Abstract
Purpose:
Glaucoma is characterized by increased intraocular pressure (IOP). The effect of nicorandil and pinacidil on IOP in experimentally induced acute and chronic models of glaucoma and the mechanism of action involved were studied.
Methods:
New Zealand white rabbits were used for the study. After the measurement of IOP, nicorandil (1%), pinacidil (1%), and pilocarpine as standard (1%) were instilled topically into the left eye. The other eye served as control. Dextrose (5%) was used to induce acute glaucoma. IOP changes were recorded every 15 minutes until the pressure became normal. Freshly prepared α-chymotrypsin solution was introduced in the posterior chamber to induce chronic glaucoma. Rabbits with ocular hypertension were selected for the study. Similar drug solutions were used to study the effect on IOP. Glibenclamide, pilocarpine, and indomethacin (1%) were used to study the mechanism of action of both drugs. The IOPs were measured just prior to drug instillation and at suitable time intervals using a tonometer.
Results:
Pretreatment with topical nicorandil and pinacidil significantly lowered the rise in IOP in the acute model. Nicorandil and pinacidil initially caused rise in IOP for 15–30 minutes in chronic glaucoma. This was followed by reduction in IOP. Pretreatment with indomethacin and pilocarpine did not modify the effect of nicorandil and pinacidil on IOP. Pretreatment with glibenclamide blocked IOP from the lowering effect of nicorandil and pinacidil.
Conclusion:
The oculohypotensive effect shown by these drugs appears to be attributable to enhancement of the aqueous humor outflow. This effect is perhaps mediated through potassium channels.
Keywords: calcium channels, IOP, nicorandil, pinacidil, potassium channels
1. Introduction
Glaucoma is a multifactorial disease with a number of elements contributing to its development. An elevation of intraocular pressure (IOP) is a prominent component in optic nerve damage, which is the hallmark of glaucoma. If the elevated IOP is inadequately treated, progressive blindness may result.
Various drugs used in treatment of glaucoma are parasympathomimetics, β-adrenoceptor antagonists, carbonic anhy-drase inhibitors, α2-adrenoceptor agonists, prostaglandin analogues, and angiotensin-converting enzyme inhibitors.1,2 Timolol eye drops are the golden standard in the treatment of glaucoma. However, timolol is also known to get into the systemic circulation and causes various systemic effects.3 Although glaucoma is known to be a serious chronic eye disease, an ideal agent to be used in this disease is still not available, and there has been a constant urge for the discovery of newer drugs.
The steady levels of IOP are the result of balanced aqueous humor formation and outflow. Calcium flux could have several effects on aqueous humor dynamics, including a hydrostatic component, a result of arterial blood pressure and ciliary body perfusion, and an osmotic component, which is a result of an effect on the active secretion of sodium, calcium, and other ions by ciliary epithelium.4 It has been reported that functional K+ dependent ATP channels are present in the trabecular meshwork, and their activation by potassium channel openers can increase outflow facility through the trabecular meshwork.5 Reports have indicated the beneficial effects of diazoxide and nicorandil on K+ dependent ATP channel-mediated IOP regulation.6 Cromakalim and nicorandil were reported as occulohypotensive in normotensive, hypotensive, and hypertensive rabbits.7 According to Food and Drug Administration reports, 0.0353% patients suffer from glaucoma after treatment with nicorandil in various disease conditions.8 In the present study, we investigated the effect of potassium channel openers, pinacidil and nicorandil, and their interaction with a potassium channel blocker, glibenclamide, for their effect on IOP. Furthermore, the possible mechanisms of action of these agents were also studied.
2. Materials and methods
2.1. Experimental animals
New Zealand white rabbits of either sex weighing 1.5–2.5 kg (Zydus Research Center, Ahmedabad, India) were housed under well-controlled conditions: temperature, 22 ± 2°C; humidity, 55 ± 5%; and 12/12-hour light/dark cycle. They were given access to food and water ad libitum. The protocol of the experiment was approved by the Institutional Animal Ethical Committee as per the guidance of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Social Justice and Empowerment, Government of India.
