Abstract
The clinical significance of papillary or follicular thyroid tissue incidentally discovered in cervical lymph nodes during pathological assessment of neck dissections for non-thyroid cancers of the upper aero-digestive tract is critically reviewed. Special emphasis is given to controversies over normal-looking, nodal, thyroid follicles. Arguments for and against the benign nature of these follicles are considered together with processes that could be involved in their formation. The admittedly limited evidence suggests that benign, thyroid follicular inclusions rarely occur in cervical lymph nodes. Histological criteria that could be helpful in recognising the inclusions, which include assessing their extent in conjunction with the size of the node, are discussed. Finally, an algorithm based on collaboration between specialists, correlating histological findings with imaging and loco-regional control of the upper aero-digestive tract cancer, is suggested for the management of patients with incidentally discovered, nodal thyroid tissue.
Keywords: Heterotopia, Metastasis, Lymph nodes, Neck dissection, Pathology, Thyroid
Introduction
Papillary or follicular thyroid tissue incidentally discovered in cervical lymph nodes during histological examination of neck dissections (NDs) performed for non-thyroid disease, is within the spectrum of the so-called “lateral aberrant thyroid” and raises questions about heterotopia, metastasis from an occult primary and further management. The issue is therefore of interest to embryologists, anatomists, pathologists and clinicians. The lateral rather than median localisation of that tissue spawned variable controversy. Although lateral papillary thyroid tissue is regarded as cancerous, it is more difficult to reach a consensus on how to categorise collections of normal-looking, colloid-containing thyroid follicles in lymph nodes. In 1981, a comprehensive review concluded that benign thyroid follicles may occur in cervical nodes [1]. The topic does not feature prominently in recent advances in head and neck surgery, but Willis’ dogma that any “lateral aberrant thyroid” reflects metastasis of a small/unsuspected thyroid carcinoma has left its mark [2]. While preparing an article on a different subject [3], it became clear that pathologists of the International Head and Neck Scientific Group held opposing views on nodal thyroid follicles, which would affect clinical decisions. It was therefore decided to revisit incidentally discovered thyroid tissue in cervical lymph nodes, review further evidence and, if possible, draw conclusions.
Brief historical survey
It seems likely that “lateral aberrant thyroid” has been known since 1779. Frantz et al. [4] and Gerard-Marchant and Caillou [1] reviewed the earlier literature, but attention is drawn to highlights.
In 1942 Frantz et al. presented their experience from 30 patients; although most of their cases corresponded with papillary thyroid carcinoma (PTC), one showed “simple thyroid tissue without abnormality” in a lymph node and was regarded as an embryological aberration [4]. Further investigations culminated in the 1960s when retrospective histological examination of large series of lymph nodes from NDs, popularised the notion of non-neoplastic thyroid tissue spreading via the lymphatic route [5, 6]. Gerard-Marchant reviewed the archives of the Gustave-Roussy Institute over a six year period (16,649 jugular nodes, 647 patients) and found thyroid follicles of benign appearance in five nodes from four patients [5]. Nicastri et al. reported similar follicles in 16 patients subjected to radical ND for malignancies other than thyroid origin at the New York Memorial Hospital for Cancer and Allied Diseases in New York between 1950 and 1965; they noted the presence of the follicles usually at cervical levels III–IV and advised a conservative approach [6]. Both Gerard-Marchant and Nicastri et al. dubbed the nodal thyroid follicles of benign appearance “inclusions” [5, 6]. The term was adopted in later influential texts [7, 8] and is used here for brevity. Some authors, however, apply it more broadly and clarification is necessary.
The 1960s also witnessed the introduction of approaches to assessing nodal thyroid inclusions, which were more rigorous than mere review of archival histological sections. In 1969, Meyer and Steinberg found such inclusions in five out of 106 autopsies (4.7%) [9]. These authors then compared nuclear dimensions between the inclusions and corresponding thyroid glands examined via serial sectioning [9]. No differences in nuclear size were observed, which reinforced the view that the inclusions were benign, though a contralateral, primary, papillary thyroid microcarcinoma was found in one of the five autopsies [9]. Meyer and Steinberg also suggested histological criteria for deciding whether nodal thyroid tissue is benign [9]. These criteria have been subsequently endorsed by others [1, 8, 10].
Routine histology had been always at the very core of the investigations, but Gerard-Marchant pioneered the use of immunohistochemistry (IHC) in studying incidentally discovered nodal thyroid tissue [1] and molecular techniques seem the latest addition [11].
With regards to pathogenesis of benign nodal thyroid, we have already alluded to the possible significance of dysembryoplasia and “benign metastasis”. These together with other possibilities are critically reviewed by Gerard-Marchant and Caillou [1].
Recent investigations
Deposits of PTC incidentally discovered have usually been interpreted as metastasis from a clinically occult thyroid primary and further management often involved thyroidectomy. However, the possibility that some of these deposits are nodal primaries deriving from benign thyroid inclusions is now considered. Table 1 catalogues cases reported since 1999 [12–20]. Although reservations may be expressed regarding efficacy of serial sectioning/thoroughness of histological examination of the entire thyroid, the fact that seven out of 27 subsequent thyroidectomies did not show evidence of PTC (26%, Table 1) makes out a case for the aforementioned possibility.
