Abstract
Brain damage following cerebral ischemia-reperfusion (I/R) is a complicated pathophysiological course, in which inflammation and oxidative stress have been suggested to serve an important role. Toll-like receptor 4 (TLR4) has been suggested to be involved in secondary inflammatory process in cerebral ischemia. Nuclear factor erythroid 2-related factor 2 (Nrf2), an important regulator of the antioxidant host defense, maintains the cellular redox homeostasis. Tissue kallikrein (TK) has been proven to elicit a variety of biological effects in ischemic stroke through its anti-inflammatory and anti-oxidant properties. However, the mechanisms underlying its beneficial effects remain poorly defined. The present study examined the hypothesis that TK attenuates ischemic cerebral injury via the TLR4/nuclear factor-κB (NF-κB) and Nrf2 signaling pathways. Using a transient rat middle cerebral artery occlusion (MCAO) model, the effects of immediate and delayed TK treatment subsequent to reperfusion were investigated. The neurological deficits, infarct size, and the expression of TLR4/NF-κB and Nrf2 pathway in ischemic brain tissues were measured at 24 following MCAO. The results indicated that TK immediate treatment significantly improved neurological deficits and reduced the infarct size, accompanied by the inhibition of TLR4 and NF-κB levels, and the activation of Nrf2 pathway. Furthermore, TK delayed treatment also exerted neuroprotection against I/R injury. However, the neuroprotective effect of TK immediate treatment was better compared with that of TK delayed treatment. In conclusion, the results indicated that TK protected the brain against ischemic injury in rats after MCAO through its anti-oxidative and anti-inflammatory effects. Suppression of TLR4/NF-κB and activation of the Nrf2 pathway contributed to the neuroprotective effects induced by TK in cerebral ischemia. Therefore, TK may provide an effective intervention with a wider therapeutic window for ischemic stroke.
Keywords: tissue kallikrein, cerebral infarction, neuroprotection, inflammation, oxidative stress
Introduction
Stroke is the leading cause of mortality and disability in adults in China (1). To date, few therapeutic options are available for the treatment of ischemic stroke, and recanalization following ischemia is the most effective method for ischemic stroke (2). However, it benefits only a small proportion of patients due to the limited thrombolysis window and the adverse reactions of thrombolytics (2,3). In addition, recanalization may cause severe cerebral ischemia-reperfusion (I/R) injury in the local region (4,5). Accumulating evidence has suggested that inflammatory and oxidative damage serves an important role in cerebral I/R injury (6–8). Excessive inflammatory and oxidative responses lead to the disruption of the blood-brain barrier (BBB), which further aggravates the progression of ischemic injury subsequent to stroke (9–12). Therefore, it is essential to identify alternative therapies for protection against I/R damage, such as the use of agents with anti-inflammatory and anti-oxidant effects. Furthermore, it is urgently necessary to establish new interventions outside of the thrombolysis time window in order to save the hypoperfused, nonfunctional, but still viable brain tissue surrounding the core area of the irreversible cerebral infarction.
TLR4 is the first reported mammalian toll-like receptor, which is located on the cell surface and is functionally similar to Drosophila Toll protein (13). Within the brain, TLR4 is mainly located on glial cells, including microglia, astrocytes and oligodendrocytes (14). During the course of brain damage caused by I/R, it is believed that heat shock proteins (HSPs), such as HSP60, HSP70 and gp90, are upregulated and leak into the extracellular compartment to induce immune response and inflammatory response. Binding of HSPs with TLR4 contributes to myeloid differentiation protein-88 (MyD88) recruitment (15). The TLR4-MyD88 association further activates IL receptor-associated kinase (IRAK), which promotes the transcription and expression of tumor necrosis factor-α (TNF-α), interleukin (IL)-1 and IL-6 (16). Several studies have revealed that targeting TLR4 may alleviate brain damage caused by cerebral I/R (17–19). For instance, TLR4 knockout mice have significantly smaller infarct volume at reperfusion 24 h after 2 h of ischemia (17). Therefore, inhibiting TLR4 and reducing its downstream signaling pathways is regarded as a promising therapeutic strategy.
