Abstract
A systematic literature review was performed evaluating articles examining the effects of pseudoexfoliation syndrome (PEX) and glaucoma (PEXG) on the cornea with a focus on the corneal endothelium. We searched for articles relevant to pseudoexfoliation syndrome, pseudoexfoliation glaucoma and corneal endothelial cell counts using Pubmed, Google Scholar Database, Web of Science and Cochrane Library databases published prior to September of 2016. We then screened the references of these retrieved papers and performed a Web of Science cited reference search. Corneal characteristics analyzed included central corneal thickness (CCT), corneal nerve density, endothelial cell density (ECD), polymegathism, and pleomorphism. These parameters were compared in the following populations: control, PEX, PEXG, and primary open angle glaucoma (POAG). Over 30 observational studies were reviewed. Most studies showed a statistically significant lower ECD in PEX and PEXG populations compared to controls. Overall, PEX eyes had a non-statistically significant trend of lower ECDs compared to PEXG eyes. No consistent trends were found when analyzing differences in CCT amongst control, PEX and PEXG groups. For the few studies that looked at corneal nerve characteristics, the control groups were found to have statistically significantly greater nerve densities than PEX eyes, which had significantly greater densities than PEXG eyes. ECD and corneal nerve densities may be potential metrics for risk-stratifying patients with PEX and PEXG. Our literature review provided further evidence of the significant negative influence PEX has on the cornea, worsening as patients convert to PEXG.
Keywords: Pseudoexfoliation Syndrome, Pseudoexfoliation Glaucoma, Endothelial Cell Density, Corneal Nerve Density
INTRODUCTION
Pseudoexfoliation syndrome (PEX) is the leading cause of secondary open angle glaucoma (OAG).[1] The prevalence of PEX over the age of 60 is roughly 10-20%,[2] increasing to 40% over the age of 80,[3] and is highly dependent on race and ethnicity.[4] PEX is a systemic, age related microfibrillopathy characterized clinically by the production and deposition of extracellular granular material in tissues, most notably in the anterior chamber of the eye.[5] The material is classically found on the lens capsule, pupillary border, the iris, non-pigmented ciliary epithelium, lens zonules, trabecular meshwork and corneal endothelial cells. The material has also been demonstrated along vascular endothelium, corneal epithelial basement membrane and corneal stroma.[5] Although the disease is systemic, it can be highly asymmetric, with material in the fellow “normal” eye only being identified on biopsy in some patients.[6] The ocular pathologies resulting from the deposition of this material include secondary open angle glaucoma, disturbances of the pre-corneal tear film, zonular weakness and dehiscence resulting in phacodonesis, angle closure glaucoma and lens dislocation, capsular rupture and vitreous release during cataract surgery, poor pupillary dilation, blood-aqueous barrier dysfunction and corneal endothelial decompensation.[5]
The rate of conversion from PEX to PEXG is 5% in patient with PEX for 5 years, 15% at 10 years[7,8] and a 15 year risk of up to 60%.[9] Compared to primary open angle glaucoma (POAG), pseudoexfoliation glaucoma (PEXG) is more severe; it is associated with an elevated risk of blindness, higher intraocular pressures (IOP) with higher IOP fluctuations, and increased glaucoma medication resistance.[10] The mechanism of damage to the high resistance trabecular meshwork in PEXG has been shown to be unique to that of POAG.[5] Additionally, it is still uncertain if factors related to PEX other than IOP elevations influence the more severe neuropathy seen in PEXG.[5] Outside of IOP measurements, qualitatively determining the amount of pseudoexfoliation material at the trabecular meshwork and measurements of flare in the anterior chamber,[11] no clinical biomarkers are currently used to quantify the severity of PEX and PEXG or determine the risk of glaucoma development and progression in these patients. Determining the frequency of monitoring and aggressiveness of treatment in this patient population can be challenging. Recent literature has suggested the use of corneal parameters as an adjunct measurement in managing patients with PEX.[12,13,14]
Several studies have shown the influence of PEX on the cornea, specifically the corneal endothelium cell density (ECD), with multiple studies showing decreased ECDs of patients with PEX and PEXG compared to control patients.[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] Additionally, patients with PEX were found to have decreased keratocyte stromal cell counts, basal corneal epithelial cell counts, and sub-basal neural integrity[13,14,23,36] which has been correlated to the decreased corneal sensitivity seen in PEX patients.[12,13] However, it is less clear as to whether patients with PEXG have more severe corneal alterations than PEX alone without secondary glaucoma, or if these corneal alterations can be utilized in the clinic to help manage patients at risk of developing or already with glaucomatous damage associated with PEX. The objective of this paper is to review the current literature evaluating the influence of PEX on the cornea, and determine the potential use of quantifiable corneal characteristics as clinical biomarkers to risk-stratify patients with PEX and PEXG.
METHODS
We searched for articles relevant to pseudoexfoliation syndrome, pseudoexfoliation glaucoma and corneal endothelial cell counts using Pubmed, Google Scholar Database, Web of Science and Cochrane Library databases published prior to September of 2016. We then screened the references of these retrieved papers and performed a Web of Science cited reference search.
Statistical analyses were not provided for all subgroups in some studies. Using means, standard deviations, and sample sizes provided by the studies, we were able to perform statistical calculations. For the cases of non-paired data, a two-tailed student's t-test for equal variance was used. For paired data, a paired two-tailed student's t-test was performed.
Devices used in the studies varied between a number of specular and confocal microscopes. Both contact and non-contact measurement techniques were used with the specular imaging. ECD analysis ranged between completely manual analysis involving a standard grid placed over developed film with manual counting,[15] to fully automatic analysis that relied on the machine to provide all the identification and calculations.[23,25] ECD was universally reported in units of cell/mm2. Measurements of pleomorphism varied. The majority of studies reported a percentage of hexagonal cells; with a few reporting a percentage of pleomorphism, an inversely related parameter. Therefore, these measurements were converted to a hexagonal cell percentage equivalent using equation 1.
Hexagonality (%) = 100% - Pleomorphism (%) (1)
Polymegathism was quantified most commonly as a coefficient of variance in cell area (CV in cell area); however, a few studies measured a percentage of polymegathism. Since these measurements were directly related we chose not to convert these values. Central corneal thickness (CCT) was provided in micrometers in most studies. Our search did not include studies specifically focusing on CCT associations with PEX or PEXG, but we evaluated this data if present in the studies.
Four studies looked at corneal nerve parameters. They used a variety of different terminology to describe similar concepts. We standardized the terminology to long nerve fiber density (LNFD), nerve branch density (NBD), total nerve density (TND), and tortuosity. Long nerve fiber density was defined as number of major nerves (or long nerve fiber bundles) per square millimeter. Nerve branch density was defined as the number of long nerves and their branches per square millimeter. Total nerve density was defined as length of nerve fiber in millimeters per square millimeter.[23] Both studies that looked at tortuosity used similar scales, one of which[36] specifically used the Oliveira-Soto and Efron scale outlined below which grades tortuosity from 0 to 4.[37]
Grade 0: nearly straight
Grade 1: slightly tortuous
Grade 2: moderately tortuous with numerous changes in the direction of the fiber
Grade 3: very tortuous
Grade 4: extremely tortuous with significant convolutions throughout their course.
RESULTS
We identified 30 studies that measured either endothelial cell density, polymegathism, pleomorphism, corneal nerve density, or CCT, and compared these values in patients with PEX and PEXG against a control group.[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,38,39,40,41,42,43] Six of these were published in a language other than English.[18,21,40,41,42,43] One study was published as an abstract only.[39]
Of the 23 articles analyzed, [Supplementary Table 1 (4.8MB, tif) ], we found only observational studies—case-control, case-series, cross-sectional and cohort studies. Control groups mostly consisted of healthy patients with no ocular disease; a few studies also used patients with senile cataracts, or unaffected fellow eyes. For the PEX and PEXG groups, most studies excluded patients with prior ocular surgery or other ocular pathology. Eight of these studies drew from a patient population with senile cataracts undergoing cataract surgery. Seven studies: Demircan et al,[17] Hayashi et al,[38] Kaljurand et al,[20] Quiroga et al,[27] Tomaszewsk et al,[30] Wali et al,[32] and Wirbelauer et al,[35] measured ECD in PEX patients and control patients before and after surgery. Ostern et al[25] measured ECD only after surgery. We further characterized the disease groups in all of these studies by the presence of both pseudoexfoliation and glaucoma. Not every study reviewed took the presence of glaucoma into account. Therefore, we categorized disease groups into pseudoexfoliation without glaucoma (PEX only), PEXG, patients with pseudoexfoliation with unspecified glaucoma status (PEX combined), and patients with only POAG. Furthermore, studies varied in if they chose patients affected by unilateral or bilateral disease. Several studies included only patients with unilateral disease and compared the diseased eye with the unaffected fellow eye.
