Abstract
Down syndrome (DS) is caused by three copies of human chromosome 21 (Hsa21) and results in phenotypes including intellectual disability and skeletal deficits. Ts65Dn mice have three copies of ~50% of the genes homologous to Hsa21 and display phenotypes associated with DS, including cognitive deficits and skeletal abnormalities. DYRK1A is found in three copies in humans with Trisomy 21 and in Ts65Dn mice, and is involved in a number of critical pathways including neurological development and osteoclastogenesis. Epigallocatechin-3-gallate (EGCG), the main polyphenol in green tea, inhibits Dyrk1a activity. We have previously shown that EGCG treatment (~10mg/kg/day) improves skeletal abnormalities in Ts65Dn mice, yet the same dose, as well as ~20mg/kg/day did not rescue deficits in the Morris water maze spatial learning task (MWM), novel object recognition (NOR) or balance beam task (BB). In contrast, a recent study reported that an EGCG-containing supplement with a dose of 2–3 mg per day (~40–60mg/kg/day) improved hippocampal-dependent task deficits in Ts65Dn mice. The current study investigated if an EGCG dosage similar to that study would yield similar improvements in either cognitive or skeletal deficits. Ts65Dn mice and euploid littermates were given EGCG [0.4 mg/mL] or a water control, with treatments yielding average daily intakes of ~50 mg/kg/day EGCG, and tested on the multivariate concentric square field (MCSF)—which assesses activity, exploratory behavior, risk assessment, risk taking, and shelter seeking—and NOR, BB, and MWM. EGCG treatment failed to improve cognitive deficits; EGCG also produced several detrimental effects on skeleton in both genotypes. In a refined HPLC-based assay, its first application in Ts65Dn mice, EGCG treatment significantly reduced kinase activity in femora but not in the cerebral cortex, cerebellum, or hippocampus. Counter to expectation, 9-week-old Ts65Dn mice exhibited a decrease in Dyrk1a protein levels in Western blot analysis in the cerebellum. The lack of beneficial therapeutic behavioral effects and potentially detrimental skeletal effects of EGCG found in Ts65Dn mice emphasize the importance of identifying dosages of EGCG that reliably improve DS phenotypes and linking those effects to actions of EGCG (or EGCG-containing supplements) in specific targets in brain and bone.
Keywords: Trisomy 21, Down syndrome, EGCG, mouse model, bone, cognition
1. Introduction
Down syndrome (DS), the leading genetic cause of intellectual disability, is caused by the three copies of human chromosome 21 (Hsa21) [1, 2]. DS has an incidence of ~1 in 700 live births and results in phenotypes that affect the central nervous and skeletal systems in all humans with DS [3–5]. Cognitive and neurodevelopmental disabilities are characterized by deficiencies in hippocampal-dependent memoryfunctions, reduced sizeof hippocampus and cerebellum, reduced dendritic and axonal number and volume, early occurrence of Alzheimer disease, motor dysfunction and altered hippocampal synaptic plasticity [6–9]. Humans with DS also exhibit skeletal structures that are generally smaller than normal and with reduced bone mineral density (BMD) that can lead to osteoporosis. [10–12].
Mouse models have been used to study the complex genotype-phenotype relationship associated with DS, assessing both the role of a chromosomal region or specific genes on the pathogenesis of DS and possible therapeutic interventions [13–16]. The Ts(1716)65Dn (Ts65Dn) mouse model is the most extensively studied and widely used animal model of DS and captures several behavioral and skeletal defects that are seen in humans with DS [13, 17–20]. We and others have shown that Ts65Dn mice exhibit behavioral and functional neurological deficits, including a variety of deficits on learning and memory tasks [20, 21]. Ts65Dn mice show deficits in novel object recognition (NOR) and spontaneous alternation [6], show increased locomotor activity [22, 23], have deficits in the Morris water maze (MWM) place learning (using the hidden platform version) and (under some testing conditions) on the cued task of the visible platform version [20, 23, 24], have gait abnormalities [25] and motor coordination deficits [23]. For skeletal parameters in DS mouse models, we and others have identified significant deficits in BMD, trabecular bone parameters (thickness, separation and number) and cortical bone measures in Ts65Dn mice compared to euploid control mice [17]. Significant deficits in mineral apposition rate (MAR), bone formation rate (BFR), and strength properties in trisomic mice compared to controls were also observed [17, 26, 27].
The gene for Dual-specificity tyrosine phosphorylation-regulated kinase 1a (DYRK1A) is found in three copies in humans with DS [28] and is thought to play a key role during CNS development and osteoclastogenesis. Notably, adverse effects may be present both with increased and decreased DYRK1A expression levels [29–31]. Dyrk1a protein levels were found to be ~1.5 fold higher than euploid control levels in the cortex, cerebellum and hippocampus of 5–6 month old Ts65Dn mice [32] and in brain of 7–8 month old Ts65Dn mice [33]. However, other studies have reported no differences in brain homogenate Dyrk1a mRNA levels in 5-month-old Ts65Dn mice [34]. In post-mortem cases of individuals with DS (age groups of 10–30 years and 40+ years old), Western blot analysis found that DYRK1A protein levels were increased 1.5 fold above age-matched controls in the frontal, temporal, occipital, and cerebellar cortices and in cerebral and cerebellar white matter; however, brains from infants (1–3 years old) in this study did not have significantly increased DYRK1A protein expression [33]. These studies support the growing possibility that Dyrk1a overexpression is both spatially and temporally regulated during development in DS. Furthermore, only a few studies have reported levels of Dyrk1a kinase activity; 15-month-old Ts65Dn mice exhibited increased Dyrk1a kinase activity in brain tissue as compared to controls [35], whereas another study reported no differences in Dyrk1a kinase activity in cerebellum hippocampus and cerebral cortex between 6 week old Ts65Dn and control mice [23]. Understanding functional Dyrk1a kinase activity differences between Ts65Dn and control mice at specific developmental periods in specific tissues is a necessary component for assessing the efficacy of pharmacotherapies targeting Dyrk1a.
Epigallocatechin-3-gallate (EGCG) is the most prevalent polyphenol found in green tea [36] and has been tested for therapeutic effects in various pathologies, including anti-cancer activity, anti-oxidant activity, anti-bacterial activity, anti-allergic activity and anti-inflammatory activities [37–41]. EGCG is a small molecular inhibitor of DYRK1A activity and is thought to function by binding to the ATP binding domain of the protein thereby inhibiting its kinase activity [42, 43]. EGCG, either alone or in supplements containing EGCG, has been tested as a potential therapy in mouse models of DS [23, 26, 44–48] and in humans with DS [45, 49, 50]. In one recent study [45] trisomic mice were given an EGCG-containing supplement (Life Extension® Mega Green Tea Extract, Lightly Caffeinated) in drinking water that was reported to deliver 2–3 mg of EGCG per day per mouse (i.e., a daily dosage of ~80–120 mg/kg [for a 25 g mouse] that would yield a final effective dosage of ~40–60 mg/kg per day after accounting for the known degradation of EGCG [23, 44]). This dosage rescued acquisition of spatial navigation and normalized thigmotaxic behavior in the MWM, and improved novel object recognition (NOR) discrimination ratios [45]. Those findings stand in contrast with studies in our laboratory that used either a three-week or a seven-week treatment of pure EGCG (concentration of 0.124 mg/mL) beginning in early adolescence that delivered a dosage of either ~10 mg/kg/day (EGCG in water) or ~20 mg/kg/day (stabilized EGCG in acidified water and corrected for degradation). The 10 mg/kg/day dosage improved skeletal deficits including femoral BMD, percent trabecular bone volume, trabecular number and trabecular thickness in Ts65Dn mice [26], but neither the 10 nor the 20 mg/kg/day dosage improved performance in the MWM, NOR, or balance beam motor coordination task [23].
The goal of the current study was to test whether the lack of EGCG effects in our previous study [23], compared to the improved phenotypes with the EGCG-containing supplement found by De la Torre and colleagues [45], may have been be due to the lower dosages of EGCG. The current study administered a dosage of pure EGCG intended to approximate the EGCG dosage that we determined was delivered in the De la Torre et al. [45] study (~50 mg/kg/day), which includes our correction for the ~50% degradation of EGCG that is known to occur when EGCG is dissolved in the drinking water [23, 44]. Thus, Ts65Dn mice were administered either EGCG [0.4mg/mL, stabilized in acidified water], which yielded a target average daily intake of ~50 mg/kg/day after correction for known degradation, or acidified water alone. Given that the current study used a higher concentration of EGCG (0.4 mg/mL) for a longer duration (7 weeks) than our previous study (0.124 mg/mL for 3 weeks) that showed that EGCG improved bone phenotypes [26], an additional goal was to determine whether this higher dosage for longer duration would yield more extensive improvement in skeletal deficits than previously observed. The current study was also the first to employ the Multivariate Concentric Square Field (MCSF) in mouse models of DS, a behavioral task originally developed by Meyerson and colleagues that assesses patterns of activity and exploration in a complex novel environment, and has been shown to identify distinct behavioral phenotypes in rodent models, quantify locomotor activity, exploratory behavior, risk-taking, risk assessment, and shelter seeking, [51]. MCSF appears to be well suited to study behavioral phenotypes associated with neurodevelopment disorders given prior results from various rat and mouse models indicating its sensitivity to effects of traumatic brain injury [52], early life maternal separation stress [53], and disparities in lines of rodents selectively bred for differences in voluntary alcohol drinking [54]. Another key goal of this study was to determine Dyrk1a protein levels using Western blot analysis and to quantify kinase activity using a modified HPLC-based assay that has been suggested to measure Dyrk1a-related activity [55]. To our knowledge, this is the first report of quantification of Dyrk1a levels and kinase activity in specific brain regions and bone assessing whether EGCG treatment altered Dyrk1a protein and activity in a manner that was linked with changes in behavioral and skeletal DS-related phenotypes.
2. Materials and Methods
2.1 Animals
All animals were housed in the secure AAALAC-accredited Science Animal Resource Center facility in the IUPUI School of Science. Ts65Dn females (approximately 50% B6 and 50% C3H background with small trisomic marker chromosome) were bred to B6C3F1 males (both obtained from the Jackson Laboratory [Bar Harbor, ME]) in rooms with a standard 12:12 light:dark cycle to generate the mice used in the study. Only male mice were used due to the subfertile nature of Ts65Dn mice, making it necessary to retain Ts65Dn females as breeders for colony maintenance. On postnatal day (PD) 21, the male mice were weaned and single-housed in standard mouse cages, and randomly assigned to the different treatment groups. The vivarium containing the mice undergoing treatment was maintained on a reverse 12:12 light:dark cycle with white light off between 0800–2000, during which time only red light was present. Experiments with animals were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and received prior approval from the IACUC committee at IUPUI (SC213R and SC255R).