Nuclear factor erythroid 2-related factor 2 (Nrf2), a regulator of the antioxidant cell defense system, is inactive in the cytoplasm by Kelch-like ECH-associated protein 1 via the formation of a covalent complex (20). Once activated, Nrf2 translocates into the nucleus, interacts with a small Maf protein, and forms a heterodimer that binds to antioxidant response element, which contributes to cytoprotection in oxidative stress-induced injury with cerebral ischemia (21). Furthermore, previous evidence suggests that mice lacking of Nrf2 are more vulnerable to the cytotoxic effects of oxidative stress-induced brain injury in comparison with wild-type mice (22). Thus, Nrf2 is fundamental to the defense against oxidative stress and may be a promising therapeutic target in stroke.
The kallikrein-kinin system (KKS), composed of kallikrein, kininogen, kinin and kinin receptors, is implicated in multiple pathological states and represents an attractive therapeutic target in ischemic stroke. As an important component of the KKS, tissue kallikrein (TK) cleaves low-molecular-weight kininogen to produce the potent vasoactive kinins (23). Intact kinins bind to the kinin B2 receptor and lead to a series of biological effects (24). It has been well documented that TK exerts protective effects against stroke (24,25). The local or systemic delivery of human TK protects against ischemic brain injury by inhibiting inflammation and oxidative stress, and by promoting angiogenesis and neurogenesis (24,25). Based on these studies, it is hypothesized that TK may protect against ischemic via the TLR4/nuclear factor-κB (NF-κB) and Nrf2 pathways. Therefore, the aim of the present study was to investigate the potential effects of TK in ischemic stroke and explore whether the therapeutic benefit of TK was associated with the activities of TLR4, NF-κB and Nrf2.
Materials and methods
Test animals
All experiments involving animals and tissue samples were performed according to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University (Nanjing, China). A total of 60 male Sprague-Dawley rats (aged 8–10 weeks; weight, 250–300 g) were provided by the Experimental Animal Center in Nanjing Medical University. Prior to the experiments, rats were housed for at least 1 week in their home cages at a constant temperature (18–22°C), with controlled illumination (12 h light/dark cycles) and humidity (30–50%), and ad libitum access to food and water.
Middle cerebral artery occlusion (MCAO) model in rats
Rats were anesthetized intraperitoneally with 10% chloral hydrate (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China; 300 mg/kg) and subjected to MCAO as previously described (26). In brief, the right common carotid artery, the external carotid artery (ECA) and the internal carotid artery (ICA) were exposed and carefully isolated. A 3–0 monofilament nylon suture with a heat-rounded tip was inserted from the lumen of the ECA to the right ICA to occlude the origin of the right middle cerebral artery. Rats were subjected to 2 h of occlusion and then the filament was withdrawn to restore blood flow. Sham-operated rats were manipulated in the same surgical procedures, but without insertion of the filament into the ICA. Body temperature was maintained at 37±5°C with a heating pad.
Drug treatments
Rats (n=15 per group) were treated with TK (Techpool Bio-Pharma Co., Ltd, Canton, China) with 0.9% saline solution through the tail-vein at a dose of 8.75×10−3 PNAU/kg within 3 min. The TK immediate treatment group received TK immediately after the reperfusion, while the TK delayed treatment group received TK at 12 h after reperfusion. In the cases of sham and MCAO model groups, equal volume 0.9% saline were administered in the same manner.
Behavioral tests
All behavioral tests in rats were conducted in a quiet, low-lit room and were evaluated by an investigator blinded to the experimental groups at 24 h after reperfusion (n=10 from each group). Neurologic deficit scores were assigned to each rat according to a previously described scoring system (26), and were as follows: 0, no neurological deficit (normal behavior); 1, mild neurological deficit (failure to fully lift forepaw); 2, moderate neurological deficit (circling to the left); 3, severe neurological deficit (falling to the left); and 4, very severe neurological deficit (failure to walk spontaneously, reduced level of consciousness). The higher the neurological deficit score, the more severe impairment the motor motion injury was. Rats with neurologic deficit scores of 0 and 4 following MCAO were excluded, as this indicated modeling failure.