Study design overwiew
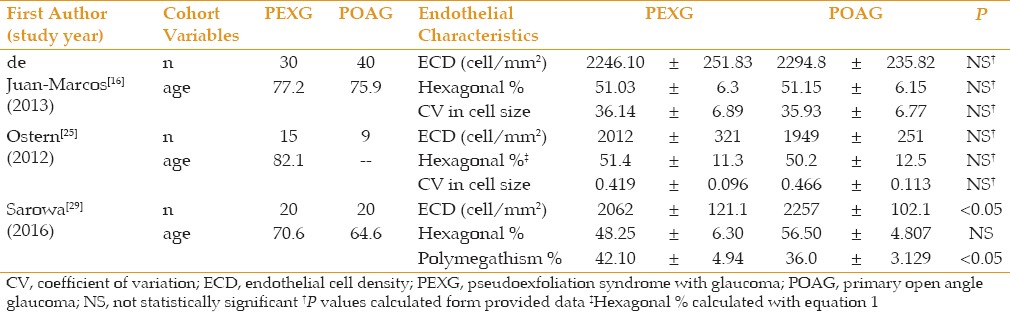
Table 1 summarizes the corneal endothelial data comparing patients with PEX combined against normal controls. All studies showed lower ECD values in patients with PEX combined; 9 of the 11 reached statistical significance. Romero et al[28] performed their analysis in stratified age groups (60-69, 70-79 and >80). They found statistically significant lower values in PEX combined patients in the two latter age groups (70-79 and >80). In the 60-69 group they saw a trend towards lower ECD in PEX combined, but without statistical significance. They did not provide data for a group that included all ages studied. Romero et al[28] was also the only study to demonstrate statistically significant increases in polymegathism and polymorphism compared to control.
Table 1.
PEX combination vs control
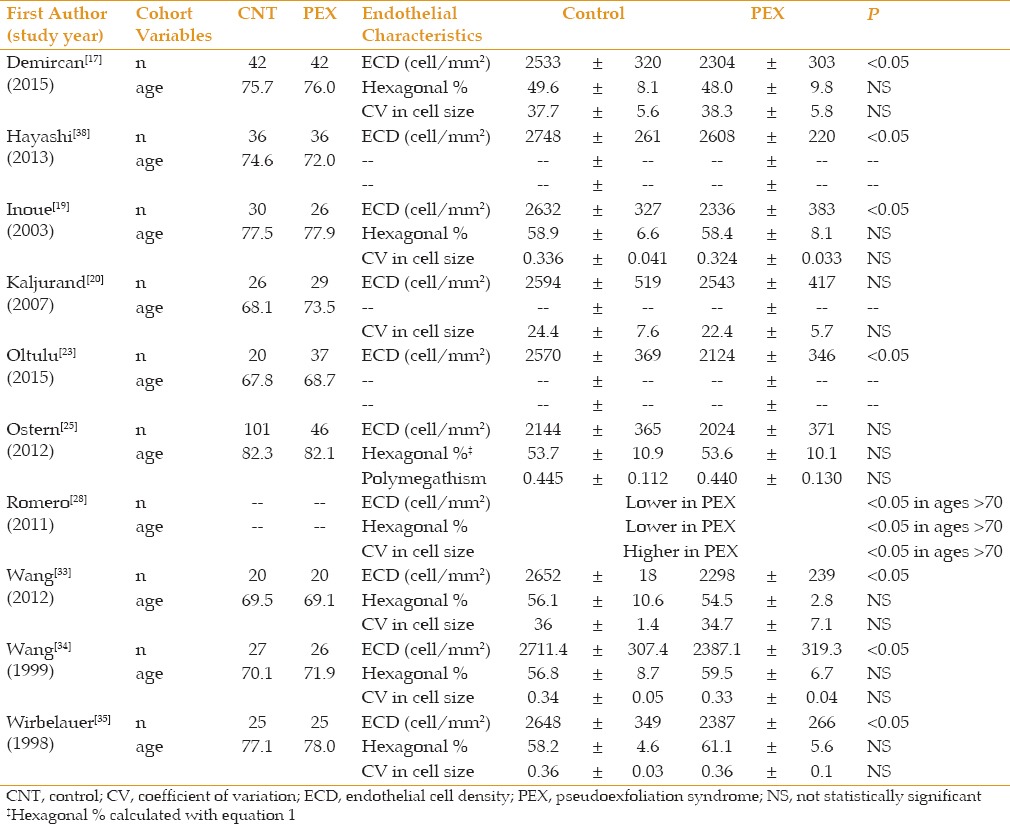
Table 2 summarizes similar data comparing patients with PEX only (without glaucoma) against normal controls. All studies showed lower ECD in the PEX only groups; 10 of the 13 studies reached statistical significance. There were stronger trends to higher values of pleomorphism (6 of 11 statistically significant) and polymegathism (5 of 11 statistically significant) in the PEX only group.
Table 2.
PEX only vs control
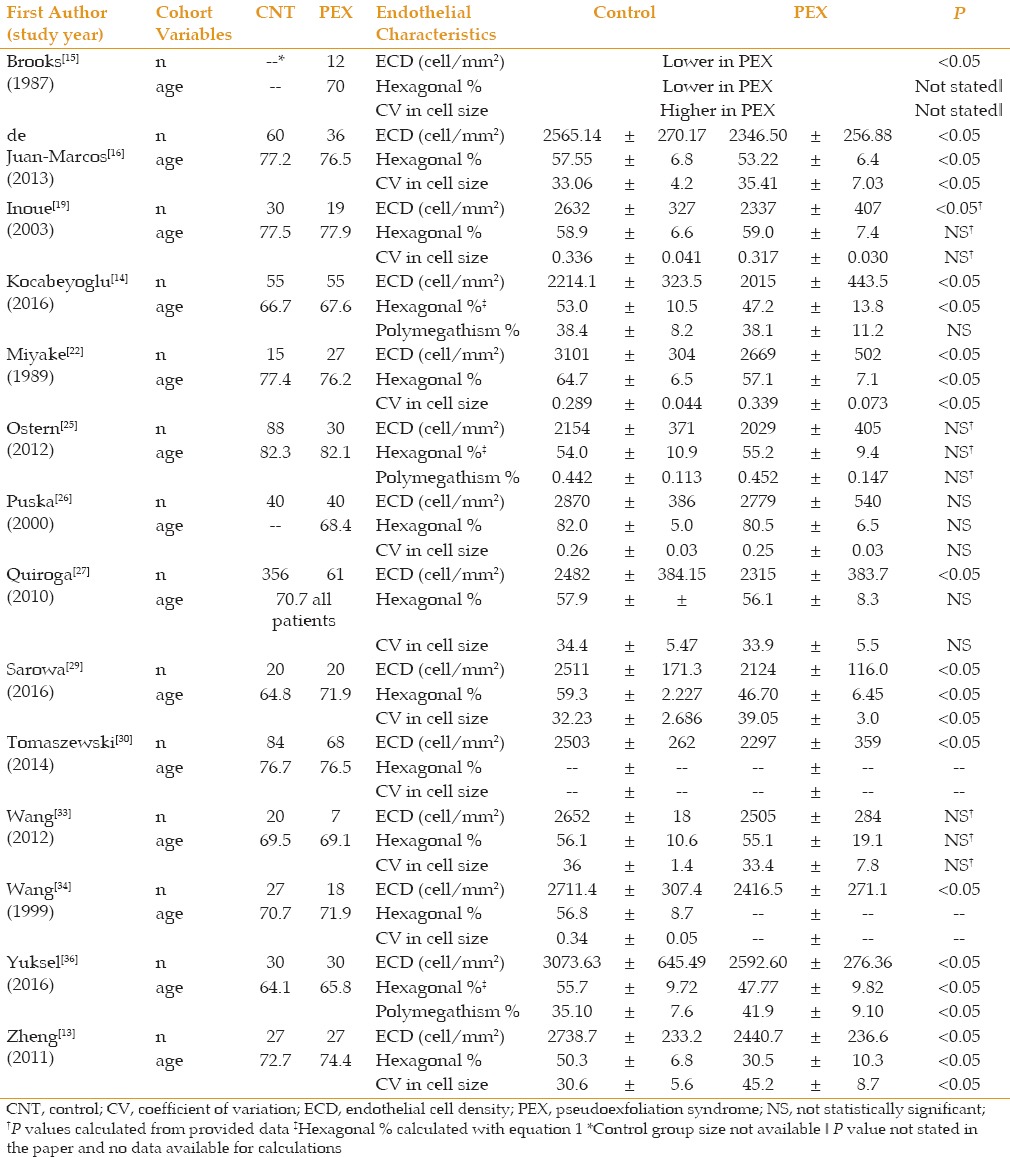
Table 3 summarizes corneal endothelial data of patients with unilateral PEX. Compared to the fellow unaffected eye, the eyes with PEX showed no clear differences in any of the corneal parameters. However, all four studies that compared clinically unaffected fellow eyes to control eyes measured a lower ECD in the unaffected fellow eye (2 of the 4 reaching statistical significance).
