Abstract
Accumulating epidemiological evidence suggests light to moderate alcohol intake reduces risk of several chronic diseases. However, there is limited information regarding the effects of low alcohol intake in animal studies. This study investigated the effect of low ethanol dosage on senescence-accelerated mouse (SAMP8), an animal model of aging and neurodegenaration. Male SAMP8 mice (11 weeks old) had free access to a commercial stock diet with drinking water containing 0, 1 or 2% (v/v) ethanol for 15 weeks. The total grading score of senescence in the 1%-ethanol group was, in large part, the lowest among the three groups. Analysis using the open-field test revealed a significant elevation (+77%, P<0.05) in the rearing activity (index of seeking behavior) in the 1%-ethanol group, but not in the 2%-ethanol group. In addition, 2% ethanol elevated spontaneous locomotor activity (+75%, P<0.05), whereas 1% ethanol did not. Scrutiny of serum parameters indicated intake of 1% ethanol significantly decreased serum insulin levels (−13%, P<0.05), whereas 2% did not. Intake of 2% ethanol significantly elevated (2.5-fold, P<0.05) S100a8 mRNA (an inflammatory signal) in the brain, but that of 1% ethanol did not. Intriguingly, 1% ethanol intake remarkably elevated (10-fold, P<0.05) mRNA of brain alcohol dehydrogenase 1 (Adh1), which metabolizes lipid-peroxidation products and is involved in the synthesis of retinoic acid, a neuroprotective factor. Of note, 2%-ethanol intake did not exert this effect. Taken together, intake of 1% ethanol is likely to be beneficial for SAMP8 mice.
Keywords: senescence-accelerated mouse prone 8, ethanol, aging, brain, alcohol dehydrogenase 1
Introduction
Several epidemiological studies show that all-cause mortality as well as the incidences of cardiovascular diseases, diabetes, liver cirrhosis and stroke are lower in people reporting moderate alcohol consumption than both non-drinkers and heavy drinkers; this suggests a J-shaped or U-shaped effect of alcohol consumption on human health (1–3). Recent epidemiological evidence has further suggested low or moderate intake of alcohol decreases the risk of brain diseases such as dementia and cognitive impairment (4,5). In epidemiological studies, however, it is difficult to completely adjust for confounding factors (e.g., ethnicity, beverage type, drinking style, socioeconomic status, lifestyle, physical activity and personality type) (6,7). Thus, epidemiological studies have limited power to conclude that moderate alcohol intake itself directly improves human health and exerts a biological effect. Animal experiments are useful for examining the direct effects of pure alcohol. However, experimental animal models have focused on high toxicological doses with forced and excessive ingestion (e.g., intragastric ethanol infusion and liquid diets) (8,9). Meanwhile, animal studies involving low alcohol intake are limited.
Research with experimental rodent models and cultured cardiac myocytes, or endothelial cells indicates that moderate alcohol exposure can promote anti-inflammatory processes involving adenosine receptors, protein kinase C (PKC), nitric oxide synthase, heat shock proteins, and others which could underlie cardioprotection (10). Decreased risks of cognitive loss or dementia in moderate, non-binge consumers of alcohol (wine, beer, liquor) have been reported, whereas increased risk has been reported only in a few studies (11). Thus, moderate alcohol exposure appears to trigger analogous mild stress-associated, anti-inflammatory mechanisms in the heart, vasculature, and brain that tend to promote cellular survival pathways (10). One study indicated that ethanol intake levels achieved by alcohol-preferring P rats as a result of chronic voluntary exposure may have favorable rather than detrimental effects on lipid profiles in this genetic line, consistent with data supporting beneficial cardioprotective and neuroprotective effects of moderate ethanol consumption (12). Our recent study has suggested that intake of 1% ethanol in drinking water improved liver function in rats maintained on a high-fat diet, but that of 2% ethanol did so to a lesser extent (13). In the present study, we examined the effect of low ethanol intake on senescence in senescence-accelerated mice (SAM). SAM are widely used as an animal genetic model for studying aging, and a techniques for evaluation of senescence degree are well established (14). The system was designed to represent changes in both behavior and appearance of these mice, which display the clinical manifestations and gross lesions associated with the aging process. The defined grading score system is one of the significant advantages in aging studies using SAM. The Senescence-Accelerated Mouse Prone 8 (SAMP8) line has further advantages, because some behavioral traits and histopathology resemble human dementia as well as its recapitulating rapid physiological senescence (15,16). Thus, the present study was conducted to examine the effects of low dose of ethanol on SAMP8 mice.
Materials and methods
Animal experiment
Eight-week-old male SAMP8 mice (Japan SLC, Shizuoka, Japan) were maintained under controlled conditions (ambient temperature, 22°C ± 2°C, 12-h light/dark cycle, lights on from 12:00 a.m. to 12:00 p.m., lights off from 12:00 p.m. to 12:00 a.m.). The animals were housed individually in plastic cages (125×200×110 mm) with free access to food (MF, Oriental Yeast, Tokyo, Japan) and water. This study was approved by the Animal Care Committee of the National Research Institute of Brewing, Japan (Ethical approval No. 25-1). After a 3-week acclimation period, the mice received deionized drinking water with 0, 1% (v/v) or 2% (v/v) ethanol (n=8 mice per group) for 15 weeks. The ethanol-consuming groups had free access to only 1 or 2% ethanol without other water being available. Licking counts of drinking water were evaluated by drinking sensors (DS-1, Shinfactory, Fukuoka, Japan) for 21 h (11:00 a.m. to 08:00 a.m.) in 20-week-old mice. Food intake was quantified using measuring the difference between the preweighed pellet in food cups and the weight of remaining pellet and spill at the end of 24-h period. Fluid intake was also determined by measuring the difference between preweighed water bottle and the weight of remaining bottle at the end of 24-h period. At the termination of experimental procedure, mice were sacrificed by decapitation under diethylether anesthesia (between 01:00 p.m. and 03:00 p.m.) after removal of food and drinking water (08:00 a.m.).
