Abstract
Estrone and 17β-estradiol are phenolic steroids that are known to be neuroprotective in multiple models of neuronal injury. Previous studies have identified the importance of their phenolic steroid A-ring for neuroprotection and have identified ortho substituents at the C-2 and C-4 positions on the phenol ring that enhance this activity. To investigate the importance of the steroid ring system for neuroprotective activity, phenolic compounds having the cyclopent[b]anthracene, cyclopenta[b]phenanthrene, benz[f]indene, benz[e]indene, indenes linked to a phenol, and a phenolic spiro ring system were prepared. New synthetic methods were developed to make some of the cyclopent[b]anthracene analogues as well as the spiro ring system. Compounds were evaluated for their ability to protect HT-22 hippocampal neurons from glutamate neurotoxicity and their activity relative to a potent neuroprotective analogue of 17β-estradiol was determined. An adamantyl substituent placed ortho to the phenolic hydroxyl group gave neuroprotective analogues in all ring systems studied.
Introduction
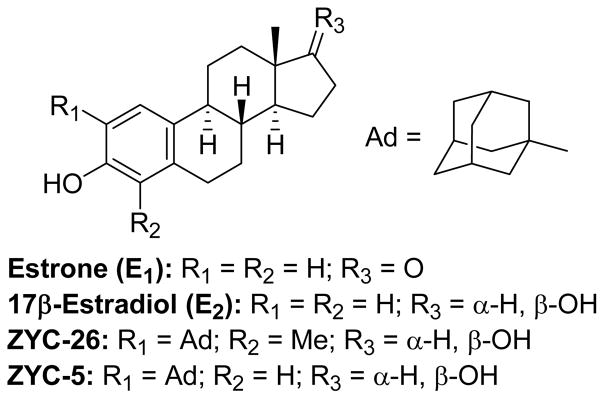
The estrogens estrone (E1) and 17β-estradiol (E2) have been shown to provide neuroprotection against oxidative damage in many different models of neuronal injury (Figure 1).1–12 These compounds are free radical scavengers.13,14 Since methylation of the aromatic hydroxyl group of an estrogen analogue eliminates neuroprotective action, it has been concluded that the antioxidative properties of phenols are responsible for neuroprotection by this class of compounds.10 In a previous structure–activity study we reported that electron donating alkyl groups positioned on either or both sides of the phenolic hydroxyl group in either E1 or E2 increase neuroprotective potency.10 ZYC-26 (Figure 1) was the most potent compound identified in that study. In addition to the electron donating properties of the adamantyl group, an NMR study of ZYC-5 supports the hypothesis that another contributing factor for the high potency of ortho-substituted adamantyl analogues is the effect that this substituent has on orienting the steroid in the membrane.15
Figure 1.
Structures of neuroprotective steroids: estrone, 17β-estradiol, ZYC-26 and ZYC-5.
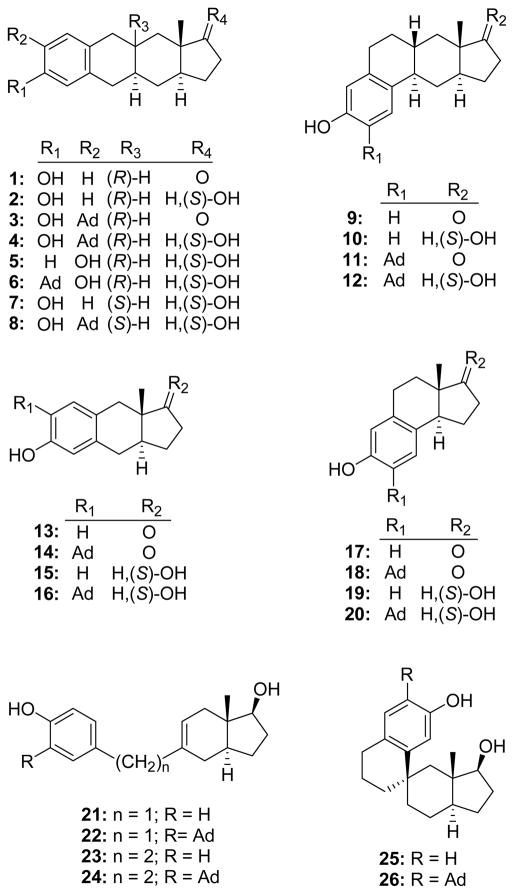
In this study we address the importance of the steroid ring system for the neuroprotective actions of phenolic analogues of E1, E2 and ZYC-26. We prepared analogues in six different ring systems (Figure 2): 1) tetracyclic cyclopent[b]anthracenes (1–8) and cyclopenta[b]phenanthrenes (9–12); 2) tricyclic benz[f]indenes (13–16) and benz[e]indenes (17–20); 3) indenes connected by a –(CH2)n– linker to a phenol (21–24); and 4) a spiro ring system (25, 26). In all ring systems we prepared analogues with an adamantyl group ortho to the phenolic hydroxyl group to evaluate the impact of this substituent on neuroprotective activity. Preparation of cyclopent[b]anthracenes 4–8 was accomplished using newly developed synthetic routes. Additionally, an efficient ring closure reaction leading to spiro compounds 25 and 26 was implemented. Neuroprotection was evaluated in a neuronal cell culture model of glutamate induced neurotoxicity. In the absence of an adamantyl group, E2 and its analogues did not have significant neuroprotective activity at the highest concentration tested (1 μM). Several analogues containing an adamantyl group had neuroprotective activity similar to that of ZYC-26 at the highest concentration evaluated (1 μM).
Figure 2.
Structures of the non-steroidal analogues synthesized and evaluated. The designation (S)-OH indicates the stereochemistry of the hydroxyl group is the same as that of a steroid 17β-OH group.
Results and Discussion
Chemistry
Four Fused Rings – cyclopent[b]anthracenes (CP[b]A) and cyclopenta[b]phenanthrenes (CP[b]P)
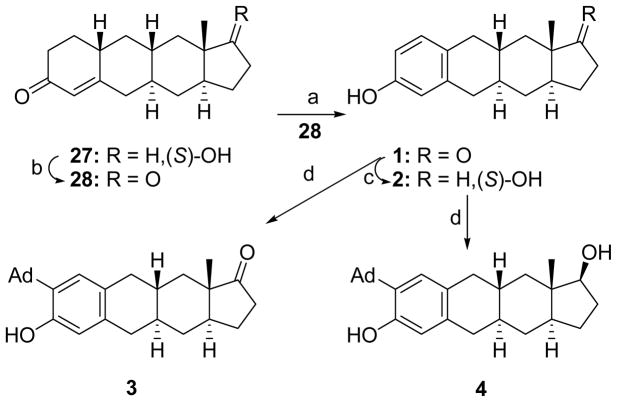
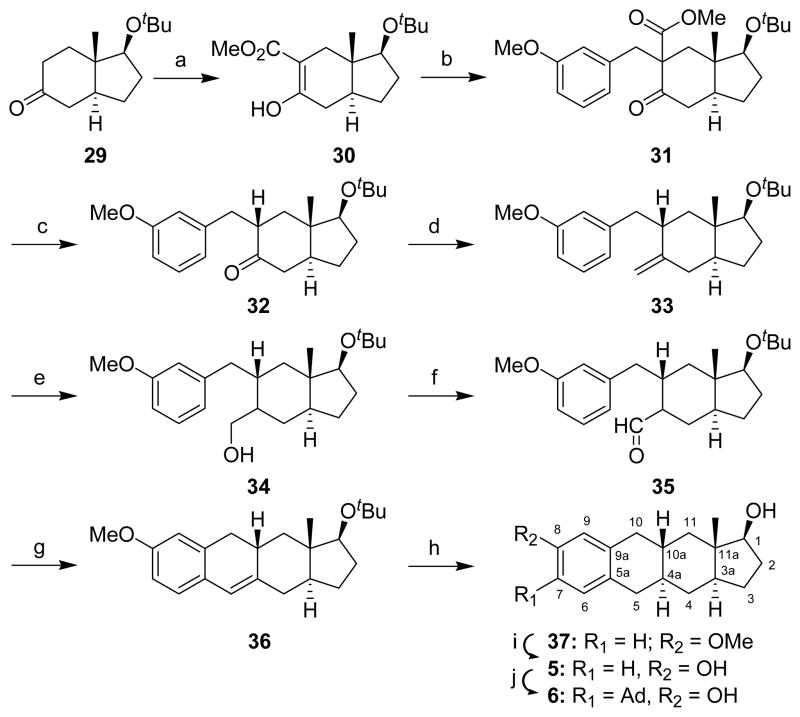
Throughout all Schemes (S)-OH indicates that the stereochemistry of the hydroxyl group is the same as that of the steroid 17β-OH. The syntheses of compounds prepared in the CP[b]A ring system are shown in Schemes 1–4. The synthesis of 1–4 from the previously prepared CP[b]A 2716 is shown in Scheme 1. Jones oxidation of CP[b]A 27 gave compound 28. The ring containing the enone group was then aromatized using CuBr2/LiBr to give compound 1. NaBH4 reduction converted compound 1 to compound 2. Reaction of compounds 1 and 2 with 1-adamantanol and BF3•Et2O gave compounds 3 and 4, respectively.
Scheme 1a.
aReagents: (a) CuBr2, LiBr, CH3CN, (83%); (b) Jones Reagent, acetone, (ca. 100%); (c) NaBH4, EtOH, (92%); (d) 1-adamantanol, BF3•Et2O, CH2Cl2, (3, 81%), (4, 75%).
Scheme 4a.
aReagents: (a) NaH, (CH3O)2CO, THF, (97%); (b) NaH, 3-Methoxybenzyl bromide, DMF/toluene, (81%); (c) LiCl, DMF, (92%); (d) NaH, methyltriphenylphosphonium bromide, benzene, (98%); (e) i: BH3•THF, THF; ii: 3 N NaOH, H2O2, (86%); (f) (COCl)2, DMSO, CH2Cl2, Et3N (ca. 100%); (g) 3 N HCl, MeOH, (85%); (h) i: Na, liq. NH3, aniline, THF; ii: 6 N HCl, MeOH (70%, 2 steps); (i) DIBAL–H, toluene, (94%); (j) 1-adamantanol, BF3•Et2O, CH2Cl2, (85%).
CP[b]A compounds 5 and 6 were prepared by a newly developed route (Scheme 2). These two analogues have the same stereochemistry at all ring fusions as the just described CP[b]A compounds 1–4. However, the position of the hydroxyl group is different. Indenone 29 was prepared from optically pure Hajos–Parrish ketone as described previously.17 Attempts to convert indenone 29 directly into benzylated indenone 32 using Pd(PPh3)4 or Pd2(dba)3 catalysts with either phosphine ligands 2-biphenyldi-tert-butyl phosphine or 2-biphenyldicyclohexyl phosphine as described in the literature for the benzylation of ketones failed.18 Accordingly, indenone 29 was first carbomethoxylated with dimethyl carbonate to give enol 30 as described previously.19,20 Benzylation then was carried out using a modified Yadav’s method21 to give compound 31. The NMR spectrum of compound 31 indicated that it was 5:1 mixture of diastereomers resulting from the new chiral center formed in the benzylation reaction. Decarbomethoxylation of compound 31 using Krapcho’s method22 gave ketone 32 as a single stereoisomer with the benzyl side chain assigned as having the thermodynamically more stable equatorial configuration. This stereochemical assignment was later confirmed (vide infra). Ketone 32 was next converted into exocyclic olefin 33 by a Wittig reaction. Hydroboration of olefin 33 using BH3•THF complex afforded primary alcohol 34 as a mixture of C-6 diastereomers (~4.5:1). Swern oxidation of diastereomeric alcohol 34 gave aldehyde 35 as a single product. Aldehyde 35 was then cyclized using aqueous 3 N HCl in MeOH to form CP[b]A 36. The stereochemistry of the groups at the C-6 stereocenter in compounds 34 and 35 was not established as this stereocenter is eliminated upon formation of product 36.
Scheme 2a.
aReagents: (a) NaH, (CH3O)2CO, THF, (93%); (b) NaH, 3-Methoxybenzyl bromide, DMF/toluene, (85%); (c) LiCl, DMF, (90%); (d) NaH, methyltriphenylphosphonium bromide, benzene, (94%); (e) i: BH3•THF, THF; ii: 3 N NaOH, H2O2, (82%); (f) (COCl)2, DMSO, CH2Cl2, Et3N, (ca. 100%); (g) 3 N HCl, MeOH, (84%); (h) i: Na, liq. NH3, aniline, THF; ii: 6 N HCl, MeOH, (62%, 2 steps); (i) DIBAL–H, toluene, (85%); (j) 1-adamantanol, BF3•Et2O, CH2Cl2, (80%).
The double bond of compound 36 was reduced using Na/liq. NH3 in the presence of added aniline. As reported previously, aniline addition was found to improve the yield of a reduction reaction of this type.23 The 1H NMR showed that the crude product was a mixture of diastereomers (4:1, 4aS:4aR) which was not separable by flash column chromatography. Separation was achieved chromatographically after removal of the tert-butyl protecting group using 3 N HCl in refluxing methanol to yield CP[b]A 37. The minor stereoisomer having the 4aR stereochemistry was not isolated in pure form or further characterized. An X-ray structure determination confirmed the structure of product 37. If the tert-butyl group was removed by HCl treatment before the Na/liq. NH3 reduction, more than half of the trisubstituted olefin 36 rearranged to the tetrasubstituted olefin. Finally, the methyl group of the methoxy ether was removed with DIBAL–H in refluxing toluene to give CP[b]A 5. If BBr3 was used as the demethylating reagent, only 64% of compound 5 was obtained. The adamantyl group of CP[b]A 6 was then added.