Table 3.
PEX vs normal fellow eye
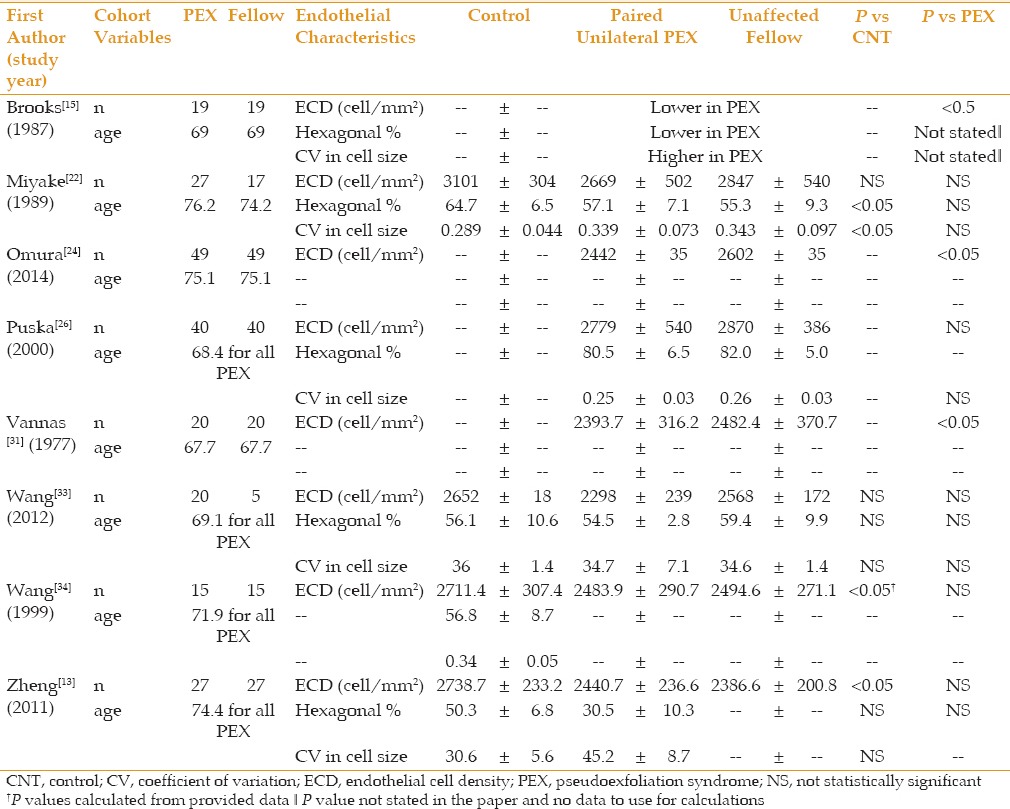
Table 4 summarizes corneal endothelial cell data comparing patients suffering from PEXG against PEX only. All studies demonstrated a trend towards lower ECD in PEXG patients versus PEX only. However, only 1 of 10 studies reached statistical significance. Average sample sizes for these groups were 31 ± 24 eyes and 26 ± 18 eyes in the PEX only and PEXG groups, respectively. There were trends toward increased polymegathism and pleomorphism in PEXG patients. In Brooks et al,[15] two patients with bilateral PEX had elevated IOPs in only one eye. When comparing these PEX eyes to the fellow PEXG eye, they saw lower ECDs and less regular cells.
Table 4.
PEX vs PEXG
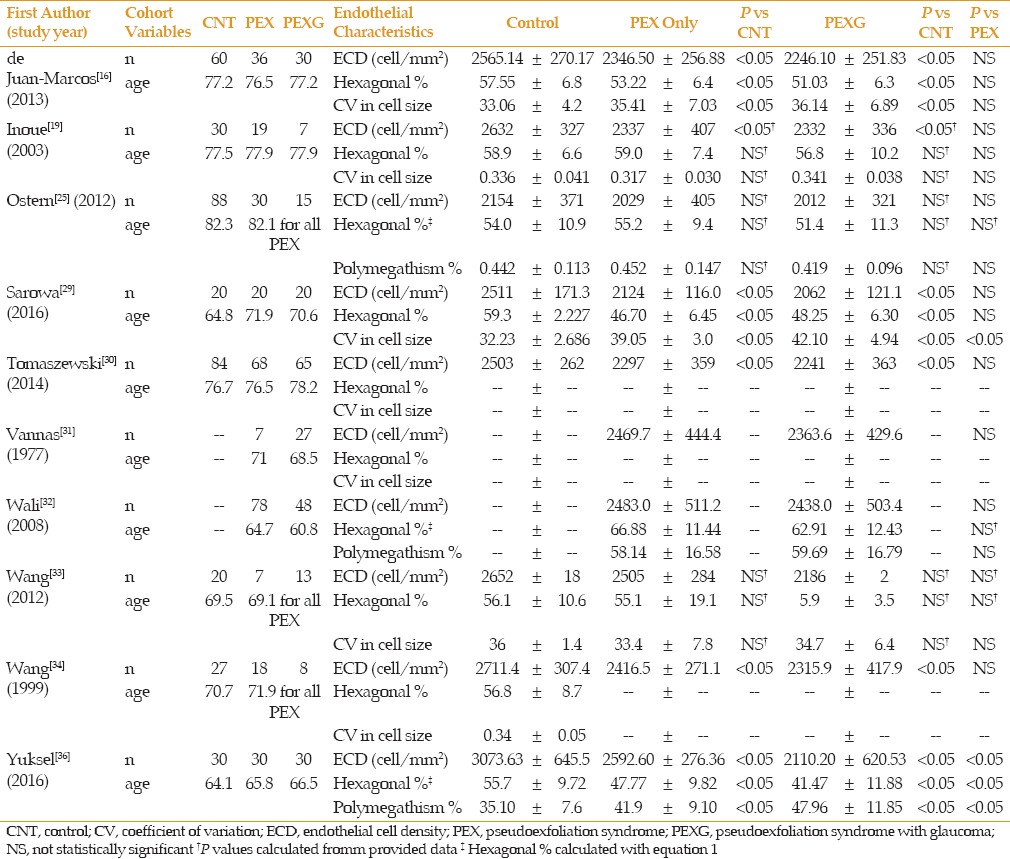
Table 5 summarizes the corneal endothelial data comparing patients with PEXG against POAG, showing no clear trend regarding the corneal parameters. Ostern et al[44] found slightly higher ECD values in the PEXG group whereas Sarowa et al[45] found higher ECD values in the POAG group.