2.2. Acute glaucoma model
The basal IOP was measured using a Schiotz-type indentation tonometer. The drug solutions were prepared in suitable solvents and were instilled topically into the left eye. The drug solutions used in the study were nicorandil (1% in phosphate buffer, pH 7.4), pinacidil (1% in PEG 400), glibenclamide (1% in PEG 400), and pilocarpine (1%; FDC Ltd., Maharastra, India). The right eye, which served as control, received the vehicle. After 15 minutes of drug administration, 5% dextrose solution (15 mL/kg) was intravenously (i.v.) infused through the marginal ear vein up to 15 minutes. Six rabbits (of either sex) were taken to study the effect of each drug. A 5% dextrose infusion induced an acute increase in IOP in both eyes of all rabbits under study. IOP was recorded every 15 minutes until the pressure became normal.9
2.3. Chronic glaucoma model
Albino rabbits were sedated with diazepam (1 mg/kg, i.v.) and anesthetized with ketamine (25 mg/kg, i.v.). Fresh α-chymotrypsin (50 units; Sigma Life Sciences, Missouri, USA) solution prepared in 0.1 mL of sterile saline was irrigated through the cannula into the posterior chamber.9 The debris of tissue blocked the pathway of the aqueous humor outflow in the trabecular meshwork to induce ocular hypertension.10 α-Chymotrypsin was administered in 36 rabbit eyes. From this, we have successfully increased IOP in 27 eyes. Rabbit with increased IOP above 30 mmHg were selected and used to determine the effect of drugs on IOP. After a steady elevated IOP was achieved, similar drug solutions as in acute glaucoma model were administered topically into the left eye, whereas the right eye served as control. The IOPs were measured at time 0 (just before eye drop instillation), 15, 30, 45, 60, 75, 90, 120, 180, 240, 300, and 360 minutes using a tonometer.
2.4. Studies on interaction of glibenclamide with nicorandil and pinacidil in acute glaucoma model in rabbits
The basal IOP was measured in both eyes. After 15 minutes of glibenclamide instillation in the left eye, nicorandil or pinacidil were instilled topically. The right eye served as control. The acute glaucoma model was produced using the procedure described above.9 IOP was recorded with a tonometer every 15 minutes until the pressure became normal.
2.5. Studies on interaction of glibenclamide with nicorandil and pinacidil in chronic glaucoma model in rabbits
Rabbits with α-chymotrypsin-induced glaucoma were selected for the study. The IOP was initially recorded with the help of a tonometer. The interaction study was carried out similarly as in the acute model.
2.6. Studies on interaction of indomethacin and pilocarpine with nicorandil and pinacidil in chronic glaucoma model in rabbits
Rabbits with α-chymotrypsin-induced glaucoma were selected for the study. The IOP was initially recorded with the help of a tonometer. Indomethacin (1%; Sterfil Laboratories Pvt. Ltd., Maharastra, India) prostaglandin inhibitor was topically administered to the left eye. The right eye served as control. After 45 minutes of administration of indomethacin, nicorandil or pinacidil was instilled topically. The changes in IOPs were recorded at suitable time intervals using a tonometer. Indomethacin was replaced with pilocarpine to study the interaction of pilocarpine with nicorandil and pinacidil.
These formulations were first tested in rabbit's eyes for ocular safety. No ill effects were observed.
2.7. Statistical analysis
Paired Student t test was used for determining the statistical significance of most of the data at the probability level of 95%. A split-plot analysis of variance was carried out for studying the time-dependent interaction between the drugs under study and other drugs.
3. Results
An acute elevation in IOP of up to 30–35 mmHg was observed when 5% dextrose (15 mL/kg) was administered intravenously. Potassium channel blocker, glibenclamide (1%) partially reversed IOP lowering effect of nicorandil (1%) and pinacidil (1%) in acute glaucoma in rabbits (Figures 1 and 2, respectively).
Figure 1.
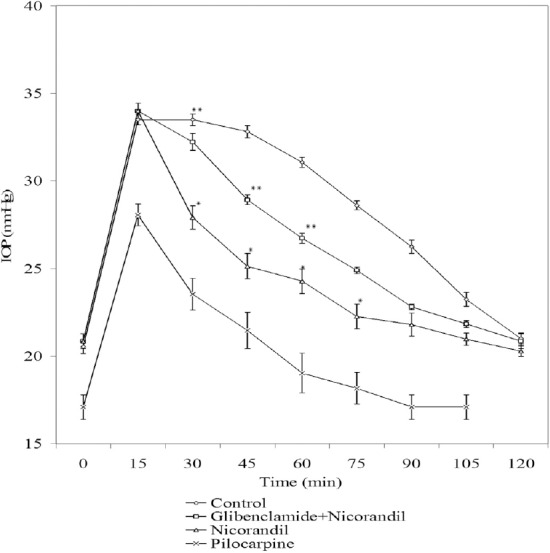
Effect of nicorandil (1%), [nicorandil (1%) + glibenclamide (1%)], and pilocarpine (1%) on IOP in acute glaucoma model in rabbits. Each point and bar represents mean ± SEM of six observations. * Significantly different from control (p < 0.05). ** Significantly different from nicorandil (p < 0.05). IOP = intraocular pressure; SEM = standard error of the mean.
