Abstract
The diverse cell-types of the basal forebrain control sleep-wake states, cortical activity and reward processing. Large, slow-firing, cholinergic neurons suppress cortical delta activity and promote cortical plasticity in response to reinforcers. Large, fast-firing, cortically-projecting GABAergic neurons promote wakefulness and fast cortical activity. In particular, parvalbumin/GABAergic neurons promote neocortical gamma band activity. Conversely, excitation of slower-firing somatostatin/GABAergic neurons promotes sleep through inhibition of cortically-projecting neurons. Activation of glutamatergic neurons promotes wakefulness, likely by exciting other cortically-projecting neurons. Similarly, cholinergic neurons indirectly promote wakefulness by excitation of wake-promoting, cortically-projecting GABAergic neurons and/or inhibition of sleep-promoting somatostatin/GABAergic neurons. Both glia and neurons increase the levels of adenosine during prolonged wakefulness. Adenosine presynaptically inhibits glutamatergic inputs to wake-promoting cholinergic and GABAergic/parvalbumin neurons, promoting sleep.
INTRODUCTION
The basal forebrain (BF) is a large heterogeneous structure located close to the ventral surface of the rostral telencephalon (Figure 1) which is involved in sleep-wake control, attention and reward processing [1–3]. Until relatively recently, most of these functions were ascribed to the BF cholinergic neurons which degenerate in Alzheimer’s disease and other dementias [4]. However, recent technical advances which allowed the specific targeting of GABAergic and glutamatergic BF neurons have revealed important roles for these neurons and have refined our understanding of cholinergic neurons [2]. Thus, in this review we summarize our current knowledge of these different neural species within the BF menagerie. We discuss their cellular properties (what they look like), their functions (how they behave) and how they interact (who talks to whom). We focus on the intermediate/caudal part of BF which contains neurons projecting to the neocortex (Figure 1) and on studies conducted in mice, since recent optogenetic and chemogenetic studies have used this species.
Figure 1.
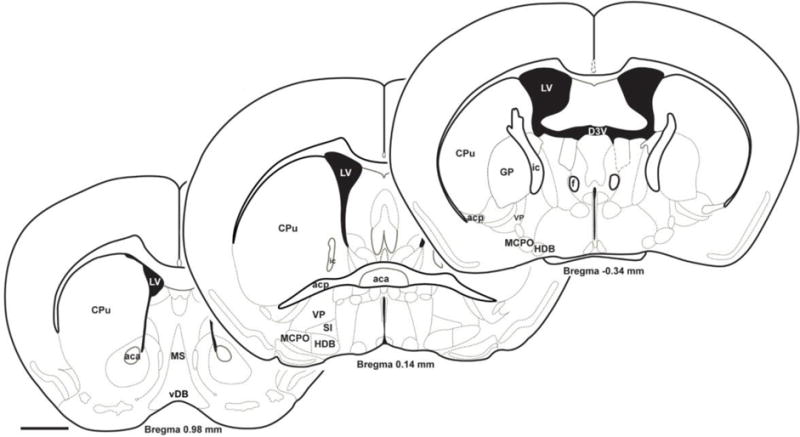
The basal forebrain (BF) is a large, heterogeneous structure located adjacent to the ventral surface of the forebrain. It is typically defined by the presence of cholinergic projection neurons [8] although as we discuss here, GABAergic and glutamatergic neurons play important functional roles. Rostral BF regions (MS, vDB) contain neurons which project to the hippocampus and associated archaecortical regions. This review is mainly focused on intermediate BF subregions with neurons projecting to neocortex, including MCPO, HDB, VP and SI as well as caudally-located cholinergic and GABAergic projection neurons in the nucleus basalis (a term often used interchangeably with basal forebrain in the human literature) located within the boundaries of the globus pallidus (GP). This figure was adapted with permission from figures previously published in “The mouse brain in stereotaxic coordinates” (ISBN: 9780123910578) by George Paxinos & Keith Franklin (2008) 3rd ed. New York, Academic Press (Copyright Elsevier). Abbreviations: aca: anterior part of anterior commissure; acp: posterior part of anterior commissure; CPu: caudate putamen; D3V: dorsal 3rd ventricle; f: fornix; GP: globus pallidus; HDB: horizontal limb of the diagonal band; ic: internal capsule; LV: lateral ventricle; MCPO: magnocellular preoptic nucleus; MS: medial septum; SI: substantia innominata; vDB: ventral limb of the diagonal band; VP: ventral pallidum. Scale bar=1mm.
HOW MANY NEURAL SPECIES ARE THERE AND WHAT DO THEY LOOK LIKE?
The BF contains three largely non-overlapping groups of neurons [5–8] which can be distinguished based on their neurotransmitter phenotype i.e. cholinergic, GABAergic and glutamatergic neurons. GABAergic and glutamatergic neurons can be further subdivided according to their projections, their expression of calcium-binding proteins, neuropeptides/neuropeptide receptors, ion channels and their intrinsic electrical properties, as described next. Figure 2 gives an overview.
Figure 2. Basal forebrain neuronal subtypes and their discharge properties across sleep-wake states.