Table 5.
PEXG vs POAG
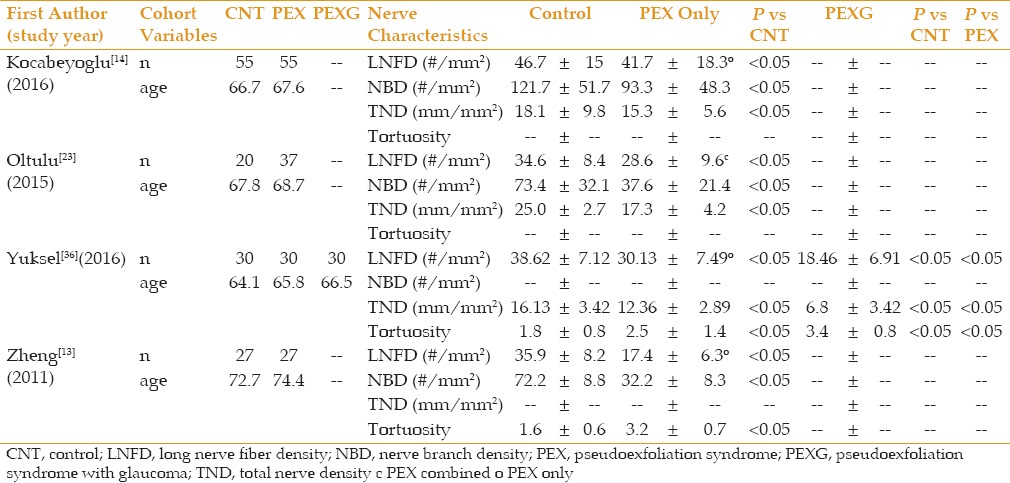
Eleven of the studies measured CCT. This data is summarized in Table 6. There was no consistent trend as to the influence of PEX or PEXG on CCT. Four studies also analyzed corneal nerve parameters, as seen in Table 7. In all studies, the nerve parameters in the control group showed significantly greater nerve density than PEX which was significantly higher than PEXG.
Table 6.
Central Corneal Thickness Data
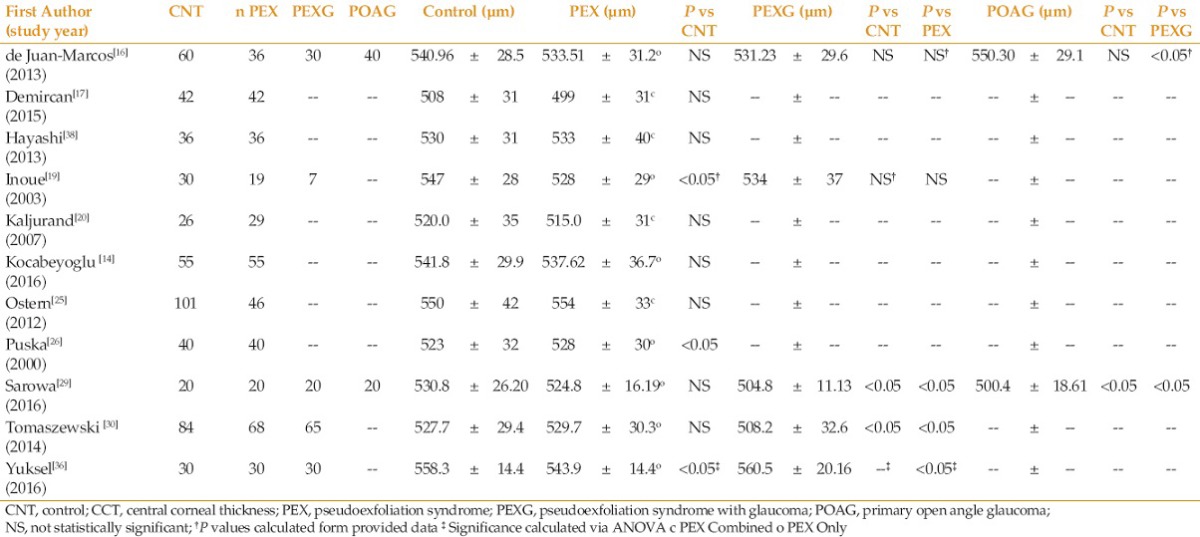
Table 7.
Corneal nerve parameters
DISCUSSION
Our literature review has consistently shown reduced ECD in PEX patients with further reductions in PEXG patients. In association with decreasing ECD, was increasing pleomorphism and polymegathism. Expectedly, polymegathism and pleomorphism measurements showed an inverse relationship to ECD. Pathophysiologically, this agrees with our understanding of the corneal endothelial response to damage. As endothelial cells are lost, the remaining cells must grow to compensate and during this process they lose their characteristic hexagonal shape and homogeneous size. The trend towards further reduction in ECD as patients progress from PEX to PEXG is likely related to endothelial damage from both elevated IOP and increased PEX severity, as endothelial loss has also been shown in patients with POAG.[46] Sarowa et al found a statistically significant lower ECD in PEXG eyes compared to POAG eyes, suggesting that elevated IOP and PEX are likely independent factors damaging the endothelium via separate mechanisms.[29]
Pseudoexfoliation associated corneal damage is likely multifactorial in etiology. Several theories exist as to why this endotheliopathy develops, including the penetration of pseudoexfoliation material towards Descemets membrane breaking the hexagonal connections and signaling of the endothelial layer and promoting apoptosis,[47,48] hypoxia to the anterior chamber with increased antioxidant stress and reduced ascorbic acid levels,[49,50,51,52,53] Changes in the blood-aqueous barrier and vascular endothelial dysfunction,[51,54,55] Compression of endothelial cells from elevated IOP,[46] Changes in levels of transforming growth factors (increased TGF-α1 and TGF-β) and ratios of MMPs (matrix metalloproteinase) and TIMPs (tissue inhibitor of metalloproteinases) promoting matrix accumulation in the affected tissues, and changes in cytokine/chemokines in the anterior chamber and cornea.[56,5] It is possible that similar or other unknown mechanisms, beyond IOP elevations, are also responsible for the increased severity of glaucomatous damage seen in PEXG relative to POAG.
Although the more severe progression of PEXG compared to POAG may be related to higher IOPs with larger fluctuations in PEXG eyes, it is still unclear if other mechanisms influence damage to the optic nerve in PEXG patients. Linner et al found increased nerve pallor in PEX patients versus control patients despite statistically equal IOP measurements.[57] The optic disc surface area in PEX eyes (both with and without glaucoma) has been reported as being smaller compared to controls.[58,59] Pulsatile ocular blood flow has been shown to be reduced in unilateral PEX compared to the unaffected fellow eye,[60] in addition to reduced laminar blood flow with progression of PEXG.[61] Several studies have linked oxidative stress, thought to be more abundant in PEX eyes, to glaucomatous progression.[62,49,63,64] More recently, PEX and PEXG eyes have been found to have significantly lower posterior choroidal thicknesses, thought to be related to increased vascular resistance, compared to fellow unaffected eyes and healthy control eyes.[65,66,67,68,69,70] As we better understand the molecular makeup of pseudoexfoliation material and its influence on the eye, it will be interesting to evaluate its role in glaucomatous optic neuropathy in PEX patients.
Brooks et al and Vannas et al were the only studies that compared ECD in PEX only eyes to a fellow PEXG eye.[15,31] Vannas et al was the only study to attempt to correlate ECD with glaucoma severity based on visual field defects.[31] Vannas et al was unable to find a correlation between ECD and the severity or length of treatment in patients with unilateral PEXG, despite finding reduced ECDs in PEXG eye in the majority of patients. In the 7 patients that were measured with both PEX and PEXG in this study, all 7 had lower or equal ECD values in the eye with PEXG compared to the eye with PEX only. This study, likely lacked the sample size and adequate enough glaucoma characterization to show correlations between PEXG severity and ECD. Future prospective studies examining correlations between corneal parameters, such as ECD, lens dislocation and PEXG severity should include not only CDRs but visual field data and OCT of the optic nerve and retinal nerve fiber layer.