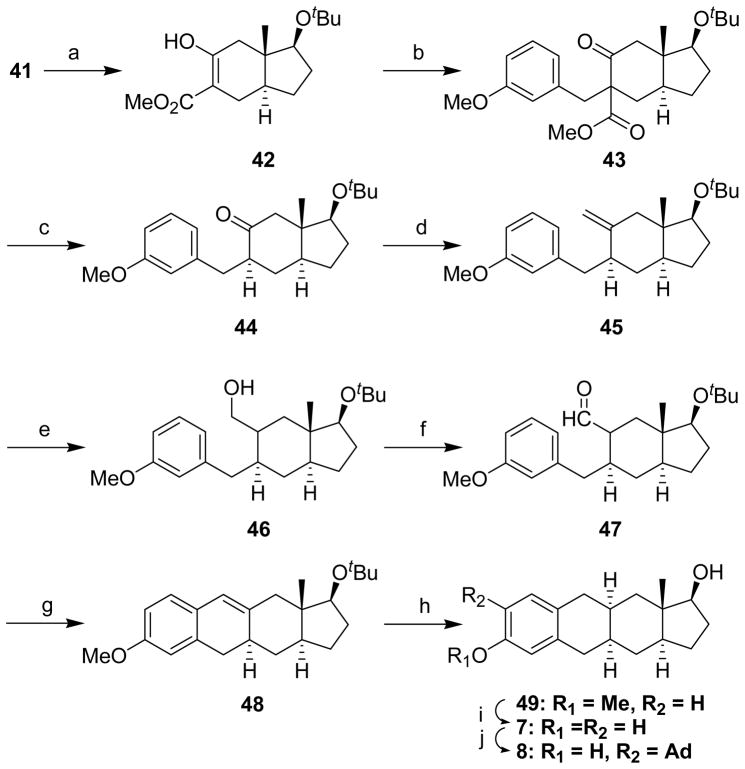
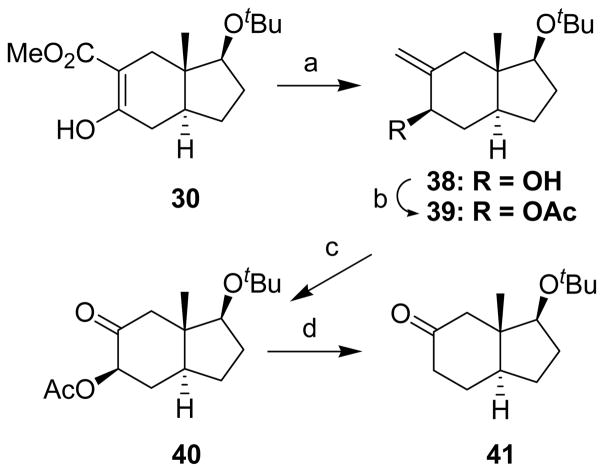
The synthetic routed used to prepare CP[b]A 27 as a starting material for the synthesis of CP[b]A 1 is not optimal. That synthetic route requires the separation of CP[b]A 27 from another product formed in the CP[b]P ring system.16 Hence, realizing that if indenone 29 could be efficiently converted into indenone 41, it might be possible to obtain CP[a]A 27 in improved yield (i.e., products in the CP[b]P ring system would not also be formed), we explored this possibility (Schemes 3 and 4). Although indenone 41 has been prepared previously,24 we developed a new route for its synthesis from enol 30. LiAlH4 reduction of enol 30 formed allylic alcohol 3825 which was subsequently acetylated to form compound 39. Ozonolysis then gave α-acetoxyketone 40, and reduction of compound 40 with SmI2, using a procedure we reported previously,26,27 afforded the desired indenone 41.
Scheme 3a.
aReagents: (a) LiAlH4, THF, (88%); (b) (AcO)2O, Et3N, DMAP, CH2Cl2 (ca. 100%); (c) i: O3, MeOH/EtOAc; ii: Me2S, (ca. 100%); (d) SmI2, THF/MeOH, (85%)
Indenone 41 was then converted into CP[b]A 48 (Scheme 4) using the same sequence of reactions shown in Scheme 2 for the conversion of indenone 29 into CP[b]A 36. Carbomethoxylation of indenone 41 gave enol 42. Benzylation of enol 42 gave product 43, and decarbomethyoxylation of compound 43 gave ketone 44 as a single stereoisomer with the benzyl group assigned the more thermodynamically stable equatorial configuration. This stereochemical assignment was later confirmed (vide infra). Ketone 44 was transformed to exocyclic olefin 45 by a Wittig reaction using THF as the solvent. If the Wittig reaction was run in benzene, no desired olefin was formed and starting material was fully recovered. Olefin 45 was hydroborated using BH3•THF to afford primary alcohol 46 as essentially a single C-6 stereoisomer rather than a mixture of diastereomers at this newly formed stereocenter. Swern oxidation of alcohol 46 gave aldehyde 47 and cyclization of aldehyde 47 using 3 N HCl in MeOH yielded product 48. The stereochemistry of the groups at the C-6 stereocenter in compounds 46 and 47 was not established as this stereocenter is eliminated upon formation of product 48.
The double bond of compound 48 was reduced using Na/liq. NH3 in the presence of added aniline. The 1H NMR showed that the crude product was a mixture of diastereomers (12:1, 10aS:10aR) which was not separable by flash column chromatography. CP[b]A 49 was obtained by chromatographic purification after removal of the tert-butyl protecting group. The minor stereoisomer having the 10aR stereochemistry was not isolated in pure form or further characterized. In analogy to what we found for the Na/liq. NH3 reduction of CP[b]A 36, we expected that the double bond reduction product with the 10aR configuration (trans 4aR,10aR ring fusion) would be the major product, not the product with the 10aS configuration (cis 4aR,10aS ring fusion). In an attempt to produce the double bond reduction product with the 10aR configuration, we carried out a Pd/C catalyzed hydrogenation of compound 48. No detectable reduction product with the 10aR configuration was detected by either 1H NMR or 13C NMR. We hypothesize that a steric effect of the 11aS methyl group explains the stereochemical outcome found for both double bond reduction methods. Thus, indenone 41 could not be efficiently converted into a CP[b]A having the trans 4aR,10aR ring fusion. However, instead, indenone 41 did provide ready access to CP[b]A analogues having the cis 4aR,10aS ring fusion. Consequently, the methoxy group of compound 49 was cleaved with DIBAL–H in refluxing toluene to give CP[b]A 7. Compound 7 was then converted into its adamantyl substituted analogue 8. An X-ray structure determination for compound 7 confirmed the stereochemistry of all chiral centers.
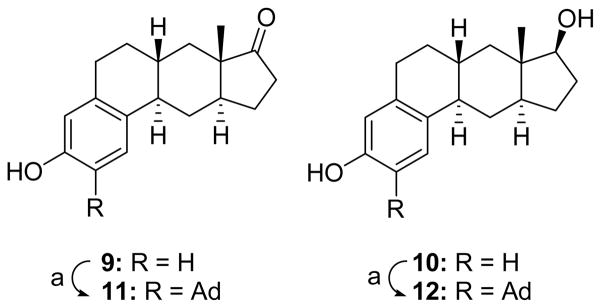
Compounds 9 and 10 in the CP[b]P ring system (Scheme 5) were prepared as described previously.20 The adamantyl groups were then added to CP[b]Ps 9 and 10 to obtain compounds 11 and 12, respectively.
Scheme 5a.
aReagents:(a) 1-adamantanol, BF3•Et2O, CH2Cl2, (11,71%), (12, 71%).
Three Fused Rings – benz[f]indenes and benz[e]indenes
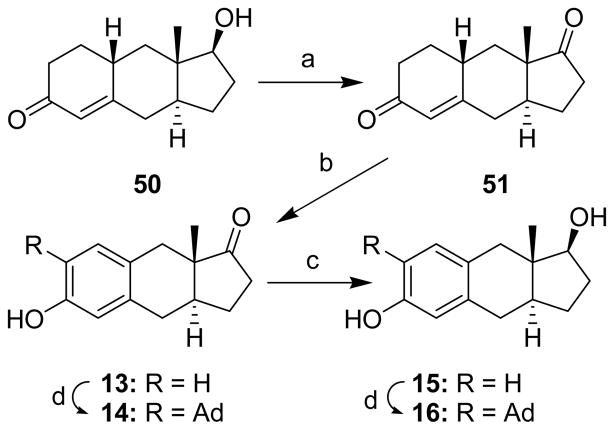
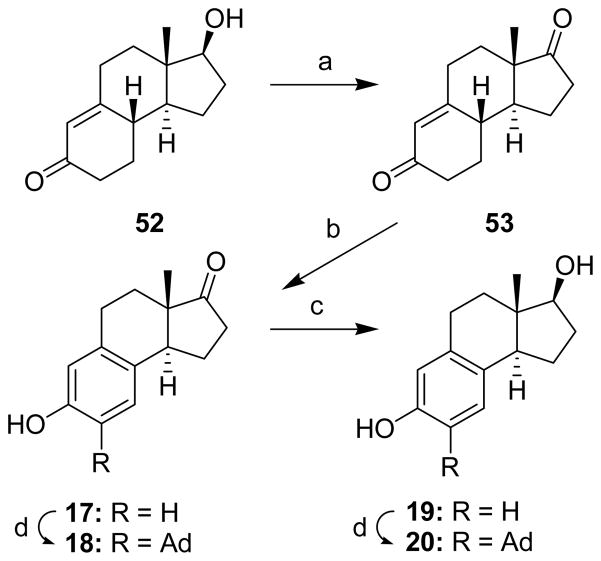
Compounds 50 (Scheme 6) and 52 (Scheme 7), the starting materials for the preparation of tricyclic compounds 13–16 and 17–20, respectively, were prepared as previously described.25,28 Each starting material was oxidized to a diketone (51 and 53, Schemes 6 and 7, respectively) before the enone ring was aromatized to yield compounds 13 and 17. The adamantyl group was then introduced to form compounds 14 and 18. Reduction of the ketone group of compounds 13 and 17 yielded compounds 15 and 19 and addition of the adamantyl group to 15 and 19 gave compounds 16 and 20, respectively.
Scheme 6a.
aReagents: (a) Jones Reagent, acetone, (97%); (b) CuBr2, LiBr, CH3CN, (74%); (c) NaBH4, EtOH, (81%); (d) 1-adamantanol, BF3•Et2O, CH2Cl2, (14, 71%), (16, 61%).
Scheme 7a.
aReagents: (a) Jones Reagent, acetone, (97%); (b) CuBr2, LiBr, CH3CN, (58%); (c) NaBH4, EtOH, (83%); (d) 1-adamantanol, BF3•Et2O, CH2Cl2, (18, 50%), (20, 56%).
Indenes connected to a phenol and a spiro ring system
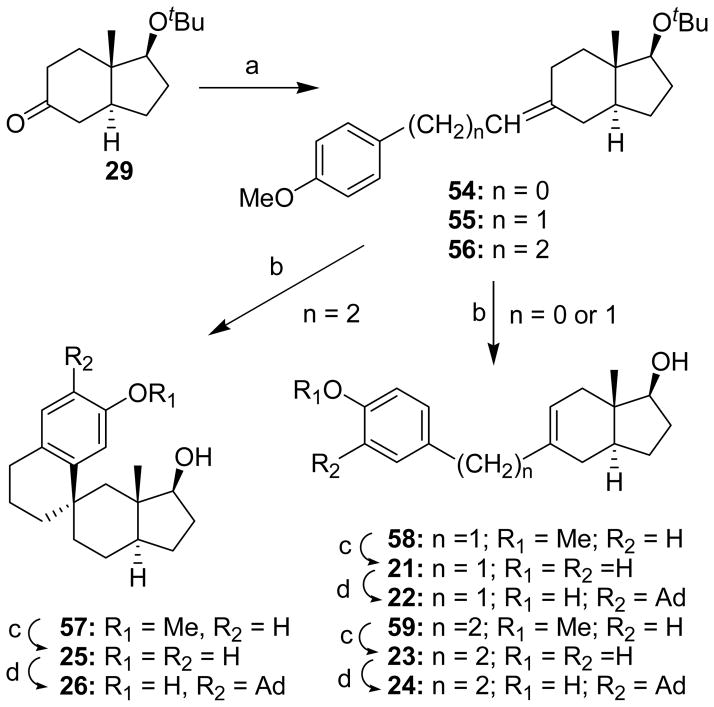
A Wittig reaction was used to connect a p-methoxybenzene group with the ketone group of indenone 29 to form compounds 54, 55 and 56 (Scheme 8). For compounds 54 and 55, the exocyclic double bond was isomerized to the endocyclic double bond using HCl/MeOH to yield compounds 58 and 59, respectively. BBr3 cleavage of the p-methoxy group yielded compounds 21 and 23. An X-ray structure determination for compound 21 established the position of the double bond. The position of the double bond in compounds 58, 59 and 23, was assigned by inference to be the same as it was in compound 21. The 13C NMR spectrum of compound 23 revealed that it consisted of two rotamers that were not interconverting at room temperature on the NMR time scale. The adamantyl group was then added to compounds 21 and 23 to obtain compounds 22 and 24, respectively.
Scheme 8a.
aReagents: (a) 54, 4-MeOC6H4CH2PPh3Br, Na, EtOH, (64%); 55, 4-MeOC6H4CH2CH2PPh3Br, NaH, THF, (80%); 56, 4-MeOC6H4CH2CH2CH2PPh3Br, NaH, THF (92%); (b) 6 N HCl, MeOH, (57, 72%), (58, 70%), (59, 89%); (c) BBr3, CH2Cl2, (21,77%); DIBAL–H, toluene, (23, 72%); DIBAL-H, toluene, (25, 94%); (d) 1-adamantanol, BF3•Et2O, CH2Cl2 (22, 79%) (24, 73%), (26, 83%).
Similar treatment of compound 56 with HCl/MeOH resulted in a ring closure reaction to form spiro compound 57 whose structure was established by an X-ray diffraction analysis. BBr3 cleavage of the p-methoxy group yielded compound 25, and subsequent addition of the adamantyl group yielded compound 26.
Biological Evaluation
The compounds were evaluated for neuroprotective activity in a cell culture model that used HT-22 hippocampal neurons (Table 1). Glutamate (3 mM) and each test compound (100 nM or 1μM) were co-incubated with the cells for 24 h and then cell viability was determined by a fluorescence assay (see Experimental for details). The neuroprotective activity of all compounds was compared to that of ZYC-26 at 1 μM.
Table 1.