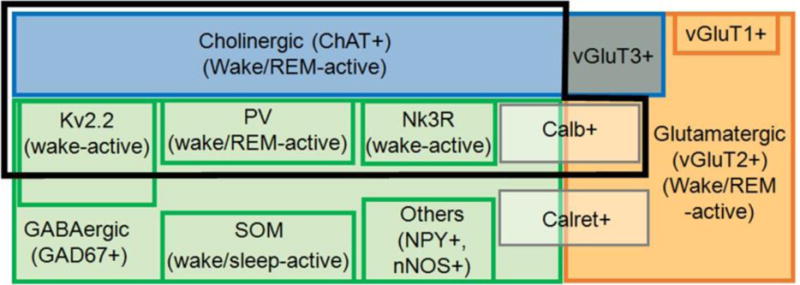
Blue represents the well-known cholinergic (ChAT+) population representing ~10–20 % of all BF neurons, depending on the subregion [8]. Green represents the GABAergic (GAD67+) population, the largest group of BF neurons, ~5 times the number of cholinergic neurons [19]. Orange represents the smaller glutamatergic population (mainly vGluT2+ with small numbers of vGluT1+ and vGluT3+ neurons; [30]). A few vGluT3 neurons are cholinergic (grey/blue box) and project to the amygdala [15]. The black frame represents cortically-projecting neuronal subtypes, including most cholinergic neurons, three different types of GABAergic neurons and glutamatergic (vGluT2) neurons. Cortically-projecting neurons are active during wakefulness and/or REM-sleep in association with cortical fast-activity [7,18,50,51]. GABAergic PV neurons are wake/REM active [7,22]. They represent ~7 % of all BF GABA neurons but ~25 % of large (>20 μm, likely cortically-projecting neurons) [6]. GABAergic Kv2.2+ neurons represent ~60 % of all BF GABAergic neurons in HDB and MCPO [28], including large putative cortically-projecting neurons. Nk3R+ neurons represent a third group of cortically-projecting GABAergic neurons [29]. Other subgroups of GABAergic neurons containing SOM, NPY, nNOS and calretinin project caudally and/or locally within BF [7,8,11,29]. Some SOM and NPY neurons are likely to be sleep-active [7,50,51]. The discharge of vGluT2 neurons is weakly modulated by behavioral state [7]. They discharge faster during wakefulness and REM sleep. The calcium binding proteins Calb and Calret are expressed in subsets of glutamatergic as well as GABAergic neurons. Calb+/vGluT2+ neurons may be cortically projecting whereas calretinin neurons are not [20]. Abbreviations: Calb: calbindin; Calret: calretinin; ChAT: Choline acetyltransferase; GAD: glutamic acid decarboxylase; Kv: voltage-gated delayed-rectifier potassium channel; Nk3R: Neurokinin B receptors, type 3; nNOS+: neuronal nitric oxide synthase; NPY: neuropeptide Y; PV: parvalbumin; REM: rapid eye movement; SOM: somatostatin; vGluT1/2/3: vesicular glutamate transporter type 1/2/3.
Properties and subtypes of cholinergic neurons
Cholinergic neurons are typically identified by their expression of choline acetyltransferase [9], the enzyme which synthesizes acetylcholine. Although they are the best known of the different BF neuronal species, cholinergic neurons represent a minority of BF neurons (10–20%, depending on the subregion) [8]. Cholinergic neurons are mostly large neurons (>20 μm), and are present throughout all BF subregions [6,10]. Most BF cholinergic neurons express the low-affinity neurotrophin receptor, p75 [8], and densely innervate the entire cortical mantle in a selective and topographically specific manner [8,11–14]. A smaller subset of cholinergic neurons lacks the p75 receptor and projects to the amygdala [8,15,16]. Some of these neurons also express the vesicular glutamate transporter, subtype 3 [15]. BF cholinergic neurons receive dense inputs from subcortical nuclei related to motivation and stress such as the amygdala, lateral hypothalamus and dorsal raphe [17].
In vitro, BF cholinergic neurons are often silent at rest (resting membrane potential ~−70 mV), discharge slowly with large and long-lasting afterhyperpolarizations when depolarized, and have a low tonic maximal firing rate of ~14 Hz [6], although higher rates are observed within burst discharges in vivo [18]. BF cholinergic neurons are excited by most wake-promoting neurotransmitters, but are inhibited by serotonin [1].
Properties and subtypes of GABAergic neurons
BF GABAergic neurons represent the largest group of BF neurons. Stereological estimates in rats suggest there are ~5 times more GABAergic than cholinergic neurons [19]. The recent availability of transgenic mouse models, such as the glutamic acid decarboxylase 67 (GAD67)-GFP knock-in and vesicular-GABA-transporter (vGAT)-Cre Recombinase mice, has helped overcome previous technical challenges in targeting them, and allowed the precise mapping and determination of their properties [5–7]. Most are small or medium-sized (<20 μm) neurons but ~12% are large sized (>20 μm) projection neurons [6,20]. The densest cluster of large-sized BF GABAergic neurons in the mouse is located laterally in the magnocellular preoptic nucleus [6]. Cortical projections of BF GABAergic neurons preferentially target cortical interneurons containing parvalbumin or somatostatin [21,22]. Other prominent BF GABAergic outputs target the thalamic reticular nucleus [5,23], midline thalamus [5], lateral hypothalamus [24] and lateral habenula [5,11,25]. A recent study identified a GABAergic BF projection to the lateral habenula which modulates aggression reward [25].