Figure 2.
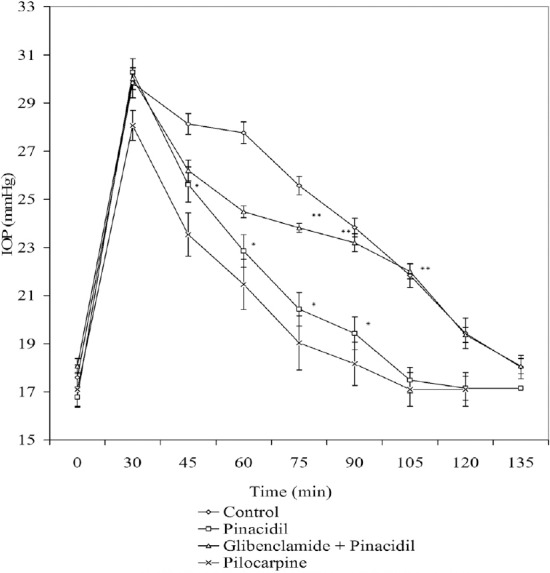
Effect of pinacidil (1%), [pinacidil (1%) + glibenclamide (1%)], and pilocarpine (1%) on IOP in acute glaucoma model in rabbits. Each point and bar represents mean ± SEM of six observations. * Significantly different from control (p < 0.05). ** Significantly different from pinacidil (p < 0.05). IOP = intraocular pressure; SEM = standard error of the mean.
The topical administration of nicorandil in animals with α-chymotrypsin-induced ocular hypertension produced a significant drop in IOP (from 33.67 ± 0.31 mmHg to 20.10 ± 0.01mmHg) after an initial rise (from 33.67 ± 0.31 mmHg to 42.17 ± 0.07 mmHg), and the topical administration of pinacidil in rabbits with α-chymotrypsin-induced occular hypertension produced a significant drop in IOP (from 33.93 ± 0.43 mmHg to 21.30 ± 0.30 mmHg) after an initial rise (from 33.93 ± 0.43 mmHg to 38.77 ± 0.84 mmHg) in IOP (Figures 3 and 4, respectively).
Figure 3.
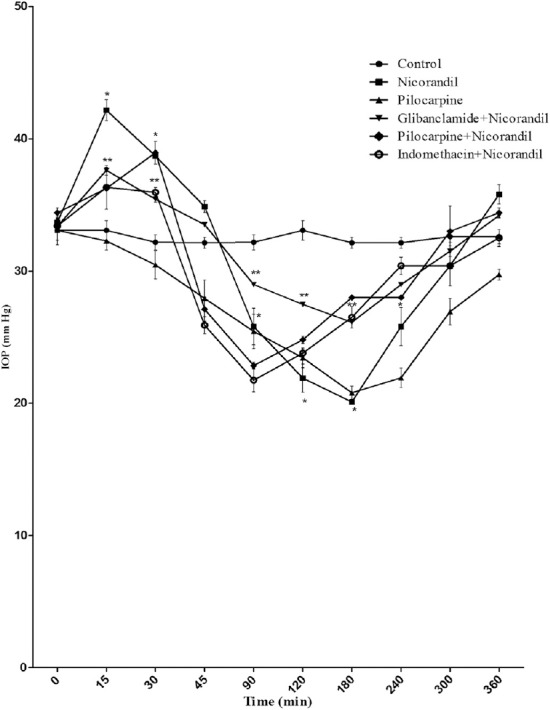
Effect of nicorandil (1%), [glibenclamide (1%) + nicorandil (1%)], [pilocarine (1%) + nicorandil (1%)], [indomethacine (1%) + nicorandil (1%)], and pilocarpine (1%) on IOP in rabbits with α-chymotrypsin-induced ocular hypertension. Each point and bar represents mean ± SEM of six observations. * Significantly different from control (p < 0.05). ** Significantly different from nicorandil (p < 0.05). IOP = intraocular pressure; SEM = standard error of the mean.