Neuroprotective activity against glutamate-induced neurotoxicity for E2 and the non-steroidal analogues in cultures of the HT-22 hippocampal cell line.
| Cmpd | Ketone or Hydroxyla | No Cmpd | Cmpd @ 100 nM | Cmpd @ 1 μM | ZYC-26 @ 1 μM |
|---|---|---|---|---|---|
| % Survival from 3mM glutamate toxicity (mean±SEM)b | |||||
| Compounds Without Adamantyl Group Compared to ZYC-26 | |||||
| Four Fused Rings | |||||
| E2 | OH | 37.3 ± 1.0 | 39.7 ± 2.3 | 43.6 ± 2.3 | 99.5 ± 2.6* |
| 1 | =O | 33.2 ± 3.4 | 42.5 ± 2.9 | 40.9 ± 2.5 | 102 ± 7.7*N |
| 2 | OH | 33.2 ± 3.4 | 38.1 ± 3.0 | 34.9 ± 2.4 | 102 ± 7.7*N |
| 5 | OH | 31.8 ± 3.5 | 38.8 ± 0.5 | 38.0 ± 1.3 | 87.9 ± 5.0*N |
| 7 | OH | 30.4 ± 2.4 | 39.8 ± 3.9 | 41.5 ± 4.6 | 112 ± 10.4*L |
| 9 | =O | 34.8 ± 5.3 | 48.6 ± 4.2 | 42.2 ± 3.5 | 113 ± 6.4*N |
| 10 | OH | 34.8 ± 5.3 | 62.8 ± 5.7*N | 43.3 ± 4.8 | 113 ± 6.4*N |
| Three Fused Rings | |||||
| 13 | =O | 43.6 ± 2.7 | 41.3 ± 2.2 | 43.0 ± 1.9 | 102±6.5*N |
| 15 | OH | 43.6 ± 2.7 | 38.7 ± 2.3 | 40.3 ± 2.4 | 102±6.5*N |
| 17 | =O | 39.6 ± 1.6 | 37.9 ± 1.3 | 39.6 ± 1.4 | 96.5 ± 2.2*N |
| 19 | OH | 41.2 ± 2.1 | 46.0 ± 3.4 | 45.1 ± 2.9 | 99.1 ± 3.0* |
| Phenolic Rings Connected via Linkers to Indenes; Spiro Rings | |||||
| 21 | OH | 38.5 ± 2.1 | 34.9 ± 1.8 | 40.9 ± 1.9 | 103 ± 5.2*L |
| 23 | OH | 42.5 ± 3.6 | 43.2 ± 3.0 | 49.2 ± 5.3 | 101 ± 5.6* |
| 25 | OH | 34.2 ± 1.5 | 35.8 ± 1.4 | 34.5 ± 1.5 | 102 ± 2.8* |
| Compounds with Adamantyl Group Compared to ZYC-26 | |||||
| Four Fused Rings | |||||
| 3 | =O | 35.4 ± 1.5 | 55.1 ± 3.5*L | 117 ± 6.0*L | 121 ± 5.2*L |
| 4 | OH | 35.4 ± 1.5 | 52.9 ± 3.2*L | 108 ± 4.9*L | 121 ± 5.2*L |
| 6 | OH | 31.8 ± 3.5 | 37.5 ± 0.8 | 45.9 ± 1.5 | 87.9 ± 4.7*N |
| 8 | OH | 30.4 ± 2.4 | 55.8 ± 6.6 | 106 ± 9.3*N | 112 ± 10.4*N |
| 11 | =O | 29.3 ± 4.9 | 52.3 ± 2.9 | 105 ± 6.1*N | 127 ± 2.2*N |
| 12 | OH | 29.3 ± 4.9 | 54.1 ± 2.3 | 133 ± 2.8*N | 127 ± 2.2*N |
| Three Fused Rings | |||||
| 14 | =O | 51.4 ± 2.1 | 54.2 ± 4.8 | 55.5 ± 3.1 | 101 ± 4.6*N |
| 16 | OH | 51.4 ± 2.1 | 56.5 ± 2.8 | 99.1 ± 5.5*L | 101 ± 4.6*L |
| 18 | =O | 39.6 ± 1.6 | 39.6 ± 1.4 | 50.0 ± 1.6*N | 96.5 ± 2.2*N |
| 20 | OH | 41.2 ± 2.1 | 45.7 ±2.4 | 93.5±4.5* | 99.1 ± 3.0* |
| Phenolic Rings Connected via Linkers to Indenes; Spiro Rings | |||||
| 22 | OH | 38.5 ± 2.1 | 36.5 ± 2.0 | 61.5 ± 4.7*N | 103 ± 5.2*L |
| 24 | OH | 42.5 ± 3.6 | 49.2 ± 5.3 | 82.6 ± 4.7* | 101 ± 5.6* |
| 26 | OH | 34.2 ± 1.5 | 54.2 ± 2.1* | 105 ± 3.5* | 102 ± 2.8* |
Ketone or hydroxyl refers to the substituent on the five membered ring in the analogues.
Symbols used to identify significant comparisons indicate the following: * = statistically significant (p<0.05) difference between marked group and the No Cmpd comparison group using ANOVA; *L = statistically significant (p<0.05) difference between marked group and the No Cmpd comparison group using ANOVA applied to data that had been log-transformed to correct a violation of homogeneity of variance (as indicated by significant Bartlett’s tests); *N = statistically significant (p<0.05) difference between marked group and the NoCmpd comparison group using non-parametric testing (Kruskal-Wallis tests) applied to data that violated the normality assumption (as indicated by significant Shapiro-Wilk tests). See Statistical Analysis in Experimental section for further details.
The purpose of comparing the analogues without the adamantyl group to E2 was to determine if any of the ring systems among the different analogues was superior to the steroid ring system of E2. No ring system was superior to that of the steroid ring system. Differences in potency may be found at concentrations above 1 μM, but we did not test higher concentrations because compounds with weaker effects than those of ZYC-26 were not of interest.
To determine if any of the ring systems lacked neuroprotective activity, an adamantyl group was introduced into the analogues. For compounds with four fused rings, the effect of the adamantyl group is apparent when the following compounds are compared: 1 and 3; 2 and 4; 5 and 6; 7 and 8; 9 and 11; and 10 and 12. Only the activity of compound 6, relative to that of compound 5, failed to gain activity by introduction of the adamantyl group. At a concentration of 1 μM, compounds 3, 4, 8, 11 and 12, like ZYC-26, fully protected the neurons against death in the assay. The planarity of compounds in the CP[b]A ring system appeared less important as a determinant of activity than the position of the hydroxyl group on the aromatic ring (compare compounds 4 and 8; and compounds 4 and 6). The importance of the position of the aromatic hydroxyl group (C-3 vs. C-2) and the lack of a strict requirement for compound planarity was also noted in an earlier study of E2 analogues.10
For the compounds with three fused rings, the effect of the adamantyl group can be observed by comparing compounds 13 and 14; 15 and 16; 17 and 18; and 19 and 20. The adamantyl group increased activity more markedly when the functional group on the five-membered ring was a hydroxyl group (16 and 20) than when it was a carbonyl group (14 and 18). The neuroprotective activity of compound 14 failed to achieve statistical significance because of the high degree of neuronal death found for the no compound control. Overall, these result parallels results in the steroid ring system where the activity of compounds with a 17β-OH group are higher than those of the corresponding steroid analogues with the 17-carbonyl group.10 At a concentration of 1μM, compounds 16 and 20 were as neuroprotective as ZYC-26. We conclude that these tricyclic ring systems can be neuroprotective when properly substituted.
For the compounds in the linked and spiro ring systems, the effect of the adamantyl group can be observed by comparing compounds 21 and 22; 23 and 24; and 25 and 26. Although the adamantyl group increased the activity of compounds 21 and 23, the corresponding adamantyl analogues 22 and 24 were not as neuroprotective at 1 μM as ZYC-26 was at this concentration. This suggests that the flexibility of these two linked systems is unfavorable for high neuroprotective activity. For the spiro ring system, compounds 25 and 26 can be compared. The adamantyl group greatly increased activity. Unlike the adamantyl compounds 22 and 24 in the linked system, adamantyl spiro compound 26 is rigid and the compound at 1 μM fully protects the neurons. This occurs even though two of the rings are orthogonal to the other two rings.
Conclusions
In summary, we have reported two new synthetic routes to compounds in the CP[b]A ring systems and a spiro ring system. These compounds, in conjunction with compounds in the other ring systems evaluated, have provided new information on the role that the steroid ring system has on the neuroprotective actions of A-ring phenolic steroids.
Experimental
General Methods
Solvents were either used as purchased or dried and purified by standard methodology. Extraction solvents were dried with anhydrous Na2SO4 and, after filtration, removed on a rotary evaporator. Flash column chromatography was performed using silica gel (32–63 μm) purchased from Scientific Adsorbents (Atlanta, GA). Melting points were determined on a Kofler micro hot stage and are uncorrected. Infrared spectra were recorded as films on a NaCl plate on a Perkin–Elmer Spectrum One FT-IR spectrometer. Optical rotations were measured on a Perkin–Elmer Model 341 Polarimeter in the solvent indicated. NMR spectra were recorded on a Varian NMR spectrometer in CDCl3, acetone-d6, or DMSO-d6 at ambient temperature at 300 MHz (1H) or 75 MHz (13C). Chemical shifts are reported as δ values relative to internal chloroform (δ = 7.27) for 1H and chloroform (δ = 77.0) for 13C. Elemental analyses were performed by M-H-W Laboratories (Phoenix, AZ).
(3aS,4aS,10aR,11aS)-2,3,3a,4,4a,5,10,10a,11,11a-Decahydro-7-hydroxy-11a-methyl-1H-cyclopent[b]anthracen-1-one (1)
CuBr2 (892 mg, 4 mmol) and LiBr (209 mg, 2.4 mmol) were added to compound 27 (544 mg, 2 mmol) in CH3CN (20 mL). The reaction was stirred at room temperature and monitored by TLC. After 3 h at room temperature, the reaction was quenched with 3 N HCl (50 mL) and the products were extracted into EtOAc (50 mL × 3), dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel, eluted with 20% EtOAc in hexanes) to give compound 1 (448 mg, 83%): mp 268–270 °C; [α]D25 +164.8 (c = 0.29, DMSO); 1H NMR (300 MHz, DMSO-d6) δ 8.87 (s, br, 1H), 6.71 (d, J = 8.3 Hz, 1H), 6.40–6.34 (m, 2H), 2.61–0.82 (m, 15H), 0.74 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ 219.5, 155.0, 137.0, 129.1, 126.3, 114.5, 113.0, 47.7, 44.5, 38.9, 38.7, 36.7, 36.5, 35.2, 34.1, 31.8, 23.6, 13.2; IR (film, cm−1) 3431, 1725. Anal. Calcd for C18H22O2: C, 79.96; H, 8.20; found: C, 79.89; H, 7.96.
(1S,3aS,4aS,10aR,11aS)-2,3,3a,4,4a,5,10,10a,11,11a-Decahydro-11a-methyl-1H-cyclopent[b]anthracene-1,7-diol (2)
NaBH4 (120 mg, 3 mmol) was added to a solution of compound 1 (250 mg, 0.93 mmol) in EtOH (20 mL) at room temperature. After 1 h, 3 N HCl (20 mL) was added and the product was extracted into CH2Cl2 (50 mL × 2). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 25% EtOAc in hexanes) to give compound 2 (233 mg, 92%): mp 260–262 °C; [α]D20 +118.5 (c = 0.27, DMSO); 1H NMR (300 MHz, DMSO-d6) δ 8.90 (d, J = 1.3 Hz, 1H), 6.71 (d, J = 8.2 Hz, 1H), 6.69–6.33 (m, 2H), 4.40 (d, J = 4.9 Hz, 1H), 3.43–3.36 (m, 1H), 2.57–0.62 (m, 15H), 0.58 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ 154.9, 137.2, 129.1, 126.6, 114.5, 112.9, 80.0, 44.3, 44.1, 43.2, 38.7, 37.1, 36.8, 34.5, 32.6, 29.7, 25.2, 11.3; IR (film, cm−1) 3462, 1500, 1258. Anal. Calcd for C18H24O2: C, 79.37; H, 8.88; found: C, 79.19; H, 8.63.
(3aS,4aS,10aR,11aS)-2,3,3a,4,4a,5,10,10a,11,11a-Decahydro-8-(adamant-1-yl)-7-hydroxy-11a-methyl-1H-cyclopent[b]anthracen-1-one (3)
BF3•Et2O (1 mL) was added to a solution of compound 1 (100 mg, 0.37 mmol) and 1-adamantanol (60 mg, 0.4 mmol) in CH2Cl2 (10 mL) at 0 °C. After 30 min, the reaction was warmed to room temperature for 2 h, and then water (20 mL) was added. The product was extracted into CH2Cl2 (50 mL × 2). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 20% EtOAc in hexanes) to give compound 3 (122 mg, 81%): mp 256–258 °C; [α]D20 +110.0 (c = 0.32, CHCl3); 1H NMR (300 MHz, DMSO-d6) δ 8.74 (s, 1H), 6.58 (s, 1H), 6.32 (s, 1H), 2.50–0.86 (m, 30H), 0.73 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ 219.4, 153.6, 133.8, 133.1, 125.9, 125.8, 115.6, 47.6, 44.5, 40.1 (3 × C), 39.8, 38.7, 36.7 (3 × C), 36.0, 35.7, 35.2, 34.2, 31.9, 28.4 (3 × C), 23.6,13.2; IR (film, cm−1) 3422, 1725, 1417. Anal. Calcd for C28H36O2: C, 83.12; H, 8.97; found: C, 83.21; H, 9.09.
(1S,3aS,4aS,10aR,11aS)-2,3,3a,4,4a,5,10,10a,11,11a-Decahydro-8-(adamant-1-yl)-11a-methyl-1H-cyclopent[b]anthracene-1,7-diol (4)
Compound 4 (100 mg, 75%) was prepared from compound 2 (90 mg, 0.33 mmol) using the procedure described for the preparation of compound 3. Compound 4 had: mp 144–146 °C; [α]D20 +78.5 (c = 0.26, DMSO); 1H NMR (300 MHz, CDCl3) δ 6.89 (s, 1H), 6.39 (s, 1H), 5.17 (s, br, 1H), 3.75–3.73 (m, 1H), 2.72–0.88 (m, 31H), 0.80 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 152.3, 134.9, 134.1, 128.4, 127.0, 116.3, 81.9, 44.6, 44.3, 43.6, 40.7 (3 × C), 39.7, 37.4, 37.1 (3 × C), 36.7, 36.3, 34.9, 32.8, 30.3, 29.0 (3 × C), 25.4, 11.0; IR (film, cm−1) 3358, 1698, 1418. Anal. Calcd for C28H38O2: C, 82.71; H, 9.42; found: C, 82.59; H, 9.42.
(1S,3aS,4aS,10aR,11aS)-2,3,3a,4,4a,5,10,10a,11,11a-Decahydro-11a-methyl-1H-cyclopent[b]anthracene-1,8-diol (5)
DIBAL–H (20 mL, 1.0 M in toluene, 20 mmol) was slowly added to a solution of compound 37 (1.65 g, 5.77 mmol) in toluene (30 mL). After gas evolution ceased, the reaction was refluxed for 24 h. The reaction was slowly quenched with aqueous NH4Cl, followed by aqueous 6 N HCl until both phases became clear. The product was extracted into EtOAc (150 mL × 3). The combined extracts were dried, filtered, and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 25% EtOAc in hexanes) to give compound 5 (1.34 g, 85%): mp 192–194 °C; [α]D20 +76.0 (c = 0.35, acetone); 1H NMR (300 MHz, acetone-d6) δ 7.89 (s, 1H), 6.78 (d, J = 8.0 Hz, 1H), 6.49–6.44 (m, 2H), 3.61–3.55 (m, 2H), 2.64–1.06 (m, 15H), 0.72 (s, 3H); 13C NMR (75 MHz, acetone-d6) δ 155.2, 137.8, 129.4, 127.5, 114.8, 113.1, 81.1, 44.8 (2 × C), 43.7, 40.5, 38.3, 36.7, 34.8, 33.0, 30.1, 25.4, 10.9; IR (film, cm−1) 3400, 1501. Anal. Calcd for C18H24O2: C, 79.37; H, 8.88; found: C, 79.48; H, 8.75.