Grading of senescence
The degree of senescence was evaluated by a grading system (14) comprising the following 11 items in four categories: Behaviors (reactivity and passivity), skin and hair (glossiness, coarseness and hair loss), eyes (ulcer, periophthalmic lesions, cataract, corneal ulcer and corneal opacity) and skeleton (lordokyphosis). The grading score was calculated by summing the scores of all 11 items from 0 to 4.
Serum biochemical analysis
The activities of serum alanine aminotransferase (ALT, EC 1.1.1.27) and aspartate aminotransferase (AST, EC 2.6.1.1) as well as levels of serum glucose, triglyceride, albumin, and total cholesterol were measured calorimetrically by the DRICHEM commercial assay system (Fujifilm, Tokyo, Japan). Serum insulin (Mercodia, Uppsala, Sweden), adiponectin and IGF-1 (both from R&D Systems, Minneapolis, MN, USA) were measured by commercial ELISA kits. Serum IL-1β, IL-12, and TNF-α were determined by the Bio-Plex cytokine assay kit in combination with the Bio-Plex Manager software (Bio-Rad, Hercules, CA, USA).
Open-field test
Open-field test was performed using a two-level infrared beam apparatus (Scanet MV-40; Melquest, Toyama, Japan), an automatic analysis system for measuring murine locomotor activity (17). Testing was performed between 01:00 p.m. and 03:00 p.m. Mice were placed into the center of the open field (44×44×30 cm) and left to explore for 10 min. Food and water were available ad libitum other than during 10-min trials. Rearing counts were evaluated as vertical activity. The field was cleaned after each session.
Spontaneous locomotor activity
Spontaneous locomotor activity was automatically measured by a laboratory animal movement analyzing system (ACTIMO-100; Shinfactory, Fukuoka, Japan). Locomotor activity was measured as ambulatory counts from a record of consecutive adjacent infrared beam breaks. Mice were housed individually in plastic cages, and food and water were available ad libitum. Mice were acclimatized to the cages for 1 h before recordings commenced and then monitored for 21 h (dark period for 11 h; 01:00 p.m. to 12:00 a.m. and light period for 10 h; 12:00 a.m. to 10:00 a.m.).
In the above behavior tests, the different treatment groups were tested in counterbalanced order with a single blinded method.
RNA extraction
Total RNA was extracted from the whole brain by using QIAzol Lysis Reagent (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Isolated RNA was purified using the RNeasy® Lipid Tissue Mini kit (Qiagen).
DNA microarray analysis
Pooled RNAs were subjected to cRNA synthesis for a DNA microarray analysis. Cyanine-3 (Cy3) labeled cRNA was prepared from 100 ng RNA using the One-Color Low Input Quick Amp labeling kit (Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer's instructions. All procedures of hybridization, slide washing, and scanning were carried out according to the manufacturer's instructions [Agilent Whole Mouse Genome Microarray kit ver2.0 (G4846A); Agilent Technologies]. The data were analyzed using GeneSpring software version 12.6.1 (Agilent Technologies).
Real-time PCR
cDNA was synthesized from total RNA using the Revertrace RT-PCR kit (Toyobo Co., Ltd., Osaka, Japan). Real-time PCR was performed on an Opticon 2 system (Bio-Rad) using SYBR qPCR mix (Toyobo Co., Ltd.) employing primers (forward/reverse) as shown in Table I. Expression of the target genes was normalized to that of GAPDH as an endogenous control gene.
Table I.
Primer sequences used for real-time PCR.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| S100a8 | ACAAGGAAATCACCATGCCCT | TCACCATCGCAAGGAACTCC |
| S100a9 | ACCAGGACAATCAGCTGAGC | ACAGCCTTTGCCATGACTGT |
| Gpr35 | TCTTCCCCCTGGAGATCTTT | CTGGGAGAAAGGAGACCACA |
| Cyp2e1 | TCCCTAAGTATCCTCCGTGA | GTAATCGAAGCGTTTGTTGA |
| Adh1 | TGTGGTTGATGCAACGGTTG | TTCGCGCATAAAAATGCCCC |
| Adh2 | AGGCCAATCTTGCCAGAGTC | GCCAAAGACAGCACAAGTGG |
| Adh3 | CTGGACGAATCCTCCTCCGTAGC | GACTGACAGGCCAACTCCTC |
| Adh4 | AGGCCAATCTTGCCAGAGTC | GCCAAAGACAGCACAAGTCC |
| Aldh1 | GCACTCAATGGTGGGAAAGT | TTTGGCCACACACTCCAATA |
| Aldh2 | GCTGGGCTGACAAGTACCAT | TTGATCAAGTTGGCCACGTA |
Statistical analysis
Data were analyzed by one-way ANOVA or two-way repeated-measures ANOVA followed by Tukey-Kramer honest significant difference (HSD) test. The level of significance was set at P<0.05. In tables and figures, the means in the row or bar with superscripts without a common letter significantly differ, P<0.05 (Tukey-Kramer HSD test).
Results
Growth and senescence grading score
Food and fluid intake, body weight, and weights of adipose tissues and gastrocnemius muscle weight were not significantly different among the three groups (Table II). Mean ethanol intake in the 2%-ethanol group was almost twice as much as that in the 1%-ethanol group (Table III). Licking counts of drinking water (access status to water bottle) in 20-week-old mice are indicated in Table IV. The temporal changes in the senescence grading score (behavior, skin and hair, eyes, spondylus, and total) are shown in Table V. In 18-week-old mice, the senescence score of behavior and total senescence score were unaffected by ethanol intake. In contrast, in 22-week-old mice, both 1% and 2%-ethanol intake significantly (P<0.05) decreased the senescence scores for behavior and total scores compared to the controls. In 25-week-old mice, 1%-ethanol intake caused lower scores of behavior and total scores than the other two groups (P<0.05). The senescence scores of skin and hair were significantly lower (P<0.05) in the 2%-ethanol group than in the control groups in 18-week-old mice, but there was no difference between the control and 1%-ethanol groups. Ethanol intake caused no influence on the senescence score of spondylus in 25-week-old mice.
Table II.