Table 1.
Cases of incidentally discovered nodal PTC in NDs for malignancies other than thyroid origin
| Authors | Nr of cases | Thyroidectomy | Thyroid histology
|
||
|---|---|---|---|---|---|
| PTC (−) | PTC(+) | Other | |||
| López-Escámez et al. [12] | 1 | 1 | 0 | 0 | granulomatous inflammation |
| Fliegelman et al. [13] | 4 | 4 | 1 | 3 | |
| Coskun et al. [14] | 3 | 2 | 1 | 1 | |
| Ansari-Lari and Westra [15] | 10 | 9 | 1 | 7 | medulary carcinoma, 1; calcification, 1 |
| Resta et al. [16] | 7 | 3 | 0 | 1 | hyperplasia, 2 |
| León et al. [17] | 5 | 3 | 2 | 1 | |
| Wang et al. [18] | 1 | 1 | 1 | 0 | |
| Yamamoto et al. [19] | 3 | 0 | |||
| Kr et al. [20] | 4 | 4 | 1 | 3 | |
| Total | 35 | 27 | 7 | 16 | |
Nr, Number; PTC, (−), absence of PTC; (+), presence of PTC
Incidental discovery of non-papillary types of thyroid cancer in cervical lymph nodes is less common. While PTC and follicular thyroid carcinoma (FTC) were equally represented in a series of eight patients [21], the PTC:FTC ratio was 7:1 in another series [15]. Although the latter regarded the FTC deposits as micrometastases, the thyroid was histologically free from tumour and the possibility that they corresponded with nodal thyroid inclusions cannot be excluded. A case of an incidental medullary carcinoma has also been reported [20].
The results of recent investigations [15, 17] regarding the occurrence of benign thyroid inclusions in cervical nodes are shown in Table 2. Earlier observations [5, 9]********** and the findings of an audit undertaken by one of the authors (AT) are also included for comparison. (Also consult Table 1 in Gerard-Marchant and Caillou [1].) The amount of detail varies between different studies and given the sampling errors inherent in routine histological assessment of NDs, the true occurrence of nodal thyroid inclusions may be underestimated. Nevertheless, Table 2 suggests that they are rare (<5% of patients, autopsies or lymph nodes). In the aforementioned audit, the nodes with thyroid inclusions were at level II. This seems rather “high”, but Nicastri et al. found a similar node even at level I [6].
Table 2.
Occurrence of thyroid inclusions in cervical lymph nodes
| Authors | Period (yrs) | Nr of patients/autopsies | Nr of NDs | Nr of nodes examined | Thyroid inclusions
|
Surgically and/or histologically explored thyroid | Thyroid tumour | |
|---|---|---|---|---|---|---|---|---|
| Nr of patients/autopsies | Nr of nodes | |||||||
| Gerard-Marchant [5] | 6 | 647 | 647 | 16649 | 4 (0.6%) | 5 (0.3%) | - | - |
| Meyer and Steinberg [9] | 106 | - | 5 (4.7%) | 5 | Contralateral, papillary microcarcinoma, 1 | |||
| Ansari-Lari and Westra [15] | 17 | 1337 | - | - | 9 (0.7%) | - | 4 | Contralateral, papillary microcarcinoma, 1 |
| León et al. [17] | 10 | 752 | 1123 | - | 6 (0.8%) | - | - | - |
| Audit (see text) | 1.5 | 118 | 135 | 2346 | 2 (1.7%) | 2 (0.1%) | - | - |
yrs, years
The controversy over nodal, thyroid inclusions: theoretical considerations
Salivary glandular inclusions and naevus cell aggregates in cervical lymph nodes are established [7, 22]. Nodal inclusions of endometriform and endometrial tissue can also be found elsewhere in the body [7]. It is therefore curious [8] that the presence of nodal thyroid inclusions is so aggressively disputed. One reason for this dispute is that the thyroglossal duct, the major thyroid primordium [1, 4, 23, 24], is median and the lymph nodes with inclusions are usually lateral, and occasionally high (see “Recent investigations”). In addition, most pathologists acknowledge difficulties in distinguishing metastatic deposits of FTC [25] and, possibly, follicular variant of PTC.
Embryological and topographical reservations can be answered. Hamilton and Mossman, noted that aberrations in the downward migration of the thyroglossal duct during development may result in heterotopic, for instance high cervical, thyroid tissue [24] and the complex events occurring during development of human neck [2, 24] could affect spatial relations. Derivatives of the paired and laterally positioned, fourth pharyngeal pouches (ultimobranchial tissue) also contribute to thyroid development [1, 4], whereas heterotopic, parathyroid derivatives of the third pharyngeal pouches can be found even in the oropharynx [26]. Aberrations during the embryonic outgrowth of the pharyngeal pouch endoderm would account for heterotopic thyroid in cervical lymph nodes, but the extent of the lateral contributions to the development of human thyroid is not established and their morphology may not correspond with that of conventional follicles. Solid cell nests or “mixed” follicles showing combinations of squamous and follicular epithelium, mucous cells and lumina containing acid mucin and colloid are regarded as thyroid structures of ultimobranchial origin [27–30].