(1S,3aS,4aS,10aR,11aS)-2,3,3a,4,4a,5,10,10a,11,11a-Decahydro-7-(adamant-1-yl)-11a-methyl-1H-cyclopent[b]anthracene-1,8-diol (6)
Compound 6 (131 mg, 80%) was prepared from compound 5 (100 mg, 0.40 mmol) using the procedure described for the preparation of compound 3. Compound 6 had: mp 294–296 °C; [α]D20 +80.0 (c = 0.23, DMSO); 1H NMR (300 MHz, DMSO-d6) δ 8.71 (s, 1H), 6.58 (s, 1H), 6.31 (s, 1H), 4.39 (d, J = 4.7 Hz, 1H), 3.41–3.3.37 (m, 1H), 2.54–0.62 (m, 30H), 0.58 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ 154.1, 134.8, 133.8, 126.6, 126.5, 116.3, 80.6, 44.9, 44.8, 40.7 (3 × C), 40.4, 37.6, 37.3 (3 × C), 37.1, 36.4, 34.9, 33.3, 30.3, 29.1 (4 × C), 25.8, 11.9; IR (film, cm−1) 3347, 1715, 1614, 1264. Anal. Calcd for C28H38O2: C, 82.71; H, 9.42; found: C, 82.53; H, 9.50.
(1S,3aS,4aS,10aS,11aS)-2,3,3a,4,4a,5,10,10a,11,11a-Decahydro-11a-methyl-1H-cyclopent[b]anthracene-1,7-diol (7)
Compound 7 (1.27 g, 94%) was prepared from compound 49 (1.40 g, 4.97 mmol) using the procedure described for the preparation of compound 5. Compound 7 had: mp 122–124 °C; [α]D20 −9.5 (c = 0.44, DMSO); 1H NMR (300 MHz, DMSO-d6) δ 8.82 (s, 1H), 6.71 (d, J = 9.1 Hz, 1H), 6.39–6.30 (m, 2H), 4.38 (d, J = 4.7 Hz, 1H), 3.40–3.30 (m, 1H), 2.79 (dd, J = 16.2 Hz, 6.6 Hz, 1H), 2.59–2.22 (m, 3H), 1.92–0.88 (m, 11H), 0.58 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ 154.5, 136.6, 127.8, 127.7, 114.3, 112.0, 80.8, 44.6, 42.4, 41.6, 35.4, 34.8, 32.5, 32.1, 29.2, 29.1, 24.8, 13.3; IR (film, cm−1) 3342, 1704, 1610, 1500, 1270. Anal. Calcd for C18H24O2: C, 79.37; H, 8.88; found: C, 79.21; H, 8.82.
(1S,3aS,4aS,10aS,11aS)-2,3,3a,4,4a,5,10,10a,11,11a-Decahydro-8-(adamant-1-yl)-11a-methyl-1H-cyclopent[b]anthracene-1,7-diol (8)
Compound 8 (121 mg, 85%) was prepared from compound 7 (95 mg, 0.35 mmol) using the procedure described for the preparation of compound 3. Compound 8 had: mp 130–132 °C; [α]D20 +9.0 (c = 0.20, acetone); 1H NMR (300 MHz, acetone-d6) δ 7.74 (s, 1H), 6.72 (s, 1H), 6.43 (s, 1H), 3.54–3.52 (m, 2H), 2.86–0.79 (m, 30H), 0.76 (s, 3H); 13C NMR (75 MHz, acetone-d6) δ 153.7, 134.1, 133.5, 128.5, 125.9, 116.2, 82.5, 46.1, 43.2, 42.7, 40.7 (3 × C), 37.2 (3 × C), 36.3, 35.4, 33.9, 33.0, 30.0, 29.9, 29.3 (3 × C), 28.3, 25.6, 13.9; IR (film, cm−1) 3347, 1715, 1614, 1264. Anal. Calcd for C28H38O2: C, 82.71; H, 9.42; found: C, 82.53; H, 9.30.
(6aR, 7aS, 10aS, 11aR) -5, 6, 6a, 7, 7a, 9, 10, 10a, 11, 11a-Decahydro-3-hydroxy-7a-methyl-8H-cyclopenta[b] phenanthren-8-one (9)
Compound 9 was prepared as described previously.20
(6aR, 7aS, 8S, 10aS, 11aR) -6, 6a, 7, 7a, 8, 9, 10, 10a, 11, 11a-Decahydro-7a-methyl-5H-cyclopenta[b] phenanthrene-3, 8-diol (10)
Compound 10 was prepared as described previously.20
(6aR, 7aS, 10aS, 11aR) -5, 6, 6a, 7, 7a, 9, 10, 10a, 11, 11a-Decahydro-2-(adamant-1-yl)- 3-hydroxy-7a-methyl-8H-cyclopenta[b] phenanthren-8-one (11)
Compound 11 (95 mg, 71%) was prepared from compound 9 (95 mg, 0.35 mmol) using the procedure described for the preparation of compound 3. Compound 11 had: mp 268–270 °C; [α]D20 −9.5 (c = 0.20, CHCl3); 1H NMR (300 MHz, CDCl3/acetone-d6) δ 7.15 (s, 1H), 6.43 (s, 1H), 5.35 (s, 1H), 2.85–1.15 (m, 30H), 0.91 (s, 3H); 13C NMR (75 MHz, CDCl3/acetone-d6) δ 220.5, 153.4, 134.8, 133.7, 130.5, 123.2, 116.6, 48.0, 45.9, 44.4, 40.5 (3 × C), 39.1, 37.1 (3 × C), 36.6, 35.7, 35.6, 30.9, 30.4, 29.6, 29.1 (3 × C), 24.0, 13.6; IR (film, cm−1) 1734, 1413. Anal. Calcd for C28H36O2: C, 83.12; H, 8.97; found: C, 82.99; H, 8.77.
(6aR, 7aS, 8S, 10aS, 11aR) -6, 6a, 7, 7a, 8, 9, 10, 10a, 11, 11a-Decahydro-2-(adamant-1-yl)-7a-methyl-5H-cyclopenta[b] phenanthrene-3, 8-diol (12)
Compound 12 (95 mg, 71%) was prepared from compound 10 (90 mg, 0.33 mmol) using the procedure described for the preparation of compound 3. Compound 12 had: mp 248–250 °C; [α]D20 −42.9 (c = 0.21, DMSO); 1H NMR (300 MHz, CDCl3/acetone-d6) δ 7.02 (s, 1H), 6.94 (s, 1H), 6.38 (s, 1H), 3.66–3.61 (m, 1H), 2.72–0.79 (m, 31H), 0.69 (s, 3H); 13C NMR (75 MHz, CDCl3/acetone-d6) δ 153.2, 134.9, 133.6, 131.2, 123.2, 116.5, 81.5, 45.4, 44.7, 44.4, 43.3, 40.5 (3 × C), 37.1 (3 × C), 36.6, 35.9, 31.2, 30.9, 30.4, 29.9, 29.1 (3 × C), 25.5, 11.2; IR (film, cm−1) 3364, 1412. Anal. Calcd for C28H38O2: C, 82.71; H, 9.42; found: C, 82.82; H, 9.25.
(3aS,9aS)-2,3,3a,4,9,9a-Hexahydro-6-hydroxy-9a-methyl-1H-benz[f]inden-1-one (13)
Compound 13 (751 mg, 74%) was prepared from compound 51 (980 mg, 4.5 mmol) using the procedure described for the preparation of compound 1. Compound 13 had: mp 268–270 °C; [α]D25 +205.0 (c = 1.05, CHCl3); 1H NMR (CDCl3) δ 7.00 (d, J = 8.0 Hz, 1H), 6.70–6.66 (m, 2H), 6.02 (s, 1H), 2.95–1.69 (m, 9H), 0.88 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 222.6, 154.0, 136.8, 131.8, 126.8, 115.8, 114.0, 47.4, 41.9, 36.7, 36.4, 31.6, 24.5, 13.5; IR (film, cm−1) 3391, 1723. Anal. Calcd for C14H16O2: C, 77.75; H, 7.46; found: C, 77.75; H, 7.61.
(3aS,9aS)-2,3,3a,4,9,9a-Hexahydro-7- (adamant-1-yl)-6-hydroxy-9a-methyl-1H-benz[f]inden-1-one (14)
Compound 14 (175 mg, 71%) was prepared from compound 13 (150 mg, 0.7 mmol) using the procedure described for the preparation of compound 3. Compound 14 had: mp 225–227 °C; [α]D20 +115.0 (c = 0.1, CHCl3); 1H NMR (300 MHz, DMSO-d6) δ 8.83 (s, 1H), 6.67 (s, 1H), 6.41 (s, 1H), 3.24 (s, 2H), 2.69–1.52 (m, 22H), 0.64 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ 220.9, 154.4, 134.4, 133.6, 128.4, 124.9, 117.2, 47.2, 41.7, 40.5 (3 × C), 37.3 (3 × C), 37.1, 36.4, 36.3, 30.9, 29.0 (3 × C), 24.5, 13.7; IR (film, cm−1) 3400, 1651, 1050. Anal. Calcd for C24H30O2: C, 82.24; H, 8.63; found: C, 82.38; H, 8.54.
(1S,3aS,9aS)-2,3,3a,4,9,9a-Hexahydro-9a-methyl-1H-benz[f]indene-1,6-diol (15)
Compound 15 (264 mg, 81%) was prepared from compound 13 (324 mg, 1.5 mmol) using the procedure described for the preparation and of compound 2. Compound 15 had: mp 112–113 °C; [α]D20 +86.6 (c = 0.58, CHCl3); 1H NMR (300 MHz, acetone-d6) δ 7.94 (s, 1H), 6.80 (d, J = 8.0 Hz, 1H), 6.53–6.50 (m, 2H), 3.88 (d, J = 8.0 Hz, 1H), 3.77–3.71 (m, 1H), 2.66–1.29 (m, 9H), 0.63 (s, 3H); 13C NMR (75 MHz, acetone-d6) δ 155.9, 138.0, 131.6, 128.0, 116.4, 114.2, 81.7, 43.4, 42.9, 42.0, 32.9, 31.2, 26.3, 10.4; IR (film, cm−1) 3402, 1045. Anal. Calcd for C14H18O2: C, 77.03; H, 8.31; found: C, 76.83; H, 8.13.
(1S,3aS,9aS)-2,3,3a,4,9,9a-Hexahydro-7-(adamant-1-yl)-9a-methyl-1H-benz[f]indene-1,6-diol (16)
Compound 16 (150 mg, 61%) was prepared from compound 15 (150 mg, 0.7 mmol) using the procedure described for the preparation of compound 3. Compound 16 had: mp 217–219 °C; [α]D20 +55.0 (c = 0.32, CHCl3); 1H NMR (300 MHz, CDCl3) δ 6.90 (s, 1H), 6.43 (s, 1H), 6.36 (s, 1H), 3.93 (t, J = 8.3 Hz, 1H), 2.80–1.26 (m, 25H), 0.76 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 152.6, 134.6, 134.2, 128.6, 127.7, 117.1, 82.0, 42.8, 42.2, 41.3, 40.9 (3 × C), 37.3 (3 × C), 36.5, 31.4, 30.9, 29.3 (3 × C), 25.7, 10.1; IR (film, cm−1) 3324, 1042; Anal. Calcd for C24H32O2: C, 81.77; H, 9.15; found: C, 81.89; H, 9.15.
(3aS, 9bS) -1, 2, 3a, 4, 5, 9b-Hexahydro-7-hydroxy-3a-methyl-3H-benz[e] inden-3-one (17)
Known compound 1729,30 (500 mg, 58%) was prepared from compound 53 (877 mg, 3.9 mmol) using the procedure described for the preparation and purification of compound 1. Compound 17 had: mp 78–80 °C; 1H NMR (CDCl3) δ 7.00 (d, J = 8.8 Hz, 1H), 6.68–6.66 (m, 2H), 4.91 (s, 1H), 2.94–1.74 (m, 9H), 0.73 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 220.8, 154.3, 138.3, 130.3, 126.4, 115.4, 113.0, 48.2, 46.1, 36.7, 29.0, 26.7, 21.6, 14.1.
(3aS, 9bS) -1, 2, 3a, 4, 5, 9b-Hexahydro-8-(adamant-1-yl)-7-hydroxy-3a-methyl-3H-benz[e] inden-3-one (18)
Compound 18 (70 mg, 50%) was prepared from compound 17 (86 mg, 0.4 mmol) using the procedure described for the preparation of compound 3. Compound 18 had: mp 210–212 °C; [α]D20 +45.8 (c = 0.45, CHCl3); 1H NMR (300 MHz, CDCl3) δ 6.93 (s, 1H), 6.47 (s, 1H), 4.70 (s, 1H), 2.95–1.24 (m, 24H), 0.76 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 221.3, 153.2, 134.6, 134.3, 129.3, 123.6, 116.8, 48.2, 46.4, 40.8 (3 × C), 37.3 (3 × C), 36.7, 36.6, 29.3 (3 × C), 29.1, 25.9, 21.7, 13.8; IR (film, cm−1) 3400, 1725, 1415. Anal. Calcd for C24H30O2: C, 82.24; H, 8.63; found: C, 82.20; H, 8.45.
(3S, 3aS, 9bS)-2, 3, 3a, 4, 5, 9b-Hexahydro-3a-methyl-1H-benz[e] indene-3, 7-diol (19)
Known compound 1931 (90 mg, 83%): was prepared from compound 17 (108 mg, 0.5 mmol) using the procedure described for the preparation of compound 2. Compound 19 had: mp 74–76 °C; 1H NMR (300 MHz, CDCl3) δ 6.86 (d, J = 8.3 Hz, 1H), 6.62–6.33 (m, 2H), 5.29 (s, br, 1H), 3.93–3.87 (m, 1H), 2.90–1.23 (m, 10H), 0.64 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 153.8, 137.8, 131.8, 126.8, 115.2, 122.7, 81.3, 45.8, 43.5, 33.9, 31.4, 27.0, 23.3, 10.7.
