Abstract
Purpose:
To evaluate the risk factors for pterygium in the dry, high altitude province of Ilam, Iran.
Methods:
The study included patients who presented to ophthalmology clinic. The patients were divided into two groups: 210 diagnosed with pterygium or pinguecula (unilateral or bilateral), and 210 healthy controls. Demographic variables, living environment, disease type, disease laterality, family history of pterygium as well as history of smoking, working outdoors, baking, welding, ocular conditions (trachoma keratopathy, glaucoma, refractive error, and dry eye), use of glasses, ultraviolet light exposure, and systemic conditions were collected from both groups and compared for risk assessment.
Results:
Univariate analysis revealed that age (P = 0.001), sex (P = 0.001), family history of pterygium (P = 0.001), positive history of smoking (P < 0.001), history of baking (P = 0.045), welding experience (P < 0.001), severe blepharitis (P < 0.001), hyperopia (P < 0.001), dry eye (P < 0.001), hypertension (P < 0.001), ischemic heart disease (P < 0.001), obesity (P = 0.038), and primary residential area (P = 0.025) had significant associations with increased incidence of pterygium. However, in multivariate analysis, only family history of pterygium, cigarette smoking, history of baking, age, and severe blepharitis were significantly associated with the incidence of pterygium (P<0.001, P<0.001, P = 0.002, P = 0.023 and P = 0.002, respectively).
Conclusion:
This study tested more risk factors related to the prevalence of pterygium compared to previous studies. It also confirmed previously established risk factors. Family history of pterygium and blepharitis were risk factors that have not been reported in previous studies and were found to be significantly associated with the development of pterygium in this study.
Keywords: Prevalence, Pterygium, Risk Factors
INTRODUCTION
Pterygium is an abnormal inflammatory fibrovascular growth extending from the interpalpebral conjunctiva to the cornea. It is a common ophthalmic disorder of unknown etiology and pathogenesis.[1,2,3,4] Pinguecula also has the same histopathological characteristics as pterygium but without corneal involvement.[5,6] Further growth of pterygium onto the cornea can cause visual impairment secondary to induced astigmatism, visual axis obstruction, and loss of corneal transparency.[6] Although the pathogenesis of pterygium is yet to be understood, many studies have reported its prevalence to range from 0.3% to 37.1% in different populations.[6,7,8,9,10] The reported prevalence of pinguecula ranges from 41% to 90%.[11,12] Reported risk factors of pterygium include: age,[11,12,13,14,15,16] sex,[11,12,14,15] exposure to ultraviolet light such as in outdoor work and lower altitudes,[17,18,19,20] and socioeconomic status, and level of education.[21,22] Environmental factors such as dust and dry weather have also been implicated as risk factors for pterygium.[23] Despite the high altitude of Ilam province, there is no available data regarding the prevalence and risk factors for pterygia in this region. The aim of this study was to identify the risk factors associated with pterygium in Ilam province in Iran.
METHODS
The study included patients who presented to the ophthalmology clinic. Patients were divided into two groups: 210 patients with pterygium or pinguecula as the case group, and 210 healthy subjects as the controls. A comprehensive questionnaire was compiled based on relevant risk factors, demographic variables, living environment, disease type, disease laterality, familial history as well as history of smoking, drug abuse, working outdoors, baking, welding, ocular conditions (trachoma keratopathy, glaucoma, refractive error, dry eye), use of glasses, ultraviolet light exposure, residential area (urban or rural), and systemic conditions. All patients underwent ophthalmological examinations, and the questionnaires were completed by one ophthalmologist. Examinations included a thorough systemic examination and a comprehensive ophthalmic examination and refraction. Severity of dry eye was evaluated by available tests and classic grading systems such as fluorescein staining pattern and tear breakup time (TBUT) tests.
At least 394 samples were needed to have a power of 80% to detect a difference as large as 10% in the prevalence of exposure between the case and control groups when the possibility for type I error was 0.05.