PEX has been described as both a unilateral entity and as bilateral but highly asymmetric.[71,72] PEX is considered a systemic microfibrillinopathy closely linked to mutations in the lysyl oxidase-like 1 (LOXL1) gene,[51] with pseudoexfoliative material being found throughout visceral organs in affected individuals.[73,74,75] Conjunctival biopsies of patients with unilateral PEX demonstrated microscopic disease in the unaffected fellow eye.[6] Our review of the literature has shown that clinically unaffected fellow eyes do not have significant differences in ECD compared to their paired eyes with PEX, with both showing similarly decreased ECD when compared to a group of age-matched controls. The bilateral decrease in ECD of these asymmetric PEX eyes supports the theory of an asymmetric bilateral process, and provides some evidence that the corneal endothelium may be one of the earliest intraocular structures with observable (and measurable) damage secondary to PEX before the presence of pseudoexfoliative material on the lens capsule or iris.
While our focus of the literature review was on the association between ECD and pseudoexfoliation, many papers also provided data evaluating the effect of PEX and PEXG on CCT and corneal nerve densities. As shown in Table 6, there was no conclusive evidence as to the influence PEX alone has on CCT. Two of the 4 studies showed a statistically significant reduction in CCT of patients with PEXG versus PEX, and 1 of the 4 a statistically significant increase in CCT in PEXG patients. The confounding influence of glaucoma and elevated IOP in these patients makes the impact of pseudoexfoliation on CCT difficult to interpret. It is possible that the more advanced damage to corneal keratocytes seen in the PEXG patients compared to PEX patients effects CCT. Keratocytes, which help regulate collagen production and spacing in the cornea, in addition to extracellular matrix (ECM) proteoglycans and glycoaminoglycans, which dictate corneal osmotic pressure, may be altered in a way that reduces corneal hydration and thickness. Alternatively, the Ocular Hypertension Treatment Study (OHTS) clearly showed that a reduced CCT is a significant risk factor in the development of POAG, which may simply translate to PEX patients with lower CCTs having a higher risk of converting to PEXG.[76] Yuksel et al found a statistical increase in CCT in PEXG patients versus PEX patients, with a potential theory being that reduced endothelial counts seen in PEXG leads to increased corneal hydration and thickness.[36] Future studies tracking the changes in CCT during PEX and PEXG progression are needed to further elucidate the influence pseudoexfoliation has on corneal thickness and hydration.
Similar and likely related to CCT changes, corneal biomechanics has also been shown to be altered in PEXG patients. Using the Ocular Response Analyzer (ORA) (Depew, NY, USA), Yazgan et al, found decreased corneal hysteresis (CH) and corneal resistance factor (CRF) in PEX and PEXG eyes compared to normal control eyes.[77] In a retrospective review, Ayala found reduced CH in PEXG eyes compared to POAG eyes.[78] This may also be a result of ECM and hydration alterations related to changes in keratocytes and disturbed endothelial counts, and could potentially indicate an alteration in the entire corneoscleral shell. If fibroblasts of the sclera are similarly reduced or altered in PEX patients, there may be structural alterations of the entire corneoscleral shell, which is significant as changes in the mechanics of the peripapillary sclera and lamina cribrosa have been shown to influence deformations of the optic nerve head with IOP elevations.[79,80] Moghimi et al found a statistically significant thinner lamina cribrosa in a group of nonglaucomatous PEX patients versus age matched controls using enhanced depth imaging spectral-domain OCT.[81] Kim et al found that despite similar IOP and glaucoma severity between the two groups, the lamina cribrosa was significantly thinner in PEXG eyes compared to POAG eyes.[82] Furthermore, Kim et al found know to no difference in lamina cribrosa thickness between the fellow normal eye and PEXG eye in patients with unilateral PEXG, indicating a possible bilateral change in structure of the posterior globe in PEX patients. Braunsmann et al, using atomic force microscopy, found significantly reduced stiffness (lower Young's modulus) of both the lamina cribrosa and peripapillary sclera in PEX patients compared to normal controls.[83] These studies suggest that PEX may be an independent risk factor for glaucomatous damage based on the mechanical function of the lamina cribrosa and peripapillary sclera alone.
All four studies analyzing nerve parameters in the cornea measured statistically significant reductions in nerve cell density and increased tortuosity in PEXG eyes, followed by PEX eyes versus normal controls. Zheng et al, elegantly found that these nerve changes correlated with decreased corneal sensitivity seen clinically,[12,13] indicating that corneal sensitivity may be a simple clinical measurement that could help the physician determine the extent of PEX damage to the eye. Yuksel et al was the only study to measure corneal nerve parameters separately in PEX and PEXG patients.[36] They found a marked reduction of approximately 50% in subbasal nerve density in PEXG patients versus PEX patients. Because topical ocular anti-hypertensive medications are known to affect nerve densities,[84] they excluded patients on ocular medications with the exception of artificial tears. This data suggests corneal nerve density may also have potential as a quantifiable risk factor in determining glaucomatous progression in PEX patients, and may be theorized to be a more reliable parameter than ECD given ECD variability and changes secondary to intraocular surgery and inflammation.
Any conclusions gathered as a result of the literature review face similar limitations. The relationship between corneal parameters and PEX/PEXG status is not widely studied. We were only able to find a total of 30 studies spanning 1977 to 2016, and the majority of these were case-control studies with limited sample sizes. Inter-study comparisons are limited by the mean age of their groups, with differences up to 15 years between studies. Furthermore, several different devices and ECD analysis techniques were used to measure corneal parameters. It has been demonstrated that different endothelial cell measuring instruments and methods have varying correlations in terms of ECD and have been shown to have statistically significant differences in absolute ECD. The interusability of these devices is even more limited when measuring hexagonality and pleomorphism. Gasser et al found that the Topcon SP3000P (Oakland, NJ, USA) and Konan Noncon Robo SP8000 (Konan, Japan) instruments correlated well with ECD but the Konan device measured systematically higher ECD.[85] The two devices varied more significantly in measurements of hexagonality and CV. Price et al showed that among Nidek Confoscan 4 (Padova, Italy), Tomey EM-3000 (Phoenix, AZ, USA) and Konan Noncon Robo SP8000 (Konan, Japan), only the Robo and EM-3000 showed automated ECD to be comparable to manual counts in normal eyes with even more divergent results in patients following Descemet stripping endothelial keratoplasy.[86] Finally, many of the reviewed studies were also limited by their cross-sectional nature, as the length of time patients have PEX or PEXG and their severity likely play a significant role in corneal damage.