(3S, 3aS, 9bS)-2, 3, 3a, 4, 5, 9b-Hexahydro-8-(adamant-1-yl)- 3a-methyl-1H-benz[e]indene-3, 7-diol (20)
Compound 20 (78 mg, 56%) was prepared from compound 19 (85 mg, 0.4 mmol) using the procedure described for the preparation of compound 3. Compound 20 had: mp 221–223 °C; [α]D20 −22.0 (c = 0.10, CHCl3); 1H NMR (300 MHz, CDCl3) δ 6.80 (s, 1H), 6.45 (s, 1H), 4.75 (s, 1H), 3.92 (t, J = 7.2 Hz, 1H), 2.85–1.24 (m, 25H), 0.67 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 152.4, 134.4, 133.9, 131.5, 124.2, 116.7, 81.3, 46.1, 43.5, 41.0 (3 × C), 37.3 (3 × C), 36.6, 34.0, 31.5, 29.3 (3 × C), 26.3, 23.4, 10.7; IR (film, cm−1) 3400, 1598; Anal. Calcd for C24H32O2: C, 81.77; H, 9.15; found: C, 81.68; H, 9.05.
(1S,3aS,7aS)-2,3,3a,4,7,7a-Hexahydro-5-(4-hydroxybenzyl)-7a-methyl-1H-inden-1-ol (21)
BBr3 in CH2Cl2 (8 mL) was added to compound 58 (194 mg, 0.72 mmol) dissolved in CH2Cl2 (8 mL) at −78 °C. After 30 min, the reaction mixture was warmed to room temperature for 2 h, and then CH3OH (3 mL) and water (20 mL) were added. The product was extracted into CH2Cl2 (100 mL × 2). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 15% EtOAc in hexanes) to give compound 21 (143 mg, 77%): mp 165–166 °C; [α]D20 +66.4 (c = 0.28, acetone); 1H NMR (300 MHz, CDCl3) δ 8.11 (s, 1H), 6.93 (d, J = 8.5 Hz, 2H), 6.69 (d, J = 8.5 Hz, 2H), 5.36 (s, 1H), 3.70–3.64 (m, 2H), 2.03–1.10 (m, 13H), 0.61 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 155.8, 137.2, 131.2, 129.7 (2 × C), 121.7, 115.2 (2 × C), 81.0, 43.3, 41.7, 41.3, 38.5, 31.0, 30.4, 25.5, 9.9; IR (film, cm−1) 3335, 1510, 1236. Anal. Calcd for C18H24O2: C, 79.03; H, 8.58; found: C, 78.98; H, 8.80.
(1S,3aS,7aS)-2,3,3a,4,7,7a-Hexahydro-5-[3-(adamantan-1-yl)-4-hydroxybenzyl]-7a-methyl-1H-inden-1-ol (22)
Compound 22 (84 mg, 79%) was prepared from compound 21 (70 mg, 0.27 mmol) using the procedure described for the preparation of compound 3. The 1H and 13C NMR spectra of compound 22 were consistent with it being a 2.5:1 mixture of rotamers. Crystals of the compound melted at two different temperatures. Compound 22 had: mp 218–220 °C and 227–229 °C; major rotamer 1H NMR (300 MHz, CDCl3) δ 8.89 (s, 1H), 6.75 (s, 1H), 6.65 (d, J = 6.0 Hz, 1H), 6.56 (d, J = 6.0 Hz, 1H), 5.30 (s, 1H), 4.41 (d, J = 4.7 Hz, 1H), 3.49 (t, J = 8.5 Hz, 1H), 3.28 (s, 1H), 2.08–0.76 (m, 25H), 0.48 (s, 3H); major rotamer 13C NMR (75 MHz, CDCl3) δ 154.7, 154.5, 137.4, 135.7, 135.6, 131.3, 130.4, 126.8, 126.7, 122.0, 116.7, 80.4, 45.1, 43.9, 43.5, 43.2, 41.8, 41.3, 41.0, 40.6, 38.8, 37.3, 36.7, 32.6, 31.5, 30.7, 29.1, 25.8, 11.1, 10.9; IR (film, cm−1) 3282, 1417, 1276. Anal. Calcd for C27H38O2: C, 82.61; H, 9.24; found: C, 82.77; H, 9.43.
(1S,3aS,7aS)-2,3,3a,4,7,7a-Hexahydro-5-(4-hydroxyphenethyl)-7a-methyl-1H-inden-1-ol (23)
Compound 23 (102 mg, 72%) was prepared from compound 59 (150 mg, 0.53 mmol) using the procedure described for the preparation of compound 21. Compound 23 had: mp 95–98 °C; [α]D20 +78.2 (c = 0.50, acetone); 1H NMR (300 MHz, CDCl3) δ 7.05 (d, J = 8.2 Hz, 2H), 6.80 (d, J = 8.2 Hz, 2H), 5.34–5.33 (m, 1H), 3.83 (t, J = 8.5 Hz, 1H), 2.73–1.26 (m, 15H), 0.72 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 154.1, 136.6, 134.2, 129.4 (2 × C), 120.5, 115.4 (2 × C), 82.1, 41.6, 41.2, 39.8, 38.2, 33.8, 31.6, 30.6, 25.6, 10.2; IR (film, cm−1) 3339, 1514. Anal. Calcd for C18H24O2: C, 79.37; H, 8.88; found: C, 79.15; H, 9.03.
(1S,3aS,7aS)-2,3,3a,4,7,7a-Hexahydro-5-[3-(adamantan-1-yl)-4-hydroxyphenethyl]-7a-methyl-1H-inden-1-ol (24)
Compound 24 (65 mg, 73%) was prepared from compound 23 (60 mg, 0.22 mmol) using the procedure described for the preparation of compound 3. Compound 24 had: mp 210–212°C; [α]D20 +19.1 (c = 0.32, acetone); 1H NMR (300 MHz, DMSO-d6) δ 8.83 (s, 1H), 6.74 (s, 1H), 6.70 (d, J = 9.4 Hz, 1H), 6.56 (d, J = 9.4 Hz, 1H), 5.16–5.14 (m, 1H), 4.41 (d, J = 4.7 Hz, 1H), 3.48–3.30 (m, 1H), 3.28 (s, 1H), 2.48–1.06 (m, 27H), 0.46 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ 154.5, 136.8, 135.5, 132.3, 126.8, 126.4, 121.1, 116.8, 80.6, 41.7, 41.0, 40.7 (3 × C), 39.9, 38.8, 37.3 (3 × C), 36.6, 34.2, 31.8, 30.7, 29.1 (3 × C), 25.9, 10.9; IR (film, cm−1) 3368, 1720. Anal. Calcd for C28H38O2: C, 82.71; H, 9.42; found: C, 82.34; H, 9.19.
(1S,3aS,4aR,7aS,)-1,2,3,3a,3′,4,4′,6,7,7a-Decahydro-7a-methyl-2′H-spiro[indene-5,1′-naphthalene]-1,7′-diol (25)
DIBAL–H (1.0 M in toluene, 3.6 mL, 3.6 mmol) was added to a solution of compound 57 (180 mg, 0.6 mmol) in toluene (15 mL) at room temperature and then the reaction was refluxed for 16 h. After cooling to room temperature, water (2 mL) and 6 N HCl (10 mL) were added and the product was extracted into CH2Cl2 (100 mL × 2). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 25% EtOAc in hexanes) to give compound 25 (160 mg, 94%): mp 161–163°C; [α]D20 +34.9 (c = 0.18, CHCl3); 1H NMR (300 MHz, CDCl3) δ 8.84 (s, 1H), 6.73 (s, 1H), 6.70 (d, J = 7.9 Hz, 1H), 6.39 (d, J = 7.9 Hz, 1H), 4.43 (d, J = 4.1 Hz, 1H), 3.50–3.48 (m, 1H), 2.45–1.06 (m, 17H), 0.75 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 160.6, 152.3, 134.7, 132.3, 118.6, 118.2, 85.2, 47.9, 45.2, 45.4, 43.4, 39.8, 38.5, 38.0, 35.2, 35.1, 30.8, 24.8, 15.5; IR (film, cm−1) 3436, 1494, 1228. Anal. Calcd for C19H26O2: C, 79.68; H, 9.15; found: C, 79.48; H, 8.97.
(1S,3aS,4aR,7aS)-1,2,3,3a,3′,4,4′,6,7,7a-Decahydro-6′-(adamantan-1-yl)-7a-methyl-2′H-spiro[indene-5,1′-naphthalene]-1,7′-diol (26)
Compound 26 (86 mg, 83%) was prepared from compound 25 (70 mg, 0.245 mmol) using the procedure described for the preparation of compound 3. Compound 26 had: mp 228–230°C; [α]D20 +25.8 (c = 0.36, acetone); mp 228–230°C; 1H NMR (300 MHz, CDCl3) δ 7.83 (s, 1H), 6.83 (s, 1H), 6.68 (s, 1H), 3.67–3.60 (m, 1H), 2.87–1.13 (m, 33H), 0.84 (s, 3H); 13C NMR (75 MHz, acetone-d6) δ 154.1, 144.3, 133.6, 127.6, 126.7, 114.8, 81.0, 43.1, 40.6 (3 × C), 40.2, 39.8, 38.0, 37.2 (3 × C), 36.2, 34.8, 33.5, 33.2, 30.4, 30.2, 29.3 (3 × C), 25.8, 20.0, 9.78; IR (film, cm−1) 3306, 1508, 1233. Anal. Calcd for C29H40O2: C, 82.81; H, 9.59; found: C, 82.66; H, 9.18.
(1S, 3aS, 4aS, 9aR, 10aR, 11aS) -1, 2, 3, 3a, 4, 4a, 5, 8, 9, 9a, 10, 10a, 11, 11a-Tetradecahydro-1-hydroxy-11a-methyl-7H-Cyclopent[b] anthracen-7-one (27)
Compound 27 was prepared as described previously.13
(3aS,4aS,9aR,10aR,11aS)-2,3,3a,4a,5,8,9,9a,10,10a,11,11a-Dodecahydro-11a-methyl-1H-cyclopenta[b]anthracene-1,7(4H)-dione (28)
Jones reagent was added at 0 °C to a solution of compound 27 (548 mg, 2 mmol) dissolved in acetone (50 mL) until the color of excess reagent persisted. After 15 min, 2-propanol (1 mL) was added to consume excess reagent. Brine (50 mL) was then added and the product was extracted into EtOAc (100 mL × 2). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) to give product 28 (544 mg, ~100%): 1H NMR (300 MHz, CDCl3) δ 5.71 (s, 1H), 2.40–0.86 (m, 20H), 0.83 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 219.7, 199.6, 165.6, 124.3, 47.8, 45.1, 43.9, 41.9, 41.0, 38.0, 37.5, 36.8, 36.4, 35.4, 31.9, 28.8, 23.5, 13.4; HRMS (ESI) calcd for [C18H24O2+Na]+: 295.1669, found: 295.1672.
(1S, 3aS, 7aS) -1- (1, 1-Dimethylethoxy)octahydro-7a-methyl-5H-inden-5-one (29)
Optically pure (>99% ee as determined by optical rotation measurement) compound 29 was prepared as described previously.17
(3S,3aS,7aS)-3-(1,1-Dimethylethoxy)-2,3,3a,4,7,7a-hexahydro-6-hydroxy-3a-methyl-1H-indene-5-carboxylic acid, methyl ester (30)
Compound 30 (13.1 g, 93%) was prepared from compound 29 (13.5 g, 150 mmol) as described previously.25 Compound 30 had: 1H NMR (300 MHz, CDCl3) δ 12.30 (s, 1H), 3.75 (s, 3H), 3.58 (t, J = 7.6 Hz, 1H), 2.38–1.24 (m, 9H), 1.17 (s, 9H), 0.72 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 173.7, 172.2, 96.8, 80.0, 72.4, 51.3, 41.5, 39.8, 35.0, 32.1, 31.4, 28.8 (3 × C), 25.6, 10.5.
(3S,3aS,7aS)-3-(1,1-Dimethylethoxy)octahydro-5-(3-methoxybenzyl)-3a-methyl-6-oxo-1H-indene-5-carboxylic acid, methyl ester (31)
NaH (800 mg, 60% in mineral oil, 20 mmol) was added to a solution of compound 30 (4.48 g, 20 mmol) in DMF/toluene (40 mL/120 mL) at room temperature and the resulting mixture was stirred until gas evolution ceased (ca. 30 min). 3-Methoxybenzyl bromide (3.6 g, 20 mmol) was added and the reaction was refluxed for 2 h. The reaction was cooled to room temperature, aqueous NH4Cl was added, and the product was extracted into EtOAc (100 mL × 3). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) to give product 31 as an unresolved mixture of two diastereomers due to the formation of the new chiral center at C-5 (6.83 g, 85%). The major diastereomer had: 1H NMR (300 MHz, CDCl3) δ 7.16 (t, J = 7.1 Hz, 1H), 6.78–6.70 (m, 3H), 3.76 (s, 3H), 3.63 (s, 3H), 3.33 (t, J = 7.1 Hz, 1H), 3.19 (d, J = 13.4 Hz, 1H), 3.01 (d, J = 13.4 Hz, 1H), 2.56–2.35 (m, 3H), 1.70–1.15 (m, 6H), 1.12 (s, 9H), 0.82 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 207.5, 173.8, 159.4, 138.5, 128.9, 123.7, 116.6, 112.7, 80.4, 72.8, 59.4, 55.2, 52.5, 45.6, 45.2, 42.5, 42.4, 41.9, 31.5, 28.7 (3 × C), 25.7, 11.6; IR (film, cm−1) 1711, 1600. Anal. Calcd for C24H34O5: C, 71.61; H, 8.51; found: C, 71.42; H, 8.27.
(1S,3aS,6R,7aS)-1-(1,1-Dimethylethoxy)octahydro-6-(3-methoxybenzyl)-7a-methyl-5H-inden-5-one (32)
LiCl (2.17 g, 51 mmol) was added to a solution of compound 31 (6.80 g, 17.0 mmol) in dry DMF (85 mL) at room temperature. The reaction was refluxed for 14 h. The reaction was quenched with aqueous NH4Cl and the product extracted into EtOAc (100 mL × 3). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) to give product 32 (5.26 g, 90%): [α]D20 +22.0 (c = 0.20, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.20 (t, J = 8.0 Hz, 1H), 6.74–6.70 (m, 3H), 3.78 (s, 3H), 3.43 (t, J = 7.4 Hz, 1H), 3.30 (dd, J = 13.7 Hz, 5.0 Hz, 1H), 2.64–1.38 (m, 11H), 1.10 (s, 9H), 0.94 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 211.8, 159.6, 142.2, 129.3, 121.6, 115.0, 111.2, 79.2, 72.2, 55.1, 53.7, 51.1, 47.2, 43.5, 35.5, 32.1, 31.1, 28.6 (3 × C), 24.9, 11.9; IR (film, cm−1) 1708, 1602, 1362. Anal. Calcd for C22H32O3: C, 76.70; H, 9.36; found: C, 76.52; H, 9.15.