To check for the normality of distribution of the data, we used the Kolmogorov-Smirnov test and quantile-quantile (q-q) plot. We presented the data as mean, standard deviation, median, range, frequency, and percentage. To evaluate the differences between groups, we used the Chi-square test, Fisher's exact test, t-test, and Mann-Whitney test. To evaluate the simultaneous effect of all variables and to obtain the adjusted odds ratio (AOR), we used multiple logistic regression. Then, we used the backward step selection method (based on likelihood ratio test) with entry criteria of 0.05 and exclusion criteria of 0.10. All statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY:IBM Corp.).
RESULTS
A total of 420 patients were enrolled in this study: 210 patients with pterygium/pinguecula (case group) and 210 healthy participants (control group). The mean ages of the case and control groups were 50.8 ± 14.8 years (range, 20 to 92 years) and 41.9 ± 20.4 years (range, 6 to 84 years), respectively. In univariate analysis, age (P = 0.001), sex (P = 0.001), working outdoors (P = 0.001), family history of pterygium (P = 0.001), positive history of smoking (P < 0.001), history of baking (P = 0.045), welding experience (P < 0.001), severe blepharitis (P < 0.001), hyperopia (P < 0.001), dry eye (P < 0.001), hypertension (P < 0.001), ischemic heart disease (P < 0.001), obesity (P = 0.038), and primary residential area (P = 0.025) were found to have significant associations with increased incidence of pterygium [Table 1].
Table 1.
Demographic data of patients in cases and controls
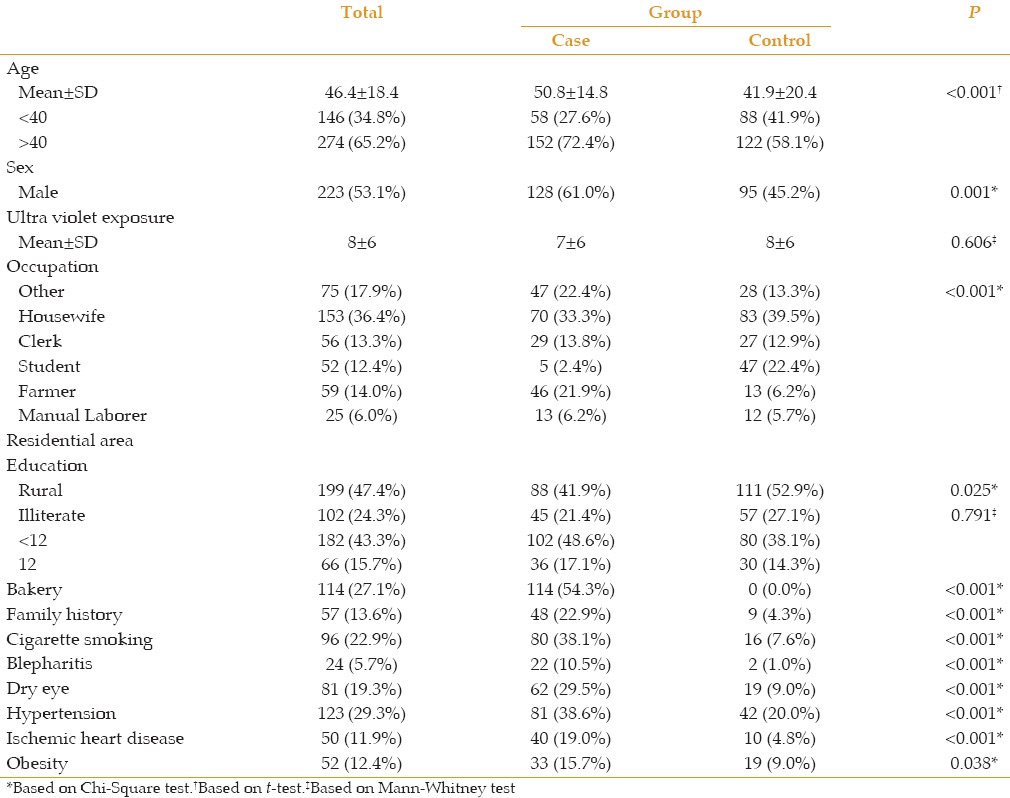
The levels of education of the patients were classified into three groups: illiterate, undergraduate diploma, and diploma or higher. There was no significant association between the level of education and prevalence of pterygium (P = 0.791).
