Abstract
Visceral (VAT) but not subcutaneous adipose tissue (SAT) is associated with obesity-related diseases including colorectal cancer (CRC). Superficial SAT (SSAT) and deep SAT (DSAT), components of SAT, also appear to independently influence disease risk. These abdominal adipose tissues (AAT) are not extensively studied in connection with CRC and have not been explored in the United States (US) despite known racial variations in body composition. We conducted a case-control study that compared associations between AAT with CRC risk and race of AA and NHW males with incident CRC matched by age, BMI and race (N=158, 79/group). Cross-sectional computed tomography (CT) images were used for assessment of AAT. Overall cases and controls had similar VAT areas (140±192 vs 149±152 cm2, p-value=0.93), however cases had lower SSAT than controls (88±39 vs 112±65 cm2, p<0.01). Among controls, AA had significantly lower VAT (114±168 vs 180±167, p<0.01) than NHW. Conditional logistic regression revealed AA males with greater SSAT had lower odds for CRC (OR: 0.24, 95%CI 0.07–0.85). Our findings indicate VAT does vary between cases and controls by race, however this variation is not a risk factor for CRC. The negative association between CRC and SSAT in AA men warrants further investigation.
Keywords: colorectal cancer, abdominal adipose tissues, visceral adipose tissue, race, computed tomography, body composition
Introduction
Obesity is significantly associated with both colon and rectal cancers in men(1). Central adiposity increases risk for colorectal cancer (CRC) (1, 2) and other obesity related chronic diseases (3–5). For many of these diseases excess visceral adipose tissue (VAT) as opposed to subcutaneous adipose tissue (SAT) quantified using computed tomography (CT) images has been linked to the increased risks (3, 4, 6). The influence of VAT vs SAT in risks for CRC in a United States (US) population has not been explored and findings from non-US populations have not been consistent(6, 7). Further, in the US, African Americans (AA), particularly AA men have higher risks for CRC than Non-Hispanic White (NHW) men, yet healthy AA men have lower VAT than their NHW counterparts(8, 9). Also, examinations of the two distinct SAT compartments, superficial SAT (SSAT) and deep SAT (DSAT) which are anatomically separated by the fascia of Scarpa may vary in their contribution to metabolic disruption (10, 11). Specifically, DSAT has been associated with insulin resistance, a risk factor associated with CRC, whereas SSAT has not (3, 11). Therefore the purpose of this study is to determine the association between excess VAT and other abdominal fat depots and CRC risk in urban males and if these risks vary by race.
Materials and Methods
Study Population, Design and Procedures
A case-control study of 79 males with incident CRC cases (51 AA and 28 NHW) matched for age (±5 years), body mass index (BMI; ±0.05kg/m2) and race to 79 cancer-free male controls with abdominal CT scans was conducted. CRC cases were selected from participants of the Chicago Colorectal Cancer Consortium Study (CCCC) at three medical centers and through medical records at these centers. The CCCC study was a prospective cohort conducted between 2009 and 2013 to examine the relationship between genetic and environmental risk factors in a race/ethnically diverse population. Participants were recruited at the time of their colonoscopy from three university medical centers in Chicago, Illinois. Males 40 years of age and older with a good quality CT image were eligible for inclusion in our case-control study. Cases with newly diagnosed with non-hereditary CRC (Stage 0-III, non-metastatic) were matched to controls that had received a CT abdominal scan for a non-cancer diagnosis during a 24 hour emergency room visit or outpatient setting (Table 1) at the CCCC hospitals during the same historical time period of cases. Patients were excluded if they had hereditary CRC, a history of inflammatory bowel disease, Stage IV CRC diagnosis, end stage renal disease, abdominal gastric surgery or organ transplant.
Table 1.
List of Diagnoses Requiring Computed Tomography Scan of Cancer-Free Controls
| Diagnosis | Frequency (n/79) |
Percent (%) |
|---|---|---|
| Abdominal/back/leg pain | 41 | 51.3 |
| Acute illness (i.e., fever, hernia repair evaluation) | 14 | 17.5 |
| Acute gastrointestinal event (i.e., perforation) | 13 | 16.3 |
| Other (i.e., transplant evaluation, aortic aneurysm) | 8 | 10.0 |
| Rule out malignancy | 4 | 5.0 |
Demographic and clinical information obtained from medical records included age, marital status, race, blood pressure, medical history of diabetes and hypertension (yes/no), history of gastrointestinal malignancies, current smoker (yes/no), current alcohol consumption (yes/no), self-reported unintentional weight loss within the past 6 months (yes/no). Height and weight for calculation of BMI was measured by CCCC staff for cases during enrollment or obtained from medical records within 3 days of CT procedure. BMI was calculated according to the Quetelet’s index as weight (kg)/stature (m2) and classified according to National Institutes of Health guidelines (18.5–24.9 for normal weight, 25.0–29.9 for overweight and 30.0 or higher for obese). Cancer staging was obtained from pathology reports and based on TNM (Tumor, Node, Metastases) cancer staging system.