The argument based on difficulties in distinguishing metastatic, well differentiated, thyroid carcinoma is less easy to refute. Most thorough histological examination of the entire thyroid would be necessary to draw definite conclusions. In this context, the data shown in Table 2 should be considered. Of the nine thyroids in Table 2, which were further investigated, seven (77.8%) did not show histological evidence of tumour. Again, the efficacy of serial sectioning/examination of the gland may be challenged; papillary microcarcinomas < 1 mm or areas of fibrosis [8, 21, 31] may be overlooked; and complete regression of a primary that has already metastasised may even be postulated. Nevertheless, some pathologists profess that detection of a primary is always possible. The finding of contralateral papillary microcarcinomas in the remainder two thyroids (Table 2, 22.2%) lend credence to the latter, but is not definite proof. Unless the thyroid tumour and nodal inclusions share a genetic profile and/or immunohistochemical phenotypes (see “Histological diagnostic approaches” below), the finding may represent concurrence of a very common tumour [8, 25, 31] with a rare embryological aberration/“benign metastasis”. Concurrence enters other biomedical debates, for instance, the alleged relationship between oral lichen planus and squamous cell carcinoma (SCC).
Substantiating investigations are therefore desirable, though may be difficult to achieve given the rarity of the inclusions and the preponderance of clinical decision making that often argues against further thyroidectomy.
Histological diagnostic approaches
Histological characterisation of papillary thyroid tissue incidentally discovered in cervical lymph nodes does not prove problematic. Identification of PTC is instant in typical cases and further IHC may be unnecessary (Fig. 1). Greater effort goes into less typical cases (Fig. 2). Attention should be paid to nuclear details and extent of nodal parenchyma replaced by thyroid tissue (see below); and, although not specific, IHC may be helpful. Depending on the pathology laboratory and availability of antibodies, immunostaining for CK19, HBME-1 (a mesothelial cell membrane antigen located in microvilli and often expressed in PTC) and galectin-3 (a lectin with affinity for β-galactosidase) could support a diagnosis of PTC if positive.
Fig. 1.
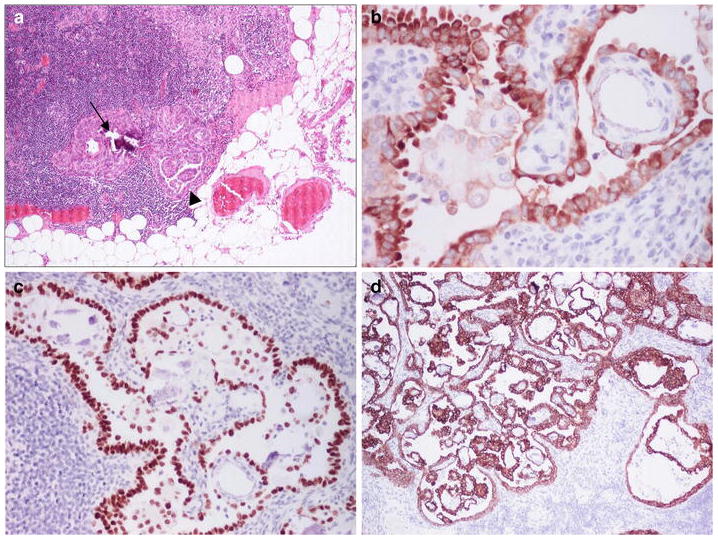
Small PTC with microcalcification (arrow) and papillae (arrowhead) in a lymph node from a ND for pT1, squamous cell carcinoma (SCC) of the left floor of mouth in a male aged 61 years (unless otherwise specified the pictures are from sections stained with haematoxylin and eosin); PTC was seen in three out of 15 nodes at levels II and III; metastatic SCC was not detected (a). IHC for thyroglobulin (b), thyroid transcription factor-1 (nuclear staining) (c) and cytokeratin (CK) 19 (d)
Fig. 2.
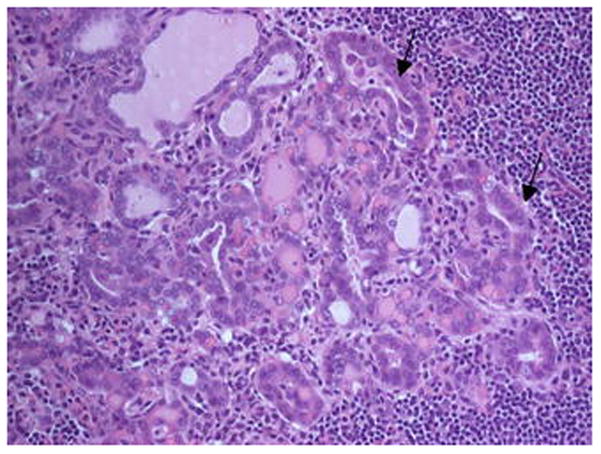
Papillae are les conspicuous here, but grooved/overlapping nuclei are arrowed. Level III lymph node from a ND for a pTX N2b, SCC in a male aged 68 years.