2.2 EGCG/Water Treatment
EGCG (>95% purity, as determined by LC/MS analyses [44]) was prepared by making a stock solution of 15 mg/mL EGCG in phosphate buffered saline (PBS). Treatments were delivered via the drinking water in a concentration of 0.4 mg/mL EGCG, prepared by diluting the stock solution in tap water, and pH balanced (5–5.5) by slowing adding phosphoric acid (approximately 100μL/100mL tap water). Treatments started on PD 24, usually 3 days after weaning. Treatments (EGCG or acidified water control) were placed in drinking tubes and the mice were allowed access ad libitum to its designated treatment as its sole source of fluid. The volumes consumed and animal weights were recorded every 48 hours at the time the treatments were changed. Treatments continued throughout the duration of behavioral testing, which ended on PD 68.
2.3 Behavioral Timeline
Figure 1 shows the behavioral timeline for the study. Starting on PD 43, mice were handled by the experimenter in the vivarium for two consecutive days. Behavioral testing began with the MCSF test on PD 45-PD 46, followed by three consecutive days of NOR (PD 47-PD 49). After two days of rest, the mice were trained for three consecutive days on the balance beam task (PD 52-PD 54), followed by the MWM task from PD 57-PD 64. A final NOR task was administered on PD 65-PD 67. The NOR and MWM tasks were all conducted using previously published protocols [23]. On PD 68, mice were euthanized using isoflurane followed by cervical dislocation. Tissue from the cerebellum, cerebral cortex and hippocampus was rapidly dissected, snap frozen, and stored at −80°C. The mouse carcass was stored at 4°C, and in the following week, femurs were dissected, wrapped in PBS-soaked gauze and frozen at −20°C until analysis was performed.
Figure 1. Behavioral Study Timeline.
Timeline for the current study beginning shortly after weaning (PD22). Animals received EGCG or water treatment throughout the behavioral testing, even on rest days.
2.3.1 Multivariate Concentric Square Field (MCSF)
Mice were tested in the MCSF on PD 45 and 46. The MCSF apparatus, adapted from Ekmark-Lewén [52], is a complex, multi-compartment novel environment in which animals may move around and explore freely among various compartments and areas, including ones that are open, elevated, or sheltered [see Supplemental Figure 1 for image]. The MCSF design consists of an outer square field [70 cm × 70 cm × 26 cm (high)] that encloses a smaller interior center square field [41 cm × 41 cm × 25 cm (high)] centered within the larger field. The center square field contains a demarcated circular zone [center circle: 16 cm diameter] to record activity in the center of the MCSF. Three of the sides of the center square contain circular openings (8 cm) that permit entry into one of three corridors that make up three sides of the perimeter of the MCSF: the north corridor (containing an entrance to the slope and bridge), the south corridor (containing an entrance to the dark corner room (DCR), and the west corridor (open sloped incline to the hurdle that contains two, 2.5-cm holes with photocells underneath the holes to measure nose pokes). The fourth side of the MCSF perimeter is side-illuminated by a fluorescent light (approximately 320 lux) and contains an elevated bridge constructed of stainless steel (10 mm) wire-mesh that is elevated 177 cm above the floor and is accessible from the north corridor. The DCR is enclosed and covered, and can only be accessed through the south corridor.
For each session, the animal was placed in the center zone facing the wall with no openings; and its activity was video recorded for each 20-minute session in dim light (15–20 lux) in all areas except for the dark corner room (1–2 lux) and the lighted bridge area (320 lux). The MCSF was thoroughly cleaned between testing sessions with 70% ethanol. Photocell counts and fecal boli were recorded after each 20-minute session by the experimenter.
All sessions were scored by three independent observers blind to experimental treatment and genotype. Behavioral measures were obtained using The Observer XT Version 8 software (Noldus Information Technology, Wageningen, The Netherlands), and included measures of frequency of entries, duration, duration per visit, and latency to initial visit for each defined location within the MCSF. Measurements of rearing were not available due to unreliable identification of defined rearing events in video playback. The three scorers’ results were averaged to obtain the score value that was used for each animal. The correlation of scores across all measures across the three raters was r >+0.99. The full listing of all behavioral measures obtained from the scoring is provided Table 1.
Table 1.
The behavioral phenotypes that were assessed from the MCSF maze (first column). Each phenotype analysis included specific behavioral measures obtained from the scoring with Observer XT and analyzed as described in the text.
| Behavioral Phenotype | Measures Comprising Behavioral Phenotypes | ||||||
|---|---|---|---|---|---|---|---|
| Total Entries | Frequency of TotCorr entries | Frequency of hurdle entries | Frequency of DCR entries | Frequency of Slope entries | Frequency of Bridge entries | Frequency of center circle entries | Frequency of center entries |
| Exploratory Behavior | Duration of hurdle visits | Hurdle duration per visit | Number of photocell counts | Latency to initial slope visit | Latency to initial hurdle visit | Center Duration per visit | |
| Risk Assessment | Latency to leave center arena | Slope duration per visit | (Latency to initial bridge visit)- (Latency to initial slope visit) | ||||
| Risk-taking havior Be | Duration of bridge visits | Bridge duration per visit | Duration of center circle visits | Center circle duration per visit | |||
| Shelter Seeking | Frequency of dark corner room | Duration of dark corner room | Duration per visit of dark corner room | Latency to initial DCR visit | |||
Meyerson and colleagues have developed rank-order analytic approaches to data from the MCSF that identify categories of behavioral phenotypes, using the sums of ranks of designated measures that were combined to form identified categories of behavioral activities in the MCSF. These combined measures (that they refer to as “trend analysis”) reflect common underlying functions expressed in different behaviors or in different behavioral strategies across the complex environment of the MCSF, classified as general activity; exploratory behavior, risk assessment, risk taking and shelter seeking [56]. The value of the MCSF with its ethological behavioral dimensions was demonstrated in that study by showing that the behavioral trends extracted from the MCSF were more sensitive to anxiolytic effects of diazepam than the standard measure of the elevated plus maze. We used similar subsets of measures modified from those of Meyerson [56], defining the five behavioral categories in the following way (see Table 1): “Total entries”—a measure of locomotor activity—included the frequency of entries into the hurdle, DCR, slope, bridge, center circle and center square, along with the summed frequency of entries into the three corridors (TotCorr). ”Exploratory behavior” included the total duration of time spent in the hurdle, the duration per visit to the hurdle, total photocell counts, latency to initial slope visit, latency to initial hurdle visit, and the duration per visit to the center square. ”Risk assessment” included the latency to leave the center square, the duration per visit to the slope, as well as the difference between latency to initial bridge visit minus the latency to initial slope visit. “Risk-taking” included the total duration of bridge visits, duration per visit to the bridge, the total duration of center circle visits, and duration per visit to the center circle. “Shelter seeking” included the frequency of DCR entries, the total duration of DCR visits, the duration per visit to the DCR, and the latency to initial visit to the DCR.
2.3.2 Novel Object Recognition (NOR)
The NOR task was performed over three consecutive days. For the first day, animals underwent habituation, in which they were placed individually inside the painted plywood test box and allowed to explore the environment for 15 minutes. On the next day (object exposure day), two matching objects (8.5–11.0 cm tall; 3.0–4.5 cm base) were placed near the NW and SE corners of the box, approximately 5 cm from the wall, and each mouse was placed in the middle of the arena and allowed 15 minutes to explore the arena and objects. On the final day (test day) one object from the previous day and one novel object were placed inside and the mice were given 15 minutes to explore. Activity on each day was recorded with video tracking using ANYMAZE software (Stoelting Co, Wooddale, IL). The discrimination ratios of the time exploring each object on the test day were determined using the following formula: discrimination ratio (%) = [(time exploring novel object- time exploring familiar object) / total exploration time for both objects] * 100.
2.3.3 Balance Beam Task
The balance beam task was performed over three consecutive days. The apparatus consisted of 1 m painted wooden beams with a surface width of either 19 mm, 12 mm, 9 mm or 6 mm, situated 58 cm above the floor. A black goal box was located at the end of the beam, and contained bedding from the animals’ cage. On the first day, a cohort of mice (2–5 mice) was brought into the testing room each mouse was trained to walk from one side of the 19mm-wide beam to a black goal box, starting at varying distances from the box. On the second day, the mice were trained to cross the entire length of the 12 mm-wide beam without stopping on three consecutive trials. On the third day, the mice were tested on three trials each on the 12 mm-, 9 mm- and 6 mm- wide beams. A Logitech camera at the opposite end of the goal box recorded these trials. The video records of the test day were scored by three trained independent scorers, blind to genotype or treatment, to quantify the number of hind paw slips, defined as either the left or right hind paw entirely missing the beam.
2.3.4 Morris Water Maze Place Learning Task (MWM)
The Morris water maze (MWM) utilized a 125 cm (diameter) white tank filled to within 25 cm of the rim with water (24–26°C) clouded with white tempera paint. Training consisted of 7 days of acquisition, followed by a single probe trial given 24 hours after the last training day. The acquisition training consisted of four trials a day, at random starting positions for each trial. In each trial, the animal was allowed to swim for 60 seconds or until it located and climbed onto the platform. If the platform was not found in the 60 seconds, the experimenter gently moved the mouse to the platform. For acquisition trials, video tracking and data collection software (HVS Image, Mountain View, CA, USA) was used to obtain measures of latency to find the platform path length (cm), time spent within 25.4 cm of the wall (thigmotaxis), time spent not moving (floating), and swimming speed (cm/sec). Mice were tested in cohorts of three to four, with an inter-trial interval of approximately 3–4 minutes. Twenty-four hours after the last training day, the mice were given a 60-sec “probe trial” in which the platform was removed and the animal’s search path was recorded and scored for spatial biases using four superimposed virtual counting discs (27 cm diameter) in the center of each quadrant (over the four possible platform positions used for training). Probe trial measures included time spent in, latency to enter, and numbers of crossings through each of the four virtual counting discs. These measures were used to quantify the spatial distribution of the search strategy of each animal by a priori comparisons of the time spent in the target location (trained during acquisition) to the mean time spent in the three non-target locations.
2.4 Micro Computed Tomography (μCT)
The left femora were thawed to room temperature and then scanned using a high-resolution μCT system (SkyScan 1172, Bruker microCT, Belgium). Daily calibrations were performed prior to scanning the bones using two cylindrical hydroxyapatite phantoms (0.25 and 0.75 g/cm3 calcium hydroxyapatite). The distal and midshaft portion of the femur were scanned using the following parameters: voltage 60 kV, resolution 6 μm, binning mode 2 k and filter Al 0.5 m. Scans were reconstructed using NRecon and CTan software (SkyScan, Bruker microCT, Belgium). After scanning, bones were wrapped in PBS-soaked gauze and stored at −20°C until mechanical testing. Bone mineral density (BMD) was calculated using the hydroxyapatite phantoms as a standard. The trabecular, and cortical analysis was performed using a previously published protocol [57]. For cortical bone analysis, a standard site was chosen at 60% of the bone’s total length away from the distal growth plate. Seven transverse slices were generated from the above site and cortical properties were obtained using a custom Matlab code (MathWorks, Inc. Natick, MA). The regions of interest for femora were kept constant across genotype and treatment groups. Trabecular analysis was performed on the distal metaphysis with a region of interest defined as 10% of total bone length, starting at the proximal end of the distal growth plate then extending proximally. The region of interest was auto-segmented to only include trabecular bone using a custom Matlab code [57]. Two bones were excluded from the cortical analysis because of extreme non-normality.