In vitro, large BF GABAergic neurons have a high spontaneous (~13 Hz) and maximal discharge rate (90 Hz or higher). Based on the amplitudes and kinetics of their hyperpolarization-activated cationic current (Ih), these large-sized BF GABAergic neurons can be further categorized into two subtypes, large Ih and small Ih [6]. Both subtypes exhibit electrical synapses linking neighboring neurons [6], which may be important for synchronizing their firing [26].
At least three types of large, cortically-projecting BF GABAergic neurons exist [27]. One functionally important subgroup (~25 % of large GABAergic neurons and ~7 % of all GABAergic neurons) [6], involved in the control of cortical gamma band oscillations [22], contains the calcium binding protein, parvalbumin (PV) [6,20]. They have very narrow action potentials and extremely high maximal discharge rates, like cortical PV interneurons [6]. Unlike cortical PV interneurons however, they exhibit prominent H-currents, a depolarized membrane potential and different pharmacology [2,6,27]. A second, large population (60 %) of BF GABAergic neurons located in HDB and MCPO expresses a particular type of delayed rectifier potassium channels (Kv2.2) [28]. Neurokinin B receptors (NK3Rs), which are involved in secretion of gonadotropin-releasing hormone, are expressed in a largely separate population (~25%) of BF cortically-projecting GABAergic neurons [29].
Many BF GABAergic neurons project caudally and/or locally within BF. Some of these smaller GABAergic neurons have intrinsic electrical properties similar to striatal medium-spiny neurons [6]. Subsets of BF GABAergic neurons co-express the neuropeptides somatostatin or neuropeptide Y, the enzyme nitric oxide synthase or the calcium binding proteins, calbindin or calretinin [8,20].
Properties and subtypes of glutamatergic neurons
Glutamatergic neurons represent the smallest of the three major groups of BF neurons. Most contain the vesicular glutamate transporter, subtype 2 (vGluT2) [8,30], with much smaller numbers expressing vGluT1 (Allen Brain Atlas) or vGluT3 [15]. The vast majority of BF vGluT3 neurons are cholinergic [15]. vGluT2 neurons comprise ~5% of BF cortically-projecting neurons [30]. Tracing experiments [11,30] suggest that vGluT2 neurons have relatively weak projections to cortex but stronger projections to many subcortical regions, including areas involved in reward processing such as the lateral habenula, as well as within the BF [7]. Cortically-projecting vGluT2 neurons are located mainly in ventromedial BF [30]. Like BF GABAergic neurons, subsets of BF glutamatergic neurons express calcium binding proteins [20]. In the rat, it was proposed that calbindin-expressing glutamatergic neurons project to cortex while calretinin-containing neurons project locally or caudally [5,20]. A subgroup (25%) of vGluT2 neurons in rostral BF express gonadotropin-releasing hormone [31]. In vitro, BF vGluT2 neurons discharge maximally around 50 Hz (Yang et al., abstract in SLEEP Abstract Supplement 2016, 39:0075). Most vGluT2 neurons exhibit moderately-sized Ih although there is subregional variability. Subsets of vGluT2 neurons have low-threshold calcium currents and show burst/cluster discharge.
HOW DO THEY BEHAVE?
In vivo discharge and role of different BF neurons in the control of sleep-wake activity, attention and reward
Behavior of BF cholinergic neurons
BF cholinergic neurons are more active during wakefulness and rapid-eye-movement (REM) sleep than during non-REM (NREM) sleep [7,18]. Furthermore, they discharge with bursts of action potentials during states associated with EEG theta activity. Behavioral studies revealed a rapid response to reinforcers [32,33]. Cholinergic signals in the cortex promote cortical activation [34], facilitate fast and dynamic plasticity of sensory perception [35], enhance the salience of stimuli [36] and promote long-lasting synaptic plasticity [37]. Thus, one main function of cholinergic neurons may be to act as a teaching and alerting signal to the cortex in the presence of behaviorally important stimuli. Caudal projections of BF cholinergic neurons whose cell bodies are located in the diagonal band are also reward-related since they modulate appetite [38].
Recent optogenetic and chemogenetic studies tested the role of BF cholinergic neurons in the promotion of wakefulness. Phasic optogenetic activation of BF cholinergic neurons increased transitions to wakefulness [39,40] or the amount of wakefulness during the period of stimulation [41], enhanced cortical theta activity [7,39–41] and suppressed delta activity [42,43]. However, short-term, wake-promoting effects were dependent on activation of neighboring BF neurons [41]. Prolonged chemogenetic activation of cholinergic neurons also reduced delta activity and increased the number of wake bouts but did not change the total amount of wakefulness [5]. Selective lesion or chemogenetic inhibition studies showed only mild changes in sleep-wake amounts [42–45]. Together, these results support the opinion that the BF cholinergic system itself has a relatively minor direct role in controlling the overall daily amounts of wakefulness but, similar to other neuromodulatory systems, enhanced activity of cholinergic neurons can temporally increase wakefulness in certain behavioral contexts.