Single archived diagnostic CT images were selected for assessment of cross-sectional area of abdominal adipose tissues at the standard landmark of L3 vertebra (12–14). Abdominal adipose tissues quantified for each image included total abdominal adipose tissue (TAT), SAT, SSAT, and DSAT. Each cross-sectional image for TAT, SAT and VAT was analyzed using three separate evaluations with SliceOmatic v4.3 (TomoVision, Montreal, QC, Canada) (Fig 1); waist circumference (WC), SSAT and DSAT were measured with IMAGEJ 1.47v (National Institutes of Health, Bethesda, USA) using the abdominal perimeter for an estimate of waist as previously published (15) and the subcutaneous and outer abdominal musculature perimeters for derivation of SSAT and DSAT areas. These medical imaging software packages permit specific tissue demarcation of abdominal adipose tissues according to Hounsfield Unit (HU) thresholds of −150 to −50 for VAT, −190 to −30 for SAT and −29 to 150 for skeletal muscle. The TAT estimate was calculated as sum of the areas of SAT + VAT. Intra-class coefficient of variations of imaging analysis between investigators was less than 2%.
Fig. 1.
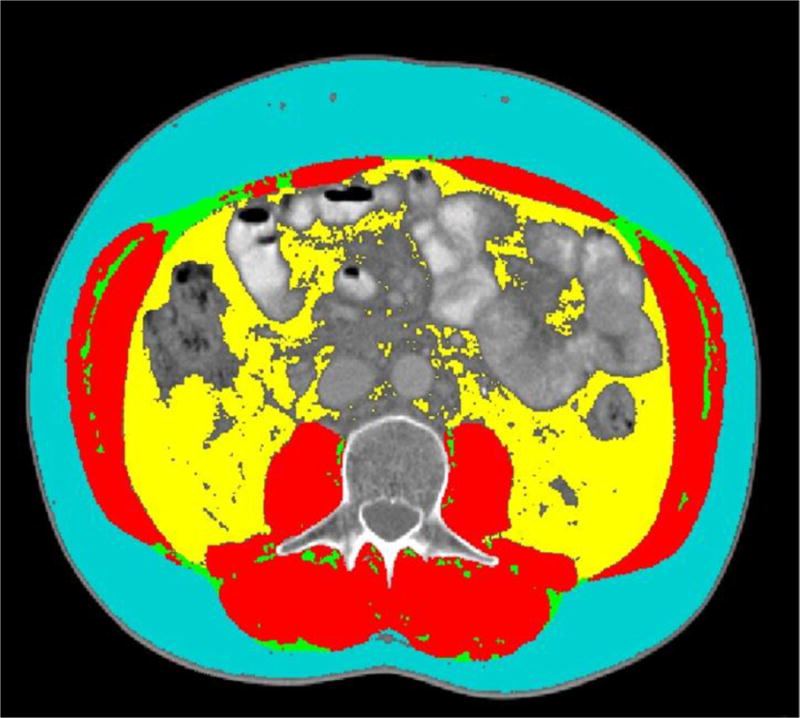
Cross-sectional image of the third lumbar (L3) analyzed for subcutaneous adipose tissue (blue), visceral adipose tissue (yellow), intermuscular adipose tissue (green) and skeletal muscle (red) using SliceOmatic medical imaging software (TomoVision, Montreal, QC, Canada).
Sample Size Estimation
Sample size was based on the area of VAT reported by Katzmarzyk et al (9) comparing NHW males (mean age: 44.9 ± 13.4 years) and AA males (mean age: 38.4±13.9 years). Sample size was calculated with PS – Power and Sample Size Version 3.0.43 using paired t-test for continuous variables (16). Based on a mean VAT area of 148.6 ± 73.4 cm2 in NHW and of 97.7± 63.9 cm2 in AA, a minimum of 19 males per race/ethnic group were needed within cases and controls to detect a difference of 50.9 cm2 with a significance level of 0.05 and a power of 0.80. Our study sample consisted of 51 AA and 28 NHW males per group (cases vs. controls).
