Abstract
Total hip arthroplasty (THA) has become one of the most reliable and patient-requested surgical interventions in all medicine. The procedure can be performed using a variety of surgical approaches, but the posterior approach, direct lateral approach, and direct anterior approach are by far the most common across the globe. This article highlights the history and technique for each of these common approaches. A review of outcomes and complications for each approach are also provided. Each approach has its own unique advantages and disadvantages, but all can be safely and successful utilized for THA. Strong, convincing, high-quality studies comparing the different approaches are lacking at this time. Surgeons are therefore recommended to choose whichever approach they are most comfortable and experienced using. Though not described here, THA can also be done using the anterolateral approach (also known as the Watson Jones approach) as well as the two-incision approach. In addition, recently, some surgeons are utilizing the so-called direct superior approach for THA. While these approaches are far less commonly utilized, they are recognized as viable alternatives to traditional approaches.
Keywords: Direct anterior approach, direct lateral approach, Hardinge approach, Moore approach, posterior approach, Surgical technique, Smith-Petersen approach, Southern approach, total hip arthroplasty, transgluteal approach
MeSH terms: Surgery, Orthopaedics, replacement, arthroplasty, hip, osteotomy
Introduction
Hip degenerative joint disease is one of the most common and debilitating musculoskeletal disorders.1,2 Approximately 28% of the population ≥45-year-old suffer from hip arthritis and this prevalence is expected to increase in coming decades.3,4,5
The development of modern total hip arthroplasty (THA) began in the 1950s with Charnley's low-friction arthroplasty.6,7 After decades of improvement, THA is now one of the most reliable and patient requested surgical interventions. In 2010, an estimated 2.5 million individuals in the USA were living with THA, and nearly 332,000 THA were being performed annually.8,9 A significant increase in THA demand is expected over the next several decades.10,11
The most commonly used approaches for THA include posterior approach (PA), direct lateral approach (DLA), and direct anterior approach (DAA). This article highlights the history and technique for each of these approaches. A review of outcomes and complications for each approach are also provided.
Posterior Approach
Although several versions of PA have been used since von Langenbeck first described in 1874, the modern PA most closely resembles Moore's approach (1957).12,13 Often also called the “Southern” or “Moore” approach, PA is reportedly the most common surgical approach used worldwide for THA.14
PA is done with the patient in lateral decubitus position on a traditional operating room (OR) table. The pelvis is stabilized in this position with a padded peg-board and four padded posts [Figure 1a]. Careful placement and appropriate height choice for each post is critical. Posts should be placed anteriorly at the level of the pubic symphysis and chest and posteriorly at the level of the sacrum and shoulder blades. Before draping, the operative hip should be ranged to ensure adequate stability while still allowing full maneuverability. The ipsilateral arm should be stabilized with a padded arm board. A padded axillary roll should be placed under the contralateral chest wall to avoid brachial plexopathy. The operative limb should be sterilely prepared and draped freely to facilitate hip dislocation and allow for limb maneuverability throughout the procedure.
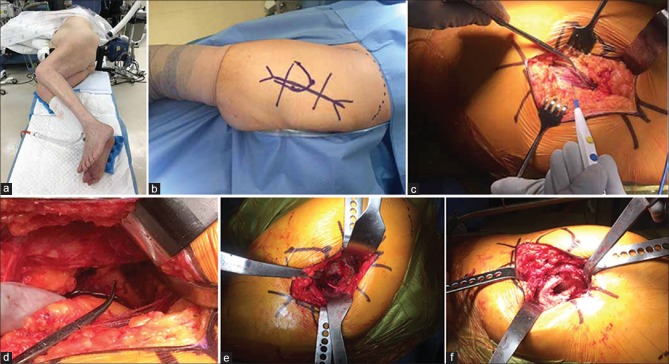
Figure 1.
Peroperative photographs showing (a) The pelvis is stabilized with a padded peg-board and four padded posts. (b) The PA incision starts approximately 5 cm distal to greater trochanter. (c) The fascia lata and ITB are incised longitudinally and proximally to split along the fibers of gluteus maximus. (d) Deep dissection identifies the piriformis and short external rotators (SERs). (e) Retractors maximize visualization of the acetabulum. (f) After cup placement, the leg is internally rotated, flexed, and adducted
The PA incision starts approximately 5 cm distal to greater trochanter (GT) at the lateral center of the femoral diaphysis. It continues proximally along the posterior border of GT and then curves toward the posterior superior iliac spine for another 5–7 cm [Figure 1b]. The skin and subcutaneous fat are incised down to the fascia lata and iliotibial band (ITB). The fascia lata and ITB are incised longitudinally and proximally to split along the fibers of gluteus maximus [Figure 1c]. A Charnley retractor can be placed to hold retraction of the split gluteus maximus. Deep dissection proceeds with hip internal rotation and identification of piriformis and the other short external rotators (SERs) [Figure 1d]. SER are then detached from GT close to their insertion. They are reflected posteriorly with stay sutures to both protect the nearby sciatic nerve and expose the posterior hip capsule. The femoral head and neck are next exposed by T-shaped capsulotomy. Further internal rotation of the leg, along with flexion, adduction, and gentle traction then allows for hip dislocation. If dislocation is difficult, additional release of the external rotators can help. The partial or full release of the gluteus maximus insertion, incision of the inferior capsule, and release of rectus femoris can also assist with femoral dislocation and retraction, particularly in severely contracted hips or revision scenarios.
