Abstract
Purpose:
To assess the safety and outcome of single-piece posterior chamber intraocular lens (PC-IOL) implantation in the ciliary sulcus following posterior capsular rupture during cataract surgery.
Methods:
Patients with posterior capsular rupture during cataract surgery with a single-piece acrylic IOL implanted into the ciliary sulcus were studied. Complete ocular examinations were performed after 6 months postoperatively.
Results:
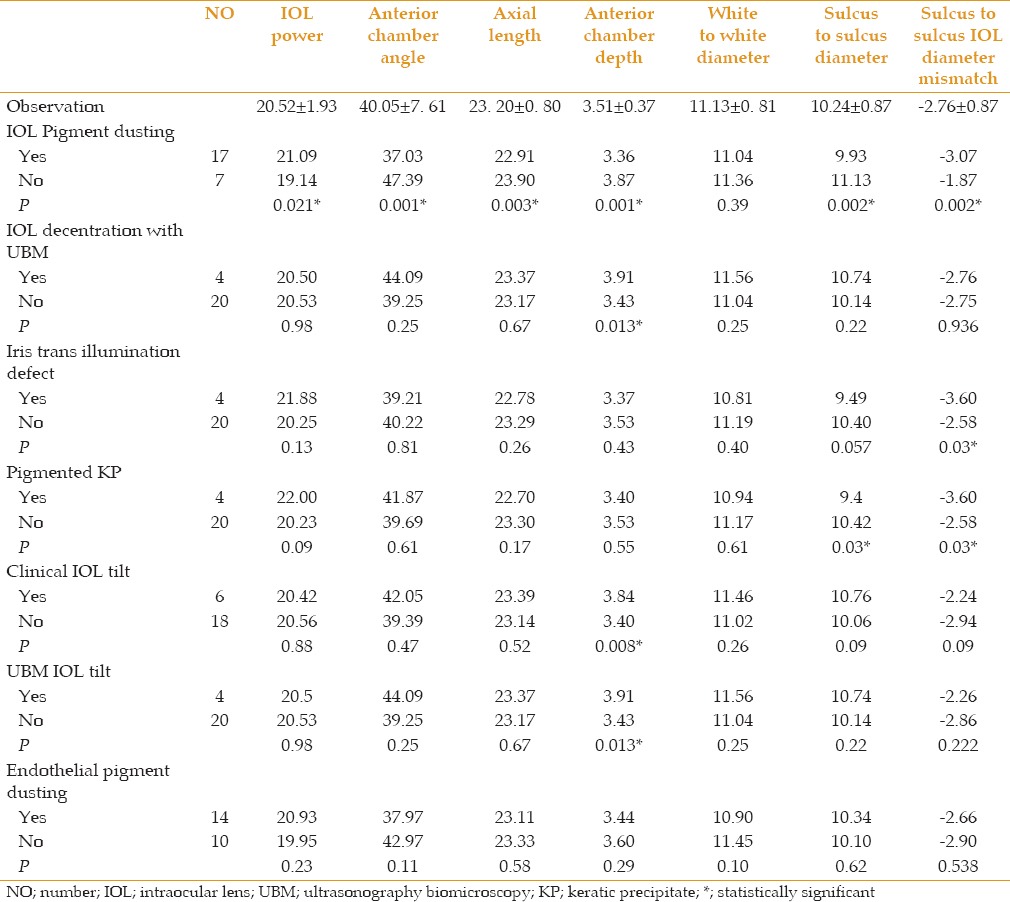
Twenty-four eyes were included. Mean follow-up duration was 8.33 ± 2.33 months. There was no significant difference between preoperative and postoperative keratometric cylinder or intraocular pressure. Visual acuity of 87.50% of patients was ≥20/40 after surgery. Complications included foveopathy (10 eyes), iris transillumination defect (4 eyes), iris chafing (2 eyes), pigmented keratic precipitate (KP) (4 eyes), clinical IOL tilt (6 eyes), endothelial pigment dusting (14 eyes), IOL pigment dusting (17 eyes), iris bowing (6 eyes), IOL decentration (4 eyes), and IOL tilt detected with ultrasonography biomicroscopy (UBM) (4 eyes). IOL pigment dusting was significantly higher in eyes with short axial lengths, high IOL power, small sulcus-to-sulcus (STS) diameter, large STS IOL diameter mismatch, and small anterior chamber depth and angle. Significant relationships were observed between pigmented KP with small STS diameter and large STS IOL diameter mismatch, UBM and clinical IOL tilt with large anterior chamber depth and between iris transillumination defect and STS IOL diameter mismatch.