Figure 4.
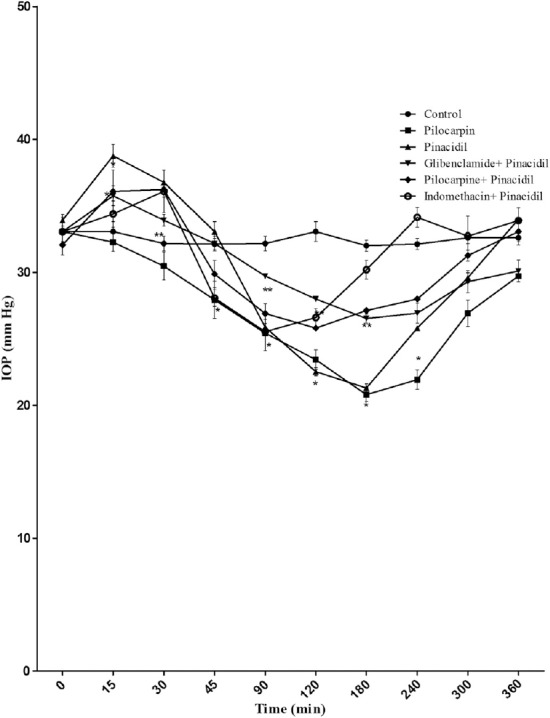
Effect of pinacidil (1%), [glibenclamide (1%) + pinacidil (1%)], [pilocarine (1%) + pinacidil (1%)], [indomethacine (1%) + pinacidil (1%)], and pilocarpine (1%) on IOP in rabbits with α-chymotrypsin-induced ocular hypertension. Each point and bar represents mean ± standard error of the mean of six observations. * Significantly different from control (p < 0.05). ** Significantly different from pinacidil (p < 0.05). IOP = intraocular pressure; SEM = standard error of the mean.
Glibenclamide reversed the OP-lowering effect of nicorandil and pinacidil in rabbits with α-chymotrypsin-induced chronic glaucoma (Figures 3 and 4, respectively). Interaction with indomethacin (1%) or pilocarpine (1%) did not produce a significant change in the IOP-lowering effect of nicorandil and pinacidil (Figures 3 and 4, respectively).
4. Discussion
Glaucoma is an eye disease with elevated IOP as a prominent and hallmark component. It has been reported that glaucoma has vascular roots, as it is more prevalent in cardiovascular and cerebrovascular diseases,11,12 and in Raynaud-like peripheral circulation disease.13
We have studied the occulohypotensive action of potassium channel openers, nicorandil and pinacidil, in experimentally induced acute and chronic glaucomatic rabbit models. Oral water loading and the more advantageous alternative, 5% dextrose infusion through the marginal ear vein, comprise one of the easiest, fastest, and reliable techniques to screen antiglaucoma agents. Oral water loading or 5% dextrose infusion leads to reduction in blood osmolality, which leads to transfer of water into the eye, causing elevation of IOP.14 We have studied the oculohypotensive effect of potassium channel openers, nicorandil and pinacidil. In the acute model of glaucoma, nicorandil and pinacidil induced a significant drop in IOP. Chronic and stable elevation of IOP was achieved by introducing α-chymotrypsin into the posterior chamber of the rabbit eye. The sustained elevation in IOP was triggered by an inflammatory reaction in the trabecular meshwork.15 In rabbits with α-chymotrypsin-induced glaucoma tic, nicorandil and pinacidil initially caused IOP to increase for 15–30 minutes, which was later followed by a reduction in IOP.