Characterisation of follicular thyroid tissue incidentally discovered in cervical lymph nodes is challenging. Criteria for recognising inclusions of benign nodal thyroid follicles are shown in Table 3; originate from Meyer and Steinberg [9]/later modifications [8, 10]; and deserve further comments.
Table 3.
Suggested criteria for nodal, follicular, thyroid inclusions
| Histology |
| Extent of thyroid follicles in conjunction with size of the lymph node: limited extent/usually small node (see text); ≤ two nodes with inclusions |
| Silhouette and localisation: various or wedge-shaped; cortical or capsular |
| Morphology of thyroid follicles: unremarkable, often small, similarly sized and oval; no papillae, crowded/hypochromatic/grooved nuclei, nuclear inclusions or tall eosinophilic epithelium |
| Absence of microcalcification (“psammoma bodies”) |
| Absence of desmoplastic stroma |
| Ancillary tests |
| Immunohistochemical and molecular profiling: inconsistent with thyroid cancer |
The limited extent of inclusions is significant. It accounts for the failure of modern imaging modalities to detect them and dependence on light microscopy. However, the optimal upper limit of that extent has not been established. Inclusions ranging in size from 0.1 to 2.3 mm [6] and containing up to 100 (average of 30) thyroid follicles [1, 5, 6, 10, 32] have been reported, but the variation in nodal size needs to be considered. Inclusions have been found in nodes ranging from 2.0 mm to 15.0 mm [5, 6, 32]. Although a “relative extent” calculated as area or number of thyroid follicles ÷ size of lymph node could be useful in defining an upper limit, validation would be impossible in the absence of thyroidectomy. However, five to 30 thyroid follicles and a lymph node often ≤ 5.0 mm seem sensible [1, 5, 32]. Pathologists should be alerted when normal-looking thyroid follicles replace a substantial portion of the lymphoid parenchyma, effect nodal enlargement or involve a few nodes. Involvement of two bilateral nodes is within the range of thyroid inclusions [5] as is the high position of a node (see “Recent investigations”). The following case may be illustrative. A “suspicious” upper cervical lymph node with calcifications was detected by ultrasound and computed tomography in an adult female. Histologically, “half of the node” had been replaced by follicular thyroid tissue and after thyroidectomy, pathology showed multiple papillary microcarcinomas [33]. The authors regarded the nodal thyroid tissue as benign [33], possibly on the basis of nuclear morphology (see below), but this interpretation should be treated with caution.
Thyroid nodal inclusions are variously silhouetted, but a wedge-shaped arrangement with the base adjacent to the nodal capsule and the apex directed towards the cortex (see Fig. 30 in Woolgar and Triantafyllou [34]) may facilitate their recognition [10]. A wedged pattern features in benign lesions like melanocytic naevi and granular cell tumours [35]. As regards localisation, except for cortex [1, 5, 32], thyroid inclusions can be seen within the nodal capsule (Fig. 3). Thyroid follicles in nodal capsule may reflect “benign metastasis” in capsular lymphatics. Alternatively, lymph nodes enlarging as a result of antigenic stimulation may encroach upon heterotopic, thyroid tissue residing in adjacent stroma. Naevus cell aggregates in the capsule of lymph nodes had been similarly explained [36]. In support of the notion that all nodal thyroid inclusions are true metastases, a synchronous/intimate relationship between descending/outgrowing thyroid primordia and lymph nodes does not feature in development [1, 4, 23, 24]. The aforementioned, metachronous involvement of ectopic thyroid by antigenically challenged lymph nodes counters this argument.
Fig. 3.
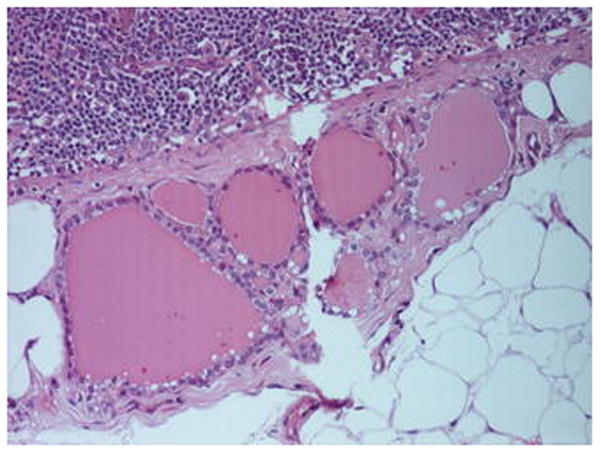
A collection of < 10, colloid containing, thyroid follicles lined by cuboidal epithelium in the capsule of a level IIA, cervical lymph node from a ND for pT4a N1, SCC of the left mandibular alveolus in a male aged 56 years. Lymphoid parenchyma and perinodal fat are seen at the upper left and lower right of the picture, respectively.