Effects of ethanol exposure on SAMP8 mice.
| Variable | Control (no ethanol) | 1% Ethanol | 2% Ethanol |
|---|---|---|---|
| Final body weight (g) | 30.6±0.8 | 28.6±0.8 | 30.4±0.7 |
| Gains in body weight (g) | 6.5±0.5 | 4.6±0.9 | 6.5±0.7 |
| Epididymal adipose tissue (g) | 0.250±0.040 | 0.156±0.028 | 0.190±0.028 |
| Perinephric adipose tissue (g) | 0.088±0.016 | 0.061±0.012 | 0.080±0.015 |
| Gastrocnemius muscle (g) | 0.111±0.006 | 0.111±0.006 | 0.117±0.004 |
| Total food intake (g) | 451±12 | 467±7 | 462±6 |
| Total fluid intake (g) | 691±30 | 680±22 | 697±31 |
Values are mean ± SE (n=8).
Table III.
Mean of ethanol ingestion.
| Ethanol ingestion (g/kg body weight−1 day−1) | ||||
|---|---|---|---|---|
| Age of mice | Control (no ethanol) | 1% Ethanol | 2% Ethanol | |
| 10-week-old | 0a | 1.72±0.07b | 3.34±0.12c | |
| 11-week-old | 0a | 1.65±0.07b | 3.17±0.13c | |
| 13-week-old | 0a | 1.62±0.05b | 3.10±0.24c | |
| 15-week-old | 0a | 1.47±0.04b | 2.93±0.13c | |
| 17-week-old | 0a | 1.47±0.04b | 2.88±0.20c | |
| 19-week-old | 0a | 1.45±0.07b | 3.17±0.43c | |
| 21-week-old | 0a | 1.47±0.06b | 2.98±0.19c | |
| 23-week-old | 0a | 1.42±0.06b | 2.69±0.15c | |
| 25-week-old | 0a | 1.41±0.06b | 2.83±0.19c | |
| 26-week-old | 0a | 1.44±0.06b | 2.89±0.14c | |
Values are mean ± SE (n=8).
Significantly different by Tukey-Kramer honest significant difference test (P<0.05).
Table IV.
Mean of licking counts of drinking water from 11:00 a.m. to 08:00 a.m.
| Licking counts (counts/h) | |||
|---|---|---|---|
| Light/dark period, time range | Control (no ethanol) | 1% Ethanol | 2% Ethanol |
| Light period, 11:00 a.m. to 12:00 p.m. | 7.1±3.4 | 12.4±1.2 | 7.9±0.2 |
| Dark period, 12:00 a.m. to 01:00 p.m. | 8.1±2.9 | 7.4±5.1 | 5.4±2.6 |
| Dark period, 01:00 p.m. to 02:00 p.m. | 26.4±8.1 | 20.6±2.9 | 13.9±4.0 |
| Dark period, 02:00 p.m. to 03:00 p.m. | 19.6±5.0 | 25.9±4.9 | 11.9±4.5 |
| Dark period, 03:00 p.m. to 04:00 p.m. | 16.8±5.4 | 19.6±6.4 | 12.7±6.0 |
| Dark period, 04:00 p.m. to 05:00 p.m. | 23.1±7.1 | 21.6±5.6 | 12.1±3.7 |
| Dark period, 05:00 p.m. to 06:00 p.m. | 17.8±5.4 | 13.6±4.9 | 21.0±4.9 |
| Dark period, 06:00 p.m. to 07:00 p.m. | 12.8±4.2 | 22.9±5.7 | 17.7±5.0 |
| Dark period, 07:00 p.m. to 08:00 p.m. | 12.1±4.7 | 18.8±7.2 | 10.4±9.7 |
| Dark period, 08:00 p.m. to 09:00 p.m. | 9.0±4.2 | 13.5±7.4 | 12.1±7.2 |
| Dark period, 09:00 p.m. to 10:00 p.m. | 4.5±3.0 | 7.5±3.5 | 10.4±6.6 |
| Dark period, 10:00 p.m. to 11:00 p.m. | 4.8±3.1 | 7.0±3.3 | 5.7±4.6 |
| Dark period, 11:00 p.m. to 12:00 a.m. | 10.8±3.8 | 1.4±3.3 | 11.3±5.9 |
| Light period, 00:00 a.m. to 01:00 a.m. | 1.6±1.5 | 6.8±0.8 | 5.4±2.7 |
| Light period, 01:00 a.m. to 02:00 a.m. | 0.0±0.0 | 0.6±2.7 | 4.3±6.8 |
| Light period, 02:00 a.m. to 03:00 a.m. | 0.0±0.0 | 0.4±0.5 | 2.9±3.7 |
| Light period, 03:00 a.m. to 04:00 a.m. | 1.8±1.6 | 2.5±0.4 | 4.1±2.8 |
| Light period, 04:00 a.m. to 05:00 a.m. | 2.8±2.2 | 0.1±2.4 | 1.7±2.9 |
| Light period, 05:00 a.m. to 06:00 a.m. | 3.1±3.1 | 0.6±0.1 | 2.4±2.2 |
| Light period, 06:00 a.m. to 07:00 a.m. | 0.3±0.2 | 0.0±0.5 | 0.0±1.7 |
| Light period, 07:00 a.m. to 08:00 a.m. | 2.4±2.4 | 0.3±0.0 | 0.0±2.4 |
Values are mean ± SE (n=8, 20-week-old).
Table V.