Conclusion:
This implantation is associated with higher incidence of complications. Single-piece acrylic IOLs are not designed for sulcus implantation. However, they may be used in eyes with longer axial length if the 3-piece IOL is not available.
Keywords: Cataract Surgery, Single-piece Intraocular Lens, Ciliary Sulcus Implantation
INTRODUCTION
Posterior capsular rupture is one of the most frequent complications of cataract surgery (1.92%-4.4%).[1,2] It imposes a dilemma in intraocular lens (IOL) implantation due to weakness of capsular support for immediate and long-term IOL stability and centration. Several options including aphakia, same-session or later scleral fixation, anterior chamber iridocorneal sulcus or iris-claw fixation, in-the-bag fixation, and ciliary sulcus fixation (with and without anterior capsule capture) are available in the event of posterior capsular rupture.[3,4] The ciliary sulcus is a suitable location due to its anatomic aspect for IOL implantation following capsular rupture.[3,4] The characteristics of a suitable IOL for sulcus implantation include large optics and thin haptics to reduce IOL decentration and improve the view of the retina in case of vitrectomy due to complications, and reduced scratching of the posterior iris surface, respectively. The iris alternation due to contact was influenced by several parameters including material, thickness and size of the IOL, implantation technique, and the haptics angle.[5] Complications may be reduced by monitoring these factors.
A single-piece IOL might result in increased complications, including uveitis, hyphema, glaucoma, and vitreous hemorrhage, due to its larger contact compared to the thin contact of a 3-piece IOL.[6,7,8,9] Subsequently, the suggestion of a 3-piece IOL for sulcus implantation was preferred to a single-piece IOL.[6,7,8,9] There were conflicting data regarding single-piece IOL in sulcus implantation. Definitive results of surgical outcomes and safety studies of sulcus implantation of single-piece IOL have not been reported. Despite this, single-piece IOL ciliary sulcus implantation after rupture of the posterior capsule is becoming popular.[10] This is partly due to lower availability of 3-piece IOLs. Several studies have reported the safety and appropriate centration of single-piece IOL in the sulcus[11,12] that are inconsistent with the results of other studies,[6,7,8,9,13] which reported several complications including vitreous hemorrhage, iris chafing, and uveitis-glaucoma-hyphema (UGH). Additional complications included elevated intraocular pressure (IOP), iris transillumination defects, intraocular hemorrhage, and cystoids macular edema.[10] In this study, we evaluated the surgical outcomes and safety of implanting a single-piece IOL in the ciliary sulcus, and explored the safety requirements.
METHODS
Setting
This retrospective observational case series was performed in a university affiliated academic institute. Surgical records from 2012 to 2014 were reviewed and patients with a posterior capsular rupture during cataract surgery who had received single-piece acrylic IOL implants in the ciliary sulcus were selected.
IOL Characteristics
The IOL was a posterior chamber hydrophobic acrylic single-piece intraocular lens. It is 6 mm in diameter, biconvex, square-edged optic, 13 mm in overall diameter, with a zero-degree angle of haptics-optic configuration, a water content <5%, and a refractive index of 1.5. The material is acrylic and hydrophobic.
Data Collection and Examinations
The selected subjects were invited for a follow-up visit after 6 months postoperatively. Demographic data, baseline keratometry, IOL power, and axial length were obtained from medical records. Patients with a history of glaucoma, uveitis, and retinal detachment before cataract surgery, an axial length less than 21 mm or longer than 25.5 mm, a calculated IOL power of 25 diopters or greater, pseudoexfoliation syndrome, or zonular defects were excluded.