Macrofollicles are reconcilable with thyroid inclusions, but widespread marked variations in size may cause concern. Papillae, crowded/hypochromatic/grooved nuclei or tall, eosinophilic cell phenotypes tip the scales towards malignancy. Nuclear features should be interpreted in conjunction with the extent of the thyroid tissue. Absence of grooved nuclei, as in the illustrative case discussed above [33], could be outweighed by substantial replacement of the lymphoid parenchyma. Squamous metaplasia is seen in PTC [8, 25] - hence, its presence in nodal thyroid would be suspicious. It is not known whether “mixed” follicles with squamous epithelium and acid mucins [28, 29] occur in cervical lymph nodes.
Microcalcification and presence of desmoplastic stroma also favour metastasis. However, metastastic adenocarcinomas in cervical lymph nodes are not always associated with desmoplasia [37].
These morphological criteria are assessable on routinely prepared/stained histological sections and could be helpful in diagnosing nodal thyroid inclusions. However, because of the controversy, there is a climate of uncertainty and many pathologists would request serial sectioning and IHC including CK19, HBME-1 or galectin-3. Some may also wish to confirm thyroid origin by means of IHC for thyroglobulin. Lymphatic channels with inspissated lymph, for instance, may simulate thyroid follicles lined by attenuated epithelium, though the former are usually isolated and not in collections. Molecular testing (see below) is already here. Logistical considerations apart, it seems difficult to condemn ancillary investigations. Frustratingly, nodal thyroid inclusions are usually small and when the embedded, usually small, nodes are serially sectioned for the purpose of those investigations, the inclusions may no longer be detected.
In the context of ancillary investigations it is noted that Mojica and Khoury found the BRAF V600E point mutation in “morphologically benign appearing thyroid inclusions of cervical lymph nodes” in an adult male [11]. Point mutation of the BRAF gene is a genetic event featuring in PTC [31] and the finding would shake the notion of benign nodal thyroid tissue. However, the “inclusions” examined by these authors do not correspond with the criteria discussed above. Their patient had PTC that metastasised in 11 out of 55 cervical lymph nodes and showed the same mutation; “inclusions” harbouring the mutation were found in eight out of the 55 nodes and largely consisted of macrofollicles; information regarding the extent of the “inclusions” is not given; and, although grooved nuclei were not seen, occasional nuclear inclusions had been detected. Possibly the follicular tissue represented metastatic PTC in follicular arrangements rather than nodal thyroid inclusions. Despite reservations, molecular testing seems to be the way forward and future developments are awaited.
Management
Discussion so far addressed and raised concepts that would assist in appreciating further management of the patient. An algorithm is suggested in Table 4 and should be compared with that provided by Fliegelman et al. [13].
Table 4.
Algorithm for management
| Incidental pathological finding of thyroid tissue in cervical lymph nodes | ||||
|---|---|---|---|---|
| ↓ | ↓ | |||
| Papillary (PTC) | Follicular | |||
| ↓ | ↓ | ↓ | ||
| Imaging of thyroid | Corresponding with criteria of Table 3 | Not corresponding with criteria of Table 3 | ||
| ↓ | ↓ | ↓ | ↓ | |
| “Negative” | “Positive” | Inclusions | Suspicious of metastasis | |
| ↓ | ↓ | ↓ | ↓ | |
| “Wait and watch”, but see text for option of further surgery | Further thyroid surgery depending on age, co-morbidities, control of primary upper aero-digestive cancer, and patient preferences | No further action, but imaging of thyroid is optional and clinical correlation is advisable | Imaging of thyroid | |
| ↓ | ↓ | |||
| “Negative” | “Positive” | |||
| ↓ | ↓ | |||
| “Wait and watch”, but see text for option of further surgery | Further thyroid surgery depending on age, co-morbidities, control of primary upper aero-digestive cancer, and patient preferences | |||
The process is initiated when a pathologist, usually in a specialising hospital/institute, incidentally detects nodal, papillary or normal-looking follicular, thyroid tissue while examining a ND for SCC, malignant melanoma and neuroendocrine carcinoma of the upper aero-digestive tract or salivary/sinonasal adenocarcinomas.
Nodal, papillary, thyroid tissue corresponds with PTC (see “Histological diagnostic approaches”) and the finding is discussed at the next meeting of the multi-disciplinary team (MDT) for head and neck cancers of the hospital/institute. In the absence of clinical findings in the thyroid, the MDT should recommend further imaging (ultrasound, computed tomography or magnetic resonance imaging) of the gland. If this shows normal appearances, the possibilities of primary, nodal PTC deriving from thyroid inclusions [18, 19] or metastasising, occult, papillary microcarcinomas should be discussed at a succeeding MDT meeting. A “wait and watch” strategy would be probably adopted, which is supported by the excellent prognosis of PTC [8, 31] and the common incidence of papillary microcarcinomas. These can be found in over one third of the autopsies [31] and it is felt that many “healthy” individuals lead a normal life unaware of hosting one or more. If imaging reveals suspicious thyroid features, the MDT should consider further action. The decision for thyroidectomy or lobectomy would be influenced by age, loco-regional control of the upper aero-digestive tract cancer that necessitated the ND (pTNM grading, stage, status of margins, extra-capsular spread, need of additional radio-/ and chemotherapy) and any co-morbidities. In addition, the wishes of the patient should be considered. A conservative approach has been suggested for differentiated thyroid cancers [21, 38] and it is likely that the upper aero-digestive tract cancer rather than the thyroid malignancy influences prognosis [39]. Information on long term follow-up is, however, lacking.