A femoral neck osteotomy is performed using an oscillating saw. Osteotomy height is determined from a pre-operative plan using the lesser trochanter as a landmark. Three retractors will now maximize visualization of the acetabulum [Figure 1e]. One retractor is placed anteriorly, above the anterior wall. A second is placed posteriorly behind the posterior wall, and a third is positioned beneath the transverse acetabular ligament. The labrum, pulvinar and any loose soft tissues should be excised, followed by routine acetabular reaming. The transverse acetabular ligament, reamer position relative to the floor, and cup-positioning guides can all be used to establish proper acetabular version and inclination during both reaming and cup insertion.
After cup placement, the leg is internally rotated, flexed, and adducted to deliver the proximal femur for preparation [Figure 1f]. Blunt “bone skids” are commonly used to help elevate the proximal femur while soft tissue around the piriformis fossa is cleared. Routine femoral preparation and broaching can then proceed. With the knee flexed and the patient's tibia pointed vertically, the leg serves as a reference for establishing femoral version during broaching and component insertion. After trialing and final component placement, the posterior capsule and SER should be repaired with sutures placed through transosseous tunnels. The fascia lata, ITB, and gluteus maximus are closed with either interrupted or running sutures followed by routine closure of the subcutaneous tissues and skin.
Direct Lateral Approach
Although several versions of DLA have been used since McFarland and Osborne described theirs in 1954, the modern DLA was popularized by Hardinge in 1982.15,16 Often also called the “Hardinge” or “Transgluteal” approach, DLA is the second most common surgical approach used worldwide for THA.14
DLA can be done in lateral decubitus position, similar to PA. It can also be performed with the patient supine, which is preferred in our institution. A standard OR table can be utilized as well as a radiolucent table if intra operative imaging is desired. For supine positioning, a bump is placed under the pelvis at the level of the anterior superior iliac spine (ASIS) to create space for the femur to be displaced into during acetabular exposure [Figure 2a]. A roller bar is placed under the ipsilateral calf. This provides a footrest to help stabilize the leg with the hip at 45°–60° of flexion and the knee at 90° of flexion. The involved limb should then be sterilely prepared and draped freely to facilitate hip dislocation and allow for limb maneuverability throughout the procedure.
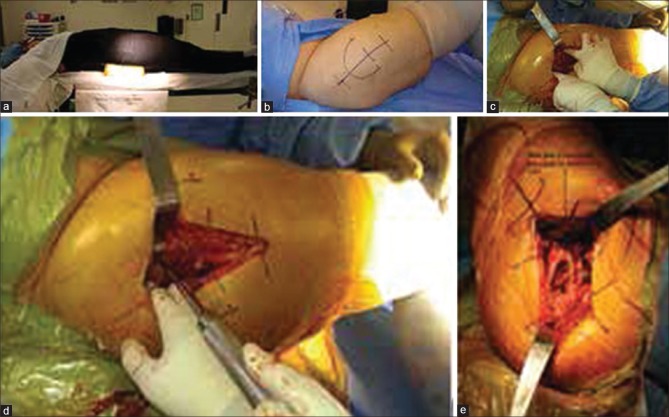
Figure 2.
Peroperative photographs showing (a) A bump is placed under the pelvis at the level of the anterior superior iliac spine. (b) The incision is started 2–4 cm proximal to the anterior-middle third of GT and extended distally in line with the femur to a point 4–6 cm distal to GT. (c) The anterior and posterior border of gluteus medius are identified. (d) Blunt dissection is used to split the muscle in line with its fibers. (e) The leg is placed in extreme adduction and external rotation to allow the surgeon excellent visualization of femoral version
The DLA incision is started 2–4 cm proximal to the anterior-middle third of GT and extended distally in line with the femur to a point 4–6 cm distal to GT [Figure 2b]. The skin and subcutaneous fat are incised to the fascia lata and ITB. The fascia is then incised longitudinally just anterior to the most lateral prominence of GT, starting approximately 3 cm proximal to GT and extending distally. The anterior portion of the fascial sleeve is retracted with a Hibbs retractor and the posterior portion is retracted with a Mueller retractor placed posterior to GT. This exposes gluteus medius and vastus lateralis. After identifying the anterior and posterior border of gluteus medius [Figure 2c], blunt dissection is used to split the muscle in line with its fibers at the junction of the anterior-middle thirds [Figure 2d]. The split is started at GT and its proximal extension should be limited to 3–5 cm.17 A blunt Hohman can then be placed extracapsular and posterior to the femoral neck to protect the posterior portion of gluteus medius while the hip capsule is incised sharply in line with the blunt muscular division. Vastus lateralis should be exposed next and split longitudinally just distal to vastus ridge, followed by placement of a Hohman anterior to the femur to retract the anterior portion of vastus lateralis. The split in vastus lateralis is then extended proximally into and through the tendinous insertion of gluteus medius until the muscular split of medius is reached. The anterior third of gluteus medius, majority of gluteus minimus, the anterior portion of the hip capsule, and anterior portion of vastus lateralis can then be elevated subperiosteally as one flap off the anterior femur. A cuff of medius tendon should remain on the anterior border of GT to allow for later repair. The dissection is facilitated by tensioning the tissue to be elevated through gentle progressive external rotation and adduction of the hip. Once the labrum is incised and the inferior femoral neck becomes visible, the hip is dislocated with traction, external rotation, and adduction. If dislocation is difficult, a bone hook can be placed anteriorly around the femoral neck to assist through anterolaterally directed traction.