Distance corrected visual acuity (DCVA) was determined. IOPs were measured. The anterior segment was examined meticulously and iris transillumination defects, pigment dispersion, posterior iris bowing, iris atrophy, pupil distortion, corneal edema, endothelial cell dusting, keratic precipitates, and IOL tilt and centration were recorded. Best effort was made to diagnose and rule out a clinical cystoid macular edema by a vitreoretinal surgeon (FG). IOL rotational orientation, tilt (displacement of one edge of the IOL's optic from the pupil margin ≥100 μm),[14] Centration (decentration was identified as displacement of the center of the IOL's optic from the center of the pupil ≥0.5 mm), haptic fixation site, iris chafing, anterior and posterior iris bowing, edema of ciliary body, zonulysis, and anterior chamber angle and depth were assessed by ultrasonography biomicroscopy (UBM).
Statistical analysis
For statistical analysis, the SPSS software version 19 was used. Normality of the data distribution was not confirmed using a Shapiro-Wilk test. The Man-Whitney U test was used to assess the relationship between the postoperative adverse outcome and the baseline characteristics such as biometric detection of pigment dusting. A Wilcoxon test was used for paired measurements, such as IOP. A P value <0.05 was considered statistically significant.
Institutional review board approval was obtained for this study from Tehran University of Medical Sciences. The Declaration of Helsinki was followed and informed consent was obtained from patients.
RESULTS
Demographic Data
Twenty-four eyes of 24 patients were included; the mean age of participants was 61.71 ± 13.23 years (range, 28-80). The proportion of male to female subjects and right eye to left eye were equal (12:12). The mean postoperative duration was 8.33 ± 2.33 months, with a range of 6-14 months.
Clinical Findings
The mean number of pre- and postoperative keratometric cylinder was -1.23 ± 1.07 and -0.79 ± 0.52, respectively (P = 0.175). The mean keratometry, axial length, and IOL power were 43.85 ± 1.65 diopters, 23.20 ± 0.80 mm, and 20.88 ± 1.77 diopters, respectively. The mean pre- and postoperative IOP were 15.00 ± 2.64 and 15.42 ± 3.91 mmHg (P = 0.58). The observed visual acuities of patients were 20/20 (12 patients), 20/25 (5 patients), 20/30 (3 patients), 20/40 (1 patient), 20/60 (1 patient), 20/100 (1 patient), and 20/400 (1 patient).
Complications
Foveopathy in the form of pigmentary changes and absence of the foveal reflex was observed in 10 patients (41.67%). This might be an indication of a resolved cystoid macular edema. Iris transillumination defect, iris chafing, keratic precipitates (KP), clinical IOL tilt, endothelial pigment dusting, IOL pigment dusting, and IOL decentration with UBM were observed in 4 (16.6%), 2 (8.3%), 4 (16.6%), 6 (25%), 14 (58.3%), 17 (70.8%), and 4 eyes (16.6%), respectively. No patient required a second operation for IOL exchange. The different anatomic characteristics between patients with and without complications are summarized in Table 1. IOL pigment dusting was significantly more common in shorter eyes with higher IOL powers, smaller sulcus-to-sulcus (STS) diameters, larger STS-IOL diameter mismatch, and smaller anterior chamber depth and anterior chamber angle. Significant relations were observed between pigmented KPs with smaller STS diameter (P=0.03) and larger STS IOL diameter mismatch (P=0.03), between UBM and clinical IOL tilt with larger ACD ((P=0.013 and 0.008 respectively), and between iris transillumination defect and STS IOL diameter mismatch (P=0.03).
Table 1.
The complications and differences of anatomic characteristics between the patients with and without complications with single-piece intraocular lens in sulcus implantation
UBM Findings
The mean ACA, ACD, white-to-white (WTW) diameter, STS diameter, and STS IOL diameter mismatch were 40.05 ± 7.61°, 3.51 ± 0.37 mm, 11.13 ± 0.81 mm, 10.24 ± 0.87 mm and -2.76 ± 0.87 mm, respectively. The IOL haptic orientation using UBM was 180° in 3 patients, 90° in 8 patients and oblique in 13 patients (considering a routine temporal approach surgery). The haptic was fixed in the sulcus in 23 eyes (95.83%) and iris root flanking in 1 eye (4.17%). Iris bowing was observed in 6 patients, including 3 patients with anterior and 3 patients with posterior bowing [Figure 1]. IOL decentration (4 eyes) and tilt with UBM (4 eyes) were observed [Figure 1]. A Soemmering ring was observed in 1 eye [Figure 1] and iris chafing was observed in 2 eyes.