(1S,3aS,6R,7aS)-1-(1,1-Dimethylethoxy)octahydro-6-(3-methoxybenzyl)-7a-methyl-5-methylene-1H-indene (33)
NaH (1.5 g, 37.5 mmol) was added to a suspension of methyltriphenylphosphonium bromide (13.39 g, 37.5 mmol) in benzene (100 mL) at room temperature and then refluxed for 30 min. Compound 32 (5.24 g, 15.2 mmol) in benzene (30 mL) was added. After 3 h, the reaction was quenched by the addition of aqueous NH4Cl and the product was extracted into EtOAc (100 mL × 3). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) to give product 33 (4.89 g, 94%): 1H NMR (300 MHz, CDCl3) δ 7.21 (t, J = 7.7 Hz, 1H), 6.76–6.71 (m, 3H), 4.77 (d, J = 23.1 Hz, 1H), 3.79 (s, 3H), 3.36 (t, J = 7.4 Hz, 1H), 3.13 (dd, J = 12.7 Hz, 4.1 Hz, 1H), 2.47–1.31 (m, 12H), 1.06 (s, 9H), 0.75 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 159.7, 153.3, 143.2, 129.2, 121.7, 114.8, 111.4, 106.3, 80.5, 72.4, 55.3, 47.4, 44.5, 43.2, 39.5, 38.9, 37.2, 31.8, 28.8 (3 × C), 25.8, 11.5; IR (film, cm−1) 1639, 1602, 1487; MS (ESI) for [C23H34O2+H]+: 343.2, found: 343.3.
(1S,3aS,6R,7aS)-1-(1,1-Dimethylethoxy)octahydro-6-(3-methoxybenzyl)-7a-methyl-1H-indene-5-methanol (34)
BH3•THF complex (15 mL, 1.0 M in THF, 15 mmol) was added to a solution of compound 33 (4.86 g, 14.2 mmol) in THF (100 mL) at 0 °C. After 30 min, the reaction was allowed to warm to room temperature. After an additional 1 h, the reaction was quenched with aqueous 3 N NaOH (40 ml) and 30% H2O2 (20 mL). The mixture was stirred at room temperature for 1 h and the product was extracted into EtOAc (100 mL × 3). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 20% EtOAc in hexanes) to give product 34 (4.22 g, 82%) as a mixture of C-5 diastereomers (ratio ~4.5:1). The major isomer had: 1H NMR (300 MHz, CDCl3) δ 7.19 (t, J = 7.7 Hz, 1H), 6.74–6.70 (m, 3H), 3.80–3.73 (m, 1H), 3.78 (s, 3H), 3.66–3.60 (m, 1H), 3.37 (t, J = 8.5 Hz, 1H), 2.62–1.11 (m, 14H), 1.09 (s, 9H), 0.94 (s, 3H); 13C NMR (CDCl3) δ 159.8, 143.0, 129.3, 121.4, 114.8, 111.2, 80.9, 72.3, 60.4, 55.3, 43.3, 40.4, 39.8, 39.0, 36.8, 31.4, 28.9 (3 × C), 28.8, 26.6, 25.9, 11.4; IR (film, cm−1) 3349, 1601, 1259; MS (ESI) for [C23H36O3+NH4]+: 378.3, found: 378.3.
(1S,3aS,6R,7aS)-1-(1,1-Dimethylethoxy)octahydro-6-(3-methoxybenzyl)-7a-methyl-1H-indene-5-carboxyaldehyde (35)
DMSO (1.13 g, 14.4 mmol) in CH2Cl2 (20 mL) was added to a solution of oxalyl chloride (1.69 g, 13.3 mmol) in CH2Cl2 (100 mL) at −78 °C. After 10 min, diastereomeric compound 34 (4.20 g, 11.7 mmol) in CH2Cl2 (40 mL) was added and the reaction was stirred at −78 °C for 1 h. Et3N (3.43 g, 34 mmol) was then added in one portion at −78 °C. After 30 min, the reaction was warmed to room temperature for 1 h. The reaction was quenched with water (40 mL) and the product was extracted into EtOAc (100 mL × 3). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) to give product 35 (4.19 g, 100%): 1H NMR (300 MHz, CDCl3) δ 9.68 (s, 1H), 7.13 (t, J = 7.7 Hz, 1H), 6.67–6.61 (m, 3H), 3.70 (s, 3H), 3.36 (t, J = 7.4 Hz, 1H), 2.88 (dd, J = 13.5 Hz, 9.3 Hz, 1H), 2.68–1.14 (m, 12H), 1.04 (s, 9H), 0.68 (s, 3H); 13C NMR (CDCl3) δ 205.1, 159.8, 142.7, 129.5, 121.4, 114.7, 111.4, 80.5, 72.4, 55.2, 49.4, 43.2, 41.4, 4.10, 39.7, 36.8, 31.3, 28.8 (3 × C), 25.8, 25.4, 11.3.
(3S,3aS,4aR,11aS)-3-(1,1-Dimethylethoxy)-2,3,3a,4,4a,5,11,11a-octahydro-7-methoxy-3a-methyl-1H-cyclopent[b]anthracene (36)
3 N HCl (50 ml) was added to a solution of compound 35 (4.15 g, 11.5 mmol) in MeOH (100 mL) at 0 °C. After 30 min, the reaction was warmed to room temperature. The reaction was complete after 1 h at room temperature. The product was extracted into EtOAc (100 ml × 3). The combined extracts were washed with aqueous NaHCO3, brine, dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) to give product 36 (3.28 g, 84%): [α]D20 −8.3 (c = 0.12, CHCl3); 1H NMR (300 MHz, CDCl3) δ 6.89–6.86 (m, 1H), 6.66–6.64 (m, 2H), 6.13 (s, 1H), 3.76 (s, 3H), 3.44 (t, J = 7.7 Hz, 1H), 2.76–1.22 (m, 12H), 1.15 (s, 9H), 0.77 (s, 3H); 13C NMR (CDCl3) δ 158.2, 140.5, 136.6, 128.2, 126.0, 122.3, 113.4, 111.1, 80.5, 72.4, 55.4, 45.6, 44.4, 43.2, 37.6, 34.4, 32.8, 31.5, 29.0 (3 × C), 26.0, 10.9; IR (film, cm−1) 1608, 1500, 1253; MS (ESI) for [C23H32O2+NH4]+: 358.3, found: 358.2.
(1S,3aS,4aS,10aR,11aS)-2,3,3a,4,4a,5,10,10a,11,11a-Decahydro-8-methoxy-11a-methyl-1H-cyclopent[b]anthracen-1-ol (37)
Na metal (1.04 g, 45 mmol) was added to an oven dried flask under N2. The flask was equipped with a Dry Ice cooled condenser and anhydrous NH3 (100 mL) was condensed in the flask. Aniline (6 ml) was then added at −78 °C. After 30 min, compound 36 (3.25 g, 9.5 mmol) in THF (40 mL) was added. The reaction was stirred for 2 h, and then quenched with solid NH4Cl until the blue color disappeared. The flask was allowed to warm up to room temperature for 14 h to allow NH3 to evaporate. Aqueous NH4Cl was added and the product was extracted into EtOAc (100 ml × 3). The combined extracts were dried, filtered and the solvents removed. The 1H NMR showed that the crude product was a mixture of diastereomers (4:1, 4aS:4aR). The unseparated diastereomeric 4aS:4aR products were dissolved in aqueous 6 N HCl in MeOH (100 mL) and refluxed 14 h. After cooling to room temperature, the product was extracted into EtOAc (100 mL × 3). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 20% EtOAc in hexanes). Purification removed the minor diastereomer (not characterized) yielding isolated pure product 37 (1.68 g, 62%, 2 steps): [α]D20 +116.5 (c = 0.17, CHCl3); 1H NMR (300 MHz, CDCl3) δ 6.92 (d, J = 8.3 Hz, 1H), 6.63–6.54 (m, 2H), 3.70 (s, 3H), 3.64 (t, J = 8.2 Hz, 1H), 2.72–1.04 (m, 15H), 0.86 (t, J = 7.7 Hz, 1H), 0.74 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 157.6, 138.0, 129.6, 129.0, 113.4, 112.0, 81.9, 55.3, 44.7, 44.5, 43.7, 40.2, 38.5, 36.7, 34.6, 32.9, 30.5, 25.5, 11.2; IR (film, cm−1) 3401, 1614, 1503. Anal. Calcd for C19H26O2: C, 79.68; H, 9.15; found: C, 79.46; H, 9.08.
(1S,3aS,7aS)-1-(1,1-Dimethylethoxy)octahydro-7a-methyl-6-methylene-1H-inden-5-ol (38)
Compound 38 (4.19 g, 88%) was prepared from compound 30 (5.64 g, 20 mmol) as described previously.25 Compound 38 had: 1H NMR (300 MHz, CDCl3) δ 5.01 (d, J = 1.4 Hz, 1H), 4.72 (d, J = 1.4 Hz, 1H), 3.94–3.89 (m, 1H), 3.41 (t, J = 7.4 Hz, 1H), 2.84 (s, br, 1H), 2.26–1.08 (m, 9H), 1.07 (s, 9H), 0.59 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 148.6, 107.3, 79.1, 72.5, 72.1, 45.0, 43.9, 43.1, 35.5, 31.6, 28.6 (3 × C), 24.8, 10.7; IR (film, cm−1) 3357, 1651, 1362; MS (ESI) for [C15H26O2+Na]+: 261.2, found: 261.2.
Acetic acid, (1S,3aS,7aS)-1-(1,1-dimethylethoxy)octahydro-7a-methyl-6-methylene-1H-inden-5-yl ester (39)
Ac2O (3.57 g, 35 mmol), Et3N (5.35 g, 52.5 mmol), and DMAP (108 mg, 0.88 mmol) were added to a solution of compound 38 (4.15 g, 17.4 mmol) in CH2Cl2 (80 mL) at room temperature. After 10 min, water (30 mL) was added and the product was extracted into CH2Cl2 (50 mL × 2). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography to afford product 39 (4.87 g, ca. 100%): 1H NMR (300 MHz, CDCl3) δ 5.15–5.10 (m, 1H), 4.83 (d, J = 27.2 Hz, 2H), 3.46 (t, J = 8.0 Hz, 1H), 2.31 (d, J = 12.9 Hz, 1H), 2.08 (s, 3H), 2.03–1.15 (m, 8H), 1.10 (s, 9H), 0.65 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 170.0, 143.7, 107.9, 79.0, 73.9, 72.2, 45.2, 43.8, 42.8, 32.0, 31.6, 28.5 (3 × C), 24.8, 21.0, 10.8; IR (film, cm−1) 1741, 1652, 1464, 1362; HRMS (ESI) calcd for [C17H28O3+Na]+: 303.1931, found: 303.1936.
Acetic acid, (1S,3aS,7aS)-1-(1,1-dimethylethoxy)octahydro-7a-methyl-6-oxo-1H-inden-5-yl ester (40)
A solution of compound 39 (4.85 g, 17.3 mmol) in MeOH (100 mL) and EtOAc (10 ml) was treated with ozone at −78 °C until a purple color persisted (ca. 30 min). Oxygen was passed through the solution for 20 min until the purple color disappeared, Me2S (10 mL) was added and the reaction was allowed to warm to room temperature for 14 h. The solvents were removed under reduced pressure and the residue was purified by flash column chromatography (silica gel eluted with 20% EtOAc in hexanes) to give product 40 (4.88 g, 17.3 mmol, ~100%): 1H NMR (300 MHz, CDCl3) δ 5.08 (t, J = 7.2 Hz, 1H), 3.56 (t, J = 8.7 Hz, 1H), 2.38 (d, J = 13.2 Hz, 1H), 2.13 (d, J = 13.2 Hz, 1H), 2.05 (s, 3H), 2.38–1.31 (m, 7 H), 1.03 (s, 9H), 0.63 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 204.0, 169.9, 78.7, 75.8, 72.6, 51.3, 47.0, 41.7, 32.2, 30.8, 28.5 (3 × C), 2.45, 20.7, 11.6; MS (ESI) for [C16H26O4+H]+: 283.2, found: 283.2.
(1S,3aS,7aS)-3-(1,1-Dimethylethoxy)octahydro-3a-methyl-5H-inden-5-one (41)
Iodine (12.7 g, 50.0 mmol) in dry THF (150 mL) was added to Sm metal filings (7.80 g, 52 mmol) by cannula under N2. The reaction was stirred at room temperature for 1 h forming a deep blue solution. Compound 40 (4.86 g, 17.2 mmol) in dry THF (75 mL) and MeOH (5 mL) was added to the SmI2-THF solution and the reaction was stirred for 30 min. The reaction was poured into 20% aqueous Na2CO3 (300 mL) and the product was extracted into EtOAc (100 mL × 3). The combined extracts were washed with water (30 mL), brine (50 mL), dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) to afford product 41 (3.27 g, 85%): [α]D20 +75.4 (c = 0.54, CHCl3); 1H NMR (300 MHz, CDCl3) δ 3.49 (t, J = 8.5 Hz, 1H), 2.25–1.19 (m, 11H), 0.96 (s, 9H), 0.54 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 212.0, 79.2, 72.4, 53.7, 46.4, 43.2, 40.6, 32.1, 28.6 (3 × C), 25.1, 24.8, 11.8; IR (film, cm−1) 1654, 1441, 1218. Anal. Calcd for C14H24O2: C, 74.95; H, 10.78; found: C, 75.01; H, 10.87.
(1S,3aS,7aS)-1-(1,1-Dimethylethoxy)-2,3,3a,4,7,7a-hexahydro-6-hydroxy-7a-methyl-1H-indene-5-carboxylic acid, methyl ester (42)
Dimethyl carbonate (3.48 g, 43.5 mmol) was added to a suspension of NaH (1.16 g, 29 mmol) in THF (100 mL) at room temperature. The reaction was then refluxed for 30 min. Compound 41 (3.25 g, 14.5 mmol) in THF (50 mL) was added and the reaction was refluxed for 14 h. After cooling to room temperature, the reaction was slowly quenched with acetic acid until pH 4–5 and water was added (50 ml). The product was extracted into EtOAc (100 mL × 3). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) to give product 42 (3.97 g, 97%): [α]D20 +73.8 (c = 0.80, CHCl3); 1H NMR (300 MHz, CDCl3) δ 12.17 (s, 1H), 3.63 (s, 3H), 3.44 (t, J = 7.6 Hz, 1H), 2.36–1.20 (m, 9H), 1.03 (s, 9H), 0.62 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 173.2, 172.0, 96.8, 80.3, 72.5, 51.4, 42.8, 41.9, 40.5, 31.6, 28.8 (3 × C), 25.7, 24.8, 11.2; IR (film, cm−1) 1654, 1609, 1361, 1218; MS (ESI) for [C16H26O4+Na]+: 305.2, found: 305.2.