We studied the interaction of glibenclamide with nicorandil and pinacidil. The topical administration of glibenclamide had significantly reversed the fall in IOP induced by nicorandil or pinacidil in acute as well as chronic models of glaucoma. This indicates that nicorandil and pinacidil may exert their oculohypotensive effect by affecting the potassium channels, which is in accordance with the reports of Ielly and Walley16 and Santafe et al.17
Prostaglandin analogs are known to reduce IOP by increasing the uveoscleral outflow.18 Pilocarpine reduces the uveoscleral outflow by providing a stretching of the scleral spur through which PGF2a reduces IOP.19 Pilocarpine is reported to decrease the uveoscleral outflow by an opposite mechanism of action to prostaglandin analogues, and it may cause a paradoxical rise in IOP in patients who depend on the secondary drainage pathway.20 To explore the IOP-lowering mechanism of nicorandil and pinacidil, we have studied the interaction of indomethacin and pilocarpine with these drugs. In the interaction of indomethacin and pilocarpine with nicorandil and pinacidil, a topical administration of indomethacin was unable to alter the fall in IOP induced by nicorandil or pinacidil. Pilocarpine failed to modify the oculohypotensive effects produced by nicorandil or pinacidil. This suggests the noninvolvement of the uveoscleral pathway in lowering the IOP by nicorandil and pinacidil.
Potassium channel openers may inhibit the ability of intracellular Ca2+ stores to refill after Ca2+ release has occurred.21 Opening of K+ channels by potassium channel openers hyperpolarizes the membrane and thus decreases the Ca2+ concentration and tendency for the membrane to become depolarized by excitatory transmitters.
Calcium channel blockers cause vasodilation and thus reduce vascular resistance. They increase the capillary blood in the optic nerve head.22,23,24 This may make them suitable for trial in the treatment of low-tension glaucoma. L-type and T-type calcium channels seem to play a role in cellular growth, and thus calcium antagonists can inhibit the growth and proliferation of vascular smooth muscle and fibroblasts. Calcium antagonists may also inhibit the synthesis of extracellular-matrix collagen proteins, suggesting a beneficial effect in glaucoma.25
Santafe et al17 reported that calcium channel blockers (CCBs) decrease aqueous humor secretion, although they also cause a slight but significant reduction of tonographic outflow facility. Moreover, the reduced outflow of aqueous humor caused by raised episcleral venous pressure may be directly increased by calcium inhibition.4 Furthermore, Gap junctions, possibly regulated by calcium, exist between nonpigmented and pigmented ciliary epithelial cells. Verapamil may interfere with these gap junctions, altering the cellular permeability of the ciliary epithelium and thus inhibiting normal aqueous humor formation.26
The synthesis of aqueous humor is dependent on the catalytic formation of HCO– from CO2 in ciliary processes, in which Na+ is possibly linked to HCO3– movement.27 Wolosin et al28 reported that the transport of HCO3– is related to the concentration of potassium ion when the extracellular potassium is increased. The intracellular pH is also increased, which causes cell depolarization and elicited a Na+-HCO– influx to decrease the formation of aqueous humor.28 Nicorandil and pinacidil are potassium channel activators, which increase the cell membrane permeability to potassium and produce membrane hyperpolarization. As the effect is opposite to cell depolarization, the formation of aqueous humor is increased. Another possible mechanism is that the aqueous humor formation is related to Na+/K+-ATPase, the enzyme distributed in the epithelium layer of ciliary body. H+ is formed by the catalysis of carbonic anhydrase and exchanged with Na+ of the extracellular fluid. Then, intracel-lular sodium is exchanged with K+ in the posterior chamber to produce an osmotic pressure. Consequently, water enters the posterior chamber of the eye to form aqueous humor.29,30 Hong et al31 reported that cromakalim indirectly activates the Na+/K+-ATPase, which is inhibited by ouabain. Thus, the mechanism of potassium channel openers under study–i.e., nicorandil and pinacidil–was found to be similar to that of cromakalim. This might explain the increase in IOP during the initial period in chronic models of glaucoma after the topical administration of nicorandil and pinacidil. The possible mechanism for the lowering of IOP may be same as that of CCBs. However CCBs are also reported for elevation of IOP.32
Because of the initial rise in IOP even in ocular hypertensive rabbits, it may not be possible to infer whether or not potassium channel openers are the right candidates for trial in glaucoma.
In conclusion, our data suggest that nicorandil and pinacidil produced an initial rise in IOP. This may be attributable to the increase in aqueous humor formation. The oculohypotensive effect shown by these drugs appears to be due to enhancement of aqueous humor outflow. This effect is perhaps mediated through potassium channels.
Acknowledgments
We thank Zydus Research Centre, India for gifting samples of nicorandil and glibenclamide, and for gratis supply of animals for this research work. We also thank Leo Pharmaceuticals Ltd., Denmark for providing us with sample of pinacidil.