Figure 1.
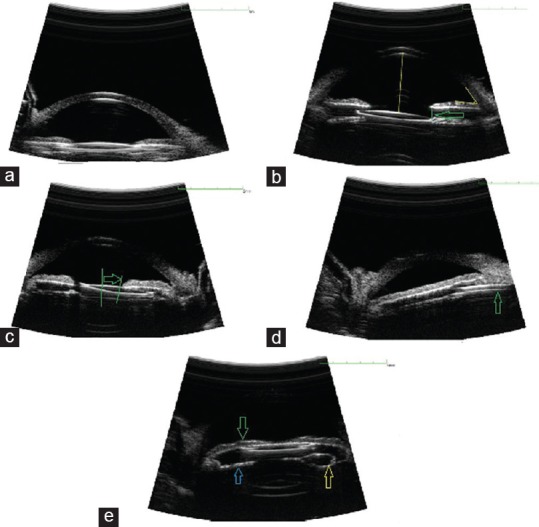
Ultrasound biomicroscopy sample images of a single-piece intraocular lens (IOL) implanted into the ciliary sulcus. (a) Well-positioned posterior chamber (PC)-IOL in the ciliary sulcus, (b) tilt of IOL's optic (displacement of one edge of IOL's optic from margin of pupil >100 µm [arrow]), (c) IOL decentration (displacement of the center of IOL's optic from the center of the pupil >0.5 mm [distance between 2 green lines]), (d) iris root flanking by the IOL haptic (arrow), (e) anterior bowing of iris (green arrow) and Soemmering ring formation (yellow and blue arrows).
DISCUSSION
The literature is paradoxical with respect to the safety of single-piece IOL implantation in the ciliary sulcus. In this investigation, 87.50% of eyes had a final visual acuity better than 20/40. Only one patient had refractory diffuse cystoid macular edema related to a diabetic state. A range of 62% to 100% of distance corrected visual acuity (DCVA) better than 20/40 was reported in previous studies.[13,15] Similar results were reported with respect to DCVA equal to or better than 20/40 in 82.02% of participants by Taskapili et al.[11] All patients in the study by Harvey et al had a DCVA better than 20/40.[13] Vision better than 20/30 in all patients was also reported by another study,[16] and this appropriate result might be related to the lack of IOL decentration or tilt. All of these results support acceptable DCVA because of sulcus implantation of a single-piece IOL. The visual outcomes of our patients were acceptable, but our follow-up time was short. Therefore, the visual outcomes of our patients were not definitive.
There was no significant difference between the pre- and postoperative mean keratometric cylinder in the current study. The mean amount of postoperative cylinder in cases with clinical IOL tilt and UBM tilt were reported to be 1.25 and 1.31 diopters, respectively. Taskapili et al[12] compared the severity of astigmatism with single-piece IOL in the sulcus with the polymethylmeth- acrylate (PMMA) IOL, and they observed a significantly higher astigmatism in the latter group (0.5 diopters compared to 1.11 diopters). The larger size of the incision and the suturing technique were stated as possible explanations of this difference.[12]
The change of postoperative IOP was not significant in this study; similar to the results of Masket[17] and Loya et al[14] One of our patients had a postoperative IOP 24 mmHg, but the preoperative IOP was the same and due to normal cup/disc ratio, antiglaucoma medication was not prescribed. A prevalence of 1.96% was reported for development of pigmentary glaucoma after sulcus implantation.[18] Although Taskapili et al[12] reported high IOP in 13.48% of single-piece IOL recipients and 6.94% of PMMA recipients, their series used anti- glaucoma medication before operation (13.48% and 6.94% respectively). Harvey et al reported a mean time of 13 months for development of secondary glaucoma with a prevalence of 15%.[13] The timing of secondary glaucoma development was uncertain; the time frame ranged from 2 weeks to as long as 7 years.[16,19] This glaucoma can be attributed to pigment dispersion that occurs in sulcus IOL implantation, and it may continue to occur, which can be determined by long-term follow-ups. The average time of secondary glaucoma development reported by Chang et al[19] was 21 months. The follow-up time of our patients was from 6 months to 14 months, which is not enough to make a definite conclusion about the risk of developing glaucoma. Therefore, performing gonioscopy to observe pigment deposition may help predict the occurrence of secondary glaucoma, so the long-term follow-up of these patients is suggested.