A standard femoral neck osteotomy is performed on the dislocated hip with an oscillating saw. A tenaculum, placed on the femoral head, assists in removal of the femoral head after neck osteotomy. Three retractors are then utilized to gain exposure of the acetabulum: Anterior, superior, and inferior. The anterior retractor should be placed carefully to avoid injury to the femoral neurovascular bundle. This can be done using a Cobb elevator to gently develop the plane between the anterior acetabular wall and the overlying anterior capsule. Positioning the hip in flexion to relax the neurovascular structures during this maneuver is also helpful. After the plane is developed, the Cobb is replaced with a blunt Hohman. A second retractor is then placed superior to the acetabulum to protect and elevate gluteus muscle away from the surgical field. A Mueller is placed posterior and inferior to the acetabulum against the ischium. Releasing the inferior hip capsule at this time between the anterior and posterior retractors helps improves acetabular visualization and assists with insertion/removal of acetabular reamers and components. The labrum, pulvinar, and any loose soft tissues are excised, followed by routine acetabular reaming. The transverse acetabular ligament, reamer position relative to the floor, and cup-positioning guides are used to establish proper version and inclination during both reaming and cup insertion.
After completing acetabular reconstruction, the leg is placed in a figure four position with the operative foot on the anterior portion of the contralateral knee and the ipsilateral knee flexed to 90°. Muellers are placed on the medial aspect of the proximal femur and posterior to GT to lateralize the proximal portion of the femur while also displacing the posterior soft tissues. Maneuvering the operative leg to a position of extreme adduction and external rotation will further direct the proximal femur toward the surgeon for excellent visualization of femoral shaft direction and femoral version [Figure 2e]. Routine femoral preparation and broaching can then proceed. After trialing and final component placement, the anterior flap (gluteus medius, gluteus minimus, anterior capsule, and anterior vastus lateralis) is repaired to its anatomic position and closed as one layer with a combination of interrupted and running sutures. If the repair appears tenuous, transosseous suture tunnels can be utilized. The fascia lata, ITB, and gluteus maximus are then closed with either interrupted or running sutures followed by routine closure of the subcutaneous tissues and skin.
Direct Anterior Approach
Smith-Petersen first described DAA to the hip in 1917.18 This initial description was for reducing congenital hip dislocations. Smith-Petersen is also credited with the first DAA for hip arthroplasty in 1949.19 Over the subsequent decades, several modifications to his technique have occurred, along with the development of new instruments to make it less invasive and easier to perform. DAA has gained popularity in recent years and is now the third most common surgical approach used worldwide for THA.14
DAA is generally considered a minimally invasive approach to the hip because of its muscle sparing nature. It is the only common approach for THA that utilizes both an intermuscular and internervous plane. Superficially, the dissection occurs between sartorius and TFL. The location of lateral femoral cutaneous nerve (LFCN) must be considered in this approach to preserve lateral thigh sensation. LFCN enters the thigh by passing under the inguinal ligament approximately 1 cm medial and below the ASIS. LFCN then travels distally in the fascia overlying the sartorius muscles belly.
DAA is done with the patient positioned supine on either a specialized traction table or regular OR table, the latter of which preferred at our institution. A radiolucent table can also be utilized if intra-operative imaging is desired. For supine positioning, similar to our DLA, a small bump is placed under the pelvis at the level of ASIS to create space for the femur to be displaced into during acetabular exposure. An arm board placed distally on the contralateral side of the table is helpful when abducting the contralateral leg and adducting the operative leg during femoral exposure [Figure 3a]. The involved limb should be sterilely prepared and draped freely to facilitate limb maneuverability throughout the procedure.
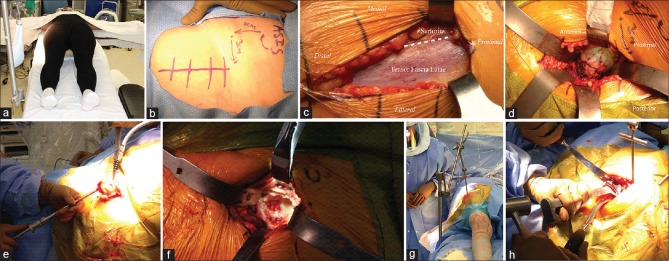
Figure 3.