2.5 Mechanical Testing
Following μCT, the mechanical properties of the femur were determined in 3-point bending (ElectroForce 3200; Eden Prairie, MN USA). The left femora were thawed to room temperature, and tested in the AP direction (anterior surface in tension). The loading spanwas set at 6 mm and a preload of 0.5 N was applied to the midpoint of the bone to establish contact. Once preloaded, the bone was monotonically tested to failure at a displacement rate of 0.025 mm/sec. Mechanical data from five bones were not obtained due to technical errors and therefore were excluded from the analysis (1 Eu+Water, 1 Eu+EGCG, 1 Ts+Water, 2 Ts+EGCG).
2.6 High Performance Liquid Chromatography (HPLC) Kinase Activity Assay
Protein was isolated from frozen (snap frozen then stored at −80°C) hippocampus, cerebellum, cerebral cortex, and femurs utilizing a protocol adapted from previous studies [58, 59]. The isolated protein was quantified using the Bradford assay to determine the concentration before proceeding with a HPLC-based kinase activity assay. The protein kinase activity was analyzed using a previously published protocol (with modifications) that was reported to quantify Dyrk1a activity in trisomic mouse models [55]. Briefly, protein samples (~100 μg) were pre-incubated for 1 minute in a 37°C water bath with a 5-carboxyfluorescein tagged Woodtide peptide (2 mg/mL) and kinase buffer. ATP (1 mM) was added to start the reaction and all samples were incubated in a 37°C water bath for 30 minutes. Following incubation, the reaction was stopped by the addition of HClO4 (15%) and centrifuged at 4 °C for 10 minutes at 14,655 g to remove any sediments. Blanks and the samples were placed in conical glass inserts inside 2mL autosampler vials for analysis on the Agilent 1260 HPLC. Separation was performed using initial conditions of 85% H2O with 0.1% formic acid (solvent A) and 15% acetonitrile with 0.1% formic acid (solvent B) held for 1 minute, followed by a stepwise gradient ending with 5% solvent A and 95% solvent B over 11 minutes. FAM-Woodtide fluorescence was measured using a fluorescence detector with an excitation wavelength of 485 nm and an emission of 530 nm. The peak height and retention time were used to determine the area under the curve for the p-FAM-Woodtide and FAM-Woodtide. Results were analyzed using OpenLab CDS Chemstation software.
2.7 Western Blot to Quantify Dyrk1a Protein
Isolated protein lysates (20μg) from the three brain regions (there was not sufficient femur protein to detect Dyrk1a protein) were resolved electrophoretically on polyacrylamide gels (Bolt 4–12% Bis Tris Plus Gels), then transferred to PVDF membranes. Membranes were blocked in 5% milk in Tris Buffered Saline with 0.1% Tween 20 (TBS-T), incubated overnight at 4°C in primary antibodies diluted in 5% milk-TBS-T as follows: rabbit anti-DYRK1A antibody, 1:500 (A303–802A, Bethyl Laboratories); mouse anti-beta-actin, 1:5000 (A2228, Sigma Aldrich), and labeled with donkey anti-rabbit IgG AlexaFluor 790 and donkey anti-mouse IgG AlexaFluor 680 secondary antibodies (1:10,000, Jackson Immunoresearch). Fluorescence was detected using a LI-COR CLx Imager. Each membrane contained at least three controls (Eu+Water) for a specific brain region (cerebellum, cerebral cortex, and hippocampus). Dyrk1a was normalized to actin controls and each normalized value was expressed as a ratio relative to the mean of the controls ran that were run within each blot. A total of eight independent blots contributed to the final data set.
2.8 Statistical Analyses
For the MCSF, the mean of each of the individual measures (e.g., latency to initial visit, duration, frequency, and duration per visit) was generated from the three independent observer scores, and scores for each of the five behavioral categories were tabulated as shown in Table 1. Five of the Ts65Dn mice with small body size were able to enter into the port with the photocell counter and remain there for extended periods of time, so their data were excluded from the MCSF analysis (2 Ts+Water; 3 Ts+EGCG). The primary hypothesis involved detection of interactive effects of treatment with genotype (i.e., normalization of Ts65Dn phenotypes with EGCG), so our approach was to apply parametric ANOVA approaches with the emphasis on identifying outcomes showing significant treatment × genotype interactions, though all significant ANOVA terms were identified. The total entries data (summed across the six included measures) constituted an underlying continuous variable and was distributed normally in all but one group (Shapiro-Wilk’s W test), so those sums were analyzed directly with a 3-way repeated measures ANOVA with genotype and treatment as grouping factors and day as a repeated measure. The other four behavioral categories (risk-taking, risk assessment, shelter seeking and exploratory behavior) involved measures using different scales (e.g., frequency; duration) and individual measures were often not normally distributed. Consequently, the data for each contributing measure (see Table 1) were first rank ordered, then the ranks for the measures contributing to a category were summed across the measures for each animal to provide a single summed score for the animal for each category. To assure that rank ordering reflected increasing amount of the target behavior in the category, the subject means of each measure were ranked in ascending order, except for the following which were ranked in descending order a) latency to leave the center (long latencies mean assessed risk is low), b) latency to initial slope visit (long latencies mean exploratory behavior is low), c) latency to initial hurdle visit (long latencies mean exploratory behavior is low), and d) latency to initial DCR visit (long latencies mean shelter seeking is low). The summed scores of subject ranks for each of these four categories formed a continuous underlying variable for each category and were normally distributed within each group in all but two cases (Shapiro-Wilk’s W test), so the data were then analyzed with a 3-way mixed measures ANOVA with genotype and treatment as grouping factors and day as a repeated measure. In the absence of main or interactive effects of day, follow-up analyses of simple main effects of genotype or treatment were performed on the data collapsed across day, Fisher’s least significant difference (LSD) tests (α=0.05) were used for post hoc comparisons between individual groups to follow up significant ANOVA main effects or interactions of genotype and treatment.
The discrimination ratios from the NOR data on the test day, were analyzed with a two-way ANOVA using genotype and treatment as a between subjects factor. Fisher’s LSD tests (α=0.05) were used for post hoc comparisons between individual groups to follow up significant ANOVA main effects or interactions.
For the MWM, the average daily latency and path length over the seven days of training were analyzed using a mixed ANOVA with day as a repeated measure and genotype and treatment as between-group factors. For each mouse, the daily latency and path length were typically strongly correlated across the acquisition period. However, ten of the 70 subjects trained in the MWM failed to perform the swimming task well across days (e.g., spending >20 sec per trial floating), such that latency and path length were poorly correlated. In those cases, (where the correlation was <0.2), the animals were excluded from both acquisition and probe MWM analyses (3 Eu+Water, 2 Eu+EGCG, 3 Ts+Water, 3 Ts+EGCG). For the probe trial, the time spent in the virtual target disc (in the target quadrant) and the average time spent in the 3 equivalent virtual non-target discs (in the other 3 quadrants) were analyzed using a mixed ANOVA with treatment group and genotype as between-group factors and disc location (target vs non-target) as a repeated measure. For the probe, four of the mice that had met the performance criteria during acquisition nevertheless floated for >26 seconds during the 60 second probe trial (> 43% of the trial) so they were excluded from the probe trial analysis (1 Eu+EGCG, 1 Ts+Water, 2 Ts+EGCG).
The number of paw slips on the balance beam was analyzed using a mixed ANOVA with treatment group and genotype as between-group factors, and beam width (12 mm, 9 mm and 6 mm) as a repeated measure.
For skeletal measurements, many of the measures were not normally distributed and transformations were made to achieve normality. Where the transformed measures did not achieve normality, non-parametric tests were used. The bone parameters (Tables 3 and 4) were analyzed with a two-way ANOVA using genotype and treatment as a between subjects variable.
Table 3.
| Skeletal Structure | Euploid (n=14) | Ts65Dn (n=10) | Ts65Dn + EGCG (n=12) | Euploid + EGCG (n=11) |
|---|---|---|---|---|
| Trabecular Bone | ||||
| Bone mineral density (BMD) (g/cm2)A | 0.248 ± 0.009 | 0.35 ± 0.013 | 0.222 ± 0.011 | 0.261 ± 0.010 |
| Percent bone volume (BV/TV) (%)A | 30.26 ± 1.38 | 27.14 ± 2.01 | 26.10 ± 1.18 | 30.39 ± 1.34 |
| Trabecular thickness (Tb.Th) (μ) | 0.072 ± 0.001 | 0.072 ± 0.002 | 0.071 ± 0.001 | 0.074 ± 0.001 |
| Trabecular separation (Tb.Sp) (mm) A | 0.145 ± 0.005 | 0.166 ± 0.009 | 0.170 ± 0.007 | 0.150 ± 0.006 |
| Trabecular number (Tb.N) (1/mm) A | 4.204 ± 0.165 | 3.756 ± 0.220 | 3.663 ± 0.155 | 4.103 ± 0.162 |
| Cortical Bone | ||||
| Total cross sectional area (CSA) (mm2)A | 1.73 ± 0.05 | 1.57 ± 0.06 | 1.51 ± 0.03 | 1.66 ± 0.05 |
| Marrow Area (mm2) | 0.71 ± 0.03 | 0.67 ± 0.04 | 0.67 ± 0.02 | 0.71 ± 0.03 |
| Cortical Area (mm2)A,B | 1.03 ± 0.03 | 0.90 ± 0.04 | 0.85 ± 0.02 | 0.95 ± 0.03 |
| Cortical Thickness (mm)A,B | 0.27 ± 0.01 | 0.25 ± 0.01 | 0.23 ± 0.01 | 0.25 ± 0.01 |
| Periosteal BS (mm) | 5.63 ± 0.07 | 5.37 ± 0.10 | 5.36 ± 0.07 | 5.46 ± 0.07 |
| Endocortical BS (mm) | 3.70 ± 0.07 | 3.61 ± 0.11 | 3.63 ± 0.06 | 3.72 ± 0.06 |
| I max (mm4)A | 0.30 ± 0.02 | 0.24 ± 0.02 | 0.23 ± 0.01 | 0.26 ± 0.01 |
| I min (mm4)A | 0.14 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.00 | 0.13 ± 0.01 |
| Tissue mineral density TMD (g/cmˆ3 HA) | 1.21 ± 0.02 | 1.22 ± 0.02 | 1.20 ± 0.01 | 1.21 ± 0.02 |
Main effect of genotype,
Main effect of treatment. Parameter values are listed as averages ± (SEM).