Effects of ethanol exposure on senescence grading score in SAMP8 mice.
| Two-way repeated-measures (ANOVA; P-value) | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Week-old | Control (no ethanol) | 1% Ethanol | 2% Ethanol | Week-old effect | Ethanol effect | Interaction |
| Behavior | 18 | 0.03±0.03 | 0.03±0.03 | 0 | |||
| 22 | 0.51±0.14a | 0.08±0.04b | 0.13±0.07b | <0.01 | <0.01 | <0.01 | |
| 25 | 0.94±0.15a | 0.30±0.16b | 0.83±0.10a | ||||
| Skin and hair | 18 | 0.10±0.04a | 0.03±0.02a,b | 0b | |||
| 22 | 0.28±0.04 | 0.24±0.03 | 0.30±0.06 | <0.01 | 0.17 | 0.40 | |
| 25 | 0.71±0.11 | 0.56±0.04 | 0.70±0.03 | ||||
| Eyes | 18 | 0 | 0 | 0 | |||
| 22 | 0.15±0.12 | 0 | 0 | 0.24 | 0.25 | 0.23 | |
| 25 | 0 | 0 | 0 | ||||
| Spondylus | 18 | 0.13±0.04 | 0.10±0.03 | 0.04±0.03 | |||
| 22 | 0.19±0.03 | 0.16±0.04 | 0.15±0.03 | <0.01 | 0.16 | <0.05 | |
| 25 | 0.54±0.08 | 0.34±0.05 | 0.48±0.03 | ||||
| Total | 18 | 0.25±0.08 | 0.15±0.06 | 0.04±0.03 | |||
| 22 | 1.13±0.18a | 0.48±0.09b | 0.58±0.10b | <0.01 | <0.01 | <0.01 | |
| 25 | 2.19±0.31a | 1.20±0.20b | 2.00±0.10a | ||||
Values are mean ± SE.
Significantly different by Tukey-Kramer honest significant difference test (P<0.05).
Behavioral analyzes
In the open-field test, the rearing activity of animals in the 1%-ethanol group was significantly higher (+77%, P<0.05) than for the control and 2%-ethanol groups. There was no difference in activity between control and 2%-ethanol groups (Fig. 1), indicating that exploratory activity (index of seeking behavior) was increased in the 1%-ethanol group. Moreover, 2%-ethanol intake significantly elevated (+75%, P<0.05, Fig. 1) spontaneous locomotor activity, whereas 1%-ethanol intake did not increase such activity, implying the vitality of 2% ethanol-treated mice. The animals allowed free access to food and drinking, mainly from 01:00 p.m. to 08:00 p.m. in the dark period in this study, which was confirmed by drink sensor measurements (Table IV). The open-field test was conducted from 01:00 p.m. to 03:00 p.m., and it is unclear that the effects of ethanol exposure on the behavioral parameters are direct or indirect effects.
Figure 1.
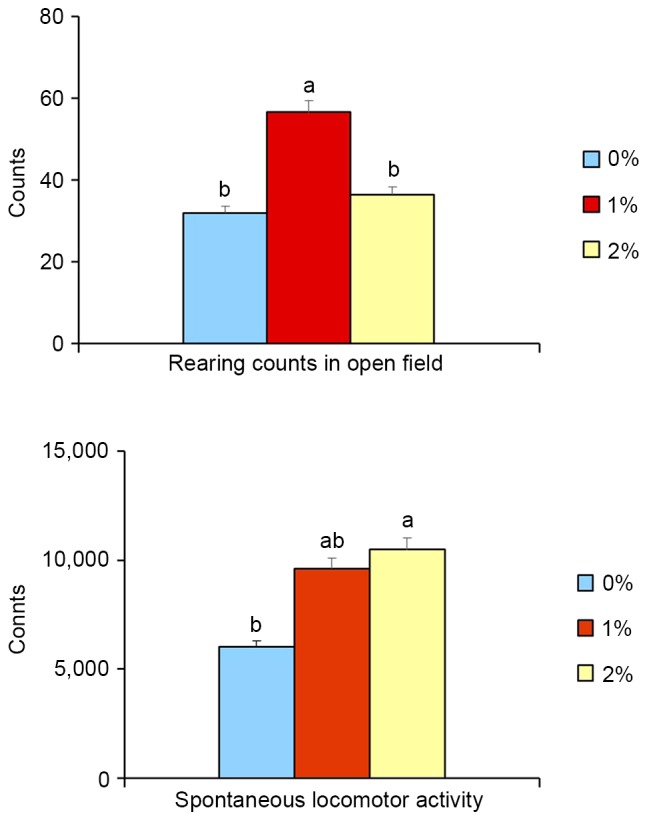
Effects of ethanol on open-field test and spontaneous locomotor activity in SAMP8 mice. Rearing counts in open-field tests at 23-week-old are shown (above). Spontaneous locomotor activity for 17-week-old animals is shown (below). Values are mean ± SE (n=8). One-way ANOVA analysis indicated significant effects of ethanol exposure on the rearing counts and spontaneous locomotor activity (P<0.05). a,bSignificantly different by Tukey-Kramer honest significant difference test (P<0.05).
Serum parameters
None of the three groups exhibited significant differences in serum triglyceride, total cholesterol, or glucose levels, and AST and ALT activities were similar among the three groups (Table VI). The serum levels of albumin were significantly lower (−8%, P<0.05) in the ethanol group than in the control group, but there was no difference between the control and 2%-ethanol groups (Table VI). Intake of 1% ethanol slightly decreased serum level of insulin (−12%, P<0.01), but that of 2% ethanol did not (Table VI). Serum levels of adiponectin, IGF-1, IL-1β, IL-12, and TNF-α were unaffected by ethanol intake (Table VI).
Table VI.
Effects of ethanol exposure on serum parameters in SAMP8 mice.
| Variable | Control (no ethanol) | 1% Ethanol | 2% Ethanol |
|---|---|---|---|
| Glucose (mmol/l) | 8.97±0.53 | 8.19±0.59 | 8.59±0.65 |
| Triglyceride (mmol/l) | 1.18±0.13 | 0.99±0.05 | 1.10±0.09 |
| Total cholesterol (mmol/l) | 2.72±0.12 | 2.65±0.10 | 2.74±0.15 |
| ALT (U/l) | 25.3±1.5 | 26.5±2.1 | 26.3±1.6 |
| AST (U/l) | 133±5 | 124±9 | 135±6 |
| Albumin (g/l) | 27.0±0.4a | 24.8±0.6b | 25.5±0.7a,b |
| Insulin (mg/l) | 0.63±0.02a | 0.55±0.01b | 0.58±0.01a,b |
| Adiponectin (mg/l) | 6.51±0.22 | 7.11±0.41 | 7.63±0.22 |
| IGF-1 (µg/l) | 310±39 | 272±35 | 237±38 |
| IL-1β (ng/l) | 407±94 | 376±94 | 308±87 |
| IL-12 (ng/l) | 128±33 | 97±33 | 80±31 |
| TNF-α (ng/l) | 312±68 | 272±68 | 211±63 |
Values are mean ± SE (n=6–8).