Ethics Approval and Consent to Participate
This study was approved by the Institutional Review Boards at the University of Illinois at Chicago (CCCC IRB Protocol # 2010-0168; IRB Protocol # 2014-0837), Rush University Medical Center (IRB Protocol # 10031003) and John H. Stroger Hospital (IRB Protocol # 10-142). A waiver of informed consent and HIPAA was obtained at each institution for retrospective use of protected health information.
Statistical Analyses
Statistical analysis was conducted using Statistical Analysis System (SAS) for Windows 9.3 (SAS Institute Inc., Cary, NC, USA). Variables were examined for presence of outliers and distributions and presented as medians with interquartile ranges in tables. Paired t-tests or Wilcoxon signed – rank tests were performed for continuous variables. Categorical data were analyzed using McNemar Chi-square test. Pearson and Spearman (non-parametric) correlation coefficients were computed to quantify the relationships between continuous and non-parametric variables. Collinearity was determined using correlation matrices. Variables with correlation >0.8 were not included in the same model. Severe collinearity was defined as a variance inflation factor greater than 4 (17). Conditional logistic regression was used to determine crude and adjusted significant predictors of CRC for the entire sample and then for each race/ethnic group using Stepwise and Forward selection. To control for the impact of unintended disease-associated weight loss the CT analysis of the abdominal adipose depots were compared in a subset of weight stable cases and controls (n=49 per group). Statistical significance set at a p-value<0.05. Bonferroni adjustment for statistical significance based on two hypotheses was calculated to be a p<0.025.
Results
The participants median and interquartile for age and BMI were 62(11) years and 27(6) kg/m2, respectively and by design cases and controls were similar for age, BMI and race (Table 2). There also had similar demographic characteristics including smoking and alcohol use and prevalence of diabetes and hypertension. As commonly observed in patients with CRC diagnosis, self-reported unintentional weight loss was significantly higher in our cases than controls.
Table 2.
Characteristics of Cases and Controls
| Cases (n= 79) |
Controls (n= 79) |
p-valuec | |
|---|---|---|---|
| Demographics & Anthropometrics | Median(IQ) or %(n) | Median(IQ) or %(n) | |
| African Americans (AA), %(n) | 64.5(51) | 64.5(51) | — |
| Non-Hispanic Whites (NHW), %(n) | 35.4(28) | 35.4(28) | — |
| Normal BMI (18.5–25), %(n) | 26.6(21) | 27.9(22) | 0.59 |
| Overweight BMI (25–30), %(n) | 49.4(39) | 43.0(34) | 0.43 |
| Obese BMI (≥30), %(n) | 24.1(19) | 29.1(23) | 0.54 |
| Cancer Stages 0-I, %(n) | 30.7(23) | — | — |
| Cancer Stage 2 (II, IIA, IIB, IIC), %(n) | 40.0(30) | — | — |
| Cancer Stage 3 (III,IIIA, IIIB, IIIC),%(n) | 29.3(22) | — | — |
| Married, yes,%(n) | 30.8(24) | 42.9(33) | 0.45 |
| Current Smoker, yes, %(n) | 27.9(22) | 25.6(20) | 0.76 |
| Alcohol consumption, yes, %(n) | 44.9(35) | 37.3(28) | 0.38 |
| Diabetes, yes. %(n) | 25.0(19) | 26.6(21) | 0.75 |
| Hypertension, yes, %(n) | 67.1(51) | 63.3(50) | 0.92 |
| Unintentional Weight Loss, yes, %(n) (within 6 months) |
27.6(21) | 7.6(6) | <0.01* |
Paired t-test & Wilcoxon-rank sum test used for continuous data; McNemar test for categorical data.
Sample size for WC, SSAT and DSAT = 68 per group.
Significant p-value < 0.05.
As anticipated VAT was highly correlated with TAT (r = 0.94, p<0.01), SAT (r = 0.71, p<0.01), SSAT (r = 0.59, p<0.01), DSAT (r = 0.68, p<0.01) and other measures of obesity [BMI (r = 0.67, p<0.01) and WC (r=0.83, p<0.01)]. However, only TAT had a correlation coefficient greater than 0.80, suggestive of high collinearity with other AAT (SAT, r = 0.89, p<0.01; SSAT r = 0.78, p<0.01; DSAT, r = 0.86, p<0.01), BMI (r = 0.76, p<0.01) and waist (r = 0.92, p<0.01) therefore, it was excluded from models. Also, as expected the SAT subtypes (SSAT and DSAT) were highly correlated (r >0.80, p< 0.01) with SAT and each other and were analyzed separately in models.