Table 4.
| Mechanical Testing | Euploid (n=13) | Ts65Dn (n=9) | Ts65Dn + EGCG (n=10) | Euploid + EGCG (n=10) |
|---|---|---|---|---|
| Yield Force (N)B | 12.62 ± 0.48 | 10.40 ± 0.82 | 9.24 ± 0.72 | 9.83 ± 0.89 |
| Ultimate Force (N)A,B | 18.91 ± 1.14 | 14.38 ± 1.14 | 12.19 ± 0.81 | 14.55 ± 0.90 |
| Displacement to Yield (μm) | 136.54 ± 11.22 | 138.00 ± 6.05 | 135.40 ± 7.70 | 115.40 ± 8.57 |
| Postyield Displacement (μm) | 688.85 ± 157.27 | 721.89 ± 234.49 | 831.80 ± 118.31 | 838.60 ± 153.17 |
| Total Displacement (μm) | 825.38 ± 164.15 | 859.89 ± 234.20 | 967.20 ± 121.95 | 954.00 ± 155.56 |
| Stiffness (N/mm)A | 119.04 ± 9.09 | 92.65 ± 9.08 | 82.48 ± 5.39 | 111.42 ± 9.45 |
| Work to Yield (mJ)B | 1.01 ± 0.11 | 0.81 ± 0.08 | 0.75 ± 0.09 | 0.68 ± 0.08 |
| Postyield Work (mJ) | 8.51 ± 1.11 | 7.61 ± 2.26 | 7.35 ± 1.25 | 8.10 ± 1.49 |
| Total Work (mJ) | 9.52 ± 1.12 | 8.42 ± 2.28 | 8.09 ± 1.32 | 8.78 ± 1.53 |
| Yield Stress (MPa) | 123.74 ± 6.54 | 120.06 ± 7.40 | 119.68 ± 9.43 | 103.89 ± 9.81 |
| Ultimate Stress (MPa)B | 181.31 ± 7.56 | 165.05 ± 5.64 | 156.57 ± 8.27 | 152.12 ± 9.32 |
| Strain to Yield (με)B | 12394.20 ± 880.50 | 12113.17 ± 544.77 | 11272.60 ± 646.70 | 10312.85 ± 710.30 |
| Total Strain (με) | 74008.75 ± 13842.05 | 77113.80 ± 22175.14 | 80917.37 ± 10486.08 | 85632.10 ± 14167.40 |
| Modulus (GPa) | 12.26 ± 0.55 | 12.03 ± 0.57 | 12.75 ± 0.73 | 12.58 ± 0.57 |
| Resilience (MPa) | 0.94 ± 0.14 | 0.83 ± 0.08 | 0.81 ± 0.10 | 0.65 ± 0.08 |
| Toughness (MPa) | 8.68 ± 1.12 | 7.98 ± 1.61 | 8.65 ± 1.35 | 8.50 ± 1.60 |
Main effect of genotype,
Main effect of treatment. Parameter values are listed as averages ± (SEM).
3. Results
3.1 Growth and EGCG intake
As shown in Table 2, Ts65Dn mice had significantly lower body weights and slower growth as compared to euploid mice over days [day × genotype interaction, F(21,1260)=6.96, p<0.001]. Both euploid and trisomic mice decreased their daily EGCG consumption (per kg body weight) over time [main effect of day, F(15,405)=13.43, p<0.001]; however, there were no significant differences in EGCG consumption between the euploid and trisomic mice (Table 2).
Table 2.
Average starting weight, final weight and EGCG consumption (corrected for degradation) for the respective tested groups.
| Group | N | Body Weight (P24) | Body Weight (P68) | Average EGCG Consumption (mg/kg/day) |
|---|---|---|---|---|
| Eu+Water | 19 | 15.9 ± 0.9 | 28.4 ± 0.6 | – |
| Eu+EGCG | 18 | 15.2 ± 0.9 | 27.4 ± 0.8 | 48.7 ± 1.1 |
| Ts+Water | 13 | 12.4 ± 0.3 | 22.8 ± 0.8 | – |
| Ts+EGCG | 15 | 11.8 ± 0.6 | 23.9 ± 0.8 | 52.9 ± 1.4 |
3.2 MCSF
Total entries
Both euploid and trisomic mice increased the frequency of total entries from day 1 to day 2 [main effect of day, F(1,54)=46.36, p<0.001] (see Figure 2), However, the trisomic mice showed significantly greater increases in total entries from day 1 to day 2, [genotype × day interaction, F(1,59)=7.71, p=0.008]. A two-way ANOVA on total entries change scores (day 2-day 1) confirmed the significantly larger increase in the Ts65Dn mice [main effect of genotype, F(1, 54)=7.7, p=0.008)]. Follow-up paired sample t-tests comparing day 1 and day 2 total scores indicated both Ts65Dn groups significantly increased total entries across days whereas for the euploid groups, only the water group significantly increased total entries.
Figure 2.
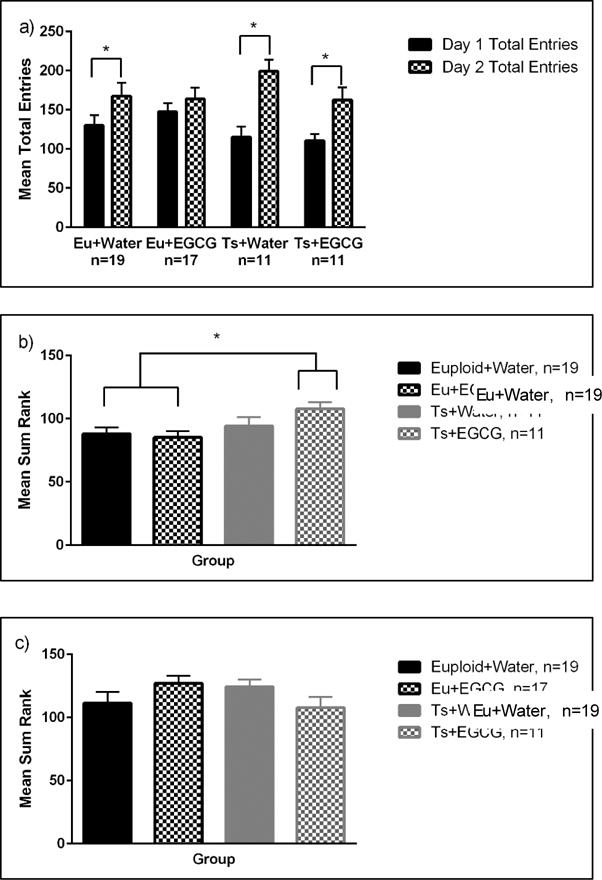
a. MCSF Total Entries. Both Euploid (Eu) and Trisomic (Ts) mice increased the number of entries from Day 1 to Day 2. As indicated by (*), Eu+Water, Ts+Water, and Ts+EGCG groups had a significantly higher number of total entries on Day 2. Data are represented by mean ± SEM.
b. MCSF Risk Assessment Behavior. The Trisomic (Ts) mice exhibited higher risk assessment behavior than the Euploid (Eu) mice (data collapsed across both days). As indicated by the (*), the Ts+EGCG group displayed significantly higher risk assessment behavior compared to both euploid groups. Data are represented by mean ± SEM.
c. MCSF Risk Taking Behavior. There was a significant genotype × treatment interaction. Data are collapsed across days since there were no effects of day. Note that EGCG treatment had opposing effects on euploid and trisomic mice. Data are represented by mean ± SEM
Exploratory behavior
There were no significant main or interactive effects found in the 2-way ANOVA on exploratory behavior (see Supplemental Table 1).
Risk Assessment
The 3-way ANOVA yielded only a main effect of genotype [F(1, 54)=6.3, p=0.015], with no main or interactive effects of treatment or day, indicating that overall the Ts65Dn mice had higher levels of risk assessment across days compared to the euploid mice. In the post hoc analysis (as shown in Figure 2b), the increased risk assessment of the Ts65Dn mice was mainly due to the significantly higher levels of the Ts65Dn group given EGCG compared to either euploid group (p<0.01).
Risk-taking behavior
The 3-way ANOVA yielded a significant genotype × treatment interaction [F(1,54)=4.07, p=0.048], with no main or interactive effects of day. As shown in Figure 2c, EGCG treatment produced opposite effects in the two genotypes, increasing risk taking in euploid mice but decreasing it in Ts65Dn mice.
Shelter Seeking behavior
There were no significant main or interactive effects found in the 2-way ANOVA on shelter seeking behavior (see Supplemental Table 1).
There were no significant main or interactive effects found in the 2-way ANOVA on fecal counts (see Supplemental Table 4).
3.3 NOR
There were no significant main or interactive effects of genotype or treatment in the discrimination ratios for either NOR test.
3.4 Balance Beam
There were no significant main or interactive effects of genotype or EGCG treatment on the balance beam task, though there was a trend for the Ts65Dn-water group to commit more paw slips than the euploid-water group. Both groups made more paw slips as the beam width decreased [main effect of width, F (2,116) = 100.84, p<0.001] (Supplemental Table 2).
3.5 Morris Water Maze
Across training days, all groups showed significant reductions in path lengths (see Figure 3a) and latencies to find the platform (Figure 3b) [main effect of day, F(6,276) = 10.2, p<0.001 for path length; F(6,276) = 7.32, p=<0.001 for latencies]. The Ts65Dn mice were significantly impaired in acquisition relative to control mice, as confirmed by the day × genotype interaction both for path length [F(6,276)=2.28, p=0.037] and for latency [F(6,276) = 2.2, p=0.041].
Figure 3.
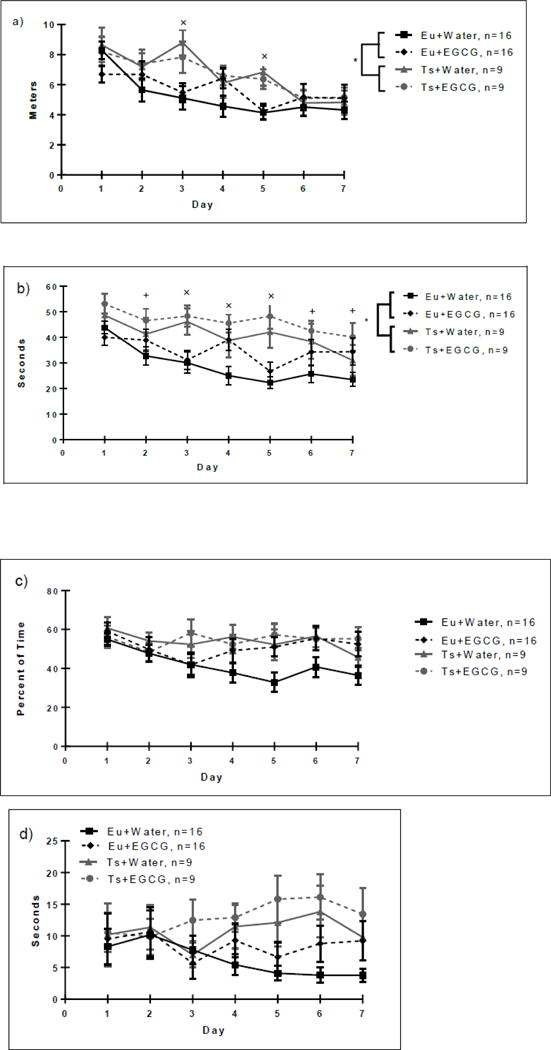
a. MWM Acquisition Path Length. Acquisition in the Morris water maze spatial learning task by the Euploid (Eu) and Trisomic (Ts) groups. Each line represents the average path length (meters) for each acquisition day. All groups showed a decrease in latency over training days. However, trisomic mice of both treatment groups displayed higher path lengths versus the euploid groups (main effect of genotype, indicated by (*). Insert graph represents the total path length summed across all 7 acquisition days. Both trisomic groups exhibited increased total latency versus euploid-water controls (as indicated by the *). LSD post-hoc analysis revealed a significant increase in Trisomic + Water and Trisomic +EGCG latencies as compared to Euploid + Water mice (as indicated by the ×). Data are represented as mean ± SEM.