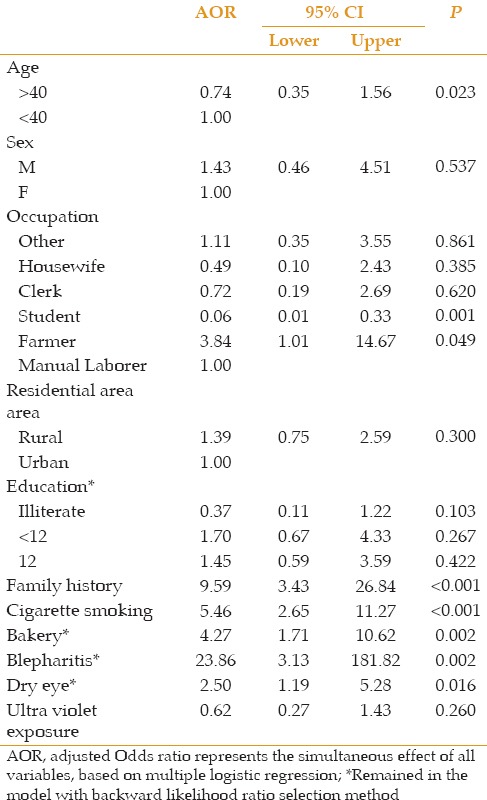
In multivariate analysis, only family history, cigarette smoking, history of baking, age, and severe blepharitis, were significantly associated with higher incidence of pterygium (P < 0.001, P < 0.001, P = 0.002, P = 0.023, and P = 0.002, respectively).
Factors such as sex (P = 0.537), residential area (P = 3.00), level of education [illiterate (P = 0.103), undergraduate diploma (P = 0.267), diploma (P = 0.422)] and ischemic heart disease (P = 0.242) were not significantly associated with increased incidence of pterygium [Table 2].
Table 2.
Significance of the risk factors based on multivariate analysis
DISCUSSION
Despite the undetermined pathogenesis of pterygium, its risk factors are well known and have been studied extensively. Considering the geography and demographics of Ilam, the estimated prevalence of pterygium in the local population is projected to be high. Even though there have been many studies on pterygium worldwide, few have been conducted in Iran, and none have been conducted on the dry, high altitude, and inbred population of Ilam.[1,2,3,4]
In this study, the prevalence of pterygium was significantly associated with increasing age, which is similar to almost all epidemiologic studies on different ethnicities.[6,9,24] Early modification of risk factors is immensely relevant for the older population since pterygium could result in significant astigmatism and visual impairment, which may affect their visual function, lifestyle, and productivity.
The evidence on the role of sex in the pathogenesis of pterygium is contradictory; some studies reported a higher prevalence in one sex, but others failed to find the association.[6,25,26,27] In univariate analysis, we found a higher prevalence of pterygium in male patients, but it was not significant in multivariate analysis. It seems that the role of sex is relevant in the social and professional context; once it is corrected for these parameters, it is no longer a risk factor.
There is no consensus regarding the pathogenesis of pterygia, but epidemiological evidence strongly supports its association with other sun-related disorders and validates the concept that ultraviolet radiation plays a major role in the development of pterygium.[2]
Sun exposure is implicated as a risk factor in low altitude, primary residential area, occupation, and current habits of population.