Unadjusted stratified analysis of cases vs controls
VAT did not differ between cases and controls (Table 3) overall, however, cases had significantly lower SAT, SSAT and DSAT than controls. Dichotomizing by race, unadjusted comparisons of abdominal adipose tissues (Table 4) revealed significantly lower SAT (p=0.02) and SSAT (p=0.002) in AA cases than AA controls but no differences in any other depot. Among NHW case-control pairs no difference in any abdominal adipose tissue was found, although a trend for lower SSAT (p = 0.09) in cases was present. Comparisons between racial groups revealed AA controls had significantly lower VAT than NHW controls, similar to those previously reported in healthy populations (8, 9). In contrast among cases the difference in VAT area trended but was not significantly lower in AA than their NHW counterparts (p = 0.06).
Table 3.
Unadjusted Comparison of Abdominal Adipose Tissues for Cases and Controlsa
| Cases (n= 79) |
Controls (n= 79) |
p-valueb | |
|---|---|---|---|
| Abdominal Depots | Median (IQR) | Median (IQR) | |
| TAT(cm2) | 294(294) | 347(259) | 0.29 |
| SAT (cm2) | 133(91) | 170(134) | 0.03* |
| SSAT (cm2)b | 88(39) | 112(65) | <0.01* |
| DSAT (cm2)b | 67(49.8) | 176(35) | 0.04* |
| VAT (cm2) | 140(192) | 149(152) | 0.93 |
| SM (cm2) | 162(38) | 176(35) | 0.10 |
| VAT/TAT (%) | 44(23) | 40(21) | 0.11 |
| VAT/SAT (%) | 91(93) | 75(75) | 0.05 |
| SAT/TAT (%) | 50(21) | 54(23) | 0.02* |
| SSAT/TAT (%) | 35(23) | 39(24) | 0.45 |
| DSAT/TAT (%) | 25(12) | 28(12) | 0.28 |
| Weight Stable Sub-Groupc | |||
| WC (cm2) | 105(18) | 109(18) | 0.40 |
| SSAT (cm2) | 95(48) | 124(58) | <0.01* |
| DSAT (cm2) | 86(47) | 88(68) | 0.22 |
| VAT (cm2) | 171(155) | 167(148) | 0.64 |
Paired t-test used for comparisons between cases and controls for continuous data.
Sample size for WC, SSAT and DSAT was 68 males per group.
Sample size for weight stable sub-group was 49 per group and 43 per group for WC, SSAT and DSAT.
Significant p-value < 0.05.
Table 4.
Demographics, Anthropometrics and Body Composition by Race/Ethnicity of Cases and Controls
| AA Cases (n=51) |
AA Controls (n=51) |
AA Cases vs AA Controls | NHW Cases (n=28) |
NHW Controls (n=28) |
NHW Cases vs NHW Controls | AA Cases vs NHW Cases | AA Controls vs NHW Controls | |
|---|---|---|---|---|---|---|---|---|
| Demographics and Anthropometrics | Median (IQ) | Median (IQ) | a p-value | Median (IQ) | Median (IQ) | a p-value | b p-value | b p-value |
| BMI (kg/m2) | 27(7) | 27(7) | 0.45 | 28(5) | 28(5) | 0.59 | 0.40 | 0.27 |
| WC (cm)c | 99(14) | 106(19) | 0.16 | 104(23) | 111(17) | 0.41 | 0.08 | 0.03* |
| Abdominal Depots | ||||||||
| TAT (cm2) | 263(264) | 320(288) | 0.45 | 366(253) | 369(239) | 0.46 | 0.05* | 0.02* |
| SAT (cm2) | 127(72) | 165(146) | 0.02* | 144(101) | 185(107) | 0.71 | 0.25 | 0.18 |
| SSAT (cm2)c | 88(40) | 111(68) | <0.01* | 88(41) | 112(50) | 0.09 | 0.85 | 0.54 |
| DSAT (cm2) | 62(50) | 71(72) | 0.06 | 77(65) | 96(73) | 0.39 | 0.10 | 0.06 |
| VAT (cm2) | 98(178) | 114(168) | 0.47 | 190(162) | 180(167) | 0.42 | 0.06 | <0.01* |