In contrast to its relatively minor role in controlling spontaneous sleep/wake behavior, considerable work supports a key role of the BF cholinergic system in the homeostatic sleep response. Specific lesions of cholinergic neurons expressing the p75 neurotrophin receptor abolished increases in sleep and EEG delta power following sleep deprivation [44,46]. In addition, increases in the inhibitory neuromodulator, adenosine [47,48], during prolonged wakefulness [49] were blocked by cholinergic lesions [46].
Behavior of BF GABAergic neurons
Unlike the relatively homogeneous cholinergic neurons, the discharge of identified BF GABAergic neurons shows considerable diversity [50]. One third of BF GABAergic neurons which show state-dependent modulation, likely the cortically-projecting GABAergic neurons, discharge maximally during wakefulness and REM sleep and in positive correlation with gamma EEG activity [50]. Identified PV neurons also show this pattern of activity [7,22,51]. Interestingly, single-unit recordings by Hangya and colleagues suggested that the discharge of a non-cholinergic BF neuronal population with discharge rates similar to identified wake/REM-active GABAergic or PV neurons were correlated with attention [32]. Another third of GABAergic neurons discharged maximally during NREM sleep and in positive correlation with EEG delta activity. At least some of these GABAergic neurons contain somatostatin [7] or NPY [51]. The final third discharge maximally during REM sleep and in negative association with electromyographic (EMG) activity [50]. Additional markers to identify this subgroup of GABAergic neurons are lacking at present. A subpopulation of slowly-discharging, non-cholinergic BF neurons, possibly GABAergic, which lack strong state-dependent modulation of discharge encode motivational salience [2,52].
Chemogenetic activation of BF GABAergic neurons in vesicular GABA transporter-Cre Recombinase (vGAT-cre) mice strongly increased total amounts of wakefulness and high-frequency cortical rhythms (>30 Hz) [5,7,22] whereas chemogenetic inhibition increased the percentage of time spent asleep [5], suggesting an essential role for a major subset of BF GABAergic neurons in promoting wakefulness. However, the role of specific subtypes of BF GABAergic neurons is still an active area of investigation. Optogenetic stimulation of BF PV neurons preferentially enhanced cortical gamma oscillations (~40 Hz) [22] likely by synchronizing cortical PV interneurons [21]. This effect was independent of BF cholinergic neurons. Furthermore, bilateral optogenetic inhibition of BF PV neurons impaired the 40 Hz auditory steady-state cortical response [22]. Optogenetic stimulation of PV neurons induces short-latency arousals from sleep (McKenna, Thankachan et al., abstract in Soc. Neurosci Abs 2016, 83.10) and increased total amounts of wakefulness [7]. However, loss-of-function experiments with respect to sleep-wake behavior have not been performed to date. C-fos immunostaining suggests that BF Kv2.2-expressing GABAergic neurons are also likely to be wake-active neurons [53]. Furthermore, global knockout of Kv2.2 reduced cortical delta power during sleep [53]. To date, single-unit recordings from cortically-projecting Kv2.2/GABAergic or NK3R/GABAergic neurons have not been performed.
Somatostatin/GABAergic neurons are heterogeneous with respect to their discharge pattern. Some are wake/REM active whereas others discharge faster during NREM sleep [7]. Despite only a portion of BF Somatostatin neurons being more active during NREM sleep, optogenetic stimulation of these neurons increased NREM sleep and decreased wakefulness [7].
Behavior of BF glutamatergic neurons
The discharge of BF vGluT2 neurons is weakly modulated across brain states. They are primarily wake and REM active [7]. Strong optogenetic stimulation of BF vGluT2 neurons potently promotes wakefulness [7], however weaker chemogenetic activation of BF vGluT2 neurons only decreased NREM delta power without affecting sleep-wake quantity, consolidation or sleep latency [5]. Loss-of-function studies of BF vGluT2 neurons have not been performed to date so it is unclear whether they are necessary for sleep-wake control. Recordings of calcium signals from vGluT2 neurons in vivo revealed that they, like other BF cell-types, are activated by punishers [33]. Rostral vGluT2 neurons control hippocampal theta oscillations and locomotion [54,55].
Basal forebrain glial cells
Very little is known about the types and physiology of glia in BF. Microglia play an important role in the differentiation, development and survival of BF cholinergic neurons [56]. Importantly for sleep-wake control, astrocytes contribute to sleep-deprivation induced increases in extracellular adenosine, through release of ATP [57]. ATP released as a co-transmitter and adenosine released from neurons via adenosine transporters are also important contributors to sleep deprivation-induced increases in extracellular adenosine [58].
WHO TALKS TO WHOM? THE BF LOCAL CELLULAR NETWORK
Understanding the interactions of different BF neuronal species (Figure 3) is key to the interpretation of studies which investigate the role of specific subsets of BF neurons in behavior. For instance, recent experiments revealed a strong excitatory effect of cholinergic neurons on cortically-projecting GABAergic neurons mediated by nicotinic and muscarinic M1/M3 receptors [7,10], whereas the most prominent effect on vGluT2 neurons is a strong, long-lasting inhibition [7]. In vivo optodialysis experiments revealed that these local interactions, most likely the excitation of GABAergic neurons, were necessary for stimulation of cholinergic neurons to induce wakefulness [41].