The iris transillumination defect was observed in 16.67% of our patients, comparable to the results of other studies, with a prevalence of 3% to 20%.[17,18] The STS-IOL total diameter mismatch was greater in patients with compared to patients without this complication. The prevalence of iris transillumination varied (0% to 80%).[10,16] A previous investigation suggested the thick iris of Asian populations might explanation the reported lack of iris defects, despite observations of pigment dispersion. Decreased manipulation during surgery may reduce the iris trauma and may prevent occurrence of this complication. As the single-piece acrylic IOLs are not designed for sulcus implantation, it may be better not to implant them in the ciliary sulcus.
The IOL and endothelial pigment dusting and pigmented KPs were observed in 70.83%, 58.33%, and 16.67% of our patients, respectively. A significant correlation was found between IOL pigment dusting and shorter axial length, higher IOL power, smaller ACA, and ACD, shorter STS, and greater STS-IOL total diameter mismatch. The pigmented KPs occurred more frequently in the shorter STS. These observations denote that more crowding of the anterior segment was associated with a higher rate of pigment dispersion. Pigment dispersion occurred as a result of continuous mechanical contact between the posterior surface of the iris by haptics, and can be considered a predisposing factor for development of secondary glaucoma.[16] The range of 20% to 100% was reported for development of pigment dispersion.[16,17] This discrepancy might be explained by a different identification method of pigment dispersion in different studies. Some argue that a close apposition between the haptics and the most peripheral area of the iris may prevent or reduce active friction and pigment dispersion.[16] However, rate of pigment release was high in our patients, and the possibility of resultant development of secondary glaucoma in the future may become problematic. We also assessed the determinants of pigment dispersion and found that smaller STS IOL diameter and higher mismatch were risk factors.
IOL decentration with a DCVA of 20/25 occurred in 4 patients, so the correction or exchange of IOL was not indicated. The approximate prevalence of 4% was reported by other studies.[11,12] The dislocation of IOL in one eye was reported by Loya et al[14] The incidence of 70% for IOL decentration was reported by Chang.[10] This high percentage was explained by their inclusion criteria; they only selected patients with complications. IOL tilt in UBM was observed in 4 patients in our study. This complication had a significant association with larger ACD. Loya et al[14] reported an incidence of 56% for IOL tilt in UBM. The intact anterior rhexis with adequate support for IOL and pupil constriction after IOL implantation were suitable for preventing IOL tilt.[16]
One can think of two detrimental processes and conditions; a significant total IOL diameter-STS diameter mismatch causes two scenarios, oversizing or undersizing. The former happens mostly in crowded eyes (similar to our pigment dispersion cases); the IOL is physically stable but the iris is dynamic and rubs against the IOL. The latter results in poor IOL fixation and possibly in constant instability in the ciliary sulcus. We could not provide a distinct observation for this subgroup.
Iris bowing was observed in 25% of our patients. In a prior study, 20% of eyes suffered forward bulging of peripheral iris that resulted in closing anterior chamber angle less than 2 hours from surgery.[16] The foveopathy (due to a presumed resolved cystoid macular edema) was observed in 10 patients in our investigation. Only 1 patient had severe visual loss. The others had only mild macular pigmentary changes without considerable effect on vision. The cystoid macular edema was reported to be 86.7% in single-piece IOL versus 16.66% in PMMA group by Taskapili et al[12] The more accurate anterior vitrectomy in the latter group was counted as a reason for this low incidence. Cystoid macular edema was related to posterior capsular rupture and accurate anterior vitrectomy other than the location of IOL implantation.