| SM (cm2) | 172(39) | 178(33) | 0.30 | 150(31) | 172(46) | 0.20 | <0.01* | 0.18 |
| VAT/TAT (%) | 42(25) | 34(25) | 0.01* | 47(21) | 48(17) | 0.58 | 0.28 | <0.01* |
| VAT/SAT (%) | 81(89) | 55(65) | 0.01* | 99(84) | 103(75) | 0.97 | 0.25 | <0.01* |
| SAT/TAT (%) | 52(21) | 61(20) | <0.01* | 47(20) | 46(18) | 0.78 | 0.24 | <0.01* |
| SSAT/TAT (%)c | 35(23) | 43(25) | 0.20 | 34(20) | 30(8) | 0.58 | 0.13 | <0.01* |
| DSAT/TAT (%) | 24(13) | 30(12) | 0.01* | 25(12) | 25(12) | 0.18 | 0.95 | 0.03* |
| Weight Stable Sub-Groupd | ||||||||
| WC (cm2) | 104.6(16) | 109(23) | 0.96 | 106(21) | 111(13) | 0.28 | 0.63 | 0.25 |
| SSAT (cm2) | 96(37) | 123(76) | 0.05 | 91(38) | 124(47) | 0.07 | 0.26 | 0.80 |
| DSAT (cm2) | 78(42) | 86(76) | 0.32 | 88(63) | 91(68) | 0.47 | 0.53 | 0.53 |
| VAT (cm2) | 170(148) | 138(147) | 0.07 | 172(181) | 186(163) | 0.18 | 0.83 | 0.04* |
Significant p-value <0.05.
Paired t-test used for comparisons between cases and controls for continuous data.
Dependent t-test & Wilcoxon-rank sum test used for within cases or controls based on normality.
Sample size for WC, SSAT and SSAT/TAT = 45 for AA and 23 NHW per group.
Sample size for weight stable sub-group: AA = 30 per group (sample for WC and SSAT = 26) and NHW = 19 per group (sample for WC and SSAT = 17).
Conditional logistic regression analysis
As shown in Table 5, significant predictors of CRC for the full sample included SSAT (OR = 0.22, 95%CI: 0.07 – 0.70) and self-reported unintentional weight loss (OR = 3.92, 95%CI: 1.43 – 10.74). After exploring significant predictors of CRC for each of the racial groups separately, the odds ratio for SSAT suggests that AAs with higher SSAT had 24% lower risk of CRC (OR = 0.24, 95%CI: 0.07 – 0.85).
Table 5.
Predictors of Colorectal Cancer for Overall Sample and Race/Ethnic Groups using Conditional Logistic Regression
| CRC (yes/no)
|
||||||
|---|---|---|---|---|---|---|
| Model | B | OR | Lower CI | Upper CI | p-value | |
| Full Sample (n=158, 78 pairs) |
Median SSATa | −1.51 | 0.22 | 0.07 | 0.70 | 0.01* |
| Unintentional Weight Loss | 1.37 | 3.92 | 1.43 | 10.74 | 0.01* | |
|
| ||||||
| African Americans Only (n=102, 51 pairs) |
Median SSATa | −1.52 | 0.24 | 0.070 | 0.852 | 0.03* |
| Unintentional Weight Loss | 1.76 | 7.91 | 2.559 | 24.438 | <0.01* | |
|
| ||||||
| Non-Hispanic Whites Only (n=56, 28 pairs) |
Alcohol | 1.6094 | 5.00 | 1.096 | 22.82 | 0.04* |
Significant p-value <0.05.
Median splits defined as: SSAT = 109.9cm2
Subset analysis in weight stable participants (n= 49 pairs)
Among weight stable pairs (n= 49 pairs), the median difference for waist (p=0.40), VAT, (p=0.40), and DSAT (p=0.22) did not differ between cases and controls (Table 3), however median SSAT was significantly higher in controls than cases (p<0.01, n = 43 per group). When this group was dichotomized by race (Table 4), only AA controls had significantly lower VAT than NHW controls (p=0.04). A trend for higher VAT was noticed between AA cases compared to AA controls (p=0.07) and higher SSAT in AA controls compared to AA cases (p=0.05).