Significantly different by Tukey-Kramer honest significant difference test (P<0.05). ALT, alanine aminotransferase; AST, aspartate aminotransferase; IGF, insulin-like growth factor; IL, interleukin; TNF, tumor necrosis factor.
Gene expression in brain
In our preliminary study, DNA microarray analysis indicated alterations in the gene expression of S100a8, S100a9, GPR35, Cyp2e1, Adh1, and Adh4 by ethanol intake. Thus, real-time PCR analysis was used in the present study to confirm these results. Gene expression of other ethanol-metabolizing enzymes was also determined. Intake of 2% ethanol resulted in a 2.5-fold elevation (P<0.05; Fig. 2) of S100a8 mRNA, but 1%-ethanol intake did not. S100a9, GPR35 and Cyp2e1 expression levels were unaffected in the 2%-ethanol intake group. Intake of 1% ethanol caused a marked elevation (10-fold, P<0.05; Fig. 3) in Adh1 expression, but that of 2% ethanol did not. Intake of 1 and 2% ethanol caused no influence on Adh2, Adh3, Adh4, Aldh1, and Aldh2 expression (Fig. 3). Adh1 expression was significantly correlated with the rearing activity of the mice (r=0.598, P<0.01; Fig. 4) and with the total senescence score at 22 weeks (r=−0.497, P<0.05), but not with the total senescence score at 25 weeks (r=−0.412, P=0.09). The expression of Adh1 was not correlated (P>0.05) with any of the serum factors or behavioral results, with the exception of rearing activity. In addition, the serum results were not correlated with the rearing activity and total senescence scores (P>0.05). Adh2, Adh3, Aldh1 and Aldh2 expression levels were unaffected by ethanol intake. DNA microarray analysis also indicated the elevated gene expression of several olfactory receptors as a consequence of 1% ethanol intake (Fig. 5).
Figure 2.
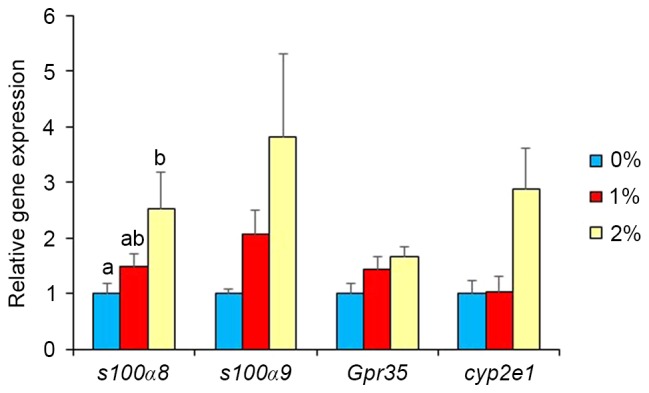
Effects of ethanol exposure on expression of genes related to inflammation and oxidative stress in the brains of SAMP8 mice. Values are mean ± SE (n=5–7). One-way ANOVA analysis indicated significant effects of ethanol exposure on S100a expression (P<0.05), but no such effects on expression of other genes. a,bSignificantly different by Tukey-Kramer honest significant difference test (P<0.05).
Figure 3.
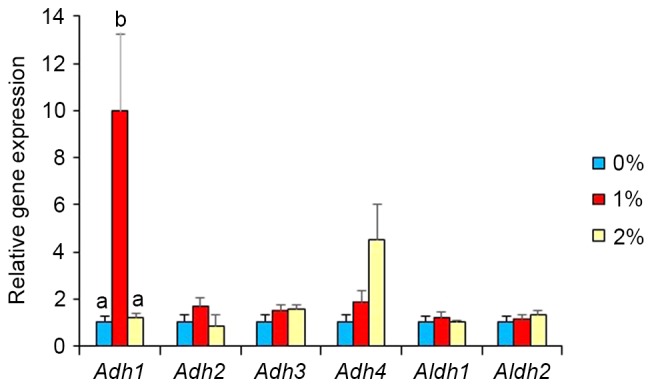
Effects of ethanol exposure on expression of genes involved in alcohol metabolism in the brain of SAMP8 mice. Values are mean ± SE (n=5–7). One-way ANOVA analysis indicated significant effects of ethanol consumption on Adh1 expression (P<0.05), but no similar effects on expression of other genes. a,bSignificantly different by Tukey-Kramer honest significant difference test (P<0.05).
Figure 4.
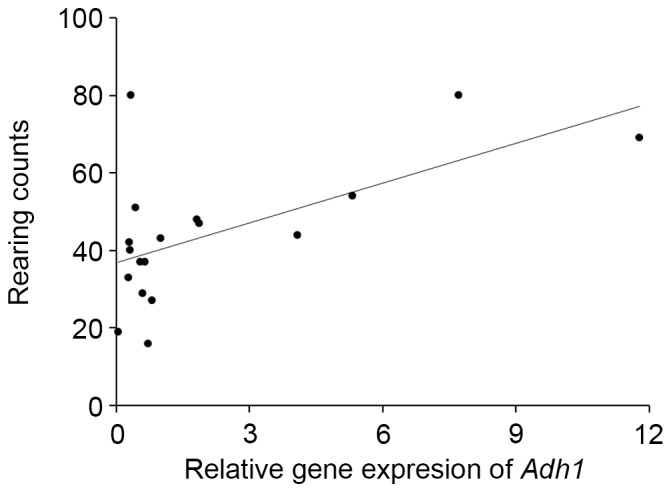
Correlation of relative gene expression of Adh1 and rearing activity in SAMP8 mice.
Figure 5.