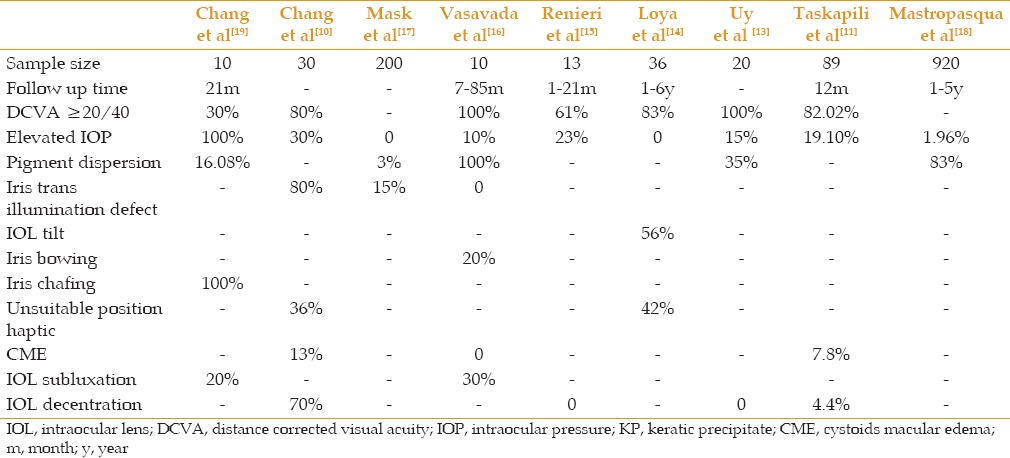
Our study design and sample size do not allow firm inferences. It is ethically complicated to randomize such subjects at the time of surgery into two groups receiving either a 3-piece or single-piece. Theoretically, it provides a best comparison scenario. At the least, a concurrent group of identical IOL design with capsular bag implantation would be very useful to determine if the adverse event frequencies are higher, and to what extent. The choice of single-piece sulcus IOL should be individualized based on the long-term rate of probable complications in different studies [Table 2].
Table 2.
The rate of different complications of using single-piece intraocular lens following capsular rupture in different studies
In conclusion, ciliary sulcus implantation of a single-piece PC IOL with a 13mm diameter remains controversial. The refractive outcome was good and seemed comparable for the first six months postoperatively.
In case of posterior capsular rupture, it is tempting to implant the IOL in the ciliary sulcus in the primary procedure provided the risk for IOL drop. However, we observed high frequency of pigment dispersion related events that causes concern over long-term ocular hypertension and uveitis. As the single piece acrylic IOLs are not designed for the sulcus implantation they must not be advised to be implanted in the ciliary sulcus in any case. However, it may be used in eyes with higher axial length if the 3-piece IOL is not available.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Desai P, Minassian DC, Reidy A. National Cataract Surgery Survey 1997-8: a report of the results of the clinical outcomes. Br J Ophthalmol. 1999;83:1336–1340. doi: 10.1136/bjo.83.12.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaycock P, Johnston RL, Taylor H, Adams M, Tole DM, Galloway P, et al. The Cataract National Dataset electronic multicentre audit of 55,567 operations: Updatingbenchmark standards of care in the United Kingdom and internationally. Eye (Lond) 2009;23:38–49. doi: 10.1038/sj.eye.6703015. [DOI] [PubMed] [Google Scholar]
- 3.Wagoner MD, Cox TA, Ariyasu RG, Jacobs DS, Karp CL. American Academy of Ophthalmology. Intraocular lens implantation in the absence of capsular support: a report by the AmericanAcademy of Ophthalmology. Ophthalmology. 2003;110:840–859. doi: 10.1016/s0161-6420(02)02000-6. [DOI] [PubMed] [Google Scholar]
- 4.Collins JF, Gaster RN, Krol WF, Colling CL, Kirk GF, Smith TJ. A. comparison of anterior chamber and posterior chamber intraocularlenses after vitreous presentation during cataract surgery. Am J Ophthalmol. 2003;136:1–9. doi: 10.1016/s0002-9394(02)01924-4. [DOI] [PubMed] [Google Scholar]
- 5.Ballin N, Weiss DM. Pigment dispersion and intraocular pressure elevation in pseudophakia. Ann Ophthalmol. 1982;14:627–630. [PubMed] [Google Scholar]
- 6.Micheli T, Cheung LM, Sharma S, Assaad NN, Guzowski M, Francis IC, Norman J, Coroneo MT. Acute haptic-induced pigmentary glaucoma with an AcrySof intraocular lens. Cataract Refract Surg. 2002;28:1869–1872. doi: 10.1016/s0886-3350(02)01644-9. [DOI] [PubMed] [Google Scholar]