In case of nodal, follicular, thyroid tissue, the pathologist should consult the criteria in Table 3 in working out whether it corresponds to inclusions or is suspicious. The rationale behind the decision, e.g. number/size of nodes with follicular thyroid tissue, extent of replacement of nodal parenchyma and nuclear features of thyroid tissue, should be included in the pathology report. Nodal, thyroid inclusions would hold only a passing interest for the MDT and usually no further action is necessary [6, 15]. Imaging is an optional extra, if the pathologist advises so, though logistics and national guidelines may be influential.
Imaging is mandatory for suspicious, nodal, follicular, thyroid tissue and further management would be similar to that discussed above (Table 4).
It is noted that when no thyroid abnormalities are seen on imaging and a “wait and watch” strategy is favoured (Table 4), further surgery should still be considered if the patient is especially concerned and circumstances (age, co-morbidities, control of primary upper aero-digestive cancer) permit.
The suggested algorithm depends on close collaboration among pathologists, radiologists, surgeons, and oncologists; is influenced by diagnostic acumen; and emphasises the significance of loco-regional control of the upper aero-digestive tract cancer. It is advisable that pathologists should avoid jargon in their reports and briefly explaining rationale in simple terms would be a good start. Radiologists, surgeons, and oncologists should respond by making an effort to appreciate the pathological nuances and areas of uncertainty associated with thyroid inclusions in lymph nodes.
Conclusions
It is hoped that this review sheds light on problems regarding the interpretation and clinical significance of incidentally discovered thyroid tissue in cervical lymph nodes. The available published evidence, though admittedly limited, suggests that inclusions of follicular thyroid tissue rarely exist in cervical lymph nodes. The morphological criteria shown in Table 3 could further assist pathologists in identifying those inclusions. Primary, nodal PTC may also exist. Table 4 offers an approach for the management of patients with incidentally discovered, nodal, thyroid tissue in NDs for upper aero-digestive tract cancers, which is based on collaboration between specialists; and correlating histological findings with imaging and loco-regional control of the upper aero-digestive tract cancer. There is need for publication of anecdotal evidence and further investigations, though attention has been drawn to potential difficulties. The application of molecular technologies in conjunction with a rigorous histological assessment would be welcome in future studies of this controversial topic.
Acknowledgments
The authors are grateful to Dr E. Leon Barnes for insightful comments.
Footnotes
This paper was written by members of the International Head and Neck Scientific Group (http://www.IHNSG.com).
Contributor Information
Asterios Triantafyllou, Oral & Maxillofacial Pathology, School of Dentistry, University of Liverpool and Pathology Department, Liverpool Clinical Laboratories, Liverpool, UK.
Michelle D. Williams, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Peter Angelos, Department of Surgery and Surgical Ethics, The University of Chicago Medicine, Chicago, IL, USA.
Jatin P. Shah, Head and Neck Oncology, Memorial Sloan Kettering Cancer Center, New York, NY, USA
William H. Westra, Departments of Pathology and Otolaryngology-Head and Neck Surgery, The Johns Hopkins Medical Institutions, Baltimore, MD, USA
Jennifer L. Hunt, Department of Pathology, University of Arkansas for Medical Sciences, Little Rock, AR, USA
Kenneth O. Devaney, Department of Pathology, Allegiance Health, Jackson, MI, USA
Alessandra Rinaldo, University of Udine School of Medicine, Udine, Italy.
Pieter J. Slootweg, Department of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands
Douglas R. Gnepp, University Pathologists, Providence, RI and Fall River, MA, USA
Carl Silver, Departments of Surgery and Otolaryngology-Head and Neck Surgery, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, USA.
Alfio Ferlito, Coordinator of the International Head and Neck Scientific Group.