Despite the limitations, this review has shown a clear association between corneal alterations and PEX, which tends to be amplified in patients with PEXG. We therefore conclude that corneal parameters such as ECD and subbasal nerve cell densities may have potential as clinical biomarkers for pseudoexfoliation syndrome to assess severity of disease and to help determine the risk of PEX patients converting to PEXG. Future prospective studies will be needed to elucidate the association between corneal parameters and risk of PEXG development with a more thorough analysis utilizing a combination of CDR, visual field data and OCT to better characterize glaucomatous damage and its association with corneal alterations.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Ritch R. Exfoliation syndrome-the most common identifiable cause of open-angle glaucoma. J Glaucoma. 1994;3:176–177. [PubMed] [Google Scholar]
- 2.Ringvold A. Epidemiology of the pseudo-exfoliation syndrome. Acta Ophthalmol Scand. 1999;77(4):371–375. doi: 10.1034/j.1600-0420.1999.770401.x. [DOI] [PubMed] [Google Scholar]
- 3.Jonasson F, Damji KF, Arnarsson A, Sverrison T, Wang L, Sasaki H, et al. Prevalence of open-angle glaucoma in Iceland: Reykjavik Eye Study. Eye. 2003;17(6):747–753. doi: 10.1038/sj.eye.6700374. [DOI] [PubMed] [Google Scholar]
- 4.Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson D, Stefansson H, et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317(5843):1397–1400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]
- 5.Ritch R, Schlötzer-Schrehardt U, Konstas AGP. Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res. 2003;22(3):253–275. doi: 10.1016/s1350-9462(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 6.Kivelä T, Hietanen J, Uusitalo M. Autopsy analysis of clinically unilateral exfoliation syndrome. Invest Ophthalmol Vis Sci. 1997;38(10):2008–2015. [PubMed] [Google Scholar]
- 7.Henry JC, Krupin T, Schmitt M, Lauffer J, Miller E, Ewing MQ, et al. Long-term follow-up of pseudoexfoliation and the development of elevated intraocular pressure. Ophthalmology. 1987;94(5):545–552. doi: 10.1016/s0161-6420(87)33413-x. [DOI] [PubMed] [Google Scholar]
- 8.Konstm A. 3rd International Glaucoma Symposium-ICS. Prague, Czech Republic: 2001. Glaucoma in eyes with exfoliation syndrome. [Google Scholar]
- 9.Jeng SM, Karger RA, Hodge DO, Burke JP, Johnson DH, Good MS. The risk of glaucoma in pseudoexfoliation syndrome. J Glaucoma. 2007;16(1):117–121. doi: 10.1097/01.ijg.0000243470.13343.8b. [DOI] [PubMed] [Google Scholar]
- 10.Konstas AG, Stewart WC, Stroman GA, Sine CS. Clinical presentation and initial treatment patterns in patients with exfoliation glaucoma versus primary open-angle glaucoma. Ophthalmic Surg Lasers. 1997;28(2):111–117. [PubMed] [Google Scholar]
- 11.Kahloun R, Attia S, Ksiaa I, Kacem I, Bouanene I, Zaouali S, et al. Anterior chamber aqueous flare, pseudoexfoliation syndrome, and glaucoma. Int Ophthalmol. 2016;36(5):671–674. doi: 10.1007/s10792-016-0169-8. [DOI] [PubMed] [Google Scholar]
- 12.Zheng X. New findings for an old disease: Morphological studies on pseudoexfoliation syndrome-related keratopathy and binocular asymmetry. Cornea. 2013;32(Suppl 1):S84–90. doi: 10.1097/ICO.0b013e3182a3657d. [DOI] [PubMed] [Google Scholar]
- 13.Zheng X, Shiraishi A, Okuma S, Mizoue S, Goto T, Kawasaki S, et al. In vivo confocal microscopic evidence of keratopathy in patients with pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci. 2011;52(3):1755–1761. doi: 10.1167/iovs.10-6098. [DOI] [PubMed] [Google Scholar]
- 14.Kocabeyoglu S, Mocan MC, Irkec M, Karakaya J. In Vivo Confocal Microscopic Evaluation of Corneas in Patients With Exfoliation Syndrome. J Glaucoma. 2016;25(2):193–197. doi: 10.1097/IJG.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 15.Brooks AM, Grant G, Robertson IF, Gillies WE. Progressive corneal endothelial cell changes in anterior segment disease. Aust N Z J Ophthalmol. 1987;15(1):71–78. doi: 10.1111/j.1442-9071.1987.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 16.de Juan-Marcos L, Cabrillo-Estévez L, Escudero-Domínguez FA, Sánchez-Jara A, Hernández-Galilea E. [Morphometric changes of corneal endothelial cells in pseudoexfoliation syndrome and pseudoexfoliation glaucoma] Arch la Soc Española Oftalmol. 2013;88(11):439–444. doi: 10.1016/j.oftal.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Demircan S, Atas M, Yurtsever Y. Effect of torsional mode phacoemulsification on cornea in eyes with/without pseudoexfoliation. Int J Ophthalmol. 2015;8(2):281–287. doi: 10.3980/j.issn.2222-3959.2015.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hattori Y. [Corneal endothelial examination of pseudoexfoliation syndrome] Nihon Ganka Gakkai Zasshi. 1990;94(10):957–963. [PubMed] [Google Scholar]
- 19.Inoue K, Okugawa K, Oshika T, Amano S. Morphological study of corneal endothelium and corneal thickness in pseudoexfoliation syndrome. Jpn J Ophthalmol. 2003;47(3):235–239. doi: 10.1016/s0021-5155(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 20.Kaljurand K, Teesalu P. Exfoliation syndrome as a risk factor for corneal endothelial cell loss in cataract surgery. Ann Ophthalmol(Skokie) 2007;39(4):327–333. doi: 10.1007/s12009-007-9012-1. [DOI] [PubMed] [Google Scholar]
- 21.Knorr HL, Jünemann A, Händel A, Naumann GO. [Morphometric and qualitative changes in corneal endothelium in pseudoexfoliation syndrome] Fortschr Ophthalmol. 1991;88(6):786–789. [PubMed] [Google Scholar]
- 22.Miyake K, Matsuda M, Inaba M. Corneal endothelial changes in pseudoexfoliation syndrome. Am J Ophthalmol. 1989;108(1):49–52. doi: 10.1016/s0002-9394(14)73259-3. [DOI] [PubMed] [Google Scholar]
- 23.Oltulu R, Satirtav G, Kayitmazbatir ET, Bitirgen G, Ozkagnici A, Karaibrahimoglu A. Characteristics of the cornea in patients with pseudoexfoliation syndrome. Arq Bras Oftalmol. 2015;78(6):348–351. doi: 10.5935/0004-2749.20150092. [DOI] [PubMed] [Google Scholar]
- 24.Omura T, Tanito M, Doi R, Ishida R, Yano K, Matsushige K, et al. Correlations among various ocular parameters in clinically unilateral pseudoexfoliation syndrome. Acta Ophthalmol. 2014;92(5):e412–3. doi: 10.1111/aos.12348. [DOI] [PubMed] [Google Scholar]
- 25.Ostern AE, Drolsum L. Corneal endothelial cells 6-7 years following cataract surgery in patients with pseudoexfoliation syndrome. Acta Ophthalmol. 2012;90(5):408–411. doi: 10.1111/j.1755-3768.2010.02012.x. [DOI] [PubMed] [Google Scholar]
- 26.Puska P, Vasara K, Harju M, Setälä K. Corneal thickness and corneal endothelium in normotensive subjects with unilateral exfoliation syndrome. Graefe's Arch Clin Exp Ophthalmol=Albr von Graefes Arch für Klin und Exp Ophthalmol. 2000;238(8):659–663. doi: 10.1007/s004170000159. [DOI] [PubMed] [Google Scholar]
- 27.Quiroga L, Lansingh VC, Samudio M, Peña FY, Carter MJ. Characteristics of the corneal endothelium and pseudoexfoliation syndrome in patients with senile cataract. Clin Experiment Ophthalmol. 2010;38(5):449–455. doi: 10.1111/j.1442-9071.2010.02313.x. [DOI] [PubMed] [Google Scholar]
- 28.Romero-Aroca P, Masip-Serra R, Martínez-Salcedo I, Salvat-Serra M, Fernández-Ballart J, Bautista-Pérez A. High prevalence of pseudoexfoliation syndrome and its complications in Tarragona in northeast Spain. Eur J Ophthalmol. 2011;21(5):580–588. doi: 10.5301/EJO.2011.6254. [DOI] [PubMed] [Google Scholar]