Footnotes
Conflicts of interest: No competing financial interest exists.
References
- 1.Whitson JT. Glaucoma: a review of adjunctive therapy and new management strategies. Expert Opin Pharmacother. 2007;8:3237–3249. doi: 10.1517/14656566.8.18.3237. [DOI] [PubMed] [Google Scholar]
- 2.Shah GB, Sharma S, Mehta AA, Goyal RI. Oculohypotensive effect of angiotensin-converting enzyme inhibitors in acute and chronic models of glaucoma. J Cardiovasc Pharmacol. 2000;36:169–175. doi: 10.1097/00005344-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Uusitalo H, Kähönen M, Ropo A, et al. Improved systemic safety and risk–benefit ratio of topical 0.1% timolol hydrogel compared with 0.5% timolol aqueous solution in the treatment of glaucoma. Graefe's Arch Clin Exp Ophthalmol. 2006;244:1491–1496. doi: 10.1007/s00417-006-0328-0. [DOI] [PubMed] [Google Scholar]
- 4.Brubaker RF. The physiology of aqueous humor formation. In: Drance SM, Neufeld AH, editors. Glaucoma. Applied Pharmacology in Medical Treatment. Orlando, FL: Grune and Stratton Inc; 1984. pp. 35–70. [Google Scholar]
- 5.Chowdhury UR, Bahler CI, Hann CR, et al. ATP-sensitive potassium (IATP) channel activation decreases intraocular pressure in the anterior chamber of the eye. Invest Ophthalmol Vis Sci. 2011;52:6435–6442. doi: 10.1167/iovs.11-7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhury UR, Holman BH, Fautsch MP. ATP-sensitive potassium (IATP) channel openers diazoxide and nicorandil lower intraocular pressure in vivo. Invest Ophthalmol Vis Sci. 2013;54:4892–4899. doi: 10.1167/iovs.13-11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang CH, Lin CH. Effects ofcromakalim and nicorandil on intraocular pressure after topical administration in rabbit eyes. J Ocul Pharmacol Ther. 1995;11:195–201. doi: 10.1089/jop.1995.11.195. [DOI] [PubMed] [Google Scholar]
- 8.Study of possible correlation between glaucoma and nicorandil. [Accessed 14.12.15]. http://factmed.com/study-NICORANDIL-causing-GLAUCOMA.php .
- 9.Mehta A, Iyer L, Parmar S, Shah G, Goyal R. Oculohypotensive effect of peri-ndopril in acute and chronic models of glaucoma in rabbits. Can J Physiol Pharmacol. 2010;88:595–600. doi: 10.1139/y10-026. [DOI] [PubMed] [Google Scholar]
- 10.Ling Z, Thompson WY, Potter DE. Multiple cyclic nucleotide phosphodiester-ases in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1999;40:1745–1752. [PubMed] [Google Scholar]
- 11.Drance SM. Anterior chamber shallowing (Letter) Arch Ophthalmol. 1973;90:512. doi: 10.1001/archopht.1973.01000050512027. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg I. Optic disc and visual field changes in primary open angle glaucoma. Aust J Ophthalmol. 1981;9:223–229. doi: 10.1111/j.1442-9071.1981.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 13.Gasser P, Flammer J. Blood-cell velocity in the nailfold capillaries of patients with normal-tension and high-tension glaucoma. Am JOphthalmol. 1991;111:585–588. doi: 10.1016/s0002-9394(14)73703-1. [DOI] [PubMed] [Google Scholar]
- 14.Bonomi L, Tomazzoli L, Jaria D. An improved model of experimentally induced ocular hypertension in the rabbit. Invest Ophthalmol. 1976;15:781–784. [PubMed] [Google Scholar]
- 15.Sears D, Sears M. Blood-aqueous barrier and alpha-chymotrypsin glaucoma in rabbits. Am J Ophthalmol. 1974;77:378–383. doi: 10.1016/0002-9394(74)90744-2. [DOI] [PubMed] [Google Scholar]