(1S,3aS,7aS)-1-(1,1-Dimethylethoxy)octahydro-5-(3-methoxybenzyl)-7a-methyl-6-oxo-1H-indene-5-carboxylic acid, methyl ester (43)
Compound 43 (4.38 g, 81%) was prepared from compound 42 (3.95 g, 14.0 mmol) using the procedure described for the preparation of compound 31 as an unresolved mixture of two diastereomers due to the formation of the new chiral center at C-5. The major diastereomer of compound 43 had: 1H NMR (300 MHz, CDCl3) δ 7.11–7.06 (m, 1H), 6.86–6.60 (m, 3H), 3.67 (s, 3H), 3.66 (s, 3H), 3.54–3.46 (m, 1H), 3.27 (d, J = 13.7 Hz, 1H), 3.00 (d, J = 13.7 Hz, 1H), 2.38–1.01 (m, 9H), 1.04 (s, 9H), 0.71 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 209.2, 172.7, 159.6, 137.7, 129.3, 122.9, 116.3, 112.4, 79.3, 72.6, 62.2, 55.1, 52.3, 51.6, 46.5, 38.7, 38.6, 31.9, 30.6, 28.7 (3 × C), 25.0, 12.1; IR (film, cm−1) 1704, 1601, 1263; MS (ESI) for [C24H34O5+H]+: 403.3, found: 403.3.
(3S,3aS,6S,7aS)-3-(1,1-Dimethylethoxy)octahydro-6-(3-methoxybenzyl)-3a-methyl-5H-inden-5-one (44)
Compound 44 (3.58 g, 92%) was prepared from compound 43 (4.35 g, 11.3 mmol) using the procedure described for the preparation and purification of compound 32. Compound 44 had: [α]D20 +12.8 (c = 0.545, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.11 (t, J = 7.7 Hz, 1H), 6.64–6.20 (m, 3H), 3.68 (s, 3H), 3.53 (t, J = 8.0 Hz, 1H), 3.20 (d, J = 9.6 Hz, 1H), 2.38–1.10 (m, 11H), 1.03 (s, 9H), 0.57 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 211.8, 159.6, 142.2, 129.3, 121.6, 114.9, 111.2, 79.2, 72.5, 55.1, 53.7, 51.1, 47.1, 43.5, 35.5, 32.1, 31.1, 28.6 (3 × C), 25.0, 11.9; IR (film, cm−1) 1705, 1602, 1256. Anal. Calcd for C22H32O3: C, 76.70; H, 9.36; found: C, 76.90; H, 9.18.
(1S,3aS,5S,7aS)-1-(1,1-Dimethylethoxy)octahydro-5-(3-methoxybenzyl)-7a-methyl-6-methylene-1H-indene (45)
Compound 45 (3.45 g, 98%) was prepared from compound 44 (3.55 g, 10.3 mmol) using the procedure described for the preparation of compound 33 except that the reaction solvent was THF instead of benzene. Compound 45 had: 1H NMR (300 MHz, CDCl3) δ 7.12 (t, J = 7.7 Hz, 1H), 6.68–6.60 (m, 3H), 4.74 (d, J = 13.5 Hz, 1H), 3.69 (s, 3H), 3.39 (t, J = 8.2 Hz, 1H), 3.08 (dd, J = 13.2 Hz, 4.1 Hz, 1H), 2.37–1.10 (m, 8H), 1.06 (s, 9H), 1.05–0.75 (m, 4H), 0.55 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 159.7, 150.4, 143.2, 129.3, 121.7, 115.1, 111.0, 108.5, 80.0, 72.4, 55.2, 48.7, 45.1, 44.6, 44.1, 39.1, 32.0, 29.9, 28.9 (3 × C), 25.6, 11.2; MS (ESI) for [C23H34O2+H]+: 343.3, found: 343.3.
(3S,3aS,6S,7aS)-3-(1,1-Dimethylethoxy)octahydro-6-(3-methoxybenzyl)-3a-methyl-1H-indene-5-methanol (46)
Compound 46 (3.06 g, 86%) was prepared from compound 45 (3.42 g, 10.0 mmol) using the procedure described for the preparation of compound 34. Compound 46 had: [α]D20 +16.0 (c = 0.43, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.21–7.15 (m, 1H), 6.77–6.70 (m, 3H), 3.89–3.83 (m, 1H), 3.78 (s, 3H), 3.68–3.62 (m, 1H), 3.35 (t, J = 7.4 Hz, 1H), 2.83 (dd, J = 13.7 Hz, 5.0 Hz, 1H), 2.46–2.39 (m, 1H), 2.06–1.14 (m, 12H), 1.11 (s, 9H), 0.72 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 159.7, 143.3, 129.2, 121.6, 115.0, 111.0, 81.7, 72.3, 64.1, 55.2, 46.3, 43.5, 42.3, 41.3, 40.1 (2 × C), 31.2, 28.9 (3 × C), 27.8, 25.9, 13.4; IR (film, cm−1) 3543, 1600, 1258; MS (ESI) for [C23H36O3+NH4]+: 378.3, found: 378.2.
(3S,3aS,6S,7aS)-3-(1,1-Dimethylethoxy)octahydro-6-(3-methoxybenzyl)-3a-methyl-1H-indene-5-carboxaldehyde (47)
Compound 47 (3.01 g, ~100%) was prepared from compound 46 (3.04 g, 8.4 mmol) using the procedure described for the preparation of compound 35. Compound 47 had: 1H NMR (300 MHz, CDCl3) δ 9.81 (s, 1H), 7.12 (t, J = 7.4 Hz, 1H), 6.66–6.60 (m, 3H), 3.69 (s, 3H), 3.33 (t, J = 6.9 Hz, 1H), 2.98–2.75 (m, 2H), 1.96–1.10 (m, 11H), 1.04 (s, 9H), 0.53 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 205.3, 159.7, 142.8, 129.4, 121.4, 114.8, 111.3, 80.8, 72.4, 55.2, 49.3, 45.8, 42.9, 42.7, 39.6, 39.5, 31.2, 29.1, 28.8 (3 × C), 25.9, 13.1.
(1S,3aS,4aS,11aS)-1-(1,1-Dimethylethoxy)-2,3,3a,4,4a,5,11,11a-octahydro-7-methoxy-11a-methyl-1H-cyclopent[b]anthracene (48)
Compound 48 (2.42 g, 85%) was prepared from compound 47 (3.00 g, 8.4 mmol) using the procedure described for the preparation of compound 36. Compound 48 had: [α]D20 +33.3 (c = 0.70, CHCl3); 1H NMR (300 MHz, CDCl3) δ 6.89–6.86 (m, 1H), 6.65–6.64 (m, 2H), 6.13 (s, 1H), 3.77 (s, 3H), 3.50 (t, J = 8.0 Hz, 1H), 2.84–2.38 (m, 4H), 1.96–1.25 (m, 8H), 1.16 (s, 9H), 0.72 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 158.2, 139.6, 136.3, 128.3, 126.0, 123.5, 113.4, 111.1, 80.0, 72.4, 55.4, 46.4, 43.8, 43.6, 37.1, 36.1, 34.5, 31.5, 28.9 (3 × C), 25.8, 11.5; IR (film, cm−1) 1609, 1251. Anal. Calcd for C23H32O2: C, 81.13; H, 9.47; found: C, 81.30; H, 9.34.
(1S,3aS,4aS,10aS,11aS)-2,3,3a,4,4a,5,10,10a,11,11a-Decahydro-7-methoxy-11a-methyl-1H-cyclopent[b]anthracen-1-ol (49)
Na metal (805 mg, 35 mmol) was added to an oven dried flask under N2. The flask was equipped with a Dry Ice cooled condenser and anhydrous NH3 (100 mL) was condensed into the flask. Aniline (5 ml) was then added at −78 °C. After 30 min, compound 48 (2.41 g, 7.1, mmol) in THF (50 mL) was added. The reaction was stirred for 2 h, and then was quenched with solid NH4Cl until the blue color disappeared. The mixture was allowed to warm to room temperature for 14 h, aqueous NH4Cl was added and the product was extracted into EtOAc (100 mL × 3). The combined extracts were dried, filtered, and the solvents removed. The 1H NMR of the crude product showed the diastereomeric 10aS:10aR products in a 12:1 ratio.
The unseparated 10aS:10aR diastereomers were dissolved in MeOH (50 mL) and aqueous 6 N HCl (25 mL) was added at room temperature. The reaction was refluxed for 3 h. After cooling to room temperature, the product was extracted into EtOAc (100 mL × 3). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 20% EtOAc in hexanes) to give compound 49 (1.42 g, 70%, 2 steps): mp 88–90 °C; [α]D20 −8.7 (c = 0.46, CHCl3); 1H NMR (300 MHz, CDCl3) δ 6.92 (d, J = 8.0 Hz, 1H), 6.64–6.59 (m, 2H), 3.97 (s, 3H), 3.63 (t, J = 7.7 Hz, 1H), 2.99 (dd, J = 16.2 Hz, 6.6 Hz, 1H), 2.73–2.42 (m, 3H), 2.15–1.10 (m, 12H), 0.76 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 157.6, 137.7, 130.4, 128.7, 113.7, 111.3, 83.0, 55.2, 45.8, 42.9, 42.5, 36.5, 35.8, 33.2, 32.9, 30.3, 29.9, 25.6, 13.8; IR (film, cm−1) 3390, 1610, 1502, 1257. Anal. Calcd for C19H26O2: C, 79.68; H, 9.15; found: C, 79.79; H, 8.98.
(1S, 3aS, 8aR, 9aS) -1, 2, 3, 3a, 4, 7, 8, 8a, 9, 9a-Decahydro-1-hydroxy-9a-methyl-6H-benz[f] inden-6-one (50)
Compound 50 was prepared as described previously.25
(3aS,8aR,9aS)-2,3,3a,4,7,8,8a,9-Octahydro-9a-methyl-3H-benz[f]indene-1,6(3aH)-dione (51)
Compound 51 (980 mg, 97%) was prepared from compound 50 (1.02 g, 4.6 mmol) using the procedure described for the preparation of compound 28. Compound 51 had: 1H NMR (300 MHz, CDCl3) δ 5.79 (s, 1H), 2.53–1.04 (m, 14H), 0.95 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 218.6, 199.4, 164.1, 126.5, 47.7, 44.7, 38.9, 37.3, 35.7, 35.2, 33.9, 30.2, 23.8, 12.9.
(3S, 3aS, 9aS, 9bS) -1, 2, 3, 3a, 4, 5, 8, 9, 9a, 9b-Decahydro-3-hydroxy-3a-methyl-7H-benz[e] inden-7-one (52)
Compound 52 was prepared as described previously.28
(3aS, 9aS, 9bS) -1, 2, 4, 5, 8, 9, 9a, 9b-Octahydro-3a-methyl-3H-benz[e] indene-3, 7(3aH) -dione (53)
Compound 53 (877 mg, 97%) was prepared from compound 52 (900 mg, 4 mmol) using the procedure described for the preparation and purification of compound 28. Compound 53 had: 1H NMR (300 MHz, CDCl3) δ 5.80 (s, 1H), 2.51–1.13 (m, 14H), 0.95 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 218.9, 199.0, 164.4, 125.9, 50.6, 47.6, 37.6, 36.6, 35.8, 30.8, 30.7, 26.7, 21.9, 13.0.
(1S,3aS,7aS)-1-(1,1-Dimethylethoxy)octahydro-5-(4-methoxybenzylidene)-7a-methyl-1H-indene (54)
Na metal (92 mg, 4.0 mmol) was dissolved in EtOH (30 mL) and then 1-(1-methylenetriphenylphosphine)-4-methoxybenzene bromide (1.85 g, 4.0 mmol) was added and the reaction was refluxed for 1 h. Compound 29 (448 mg, 2.0 mmoL) in THF (10 mL) was then added. After 16 h at reflux, the reaction mixture was cooled to room temperature, water was added (30 mL) and the product was extracted into EtOAc (100 mL × 2). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) to give product 54 (420 mg, E and Z mixture 1/1, 64%): 1H NMR (300 MHz, CDCl3) δ 7.19–7.05 (m, 2H), 6.78 (d, J = 8.2 Hz, 2H), 6.14 (s, 1H), 3.74 (s, 3H), 3.34 (t, J = 7.4 Hz, 1H), 2.72–0.84 (m, 11H), 1.07 (s, 9H), 0.80 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 157.9, 141.5, 141.4, 131.3, 131.1, 130.3, 122.9, 122.7, 113.7, 80.6, 80.5, 72.4, 55.4, 46.3, 45.6, 42.8, 42.7, 38.3, 37.7, 37.6, 33.0, 31.6, 31.4, 29.9, 29.7, 28.9, 26.1, 24.7, 10.6.
(1S,3aS,7aS)-1-(1,1-Dimethylethoxy)octahydro-5-[2-(4-methoxyphenyl)ethylidene]-7a-methyl-1H-indene (55)
NaH (80 mg, 60% in mineral oil, 2.0 mmol) suspended in THF was added to a suspension of 1-(2-ethyltriphenylphosphine)-4-methoxybenzene iodide (1.03 g, 2.0 mmol) in THF (20 mL) at room temperature. The mixture was refluxed for 1 h and then compound 29 (224 mg, 1.0 mmoL) in THF (5 mL) was added while reflux was maintained. After 1 h, the reaction was cooled to room temperature, water was added (30 mL) and the product was extracted into EtOAc (100 mL × 2). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) to give product 55 (274 mg, E and Z mixture 1/1, 80%): 1H NMR (300 MHz, CDCl3) δ 7.01 (dd, J = 1.6 Hz, 8.5 Hz, 2H), 6.75 (dd, J = 1.6 Hz, 8.5 Hz, 2H), 5.22–5.19 (m, 1H), 3.67 (s, 3H), 3.33 (t, J = 6.9 Hz, 1H), 3.27–3.20 (m, 2H), 2.49–0.73 (m, 11H), 1.05 (s, 9H), 0.75 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 157.95, 139.7, 134.0, 133.8, 129.3, 128.9, 128.7, 121.7, 121.5, 114.0, 80.7, 80.6, 72.3, 55.4, 46.4, 45.8, 43.0, 38.4, 37.8, 37.1, 33.0, 32.8, 32.5, 31.6, 31.5, 29.9, 28.9, 28.6, 26.2, 26.0, 23.9, 10.6, 10.5.