Discussion
Few studies have explored associations between obesity-related body compositional changes and cancer in racially diverse, populations at high risk for obesity and colorectal cancer. Here, we provide important initial data in this area that expands our understanding of the influence of the race/ethnic variations in abdominal adipose tissue on the risks for colorectal cancer in AAs compared to NHW males. An association between VAT and CRC risk was not found in this racially diverse case control study of urban men. Six studies have studied the relationship between VAT area and CRC (6, 7, 18–21), however none of these studies were conducted in US populations. Our findings are similar to the study reported by Choe et al, of Korean adults with and without early stage CRC (Stage 0-I) (18) and two smaller cross-sectional studies in Turkish patients (7, 19). Three other investigations in Asian populations have reported larger VAT depots in patients with CRC compared to non-CRC counterparts (6, 20, 21), however differences in study design, race and non-BMI matched comparison groups limit the implications of their findings and the comparisons that can be made with our findings.
Although the association between VAT and CRC is unclear, its association with colorectal adenomas, which are precursors of CRC (22–26) is quite well established. Insulin resistance and pro-inflammatory pathways promoted by obesity and excess VAT are speculated as the mechanisms for the increased risks (27). Thus excess VAT, a frequent consequence of obesity and mechanistically linked to inflammatory and insulin resistant pathways, remains an important risk factor for CRC via its link with adenomas. The interval between adenoma development and CRC diagnosis can range from months to years. This long interval may have impeded detection of the association between VAT and other abdominal adipose depots in our case-control study. This is particularly important in uninsured or asymptomatic populations. It is likely that abdominal adipose tissues change during this interval particularly within the intra-abdominal cavity (i.e., fluctuations of VAT deposition). To understand the role of VAT on CRC etiology, a prospective study is required that enrolls cancer-free participants assessed for body composition using CT or MRI at screening colonoscopy with subsequent study visits at regular intervals over a minimum of 10–15 years. Our findings of lower VAT in both AA cases and controls compared to their NHW BMI-matched pairs is consistent with reports between healthy AA and NHW (8, 9) and extends this relationship to men with CRC. The current study is the first to explore these relationships in a population of AA and NHW men with and without CRC, enabling robust comparisons of body composition between groups. More research is needed to clarify the biological implications of racial differences and how VAT contributes to CRC risk.
An important unexpected finding of our study was the significant negative association of SSAT with CRC observed in AA, but not NHW participants. This association has not been reported for CRC, however higher SAT to VAT body fat distribution has been found to be favorable for reduced risks for diabetes and cardiovascular disease. It is thought that the structural and functional difference between adipose depots confers the variation in risks (28, 29). Recently, evidence indicates the subcomponents of SAT, i.e. SSAT and DSAT, are distinct and may contribute to disease risk differently. DSAT metabolically resembles VAT and is more pathogenic than SSAT (30). Conversely, higher SSAT has been associated with improved blood pressure and heart rates in patients with Type 2 diabetes (31). Further, recent biopsy studies have determined SSAT has lower saturated to monounsaturated fatty acid content and lower expression of numerous pro-inflammatory genes than DSAT (11, 32). The negative association observed in our AA patients with SSAT and CRC was only apparent when the statistical analysis of the individual components of SAT (SSAT and DSAT) were separately explored; SAT was not significantly associated with CRC risk. The potential lower odds of CRC with higher SSAT in the AA participants provides an intriguing virtually unexplored area for future studies which has the potential to expand our understanding of the racial disparities in CRC.
This study had several limitations that should be considered. First, although case-control studies are prone to both subject and observation bias, given the limited knowledge in this area of and the efficiency and cost-effective nature of this study design render it ideal for the initial assessment of the disease to body composition relationship. A superior design would have enrolled cases and controls from the same pool of patients at the time of endoscopy and collected data on diet and physical activity behaviors as well as serum and mucosal tissue biopsies. Secondly, limited resources dictated selection of cases and controls from different sources and utilizing archived CT data for body composition analysis. Controls were selected from patients with a diagnostic CT for acute health conditions, primarily for abdominal, epigastric or flank pain, thus as is common in case control studies undiagnosed sub-clinical disease may have been present. Additionally, although controls with a history of any cancer were excluded, undiagnosed CRC or other types of cancer may have been unintentionally included as a control. Adenomas develop over 10–20 years(33) and patients may have polyps or undiagnosed disease for months or years, particularly in underserved populations not routinely screened with various health access disparities. Finally, the variety of cancer stages (Stage 0-III, non-metastatic) and the racial groups included in our study restricts generalizability to these populations. Our study has important strengths. It provides data of the relationship between CRC, race and abdominal body composition of a US population an area which heretofore has been largely unexplored. Additionally, the impact of body composition on CRC risks was explored with rigorous control for age, BMI and race. We used CT scans for assessment of the body fat depots which are the gold standards for body composition analysis. The analysis of CT images was conducted using detailed, standardized body composition analysis protocols, including accurate measurements of VAT (fat within kidneys, intestines were not included in estimate). CT scans are routinely performed for a variety of reasons throughout the clinical care continuum and for our study we included only those that had a CT for acute illness, mostly in an outpatient setting or with an ER visit with a discharge within 23 hours. Finally, information about weight loss in both cases and controls were identically obtained, limiting bias concerns for this measurement.