Peroperative photographs showing (a) An arm board is placed distally on the contralateral side of the table. (b) The DA incision begins approximately 3 cm lateral and 3 cm distal to ASIS. (c) The fascia overlying TFL is incised longitudinally in line with the muscle's fibers. (d) Blunt retractors are placed intracapsular around the femoral. (e) Extraction of the “napkin ring” formed by the double osteotomy allows for removal of the femoral head with a corkscrew. (f) Acetabular exposure is accomplished with three or four retractors: Lighted retractor on the anterior acetabular rim, blunt curved retractor placed just distal to the transverse acetabular ligament, and sharp Hohman placed behind the posterolateral acetabular rim. (g) A hook is placed from lateral to medial just distal to GT. This is attached to a sterile arm that applies tension to the femur during capsular release (h) Femoral preparation and broaching using offset handles
The ASIS is used to identify and mark the DAA incision. The longitudinal incision begins approximately 3 cm lateral and 3 cm distal to ASIS [Figure 3b]. The incision then continues distally toward the patient's fibular head for approximately 6–8 cm to keep it in line with the belly of TFL. The incision should be kept small at first and extended as necessary to gain additional exposure. The skin and subcutaneous fat are incised to the fascia. After confirming the identity of TFL and sartorius, as well as the interval between them, the fascia overlying TFL is incised longitudinally in line with the muscle's fibers a few millimeters lateral to the interval [Figure 3c]. Keeping the dissection within the sheath of TFL helps protect LFCN during the procedure. Blunt digital dissection of the medial fascial edge from the medial border of the TFL muscle is then performed. The fat stripe between TFL and sartorius is typically seen to verify the interval. Additional blunt dissection through this fat stripe leads to the palpable identification of the superior femoral neck. A curved, blunt retractor is then placed over the superior femoral neck. A Hohman is placed over the lateral aspect of GT, distal to the vastus ridge, to help mobilize the TFL and gluteus medius laterally. With a Hibbs retracting sartorius and rectus femoris medially, the Smith-Petersen interval is exposed. Ascending branches of the lateral femoral circumflex artery will be crossing the interval and require cauterization. A second curved, blunt retractor positioned medially around the inferior neck helps further identify the hip capsule. The plane between the anterior capsule and rectus can then be developed bluntly with a Cobb, followed by insertion of a lighted anterior retractor. Flexion of the hip can help relax the rectus and aid in safe blunt dissection and retractor placement. An anterior capsulectomy or capsulotomy is then performed, followed by intracapsular placement of curved, blunt retractors around the femoral neck [Figure 3d]. A double osteotomy of the femoral neck is next performed in situ. The first osteotomy is typically made along a line from the bony saddle at the superolateral neck to a point at the medial neck that is approximately 1 cm above lesser trochanter. A second parallel osteotomy is performed approximately 1 cm proximal to the first. Extraction of the central disk or “napkin ring” formed by the double osteotomy allows for subsequent removal of the femoral head with a corkscrew [Figure 3e].
Acetabular exposure is accomplished with three or four retractors: Lighted retractor on the anterior acetabular rim, blunt curved retractor placed just distal to the transverse acetabular ligament, and sharp Hohman placed behind the posterolateral acetabular rim. A Mueller can also be placed at the posterior acetabular rim [Figure 3f]. The labrum, pulvinar, and any loose soft tissues should be excised, followed by routine acetabular reaming. The transverse acetabular ligament, reamer position relative to the floor, and cup-positioning guides can be used to establish proper acetabular version and inclination during both reaming and cup insertion. Offset reamers and insertion handles can also be helpful for these steps.
After completing acetabular reconstruction, the leg should be adducted and externally rotated. A sharp Hohman is placed lateral to GT and a Mueller is placed over the GT tip, between the abductor muscle and lateral hip capsule. In addition, a retraction arm can be placed to assist with femoral exposure. A hook is placed from lateral to medial just distal to GT. This is attached to a sterile arm that applies tension to the femur during capsular release [Figure 3g]. The lateral capsule should be released from its attachment near the saddle of the lateral femur. The proximal femur can then be elevated with additional tension applied to the retraction arm and leverage of the Mueller. If femoral exposure is still not adequate, sequential release of the conjoint tendon and piriformis should be performed. Routine femoral preparation and broaching can then proceed, often with the use of offset handles [Figure 3h]. After trailing and final component placement, the fascia overlying TFL is closed with either interrupted or running sutures followed by routine closure of the subcutaneous tissues and skin.
Outcomes
THA generally has excellent results, with patient satisfaction ranging from 89% to 95%.20,21,22 Several studies have compared the clinical outcomes between different surgical approaches. Restrepo et al. reported improved Harris Hip Scores (HHSs), Western Ontario and McMaster Osteoarthritis Index, and Short Form-36 scores at 6-week, 6-month, and 1-year postoperatively with DAA over DLA.23 Barrett et al. similarly reported improved HHS at 6 weeks postoperatively with DAA over PA.24 However, these and other studies failed to show any long term differences in outcome measures between approaches beyond the 1st year.23,24,25,26,27,28
Infection is a rare but known complication of THA. In general, large studies report the incidence to be 0.2%–1.2% after primary THA.29,30,31 There are minimal data available directly comparing infection rates between the different approaches. Some retrospective studies found no significant difference in deep infection rates between the approaches, although Christensen et al. recently reported a greater number of wound complications with the DAA compared to PA.32,33,34,35