Figure 3. Models of the basal forebrain (BF) circuits controlling sleep-wake behavior.
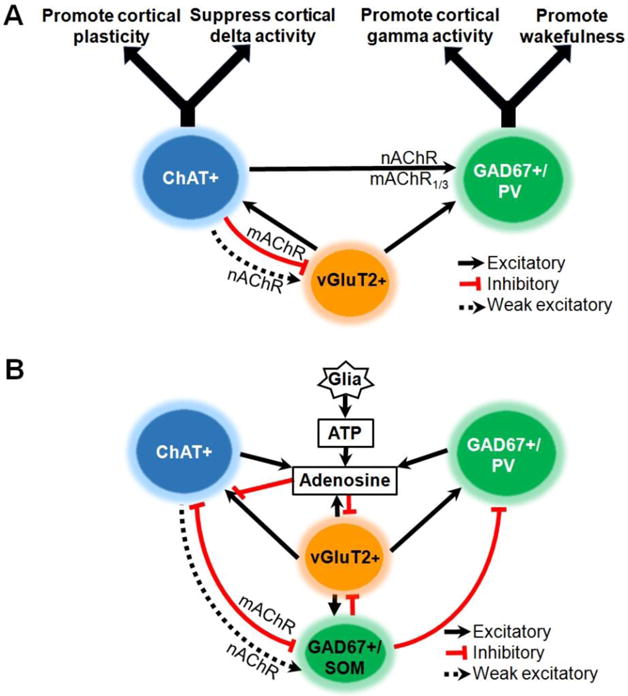
Solid lines with arrowheads indicate excitatory effects on the target neurons. The black dashed lines indicate a weak excitatory effect. Lines with flat ends indicate inhibitory effects. A. BF circuits promoting wakefulness, cortical activation and adaptive responses to behaviorally-relevant stimuli. Current data indicates that GABAergic projections neurons are the most important for promoting wakefulness. An important subset of these neurons containing parvalbumin (PV) regulates cortical gamma band activity. Cholinergic (ChAT+) and glutamatergic (vGluT2+) neurons promote wakefulness and cortical activation indirectly via excitatory effects on GABAergic/parvalbumin (GAD67+/PV) neurons, as well as via their direct cortical projections. Cholinergic neurons promote cortical plasticity in response to reinforcers. B. BF circuitry involved in sleep promotion. During prolonged wakefulness (i.e. sleep deprivation) there is accumulation of extracellular adenosine due to direct release from neurons as well as breakdown from the neurotransmitter/gliotransmitter, ATP. Adenosine inhibits BF cholinergic and GABAergic projection neurons by inhibiting their glutamatergic inputs via A1 receptors [47,48], thereby promoting a homeostatic sleep response. Activation of a subset of GABAergic neurons containing somatostatin may facilitate spontaneous transitions into non-REM sleep by direct postsynaptic inhibition of wake-promoting cholinergic and GABAergic neurons. Abbreviations: ATP: adenosine triphosphate; choline acetyltransferase; GAD: glutamic acid decarboxylase; mAChR: muscarinic acetylcholine receptors; nAChR: nicotinic acetylcholine receptor; NREM: non-rapid-eye-movement; PV: parvalbumin; SOM: somatostatin; vGluT2: vesicular glutamate transporter type 2.
In contrast to the extensive local effects of cholinergic neurons, BF PV/GABAergic neurons have relatively sparse connections to other BF neurons [7] suggesting that BF GABAergic [5] or PV neurons [22] promote wakefulness via their direct cortical projections, although interactions with other subcortical nodes of the sleep-wake circuitry [11] cannot be ruled out. The connections of Kv2.2 and NK3R GABAergic neurons are unknown at present.
Somatostatin/GABAergic neurons inhibit BF cholinergic, PV and vGluT2 neurons [7]. Thus, it is perhaps not surprising that optical stimulation of these neurons promoted sleep [7]. While the enhanced NREM discharge of a subset of these neurons would be consistent with a role in sleep promotion, loss-of-function experiments will be necessary to establish if they are necessary.
vGluT2 neurons provide excitatory inputs to all other BF neuronal subtypes [7]. Thus, the wake-promoting effects of stimulation of these neurons may be due to local interactions [7] or due to their extra-BF projections [11,30]. Loss-of-function experiments for this neuronal group have not been reported.
CONCLUSIONS
Overall, the current evidence is strongest with regards to an essential role for cortically-projecting GABAergic neurons in promoting wakefulness and cortical fast activity (Figure 3). Cholinergic neurons can increase wakefulness through their intra-BF and cortical projections and are important for cortical processing and plasticity in response to rewards and punishers. In addition, they play a key role in sleep homeostasis. The functional role of glutamatergic neurons is largely unexplored but at a minimum they act as local interneurons providing excitatory input to other BF cell-types. In the future, it will be important to dissect out the role of distinct subtypes of cholinergic, GABAergic and glutamatergic BF neurons and their postsynaptic targets.
Highlights.