- 7.LeBoyer RM, Werner L, Snyder ME, Mamalis N, Riemann CD, Augsberger JJ. Acute haptic-induced ciliary sulcus irritation associated with single-piece AcrySof intraocular lenses. Cataract Refract Surg. 2005;31:1422–1427. doi: 10.1016/j.jcrs.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 8.Toma HS, Di Bernardo C, Schein OD, Adams NA. Recurrent vitreous hemorrhage secondary to haptic-induced chafing. Can J Ophthalmol. 2007;42:312–313. [PubMed] [Google Scholar]
- 9.Masket S, editor. Consultation section. Cataract surgical problem. Cataract Refract Surg. 2007;33:1355–1361. doi: 10.1016/j.jcrs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 10.David F. Chang, MD, Samuel Masket, MD, Kevin M. Miller, MD, Rosa Braga-Mele, MD, et al. Complications of sulcus placement of single-piece acrylic intraocular lenses. Recommendations for backup IOL implantation following posterior capsule rupture. Cataract Refract Surg. 2009;35:1445–1458. doi: 10.1016/j.jcrs.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 11.Taskapili M, Engin G, Kaya G, Kucuksahin H, Kocabora MS, Yilmazli C. Single-piece foldable acrylic intraocular lens implantation in the sulcus in eyes with posterior capsule tear during phacoemulsification. 8. Cataract Refract Surg. 2005;31:1593–1597. doi: 10.1016/j.jcrs.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Taskapili M, Gulkilik G, Kocabora MS, Ozsutcu M, Yilmazli C Kaya G, Kucuksahin H. Comparison of sulcus implantation of single-piece hydrophilic foldable acrylic and polymethylmethacrylate intraocular lenses in eyes with posterior capsule tear during phacoemulsification surgery. Eur J Ophthalmol. 2007;17:595–600. doi: 10.1177/112067210701700418. [DOI] [PubMed] [Google Scholar]
- 13.Harvey Siy Uy, Pik Sha T, Chan Pigment release and secondary glaucoma after implantation of single-piece acrylic intraocular lenses in the ciliary sulcus. Am J Ophthalmol. 2006;142(2):330–332. doi: 10.1016/j.ajo.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 14.Loya N, Lichter H, Barash D, Goldenberg-cohen N, Strassmann E, Weinberger D. Posterior chamber intraocular lens implantation after capsular tear: Ultrasound biomicroscopy evaluation. J Cataract Refract Surg. 2001;27:1423–1427. doi: 10.1016/s0886-3350(01)00786-6. [DOI] [PubMed] [Google Scholar]
- 15.Giulia Renieri, Daniel Herzog, Stefan Niemann, Matthias Becker, Sabine Kurz, Hagen Thieme. Sulcus implantation of a single-piece foldable acrylic intraocular lens after posterior capsular rupture in cataract surgery. Eur J Ophthalmol. 2012;22:950–955. doi: 10.5301/ejo.5000160. [DOI] [PubMed] [Google Scholar]
- 16.Vasavada AR, Raj SM, Karve S, Vasavada V, Theoulakis P. Retrospective ultrasound biomicroscopic analysis of single-piece sulcus-fixated acrylic intraocular lenses. J Cataract Refract Surg. 2010;36(5):771–777. doi: 10.1016/j.jcrs.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Masket S. Pseudophakic posterior iris chafing syndrome. Cataract Refract Surg. 1986;12(3):252–256. doi: 10.1016/s0886-3350(86)80003-7. [DOI] [PubMed] [Google Scholar]
- 18.Mastropasqua L, Lobefalo L, Gallenga PE. Iris chafing in pseudophakia. Doc Ophthalmol. 1994;87(2):139–144. doi: 10.1007/BF01204790. [DOI] [PubMed] [Google Scholar]
- 19.Chang S, Wu W, Wu S. Late-onset secondary pigmentary glaucoma following foldable intraocular lenses implantation in the ciliary sulcus: A long-term follow-up study. BMC Ophthalmology. 2013;13:22. doi: 10.1186/1471-2415-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]