Hip instability is another potential complication after THA. Large single cohort studies have demonstrated dislocation rates of 0.6%–1.0% for DAA and 0.3%–0.6% for DLA.36,37,38,39 Conversely, PA has generally been associated with higher dislocation rates of 1.7%–5.3%.40,41,42 Although recent literature has demonstrated improved stability with posterior capsular repairs.42,43,44,45 Randomized prospective data on dislocations is lacking, but several meta analyses and large retrospective comparative studies have demonstrated stability superiority with the anterior based approaches versus PA.34,46,47,48,49,50 In recent meta analyses, Higgins et al. demonstrated significantly fewer dislocations with DAA (0.3%) over PA (1.2%) and Kwon et al. demonstrated dislocation rates of 0.70%, 0.43%, and 1.01% for anterolateral, DLA, and PA, respectively.47,48 Masonis and Bourne similarly reported dislocation rates of 0.55%, 2.18%, and 3.23% for DLA, anterolateral, and PA, respectively.49 Sheth et al., in a large registry study on 42,438 primary THAs, also reported significantly lower dislocation rates with both DAA and anterolateral approach versus PA.34 However, other studies have reported equivalent rates of dislocation for all approaches, and hence, the data are not completely clear.32,51,52,53
Intraoperative fractures, particularly at GT, can occur during THA. This may be more likely with DAA due to the need for femoral elevation during the procedure. In a study by Matta et al., 0.6% of their DAA THA done on a specialized traction table were complicated by intraoperative GT fractures.37 They also reported ankle fractures in 0.6% of cases. Other authors have reported intraoperative trochanteric fractures in 1.0%–5.7% of their DAA THA.53,54,55 GT fractures can also occur with DLA and PA as demonstrated by Hendel et al. (4.0% with DLA) and Nakata et al. (1.0% with PA), but there are fewer reports in the literature.55,56 In direct comparative studies, Malek et al. demonstrated significantly more femoral fractures with DAA (6%) over PA (0%), but a meta-analysis by Higgins et al. showed no significant difference.32,47
Because neither DLA nor PA is truly muscle sparing, one major concern often cited against them is muscle damage. Each requires the splitting and release of some muscle: Gluteus maximus and SER during PA, gluteus maximus, and medius during DLA. Abductor weakness is a particular concern after DLA THA, with a reported incidence of 4%–20%.49 It is likely caused by preoperative abductor degeneration, seen in 20.0%–25.4% of THA patients, and failed tenotomy repair.57,58,59 DAA is presumably more “muscle friendly,” thanks to the utilization of an intermuscular interval. A recent study by Bergin et al. supported this claim by reporting significantly higher levels of serum creatine kinase postoperatively in PA patients compared to DAA patients.60 However, a cadaver study by Meneghini et al. challenged that DAA is truly muscle sparing.61 Less damage occurred in gluteus minimus with DAA (mean 8% of surface area) compared to PA (18%), but TFL (31%) and the direct head of rectus femoris (12%) were also damaged during DAA. In addition, in their study the piriformis or conjoined tendon required transection in 50% of DAA.
Nerve injury is a potentially devastating complication after THA. The nerves at risk include LFCN, SGN, femoral nerve and sciatic nerve. LFCN injury most commonly occurs during DAA due to the nerve's variable course and proximity to the approach's anatomic interval. Nearly 3.4%–81.1% of patients will report at least some symptoms of LFCN neuropraxia after DAA.62,63,64 Thankfully, symptoms tend to be tolerable and most resolve with time. SGN injury most commonly occurs during DLA due to the nerve's proximity to the gluteus medius split utilized with this approach.17 2.2%–42.5% of patients reportedly have at least some degree of SGN injury after DLA THA, which typically results in temporary abductor weakness but can be persistent in rare cases.65,66,67,68 Injury to the femoral or sciatic nerves causes the most dysfunction but are uncommon. The rate of femoral nerve injury after THA is rare at 0.0%–2.3% and the rate of sciatic nerve injury is similarly low at 0.1%–1.7%.37,62,67,69,70,71 The risk of sciatic nerve injury has been shown to be significantly higher in PA versus other approaches, likely due to the nerve's proximity to the surgical field with this approach.69
Conclusion
THA can be performed using a variety of surgical approaches. PA, DLA, and DAA are by far the most common. However, other techniques, including the “Watson-Jones” approach, two-incision approach, and superior gluteal approach are currently being used as well.72,73,74,75,76 Each approach has its own unique advantages and disadvantages, but all can be safely and successfully utilized for THA. Strong, convincing, high-quality studies comparing the different approaches is lacking at this time. Surgeons are therefore recommended to choose whichever approach they are most comfortable and experienced using.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Eric A. Levicoff for providing the images in the PA section of this article [Figure 1a-f].
References
- 1.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann Intern Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–8. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: The Johnston County Osteoarthritis Project. J Rheumatol. 2009;36:809–15. doi: 10.3899/jrheum.080677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–9. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 6.Morrey BF. A historical perspective of hip arthroplasty and reconstructive surgery. In: Cashman J, Goyal N, Parvizi J, editors. The Hip: Preservation, Replacement and Revision. Brooklandville, MD: Data Trace Publishing Company; 2015. pp. 1.1–1.19. [Google Scholar]
- 7.Charnley J. Total hip replacement by low-friction arthroplasty. Clin Orthop Relat Res. 1970;72:7–21. [PubMed] [Google Scholar]
- 8.Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386–97. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.“Inpatient Surgery”. FastStats. National Center for Health Statistics. Centers for Disease Control and\Prevention. [Last accessed on 2016 Jun 26]. Available from: http://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf .
- 10.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–97. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 11.Singh JA. Epidemiology of knee and hip arthroplasty: A systematic review. Open Orthop J. 2011;5:80–5. doi: 10.2174/1874325001105010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Langenbeck B. Uber die schussverletzungen des huftgelenks. Arch Klin Chir. 1874;16:263. [Google Scholar]
- 13.Moore AT. The self-locking metal hip prosthesis. J Bone Joint Surg Am. 1957;39-A:811–27. [PubMed] [Google Scholar]
- 14.Chechik O, Khashan M, Lador R, Salai M, Amar E. Surgical approach and prosthesis fixation in hip arthroplasty world wide. Arch Orthop Trauma Surg. 2013;133:1595–600. doi: 10.1007/s00402-013-1828-0. [DOI] [PubMed] [Google Scholar]
- 15.McFarland B, Osborne G. Approach to the hip: A suggested improvement on Kocher's method. J Bone Joint Surg Br. 1954;36B:364–7. [Google Scholar]
- 16.Hardinge K. The direct lateral approach to the hip. J Bone Joint Surg Br. 1982;64:17–9. doi: 10.1302/0301-620X.64B1.7068713. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs LG, Buxton RA. The course of the superior gluteal nerve in the lateral approach to the hip. J Bone Joint Surg Am. 1989;71:1239–43. [PubMed] [Google Scholar]
- 18.Smith-Petersen MN. A new supra-articular subperiosteal approach to the hip joint. Am J Orthop Surg. 1917;15:592–5. [Google Scholar]
- 19.Smith-Petersen MN. Approach to and exposure of the hip joint for mold arthroplasty. J Bone Joint Surg Am. 1949;31A:40–6. [PubMed] [Google Scholar]
- 20.Mancuso CA, Salvati EA, Johanson NA, Peterson MG, Charlson ME. Patients’ expectations and satisfaction with total hip arthroplasty. J Arthroplasty. 1997;12:387–96. doi: 10.1016/s0883-5403(97)90194-7. [DOI] [PubMed] [Google Scholar]
- 21.Specht K, Kjaersgaard-Andersen P, Kehlet H, Wedderkopp N, Pedersen BD. High patient satisfaction in 445 patients who underwent fast-track hip or knee replacement. Acta Orthop. 2015;86:702–7. doi: 10.3109/17453674.2015.1063910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naal FD, Impellizzeri FM, Lenze U, Wellauer V, von Eisenhart-Rothe R, Leunig M. Clinical improvement and satisfaction after total joint replacement: A prospective 12-month evaluation on the patients’ perspective. Qual Life Res. 2015;24:2917–25. doi: 10.1007/s11136-015-1042-3. [DOI] [PubMed] [Google Scholar]
- 23.Restrepo C, Parvizi J, Pour AE, Hozack WJ. Prospective randomized study of two surgical approaches for total hip arthroplasty. J Arthroplasty. 2010;25:671–9.e1. doi: 10.1016/j.arth.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Barrett WP, Turner SE, Leopold JP. Prospective randomized study of direct anterior vs. postero-lateral approach for total hip arthroplasty. J Arthroplasty. 2013;28:1634–8. doi: 10.1016/j.arth.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Barber TC, Roger DJ, Goodman SB, Schurman DJ. Early outcome of total hip arthroplasty using the direct lateral vs. the posterior surgical approach. Orthopedics. 1996;19:873–5. doi: 10.3928/0147-7447-19961001-11. [DOI] [PubMed] [Google Scholar]
- 26.Witzleb WC, Stephan L, Krummenauer F, Neuke A, Günther KP. Short-term outcome after posterior versus lateral surgical approach for total hip arthroplasty – A randomized clinical trial. Eur J Med Res. 2009;14:256–63. doi: 10.1186/2047-783X-14-6-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin CT, Pugely AJ, Gao Y, Clark CR. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty. 2013;28:849–54. doi: 10.1016/j.arth.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Parvizi J, Restrepo C, Maltenfort MG. Total hip arthroplasty performed through direct anterior approach provides superior early outcome: Results of a randomized, prospective study. Orthop Clin North Am. 2016;47:497–504. doi: 10.1016/j.ocl.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Phillips CB, Barrett JA, Losina E, Mahomed NN, Lingard EA, Guadagnoli E, et al. Incidence rates of dislocation, pulmonary embolism, and deep infection during the first six months after elective total hip replacement. J Bone Joint Surg Am. 2003;85-A:20–6. doi: 10.2106/00004623-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: The incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–5. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pugely AJ, Martin CT, Gao Y, Schweizer ML, Callaghan JJ. The incidence of and risk factors for 30-day surgical site infections following primary and revision total joint arthroplasty. J Arthroplasty. 2015;30(9 Suppl):47–50. doi: 10.1016/j.arth.2015.01.063. [DOI] [PubMed] [Google Scholar]
- 32.Malek IA, Royce G, Bhatti SU, Whittaker JP, Phillips SP, Wilson IR, et al. A comparison between the direct anterior and posterior approaches for total hip arthroplasty: The role of an ‘Enhanced Recovery’ pathway. Bone Joint J. 2016;98-B:754–60. doi: 10.1302/0301-620X.98B6.36608. [DOI] [PubMed] [Google Scholar]
- 33.Namba RS, Inacio MC, Paxton EW. Risk factors associated with surgical site infection in 30,491 primary total hip replacements. J Bone Joint Surg Br. 2012;94:1330–8. doi: 10.1302/0301-620X.94B10.29184. [DOI] [PubMed] [Google Scholar]