Cholinergic, GABA and glutamatergic neurons all affect cortical activation
Cholinergic neurons suppress cortical delta activity and promote cortical plasticity
GABA/parvalbumin neurons promote gamma activity and fast arousals from sleep
GABA/somatostatin neurons inhibit wake-promoting neurons
Increases in extracellular adenosine promote sleep during prolonged wakefulness
Acknowledgments
This work was supported by the US Veterans Administration (Merit Review I01BX001356, McCarley PI), the National Institutes of Mental Health (R03 MH107650 Yang PI; R01 MH039683, McCarley PI) and the National Institute of Neurological Disorders and Stroke (R21 NS093000, Brown PI). The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have identified no conflicts of interest
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
*of special interest
**of outstanding interest
- 1.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin SC, Brown RE, Hussain Shuler MG, Petersen CC, Kepecs A. Optogenetic Dissection of the Basal Forebrain Neuromodulatory Control of Cortical Activation, Plasticity, and Cognition. J Neurosci. 2015;35:13896–13903. doi: 10.1523/JNEUROSCI.2590-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monosov IE, Leopold DA, Hikosaka O. Neurons in the Primate Medial Basal Forebrain Signal Combined Information about Reward Uncertainty, Value, and Punishment Anticipation. J Neurosci. 2015;35:7443–7459. doi: 10.1523/JNEUROSCI.0051-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grothe MJ, Heinsen H, Amaro E, Jr, Grinberg LT, Teipel SJ. Cognitive Correlates of Basal Forebrain Atrophy and Associated Cortical Hypometabolism in Mild Cognitive Impairment. Cereb Cortex. 2016;26:2411–2426. doi: 10.1093/cercor/bhv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anaclet C, Pedersen NP, Ferrari LL, Venner A, Bass CE, Arrigoni E, Fuller PM. Basal forebrain control of wakefulness and cortical rhythms. Nat Commun. 2015;6:8744. doi: 10.1038/ncomms9744. Using chemogenetic excitation and inhibition, the authors investigated the roles of basal forebrain cholinergic, GABAergic and glutamatergic neurons in sleep-wake control, and conclude that BF GABAergic neurons are the major group inducing wakefulness and high-frequency cortical rhythms associated with cognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKenna JT, Yang C, Franciosi S, Winston S, Abarr KK, Rigby MS, Yanagawa Y, McCarley RW, Brown RE. Distribution and intrinsic membrane properties of basal forebrain GABAergic and parvalbumin neurons in the mouse. J Comp Neurol. 2013;521:1225–1250. doi: 10.1002/cne.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu M, Chung S, Zhang S, Zhong P, Ma C, Chang WC, Weissbourd B, Sakai N, Luo L, Nishino S, et al. Basal forebrain circuit for sleep-wake control. Nat Neurosci. 2015;18:1641–1647. doi: 10.1038/nn.4143. Using optogenetic stimulation and single-unit recordings in vivo as well as whole-cell recordings in vitro, the authors examined the state-related discharge, effects on wakefulness and interconnections of four basal forebrain cell types (cholinergic, glutamatergic, parvalbumin/GABAergic & somatostatin/GABAergic neurons). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaborszky L, van den Pol A, Gyengesi E. The basal forebrain cholinergic projection system in mice. In: Charles W, Paxinos G, Puelles L, editors. The Mouse Nervous System. Academic Press; 2012. pp. 684–718. [Google Scholar]
- 9.Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983;216:53–68. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- 10.Yang C, McKenna JT, Zant JC, Winston S, Basheer R, Brown RE. Cholinergic neurons excite cortically projecting basal forebrain GABAergic neurons. J Neurosci. 2014;34:2832–2844. doi: 10.1523/JNEUROSCI.3235-13.2014. Using immunochemistry, whole-cell recordings and transgenic/optogenetic mouse models, the authors revealed the excitatory interaction between basal forebrain cholinergic neurons and cortically-projecting GABAergic neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Do JP, Xu M, Lee SH, Chang WC, Zhang S, Chung S, Yung TJ, Fan JL, Miyamichi K, Luo L, et al. Cell type-specific long-range connections of basal forebrain circuit. Elife. 2016:5. doi: 10.7554/eLife.13214. With optogenetic mouse models, the authors mapped the whole-brain inputs and outputs of four genetically identified basal forebrain neuronal types (cholinergic, GABAergic, parvalbumin/GABAergic and somatostatin/GABAergic neurons). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cereb Cortex. 2015;25:118–137. doi: 10.1093/cercor/bht210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Jung AH, Jeong D, Choi I, Kim K, Shin S, Kim SJ, Lee SH. Selectivity of Neuromodulatory Projections from the Basal Forebrain and Locus Ceruleus to Primary Sensory Cortices. J Neurosci. 2016;36:5314–5327. doi: 10.1523/JNEUROSCI.4333-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson A, Mooney R. The Basal Forebrain and Motor Cortex Provide Convergent yet Distinct Movement-Related Inputs to the Auditory Cortex. Neuron. 2016;90:635–648. doi: 10.1016/j.neuron.2016.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nickerson Poulin A, Guerci A, El Mestikawy S, Semba K. Vesicular glutamate transporter 3 immunoreactivity is present in cholinergic basal forebrain neurons projecting to the basolateral amygdala in rat. J Comp Neurol. 2006;498:690–711. doi: 10.1002/cne.21081. [DOI] [PubMed] [Google Scholar]