In agreement with previous studies, outdoor work (farmers) and residence in rural area were significantly associated with higher prevalence of pterygia.[28,29]
The association between low education and prevalence of pterygium is a matter of debate. While some investigators found statistically significant association between lower levels of education and pterygia, others failed to prove the connection.[27,28,30] In the present study, we did not find lower educational level to be associated with higher prevalence of pterygium.
There was a significant association between pterygium and cigarette smoking in our study. Cigarette smoking is one of the most common lifestyle-related exposures, and recently has been linked to the occurrence of pterygium.[26] The association between smoking and pterygium is inconsistent among studies, most probably due to selection bias.[28] Using a systematic approach, Rong et al, summarized the association between smoking and pterygium, and showed that cigarette smoking had a protective effect against pterygium with pooled odds ratio of 0.82 and 95% confidence interval (0.69 to 0.97).[31] In contrast to the findings of Rong et al, we found that cigarette smoking was a risk factor in the case group. Their observational study may have selection bias, which limits the ability of making a convincing conclusion. There is also an undetermined dose-response relationship between smoking and the risk of pterygium. The biological pathways that link cigarette smoking with risk reduction are not well understood, and until more compelling evidence is available, it is hard to draw concrete conclusions based on current observational studies.
Family history of pterygium was found to have a significant association with increased prevalence of pterygium in the current study. Hereditary predisposition to pterygium development has not been highlighted in other studies. Several modes of inheritance have been reported such as autosomal dominant, autosomal recessive, sex linked, and non-Mendelian modes of inheritance.[32] However, current studies rely on self-reported data, which may be biased. Thus, larger studies are needed to increase cogency.
We also found higher prevalence of pterygium among those with a history of bread baking, which has not been reported before. Baking in its traditional form in rural areas is accompanied by high levels of smoke exposure and dry conditions. Both of these are associated with inflammation and interfere with normal tissue repair. Inflammation and repetitive micro trauma have been proposed as possible causes of pterygium.[2]
Blepharitis was associated with higher risk of pterygia in our study. Although the role of blepharitis has not been reported in literature, an inflammatory reaction is a well-established pathogenesis of pterygium. We hypothesize that blepharitis induced inflammation, which increased the number of reactive oxygen species that play a role in phosphorylation of the cell membrane and increased the products of lipid metabolism.[32] Inflammatory cells are present in all pterygium samples, which indicates inflammation as a causative step in pterygium development.[19]
Ilam province is located in the west of Iran, with dry and hot weather. It is dusty for most days of the year in this province. Dust is a known risk factor for pterygium development,[16] which explains the high prevalence of pterygium in spite of the high altitude of the region.
Compared to previous studies, this study reports more risk factors related to the prevalence of pterygium. It also found blepharitis and baking as previously undetected risk factors and confirmed the already established risk factors.