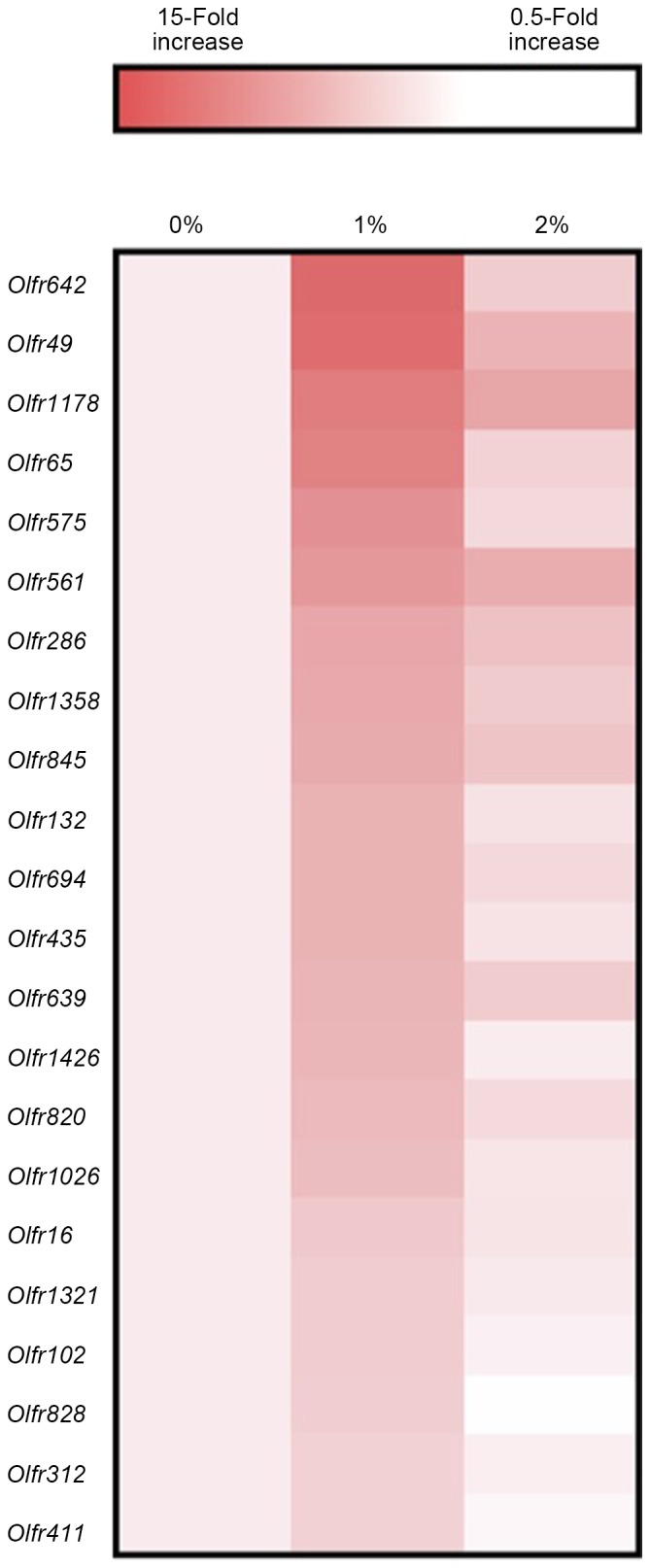
Effects of ethanol exposure on expression levels of several olfactory receptor genes in the brain of SAMP8 mice. Pooled samples from three groups were employed for the analysis using DNA microarray.
Discussion
The present results, obtained SAMP8 mice, indicate that low-ethanol intake does not exert any significant deleterious effects on the general welfare of animals. Consumption of 1% ethanol appeared to retard senescence development with respect to the eyes, skin, and hair, and behavior, whereas 2%-ethanol intake appeared to do so to a lesser extent. These results suggest that 1%-ethanol intake is beneficial for SAMP8 mice.
Here, indices of liver function in SAMP8 mice were unaffected by ethanol intake. This is in contrast to the results observed in the rats fed a high-fat diet, in which 1%-ethanol intake improved the parameters relating to the liver function (10). Although the reason for this discrepancy is unknown, our study implies a favorable effect of 1%-ethanol intake on SAMP8 animals, which may be mediated through mechanisms not involving liver function. Of interest is the finding that 1%- ethanol intake caused a significant reduction in serum insulin, which has been considered to play an important role in aging process (18), whereas 2% ethanol did not. However, serum insulin levels were not associated with the total senescence score, raising activity and Ahd1 expression. Further study is necessary to examine the effect of 1% ethanol on insulin signaling in the senescence mice.
In this study, analysis using open-field tests demonstrated a significant elevation in rearing activity in the 1%-ethanol group, but not in the 2%-ethanol group. This rearing activity has been suggested an index of exploratory behavior (19,20). Importantly, senescence has previously been reported to be associated with diminished rearing activity (21,22). Thus, at low doses of ethanol, ethanol is likely to cause positive effects on such ‘seeking-out’ behavior, which is otherwise decreased by senescence. Because senescence is associated with decreased locomotor function in SAMP1 animals (23), locomotor function was also examined. We found that 2%-ethanol intake significantly elevated (+75%) locomotor activity, whereas 1%-ethanol intake tended to promote such activity to a lesser degree (+60%). The results were consistent with the previous studies indicating low doses of ethanol stimulate locomotor activity in mice (24). Thus, intake of either 1 or 2% ethanol appears to have positive effect on the locomotor function in SAMP8 mice.
Gene expression analysis revealed significantly higher levels of brain S100a8 in the 2%-ethanol group, but not in the 1%-ethanol group. S100a9, GPR35 and Cyp2e1 expressions also tended to be higher in the 2%-ethanol group. S100a8 and S100a9 have been suggested to be involved in inflammatory signaling (25), and GPR35 is proposed to be associated with inflammation (26). Cyp2e1 is considered a source of reactive oxygen species generation (27). Thus, the dose of 2% ethanol appears to be necessary for the induction of expression of the factors responsible for inflammation and oxidative stress.