- 16.Unal CT, Pare D, Zaborszky L. Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. J Neurosci. 2015;35:853–863. doi: 10.1523/JNEUROSCI.2706-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu R, Jin S, He X, Xu F, Hu J. Whole-Brain Monosynaptic Afferent Inputs to Basal Forebrain Cholinergic System. Front Neuroanat. 2016;10:98. doi: 10.3389/fnana.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience. 2006;143:1051–1064. doi: 10.1016/j.neuroscience.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458:11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- 21.Freund TF, Meskenaite V. Gamma-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc Natl Acad Sci U S A. 1992;89:738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim T, Thankachan S, McKenna JT, McNally JM, Yang C, Choi JH, Chen L, Kocsis B, Deisseroth K, Strecker RE, et al. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci U S A. 2015;112:3535–3540. doi: 10.1073/pnas.1413625112. Using optogenetic stimulation and inhibition, the authors tested the role of basal forebrain parvalbumin neurons, and suggested that these inhibitory BF PV input controls cortical gamma band oscillations, likely by synchronizing the activity of cortical PV interneurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asanuma C, Porter LL. Light and electron microscopic evidence for a GABAergic projection from the caudal basal forebrain to the thalamic reticular nucleus in rats. J Comp Neurol. 1990;302:159–172. doi: 10.1002/cne.903020112. [DOI] [PubMed] [Google Scholar]
- 24.Henny P, Jones BE. Vesicular glutamate (VGlut), GABA (VGAT), and acetylcholine (VACht) transporters in basal forebrain axon terminals innervating the lateral hypothalamus. J Comp Neurol. 2006;496:453–467. doi: 10.1002/cne.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, Aleyasin H, Menard C, Zhang H, Hodes GE, et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534:688–692. doi: 10.1038/nature18601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tingley D, Alexander AS, Quinn LK, Chiba AA, Nitz DA. Cell assemblies of the basal forebrain. J Neurosci. 2015;35:2992–3000. doi: 10.1523/JNEUROSCI.4432-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown RE, McKenna JT. Turning a Negative into a Positive: Ascending GABAergic Control of Cortical Activation and Arousal. Front Neurol. 2015;6:135. doi: 10.3389/fneur.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermanstyne TO, Kihira Y, Misono K, Deitchler A, Yanagawa Y, Misonou H. Immunolocalization of the voltage-gated potassium channel Kv2.2 in GABAergic neurons in the basal forebrain of rats and mice. J Comp Neurol. 2010;518:4298–4310. doi: 10.1002/cne.22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furuta T, Koyano K, Tomioka R, Yanagawa Y, Kaneko T. GABAergic basal forebrain neurons that express receptor for neurokinin B and send axons to the cerebral cortex. J Comp Neurol. 2004;473:43–58. doi: 10.1002/cne.20087. [DOI] [PubMed] [Google Scholar]
- 30.Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study. J Comp Neurol. 2005;483:351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- 31.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol. 2009;587:1401–1411. doi: 10.1113/jphysiol.2008.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hangya B, Ranade SP, Lorenc M, Kepecs A. Central Cholinergic Neurons Are Rapidly Recruited by Reinforcement Feedback. Cell. 2015;162:1155–1168. doi: 10.1016/j.cell.2015.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison TC, Pinto L, Brock JR, Dan Y. Calcium Imaging of Basal Forebrain Activity during Innate and Learned Behaviors. Front Neural Circuits. 2016;10:36. doi: 10.3389/fncir.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci. 2013;16:1857–1863. doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gritton HJ, Howe WM, Mallory CS, Hetrick VL, Berke JD, Sarter M. Cortical cholinergic signaling controls the detection of cues. Proc Natl Acad Sci U S A. 2016;113:E1089–1097. doi: 10.1073/pnas.1516134113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verhoog MB, Obermayer J, Kortleven CA, Wilbers R, Wester J, Baayen JC, De Kock CP, Meredith RM, Mansvelder HD. Layer-specific cholinergic control of human and mouse cortical synaptic plasticity. Nat Commun. 2016;7:12826. doi: 10.1038/ncomms12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herman AM, Ortiz-Guzman J, Kochukov M, Herman I, Quast KB, Patel JM, Tepe B, Carlson JC, Ung K, Selever J, et al. A cholinergic basal forebrain feeding circuit modulates appetite suppression. Nature. 2016;538:253–256. doi: 10.1038/nature19789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han Y, Shi YF, Xi W, Zhou R, Tan ZB, Wang H, Li XM, Chen Z, Feng G, Luo M, et al. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr Biol. 2014;24:693–698. doi: 10.1016/j.cub.2014.02.011. Using optogenetic stimulation, the authors demonstrate that excitation of basal forebrain cholinergic neurons induces a transition to wakefulness only from slow-wave-sleep but not from rapid-eye-movement sleep. [DOI] [PubMed] [Google Scholar]