- 29.Sarowa S, Manoher J, Jain K, Singhal Y, Devathia D. Qualitative and quantitative changes of corneal endothelial cells and central corneal thickness in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Int J Med Sci Public Heal. 2016;5(12):1. [Google Scholar]
- 30.Tomaszewski BT, Zalewska R, Mariak Z. Evaluation of the endothelial cell density and the central corneal thickness in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. J Ophthalmol 2014. 2014:123683. doi: 10.1155/2014/123683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vannas A, Setälä K, Ruusuvaara P. Endothelial cells in capsular glaucoma. Acta Ophthalmol. 1977;55(6):951–958. doi: 10.1111/j.1755-3768.1977.tb05676.x. [DOI] [PubMed] [Google Scholar]
- 32.Wali UK, Al-Mujaini AS, Al-Kharusi NS, Bialasiewicz AA, Rizvi SG. Quantitative and qualitative corneal endothelial morphology of omani patients with pseudoexfoliation syndrome. Sultan Qaboos Univ Med J. 2008;8(3):300–305. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M, Sun W, Ying L, Dong X-G. Corneal endothelial cell density and morphology in Chinese patients with pseudoexfoliation syndrome. Int J Ophthalmol. 2012;5(2):186–189. doi: 10.3980/j.issn.2222-3959.2012.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Yamasita R, Hommura S. Corneal endothelial changes and aqueous flare intensity in pseudoexfoliation syndrome. Ophthalmol J Int d’ohtalmologie Int J Ophthalmol Zeitschrift für Augenheilkd. 1999;213(6):387–391. doi: 10.1159/000027460. [DOI] [PubMed] [Google Scholar]
- 35.Wirbelauer C, Anders N, Pham DT, Wollensak J. Corneal endothelial cell changes in pseudoexfoliation syndrome after cataract surgery. Arch Ophthalmol (Chicago, Ill 1960) 1998;116(2):145–149. doi: 10.1001/archopht.116.2.145. [DOI] [PubMed] [Google Scholar]
- 36.Yüksel N, Emre E, Pirhan D. Evaluation of Corneal Microstructure in Pseudoexfoliation Syndrome and Glaucoma: In vivo Scanning Laser Confocal Microscopic Study. Curr Eye Res. 2016;41(1):34–40. doi: 10.3109/02713683.2014.1002046. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20(4):374–384. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi K, Manabe S-I, Yoshimura K, Kondo H. Corneal endothelial damage after cataract surgery in eyes with pseudoexfoliation syndrome. J Cataract Refract Surg. 2013;39(6):881–887. doi: 10.1016/j.jcrs.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 39.Kovaliunas E, Stech S, Jurkute N, Cimbalas A, Asoklis R. Characteristics of the corneal endothelium and pseudoexfoliation syndrome in patients with senile cataract. [Last accessed on 2017 Jul 07];Acta Ophthalmol. 2012 90(s249) doi: 10.1111/j.1442-9071.2010.02313.x. doi:10.1111/j.1755-3768.2012.S126.x. Available from: http://onlinelibrary.wiley.com/doi/101111/j.1755-3768.2012.S126.x . [DOI] [PubMed] [Google Scholar]
- 40.Seitz B, Müller EE, Langenbucher A, Kus MM, Naumann GO. [Endothelial keratopathy in pseudoexfoliation syndrome: Quantitative and qualitative morphometry using automated video image analysis] Klin Monatsblätter für Augenheilkd. 1995;207(3):167–175. doi: 10.1055/s-2008-1035363. [DOI] [PubMed] [Google Scholar]
- 41.Wirbelauer C, Anders N, Pham DT, Holschbach A, Wollensak J. [Early postoperative endothelial cell loss after corneoscleral tunnel incision and phacoemulsification in pseudoexfoliation syndrome] Ophthalmologe. 1997;94(5):332–336. doi: 10.1007/s003470050124. [DOI] [PubMed] [Google Scholar]
- 42.Zimmermann N, Wünscher M, Schlötzer-Schrehardt U, Erb C. [Corneal endothelial cell density and its correlation with the severity of pseudoexfoliation] Klin Monatsblätter für Augenheilkd. 2014;231(2):158–163. doi: 10.1055/s-0033-1360308. [DOI] [PubMed] [Google Scholar]
- 43.Zarnowski T, Lekawa A, Dyduch A, Turek R, Zagórski Z. [Corneal endothelial density in glaucoma patients] Klin Oczna. 2005;107(7-9):448–451. [PubMed] [Google Scholar]
- 44.Ostern AE, Drolsum L. Corneal endothelial cells 6-7 years following cataract surgery in patients with pseudoexfoliation syndrome. Acta Ophthalmol. 2012;90(5):408–411. doi: 10.1111/j.1755-3768.2010.02012.x. [DOI] [PubMed] [Google Scholar]
- 45.Sarowa S, Manoher J, Jain K, Singhal Y, Devathia D. Qualitative and quantitative changes of corneal endothelial cells and central corneal thickness in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Int J Med Sci Public Heal. 2016;5(12):1. [Google Scholar]
- 46.Gagnon MM, Boisjoly HM, Brunette I, Charest M, Amyot M. Corneal endothelial cell density in glaucoma. Cornea. 1997;16(3):314–318. [PubMed] [Google Scholar]
- 47.Schlötzer-Schrehardt UM, Dörfler S, Naumann GO. Corneal endothelial involvement in pseudoexfoliation syndrome. Arch Ophthalmol (Chicago, Ill 1960) 1993;111(5):666–674. doi: 10.1001/archopht.1993.01090050100038. [DOI] [PubMed] [Google Scholar]
- 48.Naumann GO, Schlötzer-Schrehardt U. Keratopathy in pseudoexfoliation syndrome as a cause of corneal endothelial decompensation: A clinicopathologic study. Ophthalmology. 2000;107(6):1111–1124. doi: 10.1016/s0161-6420(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 49.Demirdögen BC, Ceylan OM, Işikoğlu, Mumcuoğlu T, Erel O. Evaluation of oxidative stress and paraoxonase phenotypes in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Clin Lab. 2014;60(1):79–86. doi: 10.7754/clin.lab.2013.121229. [DOI] [PubMed] [Google Scholar]
- 50.Patel S, McLaren J, Hodge D, Bourne W. Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 2001;42(2):333–339. [PubMed] [Google Scholar]
- 51.Schlötzer-Schrehardt U, Naumann GOH. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141(5):921–937. doi: 10.1016/j.ajo.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 52.Sein J, Galor A, Sheth A, Kruh J, Pasquale LR, Karp CL. Exfoliation syndrome: New genetic and pathophysiologic insights. Curr Opin Ophthalmol. 2013;24(2):167–174. doi: 10.1097/ICU.0b013e32835d5d11. [DOI] [PubMed] [Google Scholar]
- 53.Sbeity Z, Palmiero P-M, Tello C, Liebmann JM, Ritch R. Non-contact in vivo confocal scanning laser microscopy in exfoliation syndrome, exfoliation syndrome suspect and normal eyes. Acta Ophthalmol. 2011;89(3):241–247. doi: 10.1111/j.1755-3768.2009.01678.x. [DOI] [PubMed] [Google Scholar]
- 54.Ritch R. Exfoliation syndrome. Curr Opin Ophthalmol. 2001;12(2):124–130. doi: 10.1097/00055735-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Küchle M, Vinores SA, Mahlow J, Green WR. Blood-aqueous barrier in pseudoexfoliation syndrome: Evaluation by immunohistochemical staining of endogenous albumin. Graefes Arch Clin Exp Ophthalmol. 1996;234(1):12–18. doi: 10.1007/BF00186513. [DOI] [PubMed] [Google Scholar]
- 56.Schlötzer-Schrehardt U, Lommatzsch J, Küchle M, Konstas AGP, Naumann GOH. Matrix metalloproteinases and their inhibitors in aqueous humor of patients with pseudoexfoliation syndrome/glaucoma and primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2003;44(3):1117–1125. doi: 10.1167/iovs.02-0365. [DOI] [PubMed] [Google Scholar]
- 57.Linnér E, Schwartz B, Araujo D. Optic disc pallor and visual field defect in exfoliative and non-exfoliative, untreated ocular hypertension. Int Ophthalmol. 1989;13(1-2):21–24. doi: 10.1007/BF02028632. [DOI] [PubMed] [Google Scholar]
- 58.Budde WM, Jonas JB. [Morphology of the optic papilla in glaucoma. II. Secondary chronic open angle glaucoma] Klin Monbl Augenheilkd. 1999;215(4):221–227. doi: 10.1055/s-2008-1034703. [DOI] [PubMed] [Google Scholar]
