Abstract
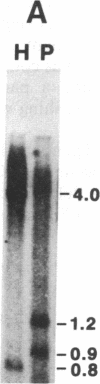
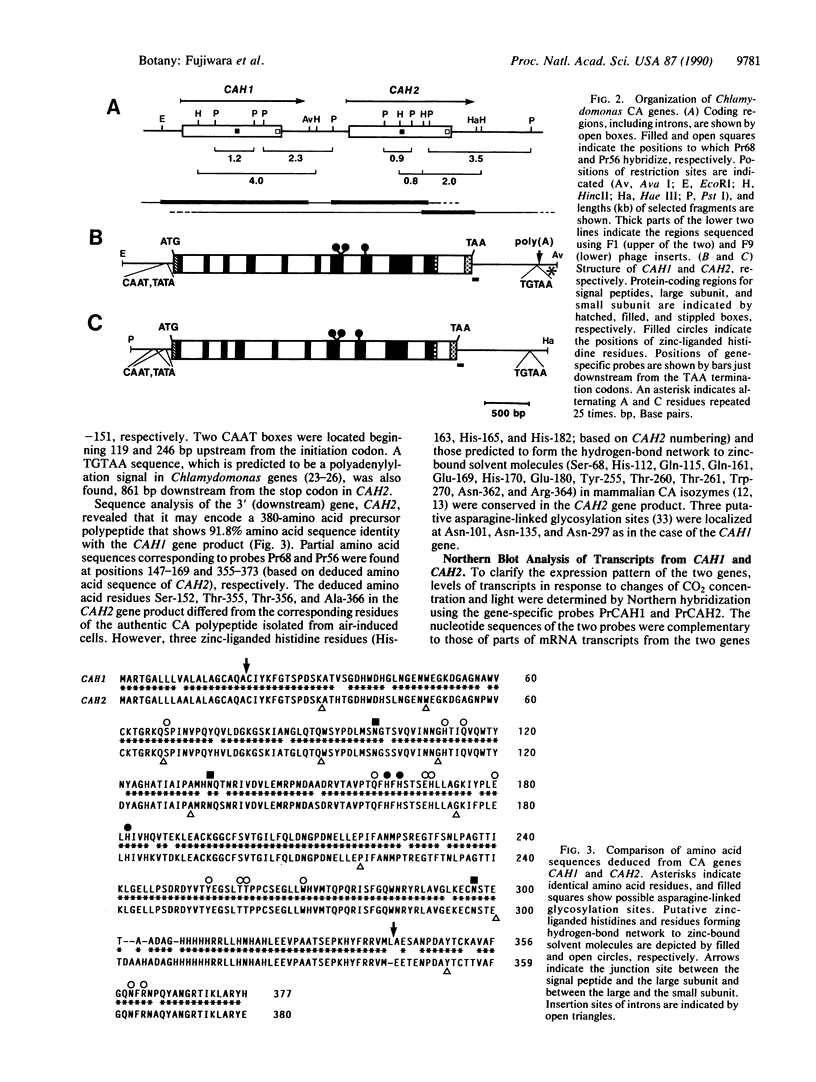
Two copies of structurally related genes (CAH1 and CAH2) for carbonic anhydrase (EC 4.2.1.1) were found to be tandemly clustered on the Chlamydomonas reinhardtii genome. The previously isolated cDNA clones for carbonic anhydrase polypeptides were derived from the upstream gene, CAH1, which has 10 introns in its coding region. The downstream gene, CAH2, also has 10 introns, at positions identical to those of CAH1. Although amino acid sequences deduced from the two genes showed 91.8% identity, partial sequences of the authentic enzyme isolated from air-induced cells were identical only to those of the CAH1 product. Northern hybridization using gene-specific probes showed that the level of 2.0-kilobase CAH1 mRNA increased in response to a decrease in CO2 concentration in the presence of light. The CAH1 mRNA did not accumulate when CO2 was lowered in the dark. In contrast, the level of 2.0-kilobase CAH2 mRNA decreased in response to lowering of CO2 and increased upon transfer to the high-CO2 condition in light. The decrease of CAH2 mRNA under the low-CO2 condition was not observed in the dark. The fully induced mRNA level was much higher for CAH1 than for CAH2. These results indicate that CAH1 is a gene coding for the major periplasmic carbonic anhydrase whose level of transcript is rapidly induced under the low-CO2 condition in the presence of light, and that CAH2 may encode another periplasmic isozyme, which is made under the high-CO2 condition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba S., Mishima H., Miyachi Y. Levels of cyclic-AMP, cyclic-GMP and betamethasone in the aqueous humor following topical administration of betamethasone in rabbit eyes. Hiroshima J Med Sci. 1983 Sep;32(3):301–304. [PubMed] [Google Scholar]
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly J., Coleman J. R. Effect of CO(2) Concentration on Protein Biosynthesis and Carbonic Anhydrase Expression in Chlamydomonas reinhardtii. Plant Physiol. 1988 Aug;87(4):833–840. doi: 10.1104/pp.87.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Coleman J. R., Grossman A. R. Biosynthesis of carbonic anhydrase in Chlamydomonas reinhardtii during adaptation to low CO(2). Proc Natl Acad Sci U S A. 1984 Oct;81(19):6049–6053. doi: 10.1073/pnas.81.19.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa H., Fujiwara S., Yamamoto Y., Dionisio-Sese M. L., Miyachi S. cDNA cloning, sequence, and expression of carbonic anhydrase in Chlamydomonas reinhardtii: regulation by environmental CO2 concentration. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4383–4387. doi: 10.1073/pnas.87.11.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Rahire M. Sequence, evolution and differential expression of the two genes encoding variant small subunits of ribulose bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. J Mol Biol. 1986 Oct 5;191(3):421–432. doi: 10.1016/0022-2836(86)90137-3. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Husic H. D., Kitayama M., Togasaki R. K., Moroney J. V., Morris K. L., Tolbert N. E. Identification of Intracellular Carbonic Anhydrase in Chlamydomonas reinhardtii which Is Distinct from the Periplasmic Form of the Enzyme. Plant Physiol. 1989 Mar;89(3):904–909. doi: 10.1104/pp.89.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamo T., Shimogawara K., Fukuzawa H., Muto S., Miyachi S. Subunit constitution of carbonic anhydrase from Chlamydomonas reinhardtii. Eur J Biochem. 1990 Sep 11;192(2):557–562. doi: 10.1111/j.1432-1033.1990.tb19261.x. [DOI] [PubMed] [Google Scholar]
- Lloyd J., Brownson C., Tweedie S., Charlton J., Edwards Y. H. Human muscle carbonic anhydrase: gene structure and DNA methylation patterns in fetal and adult tissues. Genes Dev. 1987 Aug;1(6):594–602. doi: 10.1101/gad.1.6.594. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. H., Venta P. J., Tashian R. E. Molecular analysis of G+C-rich upstream sequences regulating transcription of the human carbonic anhydrase II gene. Mol Cell Biol. 1987 Dec;7(12):4589–4593. doi: 10.1128/mcb.7.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silflow C. D., Chisholm R. L., Conner T. W., Ranum L. P. The two alpha-tubulin genes of Chlamydomonas reinhardi code for slightly different proteins. Mol Cell Biol. 1985 Sep;5(9):2389–2398. doi: 10.1128/mcb.5.9.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashian R. E. The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays. 1989 Jun;10(6):186–192. doi: 10.1002/bies.950100603. [DOI] [PubMed] [Google Scholar]
- Venta P. J., Montgomery J. C., Hewett-Emmett D., Wiebauer K., Tashian R. E. Structure and exon to protein domain relationships of the mouse carbonic anhydrase II gene. J Biol Chem. 1985 Oct 5;260(22):12130–12135. [PubMed] [Google Scholar]
- Wade R., Gunning P., Eddy R., Shows T., Kedes L. Nucleotide sequence, tissue-specific expression, and chromosome location of human carbonic anhydrase III: the human CAIII gene is located on the same chromosome as the closely linked CAI and CAII genes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9571–9575. doi: 10.1073/pnas.83.24.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoshihara C. M., Lee J. D., Dodgson J. B. The chicken carbonic anhydrase II gene: evidence for a recent shift in intron position. Nucleic Acids Res. 1987 Jan 26;15(2):753–770. doi: 10.1093/nar/15.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblom J., Schloss J. A., Silflow C. D. The two beta-tubulin genes of Chlamydomonas reinhardtii code for identical proteins. Mol Cell Biol. 1984 Dec;4(12):2686–2696. doi: 10.1128/mcb.4.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer W. E., Schloss J. A., Silflow C. D., Youngblom J., Watterson D. M. Structural organization, DNA sequence, and expression of the calmodulin gene. J Biol Chem. 1988 Dec 25;263(36):19370–19383. [PubMed] [Google Scholar]