b. MWM Acquisition Latency. Seconds spent locating the hidden platform by the Euploid (Eu) and Trisomic (Ts) groups. Each line represents the average time (latency) to find the hidden platform for each training day. All groups showed significant reductions in latencies over days (main effect of day). However, trisomic mice were significantly impaired versus controls (day × genotype interaction, p=0.041). LSD post-hoc analysis revealed a significant increase in Trisomic + Water and Trisomic +EGCG latencies as compared to Euploid + Water (as indicated by the ×), or increases in Trisomic + EGCG latencies versus Euploid + Water mice (as indicated by the +). Data are represented as mean ± SEM.
c. MWM Acquisition Thigmotaxic Behavior. Percent of time spent in thigmotaxis (within 25.4 cm of the wall) by the Euploid (Eu) and Trisomic (Ts) groups. Each line represents the average percent of time for each acquisition day. There was an overall decrease in thigmotaxic behavior over training days, most evident in the Euploid-water group; however, mice receiving EGCG displayed higher thigmotaxis versus the water groups (main effect of treatment, p=0.039), due mainly to the increased thigmotaxic behavior of the Euploid-EGCG group relative to the euploid-water group. Data are represented as mean ± SEM.
d. MWM Acquisition Floating Behavior. Seconds spent floating by the Euploid (Eu) and Trisomic (Ts) groups. Each line represents the average time (seconds) floating for each acquisition day. Trisomic mice displayed significantly less reduction in floating behavior over days than the euploid mice (day × genotype interaction, p=0.017). Data are represented as mean ± SEM.
There was a main effect of day on swimming speed [F(6,276) =11.98, p<0.001], however there were no significant effects of genotype or treatment on swimming speed, indicating that the euploid and trisomic groups did not differ on this measure of swimming performance. Mice receiving EGCG displayed higher levels of thigmotaxic behavior (Figure 3c) than those receiving water [main effect of treatment, F(6,276)=2.24, p=0.039], and thigmotaxic behavior generally declined over days [main effect of day, F(6,276)=3.65, p=0.002], most prominently in the euploid-water group. In addition, trisomic as compared to euploid mice displayed significantly less reduction in floating behavior (Figure 3d) over the course of acquisition [day × genotype interaction, F(6,276)= 2.64, p=0.017].
For the probe trial (see Figure 4), all groups had a preference for the target quadrant [main effect of location (1,42)= 32.84, p= <0.001], and there were no statistically significant main or interactive effects of genotype or treatment. A priori paired t-tests comparing time in target vs. non-target virtual discs showed that the euploid groups spent significantly more time in the target disc (p<0.05), but the differences for the trisomic groups did not reach significance. Nevertheless, there were no significant differences in time spent in the target quadrant among the four groups. There were no significant main or interactive effects of genotype or treatment on the amount of time spent floating, swimming speed, path length or the amount of time in thigmotaxis during the probe trial (see Supplemental Table 3).
Figure 4. MWM Probe Trial.
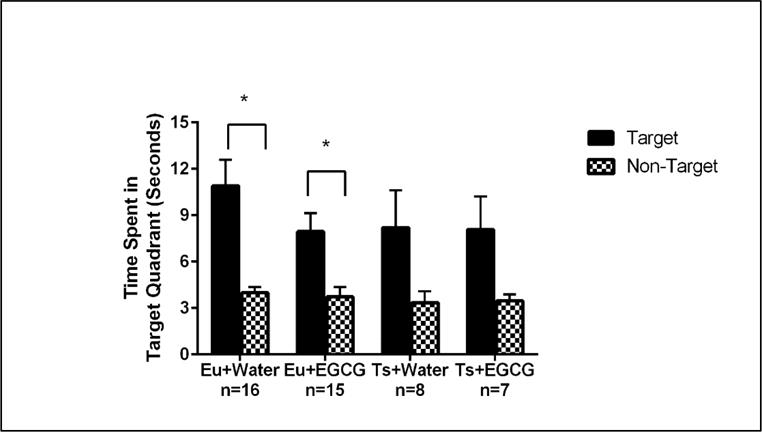
Probe trial performance of Euploid (Eu) and Trisomic (Ts) mice of the two treatment groups on the probe trial (Day 8). The (*) comparing the Target and Non-target times of the euploid groups indicates a significant spatial bias for the Target location for both euploid groups ; the similar trend for the trisomic groups did not reach statistical significance. Bars represent the average time spent in the target and non-target quadrant, with error bars represented as SEM.
3.6 Micro CT Analysis of Femora
In the cancellous bone of the distal femoral metaphysis, there were several effects of trisomy but no impact of EGCG treatment (Table 3). Bone volume fraction (BV/TV) was significantly lower in trisomic as compared to control mice [F(1,43) =6.23, p=0.017]. This decrease was likely driven by a significant decrease in trabecular number [F(1,43) =6.23, p=0.016] coupled with a significant increase in trabecular separation [F(1,43) =9.07, p=0.004] in the trisomic as compared to euploid femora.
For cortical geometry, the Ts65Dn as compared to euploid mice had significantly lower total cross sectional area [F(1,42) =9.92, p=0.005], cortical area [F(1,42) =11.99, p=0.001] and cortical thickness [F(1,42) =9.00, p=0.005] of the femora. There was also a genotype effect (trisomic mice were significantly decreased compared euploid littermates)in periosteal perimeter [F(1,42) =4.74, p=0.035], I-max [F(1,42) =6.05, p=0.018], and I-min [F(1,42) =14.51, p<0.001]. Mice that received EGCG had a significantly reduced the cortical area [F(1,42) =4.33, p=0.044] and cortical thickness [F(1,42) =5.71, p=0.02].
3.7 Mechanical Testing of Femora
Ts65Dn mice had significantly decreased structural stiffness [F(1,38) =10.17, p=0.003] and strength [F(1,38) =10.82, p=0.002] (ultimate force) in the femora as compared to their euploid counterparts (Table 4). In addition, yield force was marginally decreased [F(1,38) =3.87, p=0.056]. Treatment with EGCG did not produce positive effects on bone mechanics. Instead, EGCG’s effects were detrimental at both the structural and tissue levels in both trisomic and euploid mice. Structural strength (yield force) [F(1,38) =7.59, p=0.009] and ultimate force [F(1,38) =9.83, p=0.033]) were significantly decreased with treatment, driving a decrease in work to yield [F(1,38) =4.65, p=0.037]. At the tissue level, ultimate stress was significantly decreased [F(1,38) =5.52, p=0.024] and strain to yield was marginally decreased [F(1,38) =3.89, p=0.056] in EGCG as compared to water-treated bones. There were no effects of trisomy or treatment on any post-yield mechanical properties.
3.8 Dyrk1a Protein Levels and Kinase Activity
In protein isolated from the cerebellum, Ts65Dn mice receiving water had significantly lower Dyrk1a protein than euploid controls (genotype × treatment interaction [F(1,30)=4.51, p=0.043] (see Figure 5). There were no significant effects of genotype or treatment on Dyrk1a protein levels in the cerebral cortex, or hippocampus (Table 5).
Figure 5. Dyrk1a Protein Levels-Cerebellum.
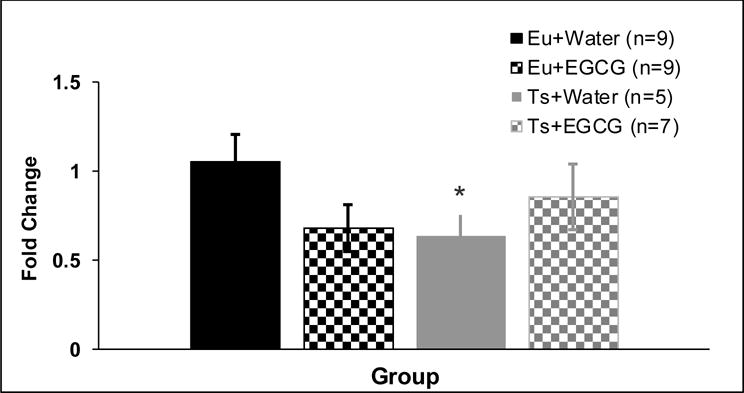
Dyrk1a protein levels in the cerebellum of ~9-week old mice given 6 weeks of treatment with water or EGCG (mean ± SEM). Ts65Dn mice receiving water had significantly less Dyrk1a protein that euploid-water controls (as indicated by the *, genotype × treatment interaction, p=0.043).
Table 5.
Ts65Dn mice receiving water had significantly less Dyrk1a protein that euploid-water controls (genotype × treatment interaction, p=0.043). There were no significant effects of genotype or treatment on Dyrk1a protein levels in the cerebral cortex, or hippocampus. Data are represented as mean ± SEM fold protein change, relative to Euploid + Water control mean.
| Group | Cerebral Cortex Mean Fold Change | Group | Hippocampus Mean Fold Change | Group | Cerebellum Mean Fold Change |
|---|---|---|---|---|---|
| Eu+Water (n=10) | 1.00 ± 0.20 | Eu+Water (n=9) | 0.99 ± 0.06 | Eu+Water (n=9) | 1.05 ± 0.15 |
| Eu+EGCG (n=10) | 1.02 ± 0.25 | Eu+EGCG (n=10) | 1.45 ± 0.19 | Eu+EGCG (n=9) | 0.68 ± 0.13 |
| Ts+Water (n=9) | 1.04 ± 0.28 | Ts+Water (n=5) | 1.16 ± 0.20 | Ts+Water (n=5) | 0.63 ± 0.12 |
| Ts+EGCG (n=6) | 0.83 ± 0.14 | Ts+EGCG (n=4) | 1.37 ± 0.36 | Ts+EGCG (n=7) | 0.85 ± 0.18 |
In protein isolated from femora of Ts65Dn and euploid mice, EGCG treatment significantly lowered kinase activity [main effect of treatment, F(1,39)= 6.68, p=0.014] (see Figure 6). The trend for an effect of genotype did not reach statistical significance (p=0.064). No significant main or interactive effects of genotype or treatment were found in kinase activity from protein isolated from cerebral cortex, cerebellum or hippocampus (Table 6).
Figure 6. Kinase activity-Femur.
Kinase activity in the femur of ~9-week old mice given 6 weeks of treatment with water or EGCG (mean ± SEM). Mice receiving EGCG treatment had significantly lower kinase activity than mice receiving water (main effect of treatment p<0.05).
Table 6.