- 16.Ielly SP, Walley TJ. Effect of calcium antagonist nifedipine on intraocular pressure in normal subjects. Br J Opthalmol. 1988;72:216–218. doi: 10.1136/bjo.72.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santafe J, Martinez-de-Ibarreta MJ, Segarra J, Melena J. A long lasting hy-potensive effect of topical diltiazem on the intraocular pressure in conscious rabbits. Naunyn-Schmiedebergs Arch Pharmacol. 1997;355:645–650. doi: 10.1007/pl00004996. [DOI] [PubMed] [Google Scholar]
- 18.Crawford I, Iaufman PL. Pilocarpine antagonizes prostaglandin F2 alpha-induced ocular hypotension in monkeys. Evidence for enhancement of Uveoscleral outflow by prostaglandin F2 alpha. Arch Ophthalmol. 1987;105:1112–1116. doi: 10.1001/archopht.1987.01060080114039. [DOI] [PubMed] [Google Scholar]
- 19.Gupta SK, Agarwal R, Galpalli ND, et al. Comparative efficacy of pilocarpine, timolol and latanoprost in experimental models of glaucoma. Methods Find Exp Clin Pharmacol. 2007;29:665–671. doi: 10.1358/mf.2007.29.10.1147765. [DOI] [PubMed] [Google Scholar]
- 20.Shioma LO, Costa VP. Parasympathomimetics. In: Hitchings RA, Shaarawy TM, Sherwood MB, Crowston, editors. Glaucoma: Medical Diagnosis and Therapy. Philadelphia: Saunders Elsevier; 2009. pp. 517–523. [Google Scholar]
- 21.Bray KM. Further studies on the actions of the K+ channel opener cromakalim and pinacidil in rabbit aorta. Pfluegers Arch. 1988:411–R202. [Google Scholar]
- 22.Netland PA, Ericson KA. Calcium channel blockers in glaucoma management. Ophthalmol Clin N Am. 1995;8:327–334. [Google Scholar]
- 23.Monica ML, Hesse RJ, Messerli FH. The effect of a calcium-channel blocking agent on intraocular pressure. Am JOphthalmol. 1983;96:814. doi: 10.1016/s0002-9394(14)71934-8. [DOI] [PubMed] [Google Scholar]
- 24.Abelson MB, Gilbert CM, Smith LM. Sustained reduction of intraocular pressure in humans with the calcium channel blocker verapamil. Am J Ophthalmol. 1988;105:155–159. doi: 10.1016/0002-9394(88)90179-1. [DOI] [PubMed] [Google Scholar]
- 25.Roth M, Eickerlberg O, Kohler E, Erne P, Block LH. Ca2+ blockers modulate metabolism of collagens within the extracellular matrix. Proc Natl Acad Sci U S A. 1996;93:5478–548. doi: 10.1073/pnas.93.11.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green K, Bountra C, Georgiou P, House CR. An electophysiologic study of rabbit ciliary epithelium. Invest Ophthalmol Vis Sci. 1985;6:371. [PubMed] [Google Scholar]
- 27.Maren TH. Carbonic anhydrase: general perspectives and advances in glaucoma research. Drug Dev Res. 1987;10:255–276. [Google Scholar]
- 28.Wolosin JM, Bonano JA, Hanzel D, Machen TE. Bicarbonate transport mechanisms in rabbit ciliary body epithelium. Exp Eye Res. 1991;5:397–407. doi: 10.1016/0014-4835(91)90035-d. [DOI] [PubMed] [Google Scholar]
- 29.Berggren L. Direct observation of secretory pumping in vitro of the rabbit eye ciliary processes: influence of ion milieus and carbonic anhydrase inhibition. Invest Ophthalmol Vis Sci. 1964;3:266–271. [PubMed] [Google Scholar]
- 30.Coltier E. Bicarbonate ATP-ase in ciliary body and a theory of Diamox effect on aqueous humor formation. Int Ophthalmol. 1979;1:123–128. doi: 10.1007/BF00154199. [DOI] [PubMed] [Google Scholar]
- 31.Hong KW, Park MG, Shin YW, Rhim BY, Ko KH. Effect of ouabain on relaxation induced by cromakalim in human and canine mesenteric arteries. Eur JPhar-macol. 1993;231:1–6. doi: 10.1016/0014-2999(93)90676-9. [DOI] [PubMed] [Google Scholar]
- 32.Beatty JF, Krupin T, Nicholas PF, Becker B. Elevation of intraocular pressure by calcium channel blockers. Arch Opthlamol. 1984;102:1072–1076. doi: 10.1001/archopht.1984.01040030866035. [DOI] [PubMed] [Google Scholar]