Conclusions
VAT area did not significantly differ between cases and controls and greater VAT area was not associated with increased CRC risks. Further, the differences observed between the racial groups in our cases and controls were very similar to those reported in healthy populations. Specifically, AA controls had significantly lower VAT than their NHW counterparts and these differences remained after exploring in a weight stable subgroup. These findings may support the accumulating evidence suggesting VAT’s role occurs earlier in the CRC pathogenic pathway, between the normal mucosa and adenoma sequence. Additionally, for AA who commonly display a low VAT phenotype other mechanisms and other abdominal adipose depots, including SSAT, should be explored in light of their increased risks for adenomas and CRC.
Acknowledgments
We would like to thank Kristen Fleming, Andrew McLeod and Caitlin Levy for their assistance with the electronic medical record review process and Julie Wang for helping with CT extractions. We also thank Liam McKeever for helping with the body composition analysis.
Funding
Research reported in this publication was supported by the national Cancer Institute of the National Institutes of Health under Award Number R25CA057699. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing Interests
The authors report no competing interests.
Author contributions
SLGP and CB designed the study and data collection procedures. SLGP analyzed CT scans. SLGP and CB drafted article and had primary responsibility for final content. GF, WM, VC and SF contributed to study methodology and scientific integrity. SF provided biostatistical supervision. VC, WM and BP supervised data collection and computed tomography extraction at each of the participating medical centers. CB, GF, VC, WM and SF approved research design and shared the responsibility of final content. All authors read, provided feedback and approved the final manuscript.
References
- 1.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. The American Journal of Clinical Nutrition. 2007;86:556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 2.Moore LL, Bradlee ML, Singer MR, Splansky GL, Proctor MH, et al. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2004;28:559–567. doi: 10.1038/sj.ijo.0802606. [DOI] [PubMed] [Google Scholar]
- 3.Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism: clinical and experimental. 2001;50:425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/circulationaha.106.675355. [DOI] [PubMed] [Google Scholar]
- 5.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–13. doi: 10.1161/circulationaha.111.067264. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto S, Nakagawa T, Matsushita Y, Kusano S, Hayashi T, et al. Visceral fat area and markers of insulin resistance in relation to colorectal neoplasia. Diabetes care. 2010;33:184–189. doi: 10.2337/dc09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erarslan E, Turkay C, Koktener A, Koca C, Uz B, et al. Association of visceral fat accumulation and adiponectin levels with colorectal neoplasia. Digestive diseases and sciences. 2009;54:862–868. doi: 10.1007/s10620-008-0440-6. [DOI] [PubMed] [Google Scholar]
- 8.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, et al. Visceral fat, waist circumference, and BMI: impact of race. Obesity (Silver Spring, Md) 2008;16:600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 9.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. The American Journal of Clinical Nutrition. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancello R, Zulian A, Gentilini D, Maestrini S, Della Barba A, et al. Molecular and morphologic characterization of superficial- and deep-subcutaneous adipose tissue subdivisions in human obesity. Obesity (Silver Spring) 2013;21:2562–70. doi: 10.1002/oby.20417. [DOI] [PubMed] [Google Scholar]
- 11.Marinou K, Hodson L, Vasan SK, Fielding BA, Banerjee R, et al. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care. 2014;37:821–9. doi: 10.2337/dc13-1353. [DOI] [PubMed] [Google Scholar]