References
- 1.Gerard-Marchant R, Caillou B. Thyroid inclusions in cervical lymph nodes. Clin Endocrinol Metab. 1981;10:337–349. doi: 10.1016/s0300-595x(81)80026-6. [DOI] [PubMed] [Google Scholar]
- 2.Willis RA. The borderland of embryology and pathology. 2. Butterworths; London: 1962. [PMC free article] [PubMed] [Google Scholar]
- 3.Woolgar JA, Triantafyllou A, Thompson LDR, Hunt JL, Lewis JS, Jr, Williams MD, Cardesa A, Rinaldo A, Barnes L, Slootweg PJ, Devaney KO, Gnepp DR, Westra WH, Ferlito A. Double reporting and second opinion in head and neck pathology. Eur Arch Otorhinolaryngol. 2014;271:847–854. doi: 10.1007/s00405-014-2879-8. [DOI] [PubMed] [Google Scholar]
- 4.Frantz VK, Forsythe R, Hanford JM, Rogers WM. Lateral aberrant thyroids. Ann Surg. 1942;115:161–183. doi: 10.1097/00000658-194202000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerard-Marchant R. Thyroid follicle inclusions in cervical lymph nodes. Arch Pathol. 1964;77:633–637. [PubMed] [Google Scholar]
- 6.Nicastri AD, Foote FW, Jr, Frazell EL. Benign thyroid inclusions in cervical lymph nodes. JAMA. 1965;194:1–4. [PubMed] [Google Scholar]
- 7.Symmers WStC. The lymphoreticular system. In: Symmers WStC., editor. Systemic pathology. 2. Vol. 2. Churchill Livingstone; Edinburgh: 1978. pp. 504–891. [Google Scholar]
- 8.Rosai J, Carcangiu ML, DeLellis RA. Atlas of tumor pathology, 3rd series, fascicle 5. Armed Forces Institute of Pathology; Washington, DC: 1992. Tumors of the thyroid gland; p. 323. [Google Scholar]
- 9.Meyer JS, Steinberg LS. Microscopically benign thyroid follicles in cervical lymph nodes. Serial section study of lymph node inclusions and entire thyroid gland in 5 cases. Cancer. 1969;24:302–311. doi: 10.1002/1097-0142(196908)24:2<302::aid-cncr2820240213>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Barnes L, Peel R. A text/atlas of differential diagnosis. Igaku-Shoin; New York: 1990. Head and neck pathology. [Google Scholar]
- 11.Mojica WD, Khoury T. Presence of the BRAF V600E point mutation in morphologically benign appearing thyroid inclusions of cervical lymph nodes. Endocr Pathol. 2006;17:183–189. doi: 10.1385/ep:17:2:183. [DOI] [PubMed] [Google Scholar]
- 12.López-Escámez JA, López-Nevot A, Moreno-García MI, Gámiz MJ, Salinero J. Cervical metastasis of occult papillary thyroid carcinoma associated with epidermoid carcinoma of the larynx. ORL J Otorhinolaryngol Relat Spec. 1999;61:224–226. doi: 10.1159/000027676. [DOI] [PubMed] [Google Scholar]
- 13.Fliegelman LJ, Genden EM, Brandwein M, Mechanick J, Urken ML. Significance and management of thyroid lesions in lymph nodes as an incidental finding during neck dissection. Head Neck. 2001;23:885–891. doi: 10.1002/hed.1128. [DOI] [PubMed] [Google Scholar]
- 14.Coskun H, Erisen L, Tolunay S, Basut O, Onart S, Tezel I. Incidental association of thyroid carcinoma and squamous cell carcinoma of head and neck. Am J Otolaryngol 2002. 2002;23:228–232. doi: 10.1053/ajot.2002.124541. [DOI] [PubMed] [Google Scholar]
- 15.Ansari-Lari MA, Westra WH. The prevalence and significance of clinically unsuspected neoplasms in cervical lymph nodes. Head Neck. 2003;25:841–847. doi: 10.1002/hed.10304. [DOI] [PubMed] [Google Scholar]
- 16.Resta L, Piscitelli D, Fiore MG, Di Nicola V, Fiorella ML, Altavilla A, Marzullo A. Incidental metastases of well-differentiated thyroid carcinoma in lymph nodes of patients with squamous cell head and neck cancer: eight cases with a review of the literature. Eur Arch Otorhinolaryngol. 2004;261:473–8. doi: 10.1007/s00405-003-0722-8. [DOI] [PubMed] [Google Scholar]
- 17.León X, Sancho FJ, García J, Sañudo JR, Orús C, Quer M. Incidence and significance of clinically unsuspected thyroid tissue in lymph nodes found during neck dissection in head and neck carcinoma patients. Laryngoscope. 2005;115:470–474. doi: 10.1097/01.mlg.0000157841.63283.87. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Qiu S, Eltorky MA, Tang WW. Histopathologic and immunohistochemical characterization of a primary papillary thyroid carcinoma in the lateral cervical lymph node. Exp Mol Pathol. 2007;82:91–94. doi: 10.1016/j.yexmp.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto T, Tatemoto Y, Hibi Y, Ohno A, Osaki T. Thyroid carcinomas found incidentally in the cervical lymph nodes: do they arise from heterotopic thyroid tissues? J Oral Maxillofac Surg. 2008;66:2566–2576. doi: 10.1016/j.joms.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Kr A, Sebastian P, Somanathan T, George NA, Jayasree K. Significance of incidentally detected thyroid tissue in lymph nodes of neck dissections in patients with head and neck carcinoma. Int J Surg Pathol. 2012;20:564–569. doi: 10.1177/1066896912449042. [DOI] [PubMed] [Google Scholar]