- 34.Sheth D, Cafri G, Inacio MC, Paxton EW, Namba RS. Anterior and anterolateral approaches for THA are associated with lower dislocation risk without higher revision risk. Clin Orthop Relat Res. 2015;473:3401–8. doi: 10.1007/s11999-015-4230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christensen CP, Karthikeyan T, Jacobs CA. Greater prevalence of wound complications requiring reoperation with direct anterior approach total hip arthroplasty. J Arthroplasty. 2014;29:1839–41. doi: 10.1016/j.arth.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 36.Siguier T, Siguier M, Brumpt B. Mini-incision anterior approach does not increase dislocation rate: A study of 1037 total hip replacements. Clin Orthop Relat Res. 2004;426:164–73. doi: 10.1097/01.blo.0000136651.21191.9f. [DOI] [PubMed] [Google Scholar]
- 37.Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;441:115–24. doi: 10.1097/01.blo.0000194309.70518.cb. [DOI] [PubMed] [Google Scholar]
- 38.Mulliken BD, Rorabeck CH, Bourne RB, Nayak N. A modified direct lateral approach in total hip arthroplasty: A comprehensive review. J Arthroplasty. 1998;13:737–47. doi: 10.1016/s0883-5403(98)90024-9. [DOI] [PubMed] [Google Scholar]
- 39.Demos HA, Rorabeck CH, Bourne RB, MacDonald SJ, McCalden RW. Instability in primary total hip arthroplasty with the direct lateral approach. Clin Orthop Relat Res. 2001;393:168–80. doi: 10.1097/00003086-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein WM, Gleason TF, Kopplin M, Branson JJ. Prevalence of dislocation after total hip arthroplasty through a posterolateral approach with partial capsulotomy and capsulorrhaphy. J Bone Joint Surg Am. 2001;83-A(Suppl 2(Pt 1)):2–7. doi: 10.2106/00004623-200100021-00002. [DOI] [PubMed] [Google Scholar]
- 41.White RE, Jr, Forness TJ, Allman JK, Junick DW. Effect of posterior capsular repair on early dislocation in primary total hip replacement. Clin Orthop Relat Res. 2001;393:163–7. doi: 10.1097/00003086-200112000-00019. [DOI] [PubMed] [Google Scholar]
- 42.Sierra RJ, Raposo JM, Trousdale RT, Cabanela ME. Dislocation of primary THA done through a posterolateral approach in the elderly. Clin Orthop Relat Res. 2005;441:262–7. doi: 10.1097/01.blo.0000194308.23105.f4. [DOI] [PubMed] [Google Scholar]
- 43.Tripuraneni KR, Munson NR, Archibeck MJ, Carothers JT. Acetabular abduction and dislocations in direct anterior vs. posterior total hip arthroplasty: A retrospective, matched cohort study. J Arthroplasty. 2016;31:2299–302. doi: 10.1016/j.arth.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Kumar V, Sharma S, James J, Hodgkinson JP, Hemmady MV. Total hip replacement through a posterior approach using a 22 mm diameter femoral head: The role of the transverse acetabular ligament and capsular repair in reducing the rate of dislocation. Bone Joint J. 2014;96-B:1202–6. doi: 10.1302/0301-620X.96B9.31831. [DOI] [PubMed] [Google Scholar]
- 45.Khan RJ, Yao F, Li M, Nivbrant B, Wood D. Capsular-enhanced repair of the short external rotators after total hip arthroplasty. J Arthroplasty. 2007;22:840–3. doi: 10.1016/j.arth.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87:2456–63. doi: 10.2106/JBJS.D.02860. [DOI] [PubMed] [Google Scholar]
- 47.Higgins BT, Barlow DR, Heagerty NE, Lin TJ. Anterior vs. posterior approach for total hip arthroplasty, a systematic review and meta-analysis. J Arthroplasty. 2015;30:419–34. doi: 10.1016/j.arth.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 48.Kwon MS, Kuskowski M, Mulhall KJ, Macaulay W, Brown TE, Saleh KJ. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop Relat Res. 2006;447:34–8. doi: 10.1097/01.blo.0000218746.84494.df. [DOI] [PubMed] [Google Scholar]
- 49.Masonis JL, Bourne RB. Clin Orthop Relat Res. 2002 Dec;(405):46–53. doi: 10.1097/00003086-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Tsukada S, Wakui M. Lower dislocation rate following total hip arthroplasty via direct anterior approach than via posterior approach: Five-year-average follow-up results. Open Orthop J. 2015;9:157–62. doi: 10.2174/1874325001509010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jolles BM, Bogoch ER. Posterior versus lateral surgical approach for total hip arthroplasty in adults with osteoarthritis. Cochrane Database Syst Rev. 2004;1:CD003828. doi: 10.1002/14651858.CD003828.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Maratt JD, Gagnier JJ, Butler PD, Hallstrom BR, Urquhart AG, Roberts KC. No difference in dislocation seen in anterior vs. posterior approach total hip arthroplasty. J Arthroplasty. 2016;31(9 Suppl):127–30. doi: 10.1016/j.arth.2016.02.071. [DOI] [PubMed] [Google Scholar]
- 53.Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop Relat Res. 2011;469:503–7. doi: 10.1007/s11999-010-1568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woolson ST, Pouliot MA, Huddleston JI. Primary total hip arthroplasty using an anterior approach and a fracture table: Short-term results from a community hospital. J Arthroplasty. 2009;24:999–1005. doi: 10.1016/j.arth.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: Two consecutive series. J Arthroplasty. 2009;24:698–704. doi: 10.1016/j.arth.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 56.Hendel D, Yasin M, Garti A, Weisbort M, Beloosesky Y. Fracture of the greater trochanter during hip replacement: A retrospective analysis of 21/372 cases. Acta Orthop Scand. 2002;73:295–7. doi: 10.1080/000164702320155284. [DOI] [PubMed] [Google Scholar]
