubMed] [Google Scholar]
- 14.Wollensak G, Spoerl E, Wilsch M, Seiler T. Endothelial cell damage after riboflavin-ultraviolet-a treatment in the rabbit. J Cataract Refract Surg. 2003;29:1786–1790. doi: 10.1016/s0886-3350(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 15.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 16.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Vinciguerra P, Albè E, Trazza S, Rosetta P, Vinciguerra R, Seiler T, et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009;116:369–378. doi: 10.1016/j.ophtha.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 18.Coskunseven E, Jankov MR, 2nd, Hafezi F. Contralateral eye study of corneal collagen cross-linking with riboflavin and UVA irradiation in patients with keratoconus. J Refract Surg. 2009;25:371–376. doi: 10.3928/1081597X-20090401-02. [DOI] [PubMed] [Google Scholar]
- 19.Caporossi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen: Preliminary refractive results in an Italian study. J Cataract Refract Surg. 200;2:837–845. doi: 10.1016/j.jcrs.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 20.Wittig-Silva C, Whiting M, Lamoureux E, Lindsay RG, Sullivan LJ, Snibson GR. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: Preliminary results. J Refract Surg. 2008;24:S720–S725. doi: 10.3928/1081597X-20080901-15. [DOI] [PubMed] [Google Scholar]
- 21.Greenstein SA, Fry KL, Hersh PS. Corneal topography indices after corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:1282–1290. doi: 10.1016/j.jcrs.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Greenstein SA, Shah VP, Fry KL, Hersh PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:691–700. doi: 10.1016/j.jcrs.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 23.Greenstein SA, Fry KL, Bhatt J, Hersh PS. Natural history of corneal haze after collagen crosslinking for keratoconus and corneal ectasia: Scheimpflug and biomicroscopic analysis. J Cataract Refract Surg. 2010;36:2105–2114. doi: 10.1016/j.jcrs.2010.06.067. [DOI] [PubMed] [Google Scholar]
- 24.Greenstein SA, Fry KL, Hersh PS. In vivo biomechanical changes after corneal collagen crosslinking for keratoconus and corneal ectasia: 1-year analysis of a randomized, controlled, clinical trial. Cornea. 2012;31:21–25. doi: 10.1097/ICO.0b013e31821eea66. [DOI] [PubMed] [Google Scholar]
- 25.Elbaz U, Yeung SN, Ziai S, Lichtinger AD, Zauberman NA, Goldich Y, et al. Collagen crosslinking after radial keratotomy. Cornea. 2014;33:131–136. doi: 10.1097/ICO.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 26.Chan TC, Chow VW, Jhanji V, Wong VW. Different topographic response between mild to moderate and advanced keratoconus after accelerated collagen cross-linking. Cornea. 2015;34:922–927. doi: 10.1097/ICO.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 27.Marino GK, Torricelli AA, Giacomin N, Santhiago MR, Espindola R, Netto MV. Accelerated corneal collagen cross-linking for postoperative LASIK ectasia: Two-Year Outcomes. J Refract Surg. 2015;31:380–384. doi: 10.3928/1081597X-20150521-04. [DOI] [PubMed] [Google Scholar]
- 28.Ng AL, Chan TC, Cheng AC. Conventional versus accelerated corneal collagen cross-linking in the treatment of keratoconus. Clin Exp Ophthalmol. 2016;44:8–14. doi: 10.1111/ceo.12571. [DOI] [PubMed] [Google Scholar]