- 59.Jonas JB, Papastathopoulos KI. Optic disk appearance in pseudoexfoliation syndrome. Am J Ophthalmol. 1997;123(2):174–180. doi: 10.1016/s0002-9394(14)71033-5. [DOI] [PubMed] [Google Scholar]
- 60.Sibour G, Finazzo C, Boles carenini A. Monolateral pseudoexfoliatio capsulae: A study of choroidal blood flow. Acta Ophthalmol Scand Suppl. 1997;224:13–14. doi: 10.1111/j.1600-0420.1997.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 61.Harju M, Vesti E. Blood flow of the optic nerve head and peripapillary retina in exfoliation syndrome with unilateral glaucoma or ocular hypertension. Graefes Arch Clin Exp Ophthalmol. 2001;239(4):271–277. doi: 10.1007/s004170100269. [DOI] [PubMed] [Google Scholar]
- 62.IZZOTTI A, BAGNIS A, SACCA S. The role of oxidative stress in glaucoma. Mutat Res Mutat Res. 2006;612(2):105–114. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Mumcu UY, Kocer I, Ates O, Alp HH. Decreased paraoxonase1 activity and increased malondialdehyde and oxidative DNA damage levels in primary open angle glaucoma. Int J Ophthalmol. 2016;9(10):1518–1520. doi: 10.18240/ijo.2016.10.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tezel G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog Retin Eye Res. 2006;25(5):490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dursun A, Ozec AV, Dogan O, Dursun FG, Toker MI, Topalkara A, et al. Evaluation of Choroidal Thickness in Patients with Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma. J Ophthalmol 2016. 2016:1–5. doi: 10.1155/2016/3545180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Demircan S, Yilmaz U, Kucuk E, Ulusoy MD, Atas M, Gulhan A, et al. The Effect of Pseudoexfoliation Syndrome on the Retinal Nerve Fiber Layer and Choroid Thickness. Semin Ophthalmol. 2017;32(3):341–347. doi: 10.3109/08820538.2015.1090611. [DOI] [PubMed] [Google Scholar]
- 67.Eroglu FC, Asena L, Simsek C, Kal A, Yılmaz G. Evaluation of choroidal thickness using enhanced depth imaging by spectral-domain optical coherence tomography in patients with pseudoexfoliation syndrome. Eye. 2015;29(6):791–796. doi: 10.1038/eye.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turan-Vural E, Yenerel N, Okutucu M, Yildiz E, Dikmen N. Measurement of Subfoveal Choroidal Thickness in Pseudoexfoliation Syndrome Using Enhanced Depth Imaging Optical Coherence Tomography. Ophthalmologica. 2015;233(3-4):204–208. doi: 10.1159/000371899. [DOI] [PubMed] [Google Scholar]
- 69.Bayhan HA, Bayhan SA, Can İ. Evaluation of the Macular Choroidal Thickness Using Spectral Optical Coherence Tomography in Pseudoexfoliation Glaucoma. J Glaucoma. 2016;25(2):184–187. doi: 10.1097/IJG.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 70.Goktas S, Sakarya Y, Ozcimen M, Sakarya R, Bukus A, Ivacik IS, et al. Choroidal thinning in pseudoexfoliation syndrome detected by enhanced depth imaging optical coherence tomography. Eur J Ophthalmol. 2014;24(6):879–884. doi: 10.5301/ejo.5000460. [DOI] [PubMed] [Google Scholar]
- 71.Schlötzer-Schrehardt U. Pseudoexfoliation syndrome: The puzzle continues. J Ophthalmic Vis Res. 2012;7(3):187–189. [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng X, Sakai H, Goto T, Namiguchi K, Mizoue S, Shiraishi A, et al. Anterior segment optical coherence tomography analysis of clinically unilateral pseudoexfoliation syndrome: Evidence of bilateral involvement and morphologic factors related to asymmetry. Invest Ophthalmol Vis Sci. 2011;52(8):5679–5684. doi: 10.1167/iovs.11-7274. [DOI] [PubMed] [Google Scholar]
- 73.Gokce SE, Gokce MI. Relationship between pseudoexfoliation syndrome and erectile dysfunction: A possible cause of endothelial dysfunction for development of erectile dysfunction. Int Braz J Urol. 2015;41(3):547–551. doi: 10.1590/S1677-5538.IBJU.2014.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Streeten BW, Li ZY, Wallace RN, Eagle RC, Keshgegian AA. Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch Ophthalmol (Chicago, Ill 1960) 1992;110(12):1757–1762. doi: 10.1001/archopht.1992.01080240097039. [DOI] [PubMed] [Google Scholar]
- 75.Schumacher S, Schlötzer-Schrehardt U, Martus P, Lang W, Naumann GO. Pseudoexfoliation syndrome and aneurysms of the abdominal aorta. Lancet (London, England) 2001;357(9253):359–360. doi: 10.1016/s0140-6736(00)03645-x. [DOI] [PubMed] [Google Scholar]
- 76.Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol (Chicago, Ill 1960) 2002;120(6):714. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 77.Yazgan S, Celik U, Alagöz N, Taş M. Corneal biomechanical comparison of pseudoexfoliation syndrome, pseudoexfoliative glaucoma and healthy subjects. Curr Eye Res. 2015;40(5):470–475. doi: 10.3109/02713683.2014.930157. [DOI] [PubMed] [Google Scholar]
- 78.Ayala M. Corneal hysteresis in normal subjects and in patients with primary open-angle glaucoma and pseudoexfoliation glaucoma. Ophthalmic Res. 2011;46(4):187–191. doi: 10.1159/000326896. [DOI] [PubMed] [Google Scholar]
- 79.Fortune B, Reynaud J, Hardin C, Wang L, Sigal IA, Burgoyne CF. Experimental Glaucoma Causes Optic Nerve Head Neural Rim Tissue Compression: A Potentially Important Mechanism of Axon Injury. Investig Opthalmology Vis Sci. 2016;57(10):4403. doi: 10.1167/iovs.16-20000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Downs JC, Roberts MD, Burgoyne CF. Mechanical environment of the optic nerve head in glaucoma. Optom Vis Sci. 2008;85(6):425–435. doi: 10.1097/OPX.0b013e31817841cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moghimi S, Mazloumi M, Johari M, Abdi P, Fakhraie G, Mohammadi M, et al. Evaluation of Lamina Cribrosa and Choroid in Nonglaucomatous Patients With Pseudoexfoliation Syndrome Using Spectral-Domain Optical Coherence Tomography. Investig Opthalmology Vis Sci. 2016;57(3):1293. doi: 10.1167/iovs.15-18312. [DOI] [PubMed] [Google Scholar]
- 82.Kim S, Sung KR, Lee JR, Lee KS. Evaluation of Lamina Cribrosa in Pseudoexfoliation Syndrome Using Spectral-Domain Optical Coherence Tomography Enhanced Depth Imaging. Ophthalmology. 2013;120(9):1798–1803. doi: 10.1016/j.ophtha.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 83.Braunsmann C, Hammer CM, Rheinlaender J, Kruse FE, Schaffer TE, Schlotzer-Schrehardt U, et al. Evaluation of Lamina cribrosa and Peripapillary Sclera Stiffness in Pseudoexfoliation and Normal Eyes by Atomic Force Microscopy. Investig Opthalmology Vis Sci. 2012;53(6):2960. doi: 10.1167/iovs.11-8409. [DOI] [PubMed] [Google Scholar]
- 84.Baratz KH, Nau CB, Winter EJ, McLaren JW, Hodge DO, Herman DC, et al. Effects of Glaucoma Medications on Corneal Endothelium, Keratocytes, and Subbasal Nerves Among Participants in the Ocular Hypertension Treatment Study. Cornea. 2006;25(9):1046–1052. doi: 10.1097/01.ico.0000230499.07273.c5. [DOI] [PubMed] [Google Scholar]
- 85.Gasser L, Reinhard T, Böhringer D. Comparison of corneal endothelial cell measurements by two non-contact specular microscopes. BMC Ophthalmol. 2015;15:87. doi: 10.1186/s12886-015-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Price MO, Fairchild KM, Price FW. Comparison of manual and automated endothelial cell density analysis in normal eyes and DSEK eyes. Cornea. 2013;32(5):567–573. doi: 10.1097/ICO.0b013e31825de8fa. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study design overwiew