- 57.Howell GE, Biggs RE, Bourne RB. Prevalence of abductor mechanism tears of the hips in patients with osteoarthritis. J Arthroplasty. 2001;16:121–3. doi: 10.1054/arth.2001.19158. [DOI] [PubMed] [Google Scholar]
- 58.Hendry J, Biant LC, Breusch SJ. Abductor mechanism tears in primary total hip arthroplasty. Arch Orthop Trauma Surg. 2012;132:1619–23. doi: 10.1007/s00402-012-1573-9. [DOI] [PubMed] [Google Scholar]
- 59.Miozzari HH, Dora C, Clark JM, Nötzli HP. Late repair of abductor avulsion after the transgluteal approach for hip arthroplasty. J Arthroplasty. 2010;25:450–7.e1. doi: 10.1016/j.arth.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 60.Bergin PF, Doppelt JD, Kephart CJ, Benke MT, Graeter JH, Holmes AS, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93:1392–8. doi: 10.2106/JBJS.J.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meneghini RM, Pagnano MW, Trousdale RT, Hozack WJ. Muscle damage during MIS total hip arthroplasty: Smith-Petersen versus posterior approach. Clin Orthop Relat Res. 2006;453:293–8. doi: 10.1097/01.blo.0000238859.46615.34. [DOI] [PubMed] [Google Scholar]
- 62.Macheras GA, Christofilopoulos P, Lepetsos P, Leonidou AO, Anastasopoulos PP, Galanakos SP. Nerve injuries in total hip arthroplasty with a mini invasive anterior approach. Hip Int. 2016;26:338–43. doi: 10.5301/hipint.5000352. [DOI] [PubMed] [Google Scholar]
- 63.Goulding K, Beaulé PE, Kim PR, Fazekas A. Incidence of lateral femoral cutaneous nerve neuropraxia after anterior approach hip arthroplasty. Clin Orthop Relat Res. 2010;468:2397–404. doi: 10.1007/s11999-010-1406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhargava T, Goytia RN, Jones LC, Hungerford MW. Lateral femoral cutaneous nerve impairment after direct anterior approach for total hip arthroplasty. Orthopedics. 2010;33:472. doi: 10.3928/01477447-20100526-05. [DOI] [PubMed] [Google Scholar]
- 65.Khan T, Knowles D. Damage to the superior gluteal nerve during the direct lateral approach to the hip: A cadaveric study. J Arthroplasty. 2007;22:1198–200. doi: 10.1016/j.arth.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 66.Picado CH, Garcia FL, Marques W., Jr Damage to the superior gluteal nerve after direct lateral approach to the hip. Clin Orthop Relat Res. 2007;455:209–11. doi: 10.1097/01.blo.0000238805.87411.e8. [DOI] [PubMed] [Google Scholar]
- 67.Oldenburg M, Müller RT. The frequency, prognosis and significance of nerve injuries in total hip arthroplasty. Int Orthop. 1997;21:1–3. doi: 10.1007/s002640050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramesh M, O’Byrne JM, McCarthy N, Jarvis A, Mahalingham K, Cashman WF. Damage to the superior gluteal nerve after the Hardinge approach to the hip. J Bone Joint Surg Br. 1996;78:903–6. doi: 10.1302/0301-620x78b6.1289. [DOI] [PubMed] [Google Scholar]
- 69.Farrell CM, Springer BD, Haidukewych GJ, Morrey BF. Motor nerve palsy following primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87:2619–25. doi: 10.2106/JBJS.C.01564. [DOI] [PubMed] [Google Scholar]
- 70.Simmons C, Jr, Izant TH, Rothman RH, Booth RE, Jr, Balderston RA. Femoral neuropathy following total hip arthroplasty. Anatomic study, case reports, and literature review. J Arthroplasty. 1991;6(Suppl):S57–66. [PubMed] [Google Scholar]
- 71.Schmalzried TP, Amstutz HC, Dorey FJ. Nerve palsy associated with total hip replacement. Risk factors and prognosis. J Bone Joint Surg Am. 1991;73:1074–80. [PubMed] [Google Scholar]
- 72.Basad E, Ishaque B, Stürz H, Jerosch J. The anterolateral minimally invasive approach for total hip arthroplasty: Technique, pitfalls, and way out. Orthop Clin North Am. 2009;40:473–8, viii. doi: 10.1016/j.ocl.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 73.Pflüger G, Junk-Jantsch S, Schöll V. Minimally invasive total hip replacement via the anterolateral approach in the supine position. Int Orthop. 2007;31(Suppl 1):S7–11. doi: 10.1007/s00264-007-0434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berger RA. Total hip arthroplasty using the minimally invasive two-incision approach. Clin Orthop Relat Res. 2003;(417):232–41. doi: 10.1097/01.blo.0000096828.67494.95. [DOI] [PubMed] [Google Scholar]
- 75.Berger RA, Duwelius PJ. The two-incision minimally invasive total hip arthroplasty: Technique and results. Orthop Clin North Am. 2004;35:163–72. doi: 10.1016/S0030-5898(03)00110-X. [DOI] [PubMed] [Google Scholar]
- 76.Leunig M, Faas M, von Knoch F, Naal FD. Skin crease ‘bikini’ incision for anterior approach total hip arthroplasty: Surgical technique and preliminary results. Clin Orthop Relat Res. 2013;471:2245–52. doi: 10.1007/s11999-013-2806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]