- 29.Shetty R, Pahuja NK, Nuijts RM, Ajani A, Jayadev C, Sharma C, Nagaraja H. Current Protocols of Corneal Collagen Cross-Linking: Visual, Refractive, and Tomographic Outcomes. Am J Ophthalmol. 2015;160:243–249. doi: 10.1016/j.ajo.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Rossi S, Orrico A, Santamaria C, Romano V, De Rosa L, Simonelli F, De Rosa G. Standard versus trans-epithelial collagen cross-linking in keratoconus patients suitable for standard collagen cross-linking. Clin Ophthalmol. 2015;18:9:503–509. doi: 10.2147/OPTH.S73991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherif AM. Accelerated versus conventional corneal collagen cross-linking in the treatment of mild keratoconus: A comparative study. Clin Ophthalmol. 2014;8:1435–1440. doi: 10.2147/OPTH.S59840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salman AG. Transepithelial corneal collagen crosslinking for progressive keratoconus in a pediatric age group. J Cataract Refract Surg. 2013;39:1164–1170. doi: 10.1016/j.jcrs.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Lim L, Wei RH, Chan WK, Tan DT. Evaluation of higher order ocular aberrations in patients with keratoconus. J Refract Surg. 2007;23:825–828. doi: 10.3928/1081-597X-20071001-13. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa T, Maeda N, Kosaki R, Hori Y, Saika M, Inoue T, et al. Higher-order aberrations due to the posterior corneal surface in patients with keratoconus. Invest Ophthalmol Vis Sci. 2009;50:2660–2665. doi: 10.1167/iovs.08-2754. [DOI] [PubMed] [Google Scholar]
- 35.Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: One year results. J Cataract Refract Surg. 2011;37:149–160. doi: 10.1016/j.jcrs.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Hafezi F, Mrochen M, Iseli HP, Seiler T. Collagen crosslinking with ultraviolet-A and hypoosmolar ribo- flavin solution in thin corneas. J Cataract Refract Surg. 2009;35:621–624. doi: 10.1016/j.jcrs.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 37.Grewal DS1, Brar GS, Jain R, Sood V, Singla M, Grewal SP. Corneal collagen crosslinking using riboflavin and ultraviolet-A light for keratoconus: One-year analysis using Scheimpflug imaging. J Cataract Refract Surg. 2009;35:425–432. doi: 10.1016/j.jcrs.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 38.Vinciguerra P, Albè E, Trazza S, Rosetta P, Vinciguerra R, Seiler T, et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009;116:369–378. doi: 10.1016/j.ophtha.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 39.Koller T, Iseli H, Hafezi F, Vinciguerra P, Seiler T. Scheimpflug imaging of corneas after collagen cross-linking. Cornea. 2009;28:510–515. doi: 10.1097/ICO.0b013e3181915943. [DOI] [PubMed] [Google Scholar]
- 40.Baumeister M, Klaproth OK, Gehmlich J, Buhren J, Kohnen T. Changes in corneal first-surface wavefront aberration after corneal collagen cross-linking in keratoconus. Klin. Monbl. Augenheilkd. 2009;226:752–756. doi: 10.1055/s-0028-1109627. [DOI] [PubMed] [Google Scholar]
- 41.Pesudovs K, Schoneveld P, Seto RJ, Coster DJ. Contrast and glare testing in keratoconus and after penetrating keratoplasty. Br J Ophthalmol. 2004;88:653–657. doi: 10.1136/bjo.2003.027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmerman AB, Lust KL, Bullimore MA. Visual acuity and contrast sensitivity testing for sports vision. Eye Contact Lens. 2011;37:153–159. doi: 10.1097/ICL.0b013e31820d12f4. [DOI] [PubMed] [Google Scholar]
- 43.Negishi K, Kumanomido T, Utsumi Y, Tsubota K. Effect of higher-order aberrations on visual function in keratoconic eyes with a rigid gas permeable contact lens. Am J Ophthalmol. 2007;144:924–629. doi: 10.1016/j.ajo.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Okamoto C, Okamoto F, Samejima T, Miyata K, Oshika T. Higher-order wave front aberration and letter-contrast sensitivity in keratoconus. Eye (Lond) 2008;22:1488–1492. doi: 10.1038/sj.eye.6702902. [DOI] [PubMed] [Google Scholar]
- 45.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]