Surprisingly, our study quantified a marked elevation in gene expression in brain tissue for Adh1 in the 1%-ethanol group, but not in the 2%-ethanol group. Alcohol dehydrogenases (ADHs) metabolize a broad spectrum of substrates such as alcohols and aldehydes endogenously produced during lipid peroxidation so as to prevent the possible toxic accumulation of these compounds (28). Because these compounds can be harmful to dopaminergic neurons, ADHs have attracted attention. Genetic variants in ADH1C have been reported to be associated with Parkinson disease (29). In fact, recent study using Adh1 knockout mice has shown lack of Adh1 leads to changes in dopamine neurons related behavior (30). Furthermore, Adhs are a critical mediator of retinoic acid synthesis from vitamin A (31,32). Retinoic acid has been suggested a protective factor against neurodegenaration via retinoid signaling (33). Our studies further indicated Adh1 expression is significantly correlated with the rearing activity. Expression of several olfactory receptor genes was also higher in the 1%-ethanol group compared with other groups. An Alzheimer's disease model rat revealed down regulation of olfactory receptor genes in the olfactory bulb (34) and olfactory dysfunction has been also reported in neurodegerative disorders such as Alzheimer's and Parkinson's diseases. Olfactory dysfunction also increases with aging. In view of these facts, it will be necessary to evaluate if perturbed expression of Adh1 expression leads to the alterations in the rearing activity. Furthermore, the elevation of Adh1 expression requires confirmation at the protein level and is being investigated in future studies.
We obtained preliminary measurement data for serum ethanol when dissected (01:00 p.m. to 03:00 p.m.) at 23-week-old, noting that no differences were observed among the three groups (Kimoto et al, unpublished data). At present, there are no supporting data from the literature to suggest what blood or brain ethanol concentrations may have been reached in this model as a result of the 1 and 2% ethanol treatments. It has been reported that consumption of 6% ethanol containing liquid diet by C56BL6 mice for 22 weeks permits the use of plasma ethanol as a confirmation of alcohol exposure model (35). Meanwhile, plasma ethanol levels of the mice fed 3% ethanol containing liquid diet did not significantly differ from the base line levels of mice without receiving ethanol (35).
In conclusion, our study provides evidence for the beneficial effect of low doses of ethanol on SAMP8 mice. In particular, 1%-ethanol intake appeared to cause a favorable effect on senescence score and rearing activity, whereas 2%-ethanol intake prompted a lesser effect. These results support the J-curve effect for ethanol exposure as suggested by a number of epidemiological studies (1–3). Of great interest is the finding of the markedly higher Adh1 expression in the brains of 1%-ethanol group, but not in those of the 2%-ethanol exposed group. Thus, our results raised the prospect that the induction of Adh1 expression by 1% ethanol intake leads to the quantified beneficial effect. Further research is necessary to examine this proposal. The molecular mechanisms modulating higher levels of Adh1 expression by 1% ethanol also warrant further investigation. At present, it is unclear whether the 1% ethanol intake exerted direct or indirect effect on the Adh1 expression and the rearing activity. Further study will be necessary to reveal this issue.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and in part by a grant from the Brewers Association of Japan. The authors would like to thank Enago (www.enago.jp) for English language review.
References
- 1.Marmot MG, Rose G, Shipley MJ, Thomas BJ. Alcohol and mortality: A U-shaped curve. Lancet. 1981;1:580–583. doi: 10.1016/S0140-6736(81)92032-8. [DOI] [PubMed] [Google Scholar]
- 2.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: An updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 3.Nova E, Baccan GC, Veses A, Zapatera B, Marcos A. Potential health benefits of moderate alcohol consumption: Current perspectives in research; Proc Nutr Soc; 2012; pp. 307–315. [DOI] [PubMed] [Google Scholar]
- 4.Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA. 2003;289:1405–1413. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- 5.Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: A systematic review. Age Ageing. 2008;37:505–512. doi: 10.1093/ageing/afn095. [DOI] [PubMed] [Google Scholar]
- 6.Fagrell B, De Faire U, Bondy S, Criqui M, Gaziano M, Gronbaek M, Jackson R, Klatsky A, Salonen J, Shaper AG. The effects of light to moderate drinking on cardiovascular diseases. J Intern Med. 1999;246:331–340. doi: 10.1046/j.1365-2796.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 7.McCann SE, Sempos C, Freudenheim JL, Muti P, Russell M, Nochajski TH, Ram M, Hovey K, Trevisan M. Alcoholic beverage preference and characteristics of drinkers and nondrinkers in western New York (United States) Nutr Metab Cardiovasc Dis. 2003;13:2–11. doi: 10.1016/S0939-4753(03)80162-X. [DOI] [PubMed] [Google Scholar]
- 8.Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989;24:197–211. [PubMed] [Google Scholar]
- 9.Izu H, Shobayashi M, Manabe Y, Goto K, Iefuji H. S-adenosylmethionine (SAM)-accumulating sake yeast suppresses acute alcohol-induced live injury in mice. Biosci Biotechnol Biochem. 2006;70:2982–2989. doi: 10.1271/bbb.60377. [DOI] [PubMed] [Google Scholar]
- 10.Collins MA, Neafsey EJ, Mukamal KJ, Gray MO, Parks DA, Das DK, Korthuis RJ. Alcohol in moderation, cardioprotection and neuroprotection: Epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res. 2009;33:206–219. doi: 10.1111/j.1530-0277.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilomaki J, Jokanovic N, Tan EC, Lonnroos E. Alcohol consumption, dementia and cognitive decline: An overview of systematic reviews. Curr Clin Pharmacol. 2015;10:204–212. doi: 10.2174/157488471003150820145539. [DOI] [PubMed] [Google Scholar]