(1S,3aS,7aS)-1-(1,1-Dimethylethoxy)octahydro-5-[3-(4-methoxyphenyl)propylidene]-7a-methyl-1H-indene (56)
NaH (80 mg, 60% in mineral oil, 2.0 mmol) suspended in THF was added to a suspension of 1-(3-propyltriphenylphosphine)-4-methoxybenzene iodide (900 mg, 2.0 mmol) in THF (20 mL) at room temperature. The mixture was refluxed for 1 h and then compound 29 (224 mg, 1.0 mmoL) in THF (5 mL) was added while reflux was maintained. After 1 h, the reaction mixture was cooled to room temperature, water was added (30 mL) and the product was extracted into EtOAc (100 mL × 2). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) to give product 56 (328 mg, E and Z mixture 1/1, 92%): 1H NMR (300 MHz, CDCl3) δ 6.99 (d, J = 8.2 Hz, 2H), 6.72 (d, J = 8.2 Hz, 2H), 5.06 (t, J = 6.6 Hz, 1H), 3.67 (s, 3H), 3.25 (t, J = 7.7 Hz, 1H), 2.56–0.72 (m, 15H), 1.03 (s, 9H), 0.69 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 157.8, 139.8, 139.6, 134.4, 129.5, 129.4, 129.3, 128.7, 128.6, 128.5, 121.8, 121.5, 113.8, 113.7, 80.5, 80.4, 79.5, 72.5, 72.1, 55.2, 46.3, 45.3, 44.7, 43.0, 42.8, 42.1, 38.4, 37.4, 37.0, 35.6, 35.3, 32.5, 31.9, 31.5, 31.4, 29.8, 29.7, 29.5, 28.8, 28.7, 28.5, 26.0, 25.9, 23.7, 10.4, 10.3.
(1S,3aS,4aR,7aS)-1,2,3,3a,3′,4,4′,6,7,7a-Decahydro-7′-methoxy-7a-methyl-2′H-spiro[indene-5,1′-naphthalen]-1-ol (57)
6 N HCl (20 mL) was added to a solution of compound 56 (356 mg, 0.92 mmol) in MeOH (20 mL) at room temperature. The reaction was refluxed for 16 h and then was cooled to room temperature. The product was extracted into CH2Cl2 (100 mL × 2). The combined extracts were dried, filtered and the solvents removed. The residue was purified by flash column chromatography (silica gel eluted with 15% EtOAc in hexanes) to give product 57 (200 mg, 72%): 1H NMR (300 MHz, CDCl3) δ 7.03 (d, J = 8.5 Hz, 2H), 6.87 (dd, J = 2.7 Hz, 8.5 Hz, 1H), 3.83 (s, 3H), 3.78 (t, J = 7.7 Hz, 1H), 2.72–2.69 (m, 1H), 2.20–1.34 (m, 17H), 0.98 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 157.8, 147.6, 129.8, 113.3, 110.7, 81.8, 55.3, 43.0, 39.9, 39.8, 38.8, 34.7, 33.3, 33.0, 30.5, 30.3, 25.8, 19.8, 10.2; HRMS (ESI) calcd for [C20H28O2+Na]+: 323.1982, found: 323.1982.
(1S,3aS,7aS)-2,3,3a,4,7,7a-Hexahydro-5-(4-methoxybenzyl)-7a-methyl-1H-inden-1-ol (58)
Compound 58 (241 mg, 70%) was prepared from compound 54 (420 mg, 1.28 mmol) using the procedure described for the preparation of compound 57. Compound 58 had: 1H NMR (300 MHz, CDCl3) δ 7.16 (d, J = 8.5 Hz, 2H), 6.87 (d, J = 8.5 Hz, 2H), 5.41–5.40 (m, 1H), 3.78 (s, 3H), 3.76 (t, J = 7.7 Hz, 1H), 3.21 (s, 1H), 2.16–1.23 (m, 11H), 0.65 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 158.1, 137.0, 132.6, 129.8 (2 × C), 121.9, 113.8 (2 × C), 81.9, 55.4, 43.5, 41.7, 41.3, 38.4, 31.2, 30.9, 25.6, 10.3; HRMS (ESI) calcd for [C18H24O2+Na]+: 295.1669, found: 295.1672.
(1S,3aS,7aS)-2,3,3a,4,7,7a-Hexahydro-5-(4-methoxyphenethyl)-7a-methyl-1H-inden-1-ol (59)
Compound 59 (150 mg, 89%) was prepared from compound 55 (200 mg, 0.6 mmol) using the procedure described for the preparation of compound 57. Compound 59 had: 1H NMR (300 MHz, CDCl3) δ 7.09 (d, J = 8.5 Hz, 2H), 6.83 (d, J = 8.5 Hz, 2H), 5.34–5.32 (m, 1H), 3.76 (s, 3H), 3.72 (t, J = 7.8 Hz, 1H), 2.68–1.24 (m, 14H), 0.68 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 157.8, 136.6, 134.6, 129.3 (2 × C), 120.6, 114.0, 113.8 (2 × C), 81.9, 55.3, 41.2, 39.8, 38.3, 33.8, 31.7, 30.8, 25.7, 10.1.
Cell culture conditions
HT-22 cells are a murine hippocampal cell line donated by David Schubert, Salk Institute, San Diego, CA. HT-22 cells were maintained in Dulbecco’s modified Eagle’s (DMEM) media (GIBCO, Gaithersburg, PA) supplemented with 10% charcoal-stripped fetal bovine serum (HyClone, Logan, UT) and 20 Ag/ml gentamicin (Sigma, St. Louis, MO) under standard cell culture conditions (5% CO2, 95% air, 37 8C). HT-22 cells were seeded into Costar 96-well plates (Corning, NY) at a density of 5,000 cells per well.
Compound neuroprotection evaluation
Compounds were administered simultaneously with the glutamate insults. HT-22 cells were incubated with 3mM glutamate for 24 h and then assessed for viability. Glutamate-induced cell death in HT-22 cells is through inhibition of the glutamate/cysteine antiporter leading to the depletion of glutathione and thus oxidative stress.32 For each compound tested, glutamate toxicity was assessed in the absence of compound, or in the presence of 100nM or 1 μM compound. As a positive control, ZYC-26, a compound that is potently neuroprotective,11 was tested at 1μM in the assay for each compound.
Cell viability determination
Cell viability was determined by calcein acetoxymethyl (AM) assay (Molecular Probes, Eugene, OR). The calcein AM assay measures cellular esterase activity and plasma membrane integrity. For complete cell death, several wells received MeOH for 15 min before calcein AM assay. Wells were rinsed with phosphate-buffered saline (PBS), after which a 2.5 AM solution of calcein AM in PBS was added. After incubation at room temperature for 15 min, fluorescence was determined (excitation 485 nm, emission 530 nm) using a fluorescence FL600 microplate reader (Biotek, Winooski, VT). We have shown a strong linear relationship between calcein fluorescence (relative fluorescence units; RFU) and cell number for HT-22 cells (range 1000 to 10,000 cells per well).
Statistical Analysis
Data from each compound represent the mean ± SEM of at least 8 independent experiments. Data were analyzed with GraphPad Prism 5.0 (La Jolla, CA) using one-way analysis of variance (ANOVA) with p <0.05 as the threshold for statistical significance. Because our interest was in comparing each of the three treatment conditions to the No Cmpd control group, we followed up significant ANOVAs with Dunnett’s post hoc test for pairwise comparisons to control for the inflated Type I error rate associated with multiple two-group comparisons. Bartlett’s tests were used to identify statistically significant (p<0.05) or marginal (0.05<p<0.1) violations of homogeneity of variance in the ANOVA tests conducted for each compound of interest. An important consequence to violations of this crucial assumption of ANOVA is an inflation of the Type I error rate, meaning an increased risk of detecting a significant effect in the sample when one does not actually exist in the population.33 In the event such violations were detected for a given compound, data were log transformed and the ANOVA/post-hoc tests were interpreted using the transformed data, provided the violation of the homogeneity of variance was corrected by the transformation. If the log transformation failed to correct the homogeneity of variance violation, non-parametric testing using the Kruskal-Wallis test, which does not rely as heavily on the assumptions underlying ANOVA, was used. As well, using the Shapiro-Wilk test, all data for each compound, even log-transformed data, were assessed for adherence to the normality assumption of ANOVA.
Supplementary Material
Acknowledgments
The Pharmacological assessment of the compounds was completed at the University of North Texas Health Science Center. The data analysis and statistical assessment was done at West Virginia University. This work was supported by NIH Grants P20 GM109098, P01 AG022550, P01 AG027956 and U54 GM1049492 to JWS; and The Taylor Family Institute for Innovative Psychiatric Research to DFC. The X-ray structure determinations were made possible by NSF Shared Instrument Grant No. CHE-042097. Support for the Washington University in St. Louis Mass Spectrometry Resource is provided by NIH grant P41 GM103422.
Footnotes
Electronic supplementary information (ESI) available: 1H NMR and 13C NMR data. Crystal data for compounds 7, 21, 37 and 57, CCDC reference numbers: 1451350, 1497517, 1451351 and 1497516, respectively.
Notes and references
- 1.Bishop J, Simpkins JW. Mol Cell Neurosci. 1994;5:303. doi: 10.1006/mcne.1994.1036. [DOI] [PubMed] [Google Scholar]
- 2.Behl C, Widmann M, Trapp T, Holsboer F. Biochem Biophys Res Commun. 1995;216:473. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- 3.Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton CJ, Holsboer F. Mol Pharmacol. 1997;51:535. [PubMed] [Google Scholar]
- 4.Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. J Neurosurg. 1997;87:724. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 5.Sawada H, Ibi M, Kihara T, Urushitani M, Akaike A, Shimohama S. J Neurosci Res. 1998;54:707. doi: 10.1002/(SICI)1097-4547(19981201)54:5<707::AID-JNR16>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 6.Goodman Y, Bruce AJ, Cheng B, Mattson MP. J Neurochem. 1996;66:1836. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 7.Moosmann B, Behl C. Proc Natl Acad Sci U S A. 1999;96:8867. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Xu W, Jiang H. Anesth Analg. 2001;92:1520. doi: 10.1097/00000539-200106000-00033. [DOI] [PubMed] [Google Scholar]
- 9.Culmsee C, Vedder H, Ravati A, Junker V, Otto D, Ahlemeyer B, Krieg JC, Krieglstein J. J Cereb Blood Flow Metab. 1999;19:1263. doi: 10.1097/00004647-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Perez E, Cai ZY, Covey DF, Simpkins JW. Drug Dev Res. 2005;66:78. [Google Scholar]
- 11.Perez E, Liu R, Yang SH, Cai ZY, Covey DF, Simpkins JW. Brain Res. 2005;1038:216. doi: 10.1016/j.brainres.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Gatson JW, Liu MM, Abdelfattah K, Wigginton JG, Smith S, Wolf S, Simpkins JW, Minei JP. J Neurotrauma. 2012;29:2209. doi: 10.1089/neu.2011.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niki E, Nakano M. Methods Enzymol. 1990;186:330. doi: 10.1016/0076-6879(90)86126-g. [DOI] [PubMed] [Google Scholar]
- 14.Lacort M, Leal AM, Liza M, Martin C, Martinez R, Ruizlarrea MB. Lipids. 1995;30:141. doi: 10.1007/BF02538267. [DOI] [PubMed] [Google Scholar]
- 15.Cegelski L, Rice CV, O’Connor RD, Caruano AL, Tochtrop GP, Cai ZY, Covey DF, Schaefer J. Drug Dev Res. 2005;66:93. [Google Scholar]
- 16.Jastrzebska I, Scaglione JB, DeKoster GT, Rath NP, Covey DF. J Org Chem. 2007;72:4837. doi: 10.1021/jo070530e. [DOI] [PubMed] [Google Scholar]
- 17.Hajos ZG, Parrish DR. Org Synth. 1984;64:26. [Google Scholar]
- 18.Martin R, Buchwald SL. Acc Chem Res. 2008;41:1461. doi: 10.1021/ar800036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins DJ, Fallon GD, Skene CE. Aust J Chem. 1992;45:71. [Google Scholar]
- 20.Qian M, Covey DF. Adv Synth Catal. 2010;352:2057. doi: 10.1002/adsc.201000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeyaraj DA, Kapoor KK, Yadav VK, Gauniyal HM, Parvez M. J Org Chem. 1998;63:287. [Google Scholar]
- 22.Krapcho AP. Synthesis. 1982:805. [Google Scholar]
- 23.Kutney JP, Winter J, McCrae W, By A. Can J Chem. 1963;41:470. [Google Scholar]
- 24.Tietze LF, Ramachandar T, Voch C. Synlett. 2003:118. [Google Scholar]
- 25.Scaglione J, Rath NP, Covey DF. J Org Chem. 2005;70:1089. doi: 10.1021/jo048561m. [DOI] [PubMed] [Google Scholar]
- 26.Qian M, Krishnan K, Kudova E, Li P, Manion BD, Taylor A, Elias G, Akk G, Evers AS, Zorumski CF, Mennerick S, Covey DF. J Med Chem. 2014;57:171. doi: 10.1021/jm401577c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Rath NP, Covey DF. Tetrahedron. 2007;63:7977. doi: 10.1016/j.tet.2007.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Y, Zorumski CF, Covey DF. J Org Chem. 1995;60:3619. [Google Scholar]
- 29.Matsuya Y, Masuda S, Itoh T, Murai T, Nemoto H. J Org Chem. 2005;70:6898. doi: 10.1021/jo051060w. [DOI] [PubMed] [Google Scholar]
- 30.Komeno T, Ishihara S, Itani H. Tetrahedron. 1972;28:4719. doi: 10.1016/s0040-4020(01)98273-0. [DOI] [PubMed] [Google Scholar]
- 31.Morales-Alanis H, Brienne MJ, Jacques J, Bouton MM, Nedelec L, Torelli V, Tournemine C. J Med Chem. 1985;28:1796. doi: 10.1021/jm00150a009. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Maher P, Schubert D. Proc Natl Acad Sci U S A. 1998;95:7748. doi: 10.1073/pnas.95.13.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keppel G, Wickens TD. Design and Analysis: A Researcher’s Handbook. Chapter 7. Prentis Hall; 2004. p. 143. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.