- 21.Vassilopoulou-Sellin R, Weber RS. Metastatic thyroid cancer as an incidental finding during neck dissection: significance and management. Head Neck. 1992;14:459–463. doi: 10.1002/hed.2880140606. [DOI] [PubMed] [Google Scholar]
- 22.Woolgar A, Triantafyllou A. Lymph node metastases in head and neck malignancies: assessment in practice and prognostic importance. Diagnostic Histopathology. 2010;16:265–275. [Google Scholar]
- 23.Sgalitzer KE. Contribution to the study of the morphogenesis of the thyroid gland. J Anat. 1941;75(Pt 4):389–405. [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton WJ, Mossman HW. Prenatal development of form and function. 4. Heffer; Cambridge: 1972. Hamilton, Boyd and Mossman’s human embryology. [Google Scholar]
- 25.Doniach I. The thyroid gland. In: Symmers WStC., editor. Systemic pathology. 2. Vol. 4. Churchill Livingstone; Edinburgh: 1978. pp. 1976–2037. [Google Scholar]
- 26.Triantafyllou A. Submucosal, oropharyngeal, heterotopia of parathyroid. Oral Surgery. 2013;6:91–93. [Google Scholar]
- 27.Harach HR. Solid cell nests of the thyroid. An anatomical survey and immunohistochemical study for the presence of thyroglobulin. Acta Anat (Basel) 1985;122:249–253. [PubMed] [Google Scholar]
- 28.Harach HR. Thyroid follicles with acid mucins in man: a second kind of follicles? Cell Tissue Res. 1985;242:211–215. doi: 10.1007/BF00225578. [DOI] [PubMed] [Google Scholar]
- 29.Harach HR. Mixed follicles of the human thyroid gland. Acta Anat (Basel) 1987;129:27–30. doi: 10.1159/000146373. [DOI] [PubMed] [Google Scholar]
- 30.Harach HR. Solid cell nests of the thyroid. J Pathol. 1988;155:191–200. doi: 10.1002/path.1711550303. [DOI] [PubMed] [Google Scholar]
- 31.LiVolsi VA, Alores-Saavedra J, Asa SL, Baloch ZW, Sobrinho-Simões M, Wenig B, Delellis RA, Cady B, Mazzaferri EL, Hay I, Fagin JA, Weber AL, Caruso P, Voutilainen PE, Franssila KO, Willams ED, Schneider AB, Nikiforov Y, Rabes HM, Akslen L, Ezzat S, Santoro M, Eng C, Harach HR. Papillary carcinoma. In: Delellis RA, Lloyd RV, Heitz PU, Eng C, editors. World Health Organization classification of tumours. Pathology and genetics of tumours of endocrine organs. IARC Press; Lyon: 2004. pp. 57–66. [Google Scholar]
- 32.Roth LM. Inclusions of non-neoplastic thyroid tissue within cervical lymph nodes. Cancer. 1965;18:105–111. doi: 10.1002/1097-0142(196501)18:1<105::aid-cncr2820180115>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 33.Lee YJ, Kim DW, Park HK, Ha TK, Kim DH, Jung SJ, Bae SK. Benign intranodal thyroid tissue mimicking nodal metastasis in a patient with papillary thyroid carcinoma: A case report. Head Neck. 2014 Oct 1; doi: 10.1002/hed.23886. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Woolgar A, Triantafyllou A. Neck dissections: a practical guide for the reporting histopathologist. Current Diagnostic Pathology. 2007;13:499–511. [Google Scholar]
- 35.Harkin JC, Reed RJ. Atlas of tumor pathology, 2nd series, fascicle 3. Armed Forces Institute of Pathology; Washington, DC: 1969. Tumors of the peripheral nervous system; p. 124. [Google Scholar]
- 36.Ioannides G. Lymph nodes with aggregates of nevus cells. In: Ackerman AB, editor. Pathology of malignant melanoma. Masson; New York: 1981. pp. 297–300. [Google Scholar]
- 37.Woolgar JA, Triantafyllou A, Lewis JS, Jr, Hunt J, Williams MD, Takes RP, Thompson LDR, Slootweg PJ, Devaney KO, Ferlito A. Prognostic biological features in neck dissection specimens. Eur Arch Otorhinolaryngol. 2013;270:1581–1592. doi: 10.1007/s00405-012-2170-9. [DOI] [PubMed] [Google Scholar]
- 38.Guzzo M, Quattrone P, Seregni E, Bianchi R, Mattavelli F. Thyroid carcinoma associated with squamous cell carcinoma of the head and neck: which policy? Head Neck. 2007;29:33–37. doi: 10.1002/hed.20474. [DOI] [PubMed] [Google Scholar]
- 39.Pacheco-Ojeda L, Micheau C, Luboinski B, Richard J, Travagli JP, Schwaab G, Marandas P. Squamous cell carcinoma of the upper aerodigestive tract associated with well-differentiated carcinoma of the thyroid gland. Laryngoscope. 1991;101(4 Pt 1):421–424. doi: 10.1002/lary.1991.101.4.421. [DOI] [PubMed] [Google Scholar]