There were no significant effects of genotype or treatment on Dyrk1a activity in the cerebral cortex, cerebellum and hippocampus. Data is represented as the area under the curve of the fluorescence emission ± SEM.
| Cerebral Cortex | Cerebellum | Hippocampus | |
|---|---|---|---|
| Eu+Water (n=8) | 143.2 ± 15.0 | 399.3 ± 21.8 | 342.6 ± 22.6 |
| Eu+EGCG (n=8) | 126.4 ± 14.0 | 404.9 ± 22.5 | 290.6 ± 34.6 |
| Ts+Water (n=6) | 171.2 ± 18.0 | 373.8 ± 34.2 | 287.7 ± 36.8 |
| Ts+EGCG (n=9) | 148.8 ± 17.7 | 424.2 ± 20.7 | 301.5 ± 21.3 |
4. Discussion
The aim of this study was to determine whether oral consumption of EGCG in the drinking water that yielded a dosage of ~50 mg/kg/day, higher than the dosage used in our previous studies (10–20 mg/kg/day) and comparable to the dosage reported by De la Torre et al. [45], would improve cognitive and skeletal phenotypes in the Ts65Dn mouse model of DS. EGCG failed to appreciably improve outcomes in trisomic mice in most cognitive/behavioral or skeletal measures. One exception to this conclusion was in the MCSF. EGCG significantly increased the risk assessment behavior of the Ts65Dn mice relative to both euploid controls together with a non-significant increase above Ts65Dn mice given water (which did not differ from controls), suggesting that EGCG treatment resulted in more cautious exploration of novel environments in the Ts65Dn mice. Consistent with this interpretation, EGCG had opposite effects on Ts65Dn and euploid mice on risk taking in the MCSF task, confirmed by the significant genotype × treatment interaction, in which EGCG decreased risk taking in Ts65Dn mice but increased risk taking in euploid controls. Although the trisomic mice did not have significantly higher risk taking than euploid controls, the interactive effect of EGCG on risk taking suggests that EGCG may have some beneficial effects for problem behaviors in DS that involve behavioral regulation [60].
No previous studies have been reported assessing the Ts65Dn mouse model using the MCSF. The test has been developed as a multidimensional assessment of distinct behavioral phenotypes in a complex environment that is rich with alternatives, in which animals are free to move around and explore among various compartments and areas [51]. The MCSF can enhance sensitivity to treatments such as brain injury or developmental manipulations that may produce changes in internal states that may be expressed as differences in behavioral patterning or profiles (e.g., in risk taking, risk assessment, shelter seeking, or exploration) [52–54, 56]. Our finding that EGCG treatment produces effects in opposite directions for risk taking behavior of trisomic and euploid mice raises the intriguing possibility that EGCG may improve the maladaptive behavior expressed in risk-related settings [51, 61], and may have similar selective effects on adaptive behavioral regulation in unfamiliar environments in individuals with DS [49].
As is generally reported, the Ts65Dn mice showed significant impairments in acquisition of the MWM, emerging over the first five days (of the seven-day training protocol) and accompanied by higher levels of thigmotaxis and floating behavior than the euploid mice given water. However, by the last two days there were no significant group differences in path length measures. In contrast to most findings (including our previous report [23]), in the current study the probe trial (on the 8th day of testing) yielded no significant group differences. Since probe trial performance typically reflects the strength of memory for the previously learned location, the lack of differences is consistent with all groups reaching comparable terminal performance on the last two days of acquisition training. Had the groups been given a probe trial test after five days of training (by which time the euploid but not the trisomic mice had acquired the spatial learning) rather than after seven training days, it is likely that the probe trial would have revealed the trisomic deficit.
The balance beam test did not yield significant deficits in this study, in contrast to the impairments reported in our previous study [62]. In the current study, there was a nonsignificant trend toward more slips in the Ts65Dn-water group compared to euploid-water group, but the lack of reliable differences in the balance beam suggests it may be susceptible to procedural variability. For example, this study introduced a third, narrower beam test that increased difficulty even for the euploid controls and thereby may have reduced the overall sensitivity of the task. In addition, we failed to see any significant differences between euploid and trisomic mice in the NOR task. This is in agreement with our previous finding when mice were given 20mg/kg/day EGCG for approximately 6 weeks [23]. While other studies have reported a trisomic deficit in this task, the many methodological differences across laboratory settings (types of objects used, light intensity, duration of sessions, testing intervals, etc.) may make NOR outcomes perhaps a less reliable or generalizable indicator of cognitive deficits.
The treatment with ~50/mg/kg day EGCG for 7 weeks caused unfavorable effects in bone structure and mechanical properties in Ts65Dn and euploid mice, including cortical area, cortical thickness, yield force, ultimate force, work to yield, ultimate stress, and strain to yield. Ts65Dn mice showed deficits in trabecular bone (percent bone volume, separation and number) cortical bone geometry (total cross sectional area, cortical area, cortical thickness, periosteal perimeter), I-max, and I-min and bone strength (ultimate force and stiffness), that were either not improved or were reduced by EGCG treatment. No post-yield mechanical properties were impacted by either trisomy or EGCG treatment. Aside from the change in ultimate stress with treatment, all mechanical changes were in whole bone properties which suggest that it was mostly changes in bone size/geometry that drove mechanical deficits as compared to inferior tissue quality. Similar skeletal deficits including decreases in ultimate force and stiffness were quantified in femurs from euploid mice with only one copy of Dyrk1a [63].
In contrast, our previous study using a three week administration of EGCG (~10mg/kg/day) showed improved skeletal deficits including femoral bone mineral density, percent trabecular bone volume, trabecular number and trabecular thickness in Ts65Dn mice [63]. These results suggest that in bone, there may be an “inverted U” function of optimal Dyrk1a activity, such that an optimal range of activity is necessary to achieve maximum skeletal strength, and falling below the threshold (as with high doses of EGCG) or exceeding an upper boundary (as with excess activity in trisomy) may be detrimental to bone strength. Additionally, when we treated Ts65Dn mice with two EGCG-containing supplements that delivered lower daily dosages (3–4 mg/kg/day EGCG), we found that the structure of the bone improved but the strength decreased [44]. These data suggested that other catechins or a combination of catechins could have a detrimental effect on skeletal strength. Our outcomes are among the first to report apparently adverse effects of EGCG treatment in association with DS phenotypes in Ts65Dn as well as in normal mice.
This study is one of only a few that has quantified Dyrk1a protein levels and kinase activity in brain and/or bone of Ts65Dn mice after undergoing EGCG treatment, and only a few studies have examined Dyrk1a protein levels in Ts65Dn mice in specific brain regions. We found that trisomic mice displayed decreased Dyrk1a protein levels in the cerebellum, but there were no significant effects of trisomy or EGCG treatment on Dyrk1a protein levels in the cerebral cortex or the hippocampus. In contrast, Ahmed et al. [64] reported increased Dyrk1a protein levels in the hippocampus, cortex and cerebellum of older Ts65Dn mice (~5–8 months of age). In addition, increased Dyrk1a protein levels in the hippocampus were found in ~3–4 month-old Ts65Dn mice [65]. The discrepancies between these findings and those of the current study may reflect differences in age, prior experience (extensive behavioral testing in our study) or other methodological differences, and highlight the need for a systematic analysis of potential changes in the spatial and temporal expression of Dyrk1a protein across the lifespan of Ts65Dn mice.
The relative lack of consistent matches between Dyrk1a brain protein levels and kinase activity measures—across trisomic and euploid mice, with and without EGCG treatment— suggests that the kinase assay may not be specific for Dyrk1a kinase activity but may be indicative of broader kinase activity. We did find a significant EGCG-induced reduction in kinase activity in femur regardless of phenotype, and this effect of EGCG treatment was associated with adverse changes in femora cortical structure and overall skeletal strength. Taken together, our current results indicate that the reduction in kinase activity in the appendicular skeleton after 7 weeks of treatment with ~50 mg/kg/day EGCG was associated with detrimental effects on bone structure and strength in trisomic and normal mice, rather than the beneficial effects seen with our previous lower-dose treatments. In addition, our finding that EGCG did not significantly alter kinase activity in any of the brain regions suggests that EGCG treatment either induced compensatory effects on brain Dyrk1a activity (or on activity of other kinases that phosphorylate the Woodtide substrate, discussed below) or that bioavailability of EGCG was less in brain than in bone.
The kinase activity assay used herein that was modified after Bui et al. [55] has been reported to detect Dyrk1a kinase activity levels. The Dyrk1a substrate, 5-carboxyfluorescein-Woodtide, is derived from amino acids 321–332 of the FOXO1 transcription factor [66] and was used to determine kinase activity from proteins isolated from the cerebral cortex, cerebellum, hippocampus, and femur. One important consideration is that in addition to being phosphorylated by Dyrk1a, this protein fragment also is phosphorylated by CK1 and may be phosphorylated by other kinases [66]. Although the reduction of kinase activity observed in the femur after EGCG treatment may be attributed to Dyrk1a inhibition, other kinases that phosphorylate Woodtide may be present and inhibited by EGCG. This possibility highlights the need for future studies to identify the specific and independent contribution of Dyrk1a phosphorylation of the target peptide.
Our findings highlight important remaining gaps in knowledge about the extent to which EGCG holds potential therapeutic value for DS phenotypes and which need to be addressed in future studies in mouse models of DS. Preclinical models need to focus on and clarify four critical aspects of EGCG treatment: 1) the timing and duration of EGCG administration, 2) the optimal dosage of administration, 3) the type of EGCG-containing supplement that is administered, and 4) the mechanisms that may account for any therapeutic effects of EGCG, including determining whether effects are mediated by inhibition of Dyrk1a.
The timing and duration of EGCG administration may determine the effectiveness of the treatment. Others have treated with EGCG beginning in adulthood, whereas this and our previous studies began treatment in adolescence. When 3-month-old Ts65Dn mice were given an EGCG-containing supplement (Life Extension® Mega Green Tea Extract, Lightly Caffeinated) in drinking water, improvements in cognitive behaviors was reported [45]. Combining treatment with an EGCG-containing supplement and environmental enrichment resulted in improvement in hippocampal-dependent MWM task in older 5–6 month-old mice [61]. Several studies have assessed EGCG treatment early in development. Treatment with pure EGCG during perinatal stages on Ts65Dn mice (25mg/kg via subcutaneous injection on PD 3–15) yielded an increase in neurogenesis and related proteins immediately after treatment cessation on PD 15, but these cellular effects were transient and were not evident at PD 45 and did not significantly improve hippocampal dependent learning and memory in trisomic mice [48]. Pregnant Ts65Dn mothers treated with 400mg/kg/day EGCG on embryonic days (E) 7 and 8 via oral gavage and saw improvements in the E9.5 1st pharyngeal arch size and cell number and in the size and shape of the cranial vault in 6 week old mice [47]. Thus, the age at which the mice are administered an EGCG-containing therapeutic appears to be a critical factor in the efficacy of targeted therapeutics. Taken together, these data suggest that a pre- or perinatal EGCG treatment, perhaps combined with other behaviorally-based therapies during development, may be the most effective approaches for EGCG therapeutics for DS phenotypes.