- 40.Irmak SO, de Lecea L. Basal forebrain cholinergic modulation of sleep transitions. Sleep. 2014;37:1941–1951. doi: 10.5665/sleep.4246. Using optogenetic stimulation, the authors demonstrated that excitation of basal forebrain cholinergic neurons during slow-wave-sleep induces cortical activation and a long-latency transition to wakefulness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zant JC, Kim T, Prokai L, Szarka S, McNally J, McKenna JT, Shukla C, Yang C, Kalinchuk AV, McCarley RW, et al. Cholinergic Neurons in the Basal Forebrain Promote Wakefulness by Actions on Neighboring Non-Cholinergic Neurons: An Opto-Dialysis Study. J Neurosci. 2016;36:2057–2067. doi: 10.1523/JNEUROSCI.3318-15.2016. Using combined optogenetic and microdialysis approaches (Opto-dialysis), the authors demonstrated that the wakefulness-promoting effect of excitation of basal forebrain cholinergic neurons requires actions on non-cholinergic neurons. This is the first study to use opto-dialysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi YF, Han Y, Su YT, Yang JH, Yu YQ. Silencing of Cholinergic Basal Forebrain Neurons Using Archaerhodopsin Prolongs Slow-Wave Sleep in Mice. PLoS One. 2015;10:e0130130. doi: 10.1371/journal.pone.0130130. Using optogenetic inhibition, the authors demonstrated that bilateral inactivation of basal forebrain prolongs slow-wave-sleep without changing the duration of wakefulness or rapid-eye-movement sleep, suggesting a main role of basal forebrain cholinergic neurons in terminating slow-wave-sleep instead of promoting wakefulness per se. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, Yin D, Wang TX, Guo W, Dong H, Xu Q, Luo YJ, Cherasse Y, Lazarus M, Qiu ZL, et al. Basal Forebrain Cholinergic Neurons Primarily Contribute to Inhibition of Electroencephalogram Delta Activity, Rather Than Inducing Behavioral Wakefulness in Mice. Neuropsychopharmacology. 2016;41:2133–2146. doi: 10.1038/npp.2016.13. Using chemogenetics, the authors showed that activation of basal forebrain cholinergic neurons strongly decreases delta power and slightly increase wakefulness while inhibition of basal forebrain cholinergic neurons increases delta power and slightly decreases wakefulness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaur S, Junek A, Black MA, Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J Neurosci. 2008;28:491–504. doi: 10.1523/JNEUROSCI.1585-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalinchuk AV, McCarley RW, Stenberg D, Porkka-Heiskanen T, Basheer R. The role of cholinergic basal forebrain neurons in adenosine-mediated homeostatic control of sleep: lessons from 192 IgG-saporin lesions. Neuroscience. 2008;157:238–253. doi: 10.1016/j.neuroscience.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawryluk JM, Ferrari LL, Keating SA, Arrigoni E. Adenosine inhibits glutamatergic input to basal forebrain cholinergic neurons. J Neurophysiol. 2012;107:2769–2781. doi: 10.1152/jn.00528.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang C, Franciosi S, Brown RE. Adenosine inhibits the excitatory synaptic inputs to Basal forebrain cholinergic, GABAergic, and parvalbumin neurons in mice. Front Neurol. 2013;4:77. doi: 10.3389/fneur.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hassani OK, Lee MG, Henny P, Jones BE. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J Neurosci. 2009;29:11828–11840. doi: 10.1523/JNEUROSCI.1259-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duque A, Balatoni B, Detari L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. J Neurophysiol. 2000;84:1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- 52.Raver SM, Lin SC. Basal forebrain motivational salience signal enhances cortical processing and decision speed. Front Behav Neurosci. 2015;9:277. doi: 10.3389/fnbeh.2015.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hermanstyne TO, Subedi K, Le WW, Hoffman GE, Meredith AL, Mong JA, Misonou H. Kv2.2: a novel molecular target to study the role of basal forebrain GABAergic neurons in the sleep-wake cycle. Sleep. 2013;36:1839–1848. doi: 10.5665/sleep.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuhrmann F, Justus D, Sosulina L, Kaneko H, Beutel T, Friedrichs D, Schoch S, Schwarz MK, Fuhrmann M, Remy S. Locomotion, Theta Oscillations, and the Speed-Correlated Firing of Hippocampal Neurons Are Controlled by a Medial Septal Glutamatergic Circuit. Neuron. 2015;86:1253–1264. doi: 10.1016/j.neuron.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Robinson J, Manseau F, Ducharme G, Amilhon B, Vigneault E, El Mestikawy S, Williams S. Optogenetic Activation of Septal Glutamatergic Neurons Drive Hippocampal Theta Rhythms. J Neurosci. 2016;36:3016–3023. doi: 10.1523/JNEUROSCI.2141-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jonakait GM, Pratt L, Acevedo G, Ni L. Microglial regulation of cholinergic differentiation in the basal forebrain. Dev Neurobiol. 2012;72:857–864. doi: 10.1002/dneu.20969. [DOI] [PubMed] [Google Scholar]
- 57.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim T, Ramesh V, Dworak M, Choi DS, McCarley RW, Kalinchuk AV, Basheer R. Disrupted sleep-wake regulation in type 1 equilibrative nucleoside transporter knockout mice. Neuroscience. 2015;303:211–219. doi: 10.1016/j.neuroscience.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