- 12.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. Journal of applied physiology (Bethesda, Md: 1985) 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 13.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. Journal of applied physiology (Bethesda, Md: 1985) 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 14.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35. doi: 10.1016/s1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Perez SL, Haus JM, Sheean P, Patel B, Mar W, et al. Measuring Abdominal Circumference and Skeletal Muscle From a Single Cross-Sectional Computed Tomography Image: A Step-by-Step Guide for Clinicians Using National Institutes of Health ImageJ. JPEN J Parenter Enteral Nutr. 2016;40:308–18. doi: 10.1177/0148607115604149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–28. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 17.Pan Y, Jackson RT. Ethnic difference in the relationship between acute inflammation and serum ferritin in US adult males. Epidemiol Infect. 2008;136:421–31. doi: 10.1017/s095026880700831x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choe EK, Kim D, Kim HJ, Park KJ. Association of visceral obesity and early colorectal neoplasia. World J Gastroenterol. 2013;19:8349–56. doi: 10.3748/wjg.v19.i45.8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erarslan E, Coskun Y, Turkay C, Koktener A, Aydogan T. IGF-I levels and visceral fat accumulation in colonic neoplasia. Clin Res Hepatol Gastroenterol. 2014;38:99–105. doi: 10.1016/j.clinre.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Lee HS, Lee DC, Chu SH, Jeon JY, et al. Visceral fat accumulation is associated with colorectal cancer in postmenopausal women. PLoS One. 2014;9:e110587. doi: 10.1371/journal.pone.0110587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh TH, Byeon JS, Myung SJ, Yang SK, Choi KS, et al. Visceral obesity as a risk factor for colorectal neoplasm. Journal of gastroenterology and hepatology. 2008;23:411–417. doi: 10.1111/j.1440-1746.2007.05125.x. [DOI] [PubMed] [Google Scholar]
- 22.Keum N, Lee DH, Kim R, Greenwood DC, Giovannucci EL. Visceral adiposity and colorectal adenomas: dose-response meta-analysis of observational studies. Ann Oncol. 2015;26:1101–9. doi: 10.1093/annonc/mdu563. [DOI] [PubMed] [Google Scholar]
- 23.Nagata N, Sakamoto K, Arai T, Niikura R, Shimbo T, et al. Visceral abdominal fat measured by computed tomography is associated with an increased risk of colorectal adenoma. Int J Cancer. 2014;135:2273–81. doi: 10.1002/ijc.28872. [DOI] [PubMed] [Google Scholar]
- 24.Nam SY, Kim BC, Han KS, Ryu KH, Park BJ, et al. Abdominal visceral adipose tissue predicts risk of colorectal adenoma in both sexes. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8:443–50. e1–2. doi: 10.1016/j.cgh.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Hu H, Cai Y, Huang J, Zhang J, Deng Y. Visceral adipose tissue and the risk of colorectal adenomas: a meta-analysis of observational studies. Eur J Cancer Prev. 2014 doi: 10.1097/cej.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 26.Hong S, Cai Q, Chen D, Zhu W, Huang W, et al. Abdominal obesity and the risk of colorectal adenoma: a meta-analysis of observational studies. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) 2012 doi: 10.1097/CEJ.0b013e328351c775. [DOI] [PubMed] [Google Scholar]
- 27.Feakins RM. Obesity and metabolic syndrome: pathological effects on the gastrointestinal tract. Histopathology. 2015 doi: 10.1111/his.12907. [DOI] [PubMed] [Google Scholar]
- 28.Bays HE, Gonzalez-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert review of cardiovascular therapy. 2008;6:343–368. doi: 10.1586/14779072.6.3.343. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 30.Walker GE, Verti B, Marzullo P, Savia G, Mencarelli M, et al. Deep subcutaneous adipose tissue: a distinct abdominal adipose depot. Obesity (Silver Spring, Md) 2007;15:1933–1943. doi: 10.1038/oby.2007.231. [DOI] [PubMed] [Google Scholar]
- 31.Golan R, Shelef I, Rudich A, Gepner Y, Shemesh E, et al. Abdominal superficial subcutaneous fat: a putative distinct protective fat subdepot in type 2 diabetes. Diabetes care. 2012;35:640–647. doi: 10.2337/dc11-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundbom J, Hakkarainen A, Lundbom N, Taskinen MR. Deep subcutaneous adipose tissue is more saturated than superficial subcutaneous adipose tissue. Int J Obes (Lond) 2013;37:620–2. doi: 10.1038/ijo.2012.72. [DOI] [PubMed] [Google Scholar]
- 33.Society AC. Colorectal Cancer Facts & Figures 2014–2016. American Cancer Society; Atlanta, GA: 2014. [Google Scholar]