It is essential to identify potential dose-related differences in outcomes with EGCG treatment, in part because different doses of EGCG have been hypothesized to affect neurocognitive phenotypes differently. For example, it has been reported that a low concentration of EGCG produces an anti-apoptotic effect to protect against neurotoxicity and a high concentration of EGCG induces a pro apoptotic effect [67]. Our laboratory has yet to find significant improvement in cognitive tasks after various doses of pure EGCG administration of 3–7 weeks to adolescent Ts65D mice (10, 20, and 50mg/kg/day). A perinatal dose of 25mg/kg did not improve hippocampal dependent learning and memory in trisomic mice [48]. These findings suggest that varying doses of pure EGCG administered to Ts65Dn mice for different periods of time have limited or no effectiveness in improving cognitive deficits.
One important consideration is that our studies finding almost no therapeutic benefit used pure EGCG, whereas other laboratories that use EGCG-containing supplements that include other catechins and unknown components have reported beneficial effects on cognitive behavior [45]. This suggests that other molecules found in these supplements may be contributing to these reported beneficial effects. We previously used a radioactive-based Dyrk1a activity assay (based on activity of antibody-specific isolation of Dyrk1a protein) to show that Dyrk1a activity in cerebellum, hippocampus or femur was not significantly affected by a three-week adolescent treatment with ~10 mg/kg/day EGCG [23, 26]. In contrast, De la Torre and colleagues [45] used a similar radioactivity assay of Dyrk1a kinase activity and found significant reductions in Dyrk1a activity in the hippocampus of transgenic TgDyrk1a mice (that have an increased copy number of Dyrk1a with no other trisomic genes) after treatment for 1 month in adulthood. Calculations of the amount of EGCG administered in any dosing regimen must account for the differences in the amount of EGCG in the various EGCG-containing supplements used, amount of degradation, and should also determine the amount of the other catechins included in those supplements. It should be determined whether other catechins (or other molecules) present in the EGCG-containing supplements could be influencing the observed behavioral improvements. Identifying the role of these other catechins in therapeutic outcomes in preclinical mouse models will be important to identify the optimal use of supplements as potential therapeutic nutraceuticals. This information will allow for better comparisons across studies, and the development more targeted therapeutics. Questions also remain about the exact bioavailability and blood-brain-barrier penetration of EGCG, given the lack of behavioral improvements and failure to inhibit brain kinase activity with pure EGCG administration in this study.
Finally, if the therapeutic effects of EGCG are mechanistically caused by inhibition of Dyrk1a, then it will be important to demonstrate conclusively that Dyrk1a overexpression and/or increased Dyrk1a activity in specific tissues or specific brain regions can be causally linked to specific trisomic phenotypes. The possibility that enduring adverse effects resulting from a time- and spatially-limited period of excessive Dyrk1a activity must also be considered. We did not detect significant differences in kinase activity between adult euploid and Ts65Dn mice in three brain regions. In addition, we did not detect any group differences in Dyrk1a protein levels in either the cerebral cortex or hippocampus, and found the counterintuitive result that Ts65Dn mice had significantly lower Dyrk1a protein expression compared to euploid controls in the cerebellum. If confirmed, this could mean that Dyrk1a overexpression/kinase activity is developmentally regulated and its status in adulthood may not correlate with or cause observed adult DS phenotypes. It may be that overexpression/over activity of Dyrk1a at an earlier age or critical period is responsible for these phenotypes or that other triplicated genes may be responsible for these deficits. The prospect remains that Dyrk1a is a viable target for improving DS phenotypes, but additional information about spatiotemporal expression of Dyrk1a and Dyrk1a inhibitors with increased bioavailability and well-characterized pharmacological activity in the brain in preclinical studies using mouse models of DS must be ascertained.
Supplementary Material
Highlights.
EGCG treatment produces minimal improvement in cognitive phenotypes in DS mice
DS mice treated with 50 mg/kg/day EGCG displayed unfavorable bone phenotypes
Dyrk1a protein and kinase activity levels revealed need to understand effects of EGCG
Significant gaps remain regarding potential therapeutic value of EGCG for DS traits
Acknowledgments
This project is supported by the Indiana Clinical and Translational Sciences Institute funded, in part by Grant Number UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award (RJR and CRG), a Research Support Funds Grant from IUPUI (RJR and CRG), and Undergraduate Research Opportunities Grant from the Center for Research and Learning at IUPUI (KS, CRG, and RJR). The authors thank Dr. Marian Logrip and Shannon Roy for their guidance in performing the Western blots and Hardeep Dillon and Prabhjot Singh for their assistance in data collection. The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lejeune J, Turpin R, Gautier M. [Mongolism; a chromosomal disease (trisomy)] Bulletin de l’Academie nationale de medecine. 1959;143:256–65. [PubMed] [Google Scholar]
- 2.Roubertoux PL, Kerdelhue B. Trisomy 21: from chromosomes to mental retardation. Behavior genetics. 2006;36:346–54. doi: 10.1007/s10519-006-9052-0. [DOI] [PubMed] [Google Scholar]
- 3.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–16. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 4.Van Cleve SN, Cannon S, Cohen WI. Part II: Clinical Practice Guidelines for adolescents and young adults with Down Syndrome: 12 to 21 Years. Journal of pediatric health care : official publication of National Association of Pediatric Nurse Associates & Practitioners. 2006;20:198–205. doi: 10.1016/j.pedhc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Van Cleve SN, Cohen WI. Part I: clinical practice guidelines for children with Down syndrome from birth to 12 years. Journal of pediatric health care : official publication of National Association of Pediatric Nurse Associates & Practitioners. 2006;20:47–54. doi: 10.1016/j.pedhc.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, et al. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–3. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- 7.Guidi S, Bonasoni P, Ceccarelli C, Santini D, Gualtieri F, Ciani E, et al. Neurogenesis impairment and increased cell death reduce total neuron number in the hippocampal region of fetuses with Down syndrome. Brain Pathol. 2008;18:180–97. doi: 10.1111/j.1750-3639.2007.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorenzi HA, Reeves RH. Hippocampal hypocellularity in the Ts65Dn mouse originates early in development. Brain Res. 2006;1104:153–9. doi: 10.1016/j.brainres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Martinez de Lagran M, Altafaj X, Gallego X, Marti E, Estivill X, Sahun I, et al. Motor phenotypic alterations in TgDyrk1a transgenic mice implicate DYRK1A in Down syndrome motor dysfunction. Neurobiol Dis. 2004;15:132–42. doi: 10.1016/j.nbd.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Baptista F, Varela A, Sardinha LB. Bone mineral mass in males and females with and without Down syndrome. Osteoporos Int. 2005;16:380–8. doi: 10.1007/s00198-004-1687-1. [DOI] [PubMed] [Google Scholar]
- 11.Center J, Beange H, McElduff A. People with mental retardation have an increased prevalence of osteoporosis: a population study. American journal of mental retardation : AJMR. 1998;103:19–28. doi: 10.1352/0895-8017(1998)103<0019:PWMRHA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Guijarro M, Valero C, Paule B, Gonzalez-Macias J, Riancho JA. Bone mass in young adults with Down syndrome. Journal of intellectual disability research : JIDR. 2008;52:182–9. doi: 10.1111/j.1365-2788.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- 13.Costa AC, Scott-McKean JJ. Prospects for improving brain function in individuals with Down syndrome. CNS drugs. 2013;27:679–702. doi: 10.1007/s40263-013-0089-3. [DOI] [PubMed] [Google Scholar]
- 14.Lana-Elola E, Watson-Scales SD, Fisher EM, Tybulewicz VL. Down syndrome: searching for the genetic culprits. Dis Model Mech. 2011;4:586–95. doi: 10.1242/dmm.008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson LE, Richtsmeier JT, Leszl J, Reeves RH. A chromosome 21 critical region does not cause specific Down syndrome phenotypes. Science. 2004;306:687–90. doi: 10.1126/science.1098992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rueda N, Florez J, Martinez-Cue C. Mouse models of Down syndrome as a tool to unravel the causes of mental disabilities. Neural plasticity. 2012;2012:584071. doi: 10.1155/2012/584071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blazek JD, Gaddy A, Meyer R, Roper RJ, Li J. Disruption of bone development and homeostasis by trisomy in Ts65Dn Down syndrome mice. Bone. 2011;48:275–80. doi: 10.1016/j.bone.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davisson MT, Schmidt C, Reeves RH, Irving NG, Akeson EC, Harris BS, et al. Segmental trisomy as a mouse model for Down syndrome. Progress in clinical and biological research. 1993;384:117–33. [PubMed] [Google Scholar]
- 19.Liu C, Belichenko PV, Zhang L, Fu D, Kleschevnikov AM, Baldini A, et al. Mouse models for Down syndrome-associated developmental cognitive disabilities. Dev Neurosci. 2011;33:404–13. doi: 10.1159/000329422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, et al. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–84. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- 21.Escorihuela RM, Fernandez-Teruel A, Vallina IF, Baamonde C, Lumbreras MA, Dierssen M, et al. A behavioral assessment of Ts65Dn mice: a putative Down syndrome model. Neurosci Lett. 1995;199:143–6. doi: 10.1016/0304-3940(95)12052-6. [DOI] [PubMed] [Google Scholar]
- 22.Sago H, Carlson EJ, Smith DJ, Rubin EM, Crnic LS, Huang TT, et al. Genetic dissection of region associated with behavioral abnormalities in mouse models for Down syndrome. Pediatr Res. 2000;48:606–13. doi: 10.1203/00006450-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Stringer M, Abeysekera I, Dria KJ, Roper RJ, Goodlett CR. Low dose EGCG treatment beginning in adolescence does not improve cognitive impairment in a Down syndrome mouse model. Pharmacology, biochemistry, and behavior. 2015;138:70–9. doi: 10.1016/j.pbb.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Netzer WJ, Powell C, Nong Y, Blundell J, Wong L, Duff K, et al. Lowering beta-amyloid levels rescues learning and memory in a Down syndrome mouse model. PLoS One. 2010;5:e10943. doi: 10.1371/journal.pone.0010943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampton TG, Stasko MR, Kale A, Amende I, Costa AC. Gait dynamics in trisomic mice: quantitative neurological traits of Down syndrome. Physiology & behavior. 2004;82:381–9. doi: 10.1016/j.physbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Blazek JD, Abeysekera I, Li J, Roper RJ. Rescue of the abnormal skeletal phenotype in Ts65Dn Down syndrome mice using genetic and therapeutic modulation of trisomic Dyrk1a. Hum Mol Genet. 2015;24:5687–96. doi: 10.1093/hmg/ddv284. [DOI] [PubMed] [Google Scholar]