In conclusion, health system plans and strategies should target the dusty and polluted environment of Ilam province to decrease the prevalence of pterygium. Since limiting sun exposure has proven protective effect against pterygium development, the enhancement of cultural acceptance of sunglasses and brimmed hats could effectively reduce the prevalence of pterygium and related visual impairments.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Buratto L, Phillips RL, Carito G, editors. Pterygium Surgery. Thorofare, NJ: Slack Inc; 2000. pp. 21–25. [Google Scholar]
- 2.Coster D. Pterygium: An ophthalmic enigma. Br J Ophthalmol. 1995;79:304–305. doi: 10.1136/bjo.79.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaros PA, DeLuise VP. Pingueculae and pterygia. Surv Ophthalmol. 1988;33:41–49. doi: 10.1016/0039-6257(88)90071-9. [DOI] [PubMed] [Google Scholar]
- 4.Taylor HR. Aetiology of climatic droplet keratopathy and pterygium. Br J Ophthalmol. 1980;64:154–163. doi: 10.1136/bjo.64.3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanski JJ. Clinical Ophthalmology: A Synopsis. 6th ed. Oxford: Butterworth-Heinemann Ltd; 2007. pp. 242–243. [Google Scholar]
- 6.Shiroma H, Higa A, Sawaguchi S, Iwase A, Tomidokoro A, Amano S, Araie M. Prevalence and risk factors of pterygium in a southwestern island of Japan: The Kumejima Study. Am J Ophthalmol. 2009;148:766–771. doi: 10.1016/j.ajo.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Wong TY, Foster PJ, Johnson GJ, Seah SK, Tan DT. The prevalence and risk factors for pterygium in an adult Chinese population in Singapore: The Tanjong Pagar survey. Am J Ophthalmol. 2001;131:176–183. doi: 10.1016/s0002-9394(00)00703-0. [DOI] [PubMed] [Google Scholar]
- 8.Fotouhi A, Hashemi H, Khabazkhoob M, Mohammad K. Prevalence and risk factors of pterygium and pinguecula: The Tehran Eye Study. Eye (Lond) 2009;23:1125–1129. doi: 10.1038/eye.2008.200. [DOI] [PubMed] [Google Scholar]
- 9.Cajucom-Uy H, Tong L, Wong TY, Tay WT, Saw SM. The prevalence of and risk factors for pterygium in an urban Malay population: The Singapore Malay Eye Study (SiMES) Br J Ophthalmol. 2010;94:977–981. doi: 10.1136/bjo.2008.150847. [DOI] [PubMed] [Google Scholar]
- 10.West S, Munoz B. Prevalence of pterygium in Latinos: Proyecto VER. Br J Ophthalmol. 2009;93:1287–1290. doi: 10.1136/bjo.2008.152694. [DOI] [PubMed] [Google Scholar]
- 11.Panchapakesan J, Hourihan F, Mitchell P. Prevalence of pterygium and pinguecula: The Blue Mountains Eye Study. Aust N Z J Ophthalmol. 1998;26(Suppl 1):S2–S5. doi: 10.1111/j.1442-9071.1998.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 12.Norn MS. Prevalence of pinguecula in Greenland and in Copenhagen, and its relation to pterygium and spheroid degeneration. Acta Ophthalmol (Copenh) 1979;57:96–105. doi: 10.1111/j.1755-3768.1979.tb06664.x. [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Wang Z, Lu P, Chen X, Zhang W, Shi K, et al. Pterygium in an aged Mongolian population: A population-based study in China. Eye (Lond) 2009;23:421–427. doi: 10.1038/sj.eye.6703005. [DOI] [PubMed] [Google Scholar]
- 14.Tan CS, Lim TH, Koh WP, Liew GC, Hoh ST, Tan CC, et al. Epidemiology of pterygium on a tropical island in the Riau Archipelago. Eye (Lond) 2006;20:908–912. doi: 10.1038/sj.eye.6702046. [DOI] [PubMed] [Google Scholar]
- 15.Ashaye AO. Pterygium in Ibadan. West Afr J Med. 1991;10:232–43. [PubMed] [Google Scholar]