- 12.Godfrey J, Jeanguenin L, Castro N, Olney JJ, Dudley J, Pipkin J, Walls SM, Wang W, Herr DR, Harris GL, Brasser SM. Chronic voluntary ethanol consumption induces favorable ceramide profiles in selectively bred alcohol-preferring (P) rats. PLoS One. 2015;10:e0139012. doi: 10.1371/journal.pone.0139012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osaki A, Okazaki Y, Kimoto A, Izu H, Kato N. Beneficial effect of low dose of ethanol on liver function and serum urate in rats fed a high-fat diet. J Nutr Sci Vitaminol (Tokyo) 2014;60:408–412. doi: 10.3177/jnsv.60.408. [DOI] [PubMed] [Google Scholar]
- 14.Hosokawa M, Kasai R, Higuchi K, Takeshita S, Shimizu K, Hamamoto H, Honma A, Irino M, Toda K, Matsumura A, et al. Grading score system: A method for evaluation of the degree of senescence in senescence accelerated mouse (SAM) Mech Ageing Dev. 1984;26:91–102. doi: 10.1016/0047-6374(84)90168-4. [DOI] [PubMed] [Google Scholar]
- 15.Takeda T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem Res. 2009;34:639–659. doi: 10.1007/s11064-009-9922-y. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Lian K, Han B, Wang Y, Kuo SH, Geng Y, Qiang J, Sun M, Wang M. Age-related alterations in the metabolic profile in the hippocampus of the senescence-accelerated mouse prone 8: A spontaneous Alzheimer's disease mouse model. J Alzheimers Dis. 2014;39:841–848. doi: 10.3233/JAD-131463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinozaki M, Takahashi Y, Mukaino M, Saito N, Toyama Y, Okano H, Nakamura M. Novel concept of motor functional analysis for spinal cord injury in adult mice. J Biomed Biotechnol. 2011;2011:157458. doi: 10.1155/2011/157458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamming DW. Diminished mTOR signaling: A common mode of action for endocrine longevity factors. Springerplus. 2014;3:735. doi: 10.1186/2193-1801-3-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Easton A, Arbuzova J, Turek FW. The circadian Clock mutation increases exploratory activity and escape-seeking behavior. Genes Brain Behav. 2003;2:11–19. doi: 10.1034/j.1601-183X.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 20.Pardo M, López-Cruz L, Valverde O, Ledent C, Baqi Y, Müller CE, Salamone JD, Correa M. Effect of subtype-selective adenosine receptor antagonists on basal or haloperidol-regulated striatal function: Studies of exploratory locomotion and c-Fos immunoreactivity in outbred and A(2A)R KO mice. Behav Brain Res. 2013;247:217–226. doi: 10.1016/j.bbr.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 21.Ikegami S, Shumiya S, Kawamura H. Age-related changes in radial-arm maze learning and basal forebrain cholinergic systems in senescence accelerated mice (SAM) Behav Brain Res. 1992;51:15–22. doi: 10.1016/S0166-4328(05)80307-9. [DOI] [PubMed] [Google Scholar]
- 22.Lalonde R, Strazielle C. Exploratory activity and motor coordination in old versus middle-aged C57BL/6 J mice. Arch Gerontol Geriatr. 2009;49:39–42. doi: 10.1016/j.archger.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Aoyama Y, Kim TY, Yoshimoto T, Niimi K, Takahashi E, Itakura C. Impaired motor function in senescence-accelerated mouse prone 1 (SAMP1) Brain Res. 2013;1515:48–54. doi: 10.1016/j.brainres.2013.03.053. [DOI] [PubMed] [Google Scholar]
- 24.Arizzi MN, Correa M, Betz AJ, Wisniecki A, Salamone JD. Behavioral effects of intraventricular injections of low doses of ethanol, acetaldehyde and acetate in rats: Studies with low and high rate operant schedules. Behav Brain Res. 2003;147:203–210. doi: 10.1016/S0166-4328(03)00158-X. [DOI] [PubMed] [Google Scholar]
- 25.Gebhardt C, Németh J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72:1622–1631. doi: 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Divorty N, Mackenzie AE, Nicklin SA, Milligan G. G protein-coupled receptor 35: An emerging target in inflammatory and cardiovascular disease. Front Pharmacol. 2015;6:41. doi: 10.3389/fphar.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seitz HK, Wang XD. The role of cytochrome P450 2E1 in ethanol-mediated carcinogenesis. Subcell Biochem. 2013;67:131–143. doi: 10.1007/978-94-007-5881-0_3. [DOI] [PubMed] [Google Scholar]
- 28.Boleda MD, Saubi N, Farrés J, Parés X. Physiological substrates for rat alcohol dehydrogenase classes: Aldehydes of lipid peroxidation, omega-hydroxyfatty acids and retinoids. Arch Biochem Biophys. 1993;307:85–90. doi: 10.1006/abbi.1993.1564. [DOI] [PubMed] [Google Scholar]
- 29.Buervenich S, Sydow O, Carmine A, Zhang Z, Anvret M, Olson L. Alcohol dehydrogenase alleles in Parkinson's disease. Mov Disord. 2000;15:813–818. doi: 10.1002/1531-8257(200009)15:5<813::AID-MDS1008>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 30.Anvret A, Ran C, Westerlund M, Gellhaar S, Lindqvist E, Pernold K, Lundströmer K, Duester G, Felder MR, Galter D, Belin AC. Adh1 and Adh1/4 knockout mice as possible rodent models for presymptomatic parkinson's disease. Behav Brain Res. 2012;227:252–257. doi: 10.1016/j.bbr.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 31.Duester G. Alcohol dehydrogenase as a critical mediator of retinoic acid synthesis from vitamin A in the mouse embryo. J Nutr. 1998;128(2 Suppl):459S–462S. doi: 10.1093/jn/128.2.459S. [DOI] [PubMed] [Google Scholar]
- 32.Molotkov A, Deltour L, Foglio MH, Cuenca AE, Duester G. Distinct retinoid metabolic functions for alcohol dehydrogenase genes Adh1 and Adh4 in protection against vitamin A toxicity or deficiency revealed in double null mutant mice. J Biol Chem. 2002;277:13804–13811. doi: 10.1074/jbc.M112039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sodhi RK, Singh N. Retinoids as potential targets for Alzheimer's disease. Pharmacol Biochem Behav. 2014;120:117–123. doi: 10.1016/j.pbb.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Zhu YY, Ni DF, Xu CM. Gene expression profiles in the olfactory bulb from a rat model of Alzheimer's disease. J Alzheimers Dis. 2009;18:581–593. doi: 10.3233/JAD-2009-1201. [DOI] [PubMed] [Google Scholar]
- 35.Emeson EE, Manaves V, Singer T, Tabesh T. Chronic alcohol feeding inhibits atherogenesis in C57BL/6 hyperlipidemic mice. Am J Pathol. 1995;147:1749–1758. [PMC free article] [PubMed] [Google Scholar]