- 27.Fowler TW, McKelvey KD, Akel NS, Vander Schilden J, Bacon AW, Bracey JW, et al. Low bone turnover and low BMD in Down syndrome: effect of intermittent PTH treatment. PLoS One. 2012;7:e42967. doi: 10.1371/journal.pone.0042967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker W, Joost HG. Structural and functional characteristics of Dyrk, a novel subfamily of protein kinases with dual specificity. Progress in nucleic acid research and molecular biology. 1999;62:1–17. doi: 10.1016/s0079-6603(08)60503-6. [DOI] [PubMed] [Google Scholar]
- 29.Arque G, Casanovas A, Dierssen M. Dyrk1A is dynamically expressed on subsets of motor neurons and in the neuromuscular junction: possible role in Down syndrome. PLoS One. 2013;8:e54285. doi: 10.1371/journal.pone.0054285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arque G, de Lagran MM, Arbones ML, Dierssen M. Age-associated motor and visuo-spatial learning phenotype in Dyrk1A heterozygous mutant mice. Neurobiol Dis. 2009;36:312–9. doi: 10.1016/j.nbd.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 31.Thomazeau A, Lassalle O, Iafrati J, Souchet B, Guedj F, Janel N, et al. Prefrontal deficits in a murine model overexpressing the down syndrome candidate gene dyrk1a. J Neurosci. 2014;34:1138–47. doi: 10.1523/JNEUROSCI.2852-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Souchet B, Guedj F, Sahun I, Duchon A, Daubigney F, Badel A, et al. Excitation/inhibition balance and learning are modified by Dyrk1a gene dosage. Neurobiol Dis. 2014;69:65–75. doi: 10.1016/j.nbd.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Dowjat WK, Adayev T, Kuchna I, Nowicki K, Palminiello S, Hwang YW, et al. Trisomy-driven overexpression of DYRK1A kinase in the brain of subjects with Down syndrome. Neurosci Lett. 2007;413:77–81. doi: 10.1016/j.neulet.2006.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi JH, Berger JD, Mazzella MJ, Morales-Corraliza J, Cataldo AM, Nixon RA, et al. Age-dependent dysregulation of brain amyloid precursor protein in the Ts65Dn Down syndrome mouse model. Journal of neurochemistry. 2009;110:1818–27. doi: 10.1111/j.1471-4159.2009.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu F, Liang Z, Wegiel J, Hwang YW, Iqbal K, Grundke-Iqbal I, et al. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:3224–33. doi: 10.1096/fj.07-104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato T, Miyata G. The nutraceutical benefit, part I: green tea. Nutrition. 2000;16:315–7. doi: 10.1016/s0899-9007(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 37.Brown MD. Green tea (Camellia sinensis) extract and its possible role in the prevention of cancer. Alternative medicine review : a journal of clinical therapeutic. 1999;4:360–70. [PubMed] [Google Scholar]
- 38.Ko CH, Lau KM, Choy WY, Leung PC. Effects of tea catechins, epigallocatechin, gallocatechin, and gallocatechin gallate, on bone metabolism. J Agric Food Chem. 2009;57:7293–7. doi: 10.1021/jf901545u. [DOI] [PubMed] [Google Scholar]
- 39.Lin YL, Lin JK. (−)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-kappaB. Mol Pharmacol. 1997;52:465–72. [PubMed] [Google Scholar]
- 40.Maeda-Yamamoto M, Inagaki N, Kitaura J, Chikumoto T, Kawahara H, Kawakami Y, et al. O-methylated catechins from tea leaves inhibit multiple protein kinases in mast cells. Journal of immunology. 2004;172:4486–92. doi: 10.4049/jimmunol.172.7.4486. [DOI] [PubMed] [Google Scholar]
- 41.Yang CS, Landau JM. Effects of tea consumption on nutrition and health. J Nutr. 2000;130:2409–12. doi: 10.1093/jn/130.10.2409. [DOI] [PubMed] [Google Scholar]
- 42.Adayev T, Chen-Hwang MC, Murakami N, Wegiel J, Hwang YW. Kinetic properties of a MNB/DYRK1A mutant suitable for the elucidation of biochemical pathways. Biochemistry. 2006;45:12011–9. doi: 10.1021/bi060632j. [DOI] [PubMed] [Google Scholar]
- 43.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. The Biochemical journal. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abeysekera I, Thomas J, Georgiadis TM, Berman AG, Hammond MA, Dria KJ, et al. Differential effects of Epigallocatechin-3-gallate containing supplements on correcting skeletal defects in a Down syndrome mouse model. Mol Nutr Food Res. 2016;60:717–26. doi: 10.1002/mnfr.201500781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De la Torre R, De Sola S, Pons M, Duchon A, de Lagran MM, Farre M, et al. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol Nutr Food Res. 2014;58:278–88. doi: 10.1002/mnfr.201300325. [DOI] [PubMed] [Google Scholar]
- 46.Guedj F, Sebrie C, Rivals I, Ledru A, Paly E, Bizot JC, et al. Green tea polyphenols rescue of brain defects induced by overexpression of DYRK1A. PLoS One. 2009;4:e4606. doi: 10.1371/journal.pone.0004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McElyea SD, Starbuck JM, Brink DT, Harrington E, Blazek JD, Ghonemia A, et al. Influence of Prenatal EGCG Treatment and Dyrk1a Dosage Reduction on Craniofacial Features Associated with Down Syndrome. Hum Mol Genet. 2016;25:4856–69. doi: 10.1093/hmg/ddw309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stagni F, Giacomini A, Emili M, Trazzi S, Guidi S, Sassi M, et al. Short- and long-term effects of neonatal pharmacotherapy with epigallocatechin-3-gallate on hippocampal development in the Ts65dn mouse model of Down syndrome. Neuroscience. 2016;333:277–301. doi: 10.1016/j.neuroscience.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 49.De la Torre R, de Sola S, Hernandez G, Farre M, Pujol J, Rodriguez J, et al. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down’s syndrome (TESDAD): a double-blind, randomised, placebo-controlled, phase 2 trial. The Lancet Neurology. 2016;15:801–10. doi: 10.1016/S1474-4422(16)30034-5. [DOI] [PubMed] [Google Scholar]
- 50.Vacca RA, Valenti D. Green tea EGCG plus fish oil omega-3 dietary supplements rescue mitochondrial dysfunctions and are safe in a Down’s syndrome child. Clinical nutrition. 2015;34:783–4. doi: 10.1016/j.clnu.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 51.Meyerson BJ, Augustsson H, Berg M, Roman E. The Concentric Square Field: a multivariate test arena for analysis of explorative strategies. Behav Brain Res. 2006;168:100–13. doi: 10.1016/j.bbr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 52.Ekmark-Lewen S, Lewen A, Meyerson BJ, Hillered L. The multivariate concentric square field test reveals behavioral profiles of risk taking, exploration, and cognitive impairment in mice subjected to traumatic brain injury. Journal of neurotrauma. 2010;27:1643–55. doi: 10.1089/neu.2009.0953. [DOI] [PubMed] [Google Scholar]
- 53.Daoura L, Hjalmarsson M, Oreland S, Nylander I, Roman E. Postpartum Behavioral Profiles in Wistar Rats Following Maternal Separation – Altered Exploration and Risk-Assessment Behavior in MS15 Dams. Frontiers in behavioral neuroscience. 2010;4:37. doi: 10.3389/fnbeh.2010.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roman E, Stewart RB, Bertholomey ML, Jensen ML, Colombo G, Hyytia P, et al. Behavioral profiling of multiple pairs of rats selectively bred for high and low alcohol intake using the MCSF test. Addiction biology. 2012;17:33–46. doi: 10.1111/j.1369-1600.2011.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bui LC, Tabouy L, Busi F, Dupret JM, Janel N, Planque C, et al. A high-performance liquid chromatography assay for Dyrk1a, a Down syndrome-associated kinase. Analytical biochemistry. 2014;449:172–8. doi: 10.1016/j.ab.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 56.Meyerson BJ, Jurek B, Roman E. A Rank-Order Procedure Applied to an Ethoexperimental Behavior Model–The Multivariate Concentric Square Field (MCSF) Test. Journal of Behavioral and Brain Science. 2013;3:350–61. [Google Scholar]
- 57.Berman AG, Clauser CA, Wunderlin C, Hammond MA, Wallace JM. Structural and Mechanical Improvements to Bone Are Strain Dependent with Axial Compression of the Tibia in Female C57BL/6 Mice. PLoS One. 2015;10:e0130504. doi: 10.1371/journal.pone.0130504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papadopoulos C, Arato K, Lilienthal E, Zerweck J, Schutkowski M, Chatain N, et al. Splice variants of the dual specificity tyrosine phosphorylation-regulated kinase 4 (DYRK4) differ in their subcellular localization and catalytic activity. J Biol Chem. 2011;286:5494–505. doi: 10.1074/jbc.M110.157909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pons-Espinal M, Martinez de Lagran M, Dierssen M. Environmental enrichment rescues DYRK1A activity and hippocampal adult neurogenesis in TgDyrk1A. Neurobiol Dis. 2013;60C:18–31. doi: 10.1016/j.nbd.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 60.van Gameren-Oosterom HB, Fekkes M, van Wouwe JP, Detmar SB, Oudesluys-Murphy AM, Verkerk PH. Problem behavior of individuals with Down syndrome in a nationwide cohort assessed in late adolescence. J Pediatr. 2013;163:1396–401. doi: 10.1016/j.jpeds.2013.06.054. [DOI] [PubMed] [Google Scholar]
- 61.Catuara-Solarz S, Espinosa-Carrasco J, Erb I, Langohr K, Notredame C, Gonzalez JR, et al. Principal Component Analysis of the Effects of Environmental Enrichment and (−)-epigallocatechin-3-gallate on Age-Associated Learning Deficits in a Mouse Model of Down Syndrome. Frontiers in behavioral neuroscience. 2015;9:330. doi: 10.3389/fnbeh.2015.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stringer M, Abeysekera I, Dria KJ, Roper RJ, Goodlett CR. Low dose EGCG treatment beginning in adolescence does not improve cognitive impairment in a Down syndrome mouse model. Pharmacology Biochemistry and Behavior. 2015;138:70–9. doi: 10.1016/j.pbb.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Blazek JD, Malik AM, Tischbein M, Arbones ML, Moore CS, Roper RJ. Abnormal mineralization of the Ts65Dn Down syndrome mouse appendicular skeleton begins during embryonic development in a Dyrk1a-independent manner. Mech Dev. 2015;136:133–42. doi: 10.1016/j.mod.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 64.Ahmed MM, Sturgeon X, Ellison M, Davisson MT, Gardiner KJ. Loss of correlations among proteins in brains of the Ts65Dn mouse model of down syndrome. J Proteome Res. 2012;11:1251–63. doi: 10.1021/pr2011582. [DOI] [PubMed] [Google Scholar]
- 65.Altafaj X, Martin ED, Ortiz-Abalia J, Valderrama A, Lao-Peregrin C, Dierssen M, et al. Normalization of Dyrk1A expression by AAV2/1-shDyrk1A attenuates hippocampal-dependent defects in the Ts65Dnmousemodel of Downsyndrome. Neurobiol Dis. 2013;52:117–27. doi: 10.1016/j.nbd.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 66.Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. The Biochemical journal. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh NA, Mandal AK, Khan ZA. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG) Nutrition journal. 2016;15:60. doi: 10.1186/s12937-016-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