- 16.Gazzard G, Saw SM, Farook M, Koh D, Widjaja D, Chia SE, et al. Pterygium in Indonesia: Prevalence, severity and risk factors. Br J Ophthalmol. 2002;86:1341–1346. doi: 10.1136/bjo.86.12.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemesure B, Wu SY, Hennis A, Leske MC. Nine-year incidence and risk factors for pterygium in the Barbados eye studies. Ophthalmology. 2008;115:2153–2158. doi: 10.1016/j.ophtha.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Saw SM, Banerjee K, Tan D. Risk factors for the development of pterygium in Singapore: A hospital-based case-control study. Acta Ophthalmol Scand. 2000;78:216–220. doi: 10.1034/j.1600-0420.2000.078002216.x. [DOI] [PubMed] [Google Scholar]
- 19.Threlfall TJ, English DR. Sun exposure and pterygium of the eye: A dose-response curve. Am J Ophthalmol. 1999;128:280–287. doi: 10.1016/s0002-9394(99)00161-0. [DOI] [PubMed] [Google Scholar]
- 20.Khoo J, Saw SM, Banerjee K, Chia SE, Tan D. Outdoor work and the risk of pterygia: A case-control study. Int Ophthalmol. 1998;22:293–298. doi: 10.1023/a:1006340822308. [DOI] [PubMed] [Google Scholar]
- 21.Lu P, Chen X, Kang Y, Ke L, Wei X, Zhang W. Pterygium in Tibetans: A population-based study in China. Clin Experiment Ophthalmol. 2007;35:828–833. doi: 10.1111/j.1442-9071.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 22.Ma K, Xu L, Jie Y, Jonas JB. Prevalence of and factors associated with pterygium in adult Chinese: The Beijing Eye Study. Cornea. 2007;26:1184–1186. doi: 10.1097/ICO.0b013e318151f9c6. [DOI] [PubMed] [Google Scholar]
- 23.Detels R, Dhir SP. Pterygium: A geographical study. Arch Ophthalmol. 1967;78:485–491. doi: 10.1001/archopht.1967.00980030487014. [DOI] [PubMed] [Google Scholar]
- 24.Asokan R, Venkatasubbu RS, Velumuri L, Lingam V, George R. Prevalence and associated factors for pterygium and pinguecula in a south Indian population. Ophthalmic Physiol Opt. 2012;32:39–44. doi: 10.1111/j.1475-1313.2011.00882.x. [DOI] [PubMed] [Google Scholar]
- 25.Viso E, Gude F, Rodriguez-Ares MT. Prevalence of pinguecula and pterygium in a general population in Spain. Eye (Lond) 2011;25:350–357. doi: 10.1038/eye.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rezvan F, Hashemi H, Emamian MH, Kheirkhah A, Shariati M, Khabazkhoob M, et al. The prevalence and determinants of pterygium and pinguecula in an urban population in Shahroud, Iran. Acta Med Iran. 2012;50:689–696. [PubMed] [Google Scholar]
- 27.Rim Th, Nam J, Kim EK, Kim TI. Risk factors associated with pterygium and its subtypes in Korea: The Korean national health and nutrition examination survey 2008-2010. Cornea. 2013;32:962–970. doi: 10.1097/ICO.0b013e3182801668. [DOI] [PubMed] [Google Scholar]
- 28.Song E, Sun HP, Xu Y, Pan CW. Cigarette Smoking and Pterygium: A Propensity Score Matching Analysis. Optom Vis Sci. 2016;93:466–470. doi: 10.1097/OPX.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 29.Sun LP, Lv W, Liang YB, Friedman DS, Yang XH, Guo LX, et al. The prevalence of and risk factors associated with pterygium in a rural adult Chinese population: The Handan Eye study. Ophthalmic Epidemiol. 2013;20:148–154. doi: 10.3109/09286586.2013.763991. [DOI] [PubMed] [Google Scholar]
- 30.Marmamula S, Khanna RC, Gullapalli RN. Population based assessment of prevalence and risk factors for pterygium in south Indian state of Andhra Pradesh: The Andhra Pradesh Eye Disease study (APEDS) Invest Ophthalmol Cis Sci. 2013 doi: 10.1167/iovs.13-12529. iovs 13-12529v1. [DOI] [PubMed] [Google Scholar]
- 31.Rong SS, Peng Y, Liang YB, Cao D, Jhanji V. Does cigarette smoking alter the risk of pterygium? A systematic review and meta-analysis. Invest Ophthalmol Vis Sci. 2014;55:6235–6243. doi: 10.1167/iovs.14-15046. [DOI] [PubMed] [Google Scholar]
- 32.Anguria P, Kitinya J, Ntuli N, Carmichael T. The role of heredity in pterygium development. Int J Ophthalmol. 2014;7:563–573. doi: 10.3980/j.issn.2222-3959.2014.03.31. [DOI] [PMC free article] [PubMed] [Google Scholar]