Determination of infarct volume
At 24 h after MCAO, rats (n=5 from each group) were sacrificed by removing the brains following anesthesia with 10% chloral hydrate (400 mg/kg, i.p.). Brains were dissected and cut into 2-mm thick slices. The slices were incubated in 2% 2,3,5-triphenyltetrazolium chloride (TTC) for 30 min at 37°C, followed by immersion-fixation with 4% paraformaldehyde. A deep red color indicated normal tissue and a pale gray color indicated the infarct area. The stained sections were photographed, and then the digital images were quantified using an Image analysis software, Image Pro-Plus 5.1 (Media Cybernetics, Inc., Rockville, MD, USA). The lesion volume was calculated by multiplying the area by the thickness of slices. Infarct volume in all slices was expressed as a percentage of the contralateral hemisphere after correction for edema, using the following formula (27): Hemisphere lesion volume (%) = [total infarct volume-(volume of intact ipsilateral hemisphere-volume of intact contralateral hemisphere)]/contralateral hemisphere volume × 100%.
Western blot analysis
For western blotting assay, total proteins from the brain cortex samples were obtained using cold RIPA Lysis Buffer (Beyotime Institute of Biotechnology, Haimen, China) containing 1 mM phenylmethylsulfonyl fluoride (PMSF). The homogenates were centrifuged at 12,000 × g for 10 min at 4°C, and the protein concentration in the supernatants was measured using a BCA protein assay kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA; catalogue number 23227). Protein samples (40 µg per lane) were separated by 12% SDS-PAGE and transferred onto polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked with 5% nonfat dried milk for 2 h at room temperature and then incubated at 4°C overnight with the following primary antibodies: Monoclonal mouse anti-TLR4 (1:400; ab30667; Abcam, Cambridge, MA, USA), rabbit anti-Nrf2 (1:1,000; ab31163; Abcam), monoclonal rabbit anti-heme oxygenase 1 (HO-1) (1:10,000; ab68477; Abcam), monoclonal rabbit anti-matrix metallopeptidase 9 (MMP-9; 1:5,000; ab76003; Abcam), rabbit monoclonal NF-κB p65 (1:1,000; cat. no. 8242; Cell Signaling Technology, Inc., Danvers, MA, USA), and β-actin (1:1,000; CMCTAG, Inc., San Diego, CA, USA; catalogue number AT0001). The membranes were subsequently washed three times with Tris-buffered saline and 0.1% Tween-20. Next, membranes were incubated with horseradish peroxidase-conjugated anti-mouse IgG (1:5,000; CWBiotech, Beijing, China; catalogue number CW0102S) or anti-rabbit IgG (1:5,000; CWBiotech; catalogue number CW01035) secondary antibodies for 2 h at room temperature. Immunoreactivity was detected using an enhanced chemiluminescence reaction (EMD Millipore; catalogue number WBKLS0500). Densitometric analysis employed Image J Pro-Plus 6.0 analysis system (NIH, Bethesda, MD, USA). Densitometry values were normalized with respect to the β-actin immunoreactivity in order to correct for any loading and transfer differences among samples. A total of 5 replicates were performed.
Enzyme-linked immunosorbent assay (ELISA)
In order to detect the IL-1β and TNF-α protein expression, tissues from the brain cortex were harvested at 24 h after MCAO and homogenized in 0.1 mM phosphate-buffered saline lysis buffer containing a cocktail of protease inhibitors (Sigma-Aldrich, St. Louis, MO, USA) and 10 nM PMSF. The homogenate was centrifuged at 17,000 × g for 15 min, and the supernatant was collected. The tissue samples were frozen at −80°C immediately until analysis of cytokine protein concentrations. Next, the tissue samples were detected by commercial ELISA kits for IL-1β (Shanghai ExCell Biology Inc., Shanghai, China; catalogue number ER008-48) and TNF-α (Shanghai ExCell Biology, Inc.; catalogue number ER006-96) according to the manufacturer's instructions. Inter- and intra-assay coefficients of variation were <10%.
Statistical analysis
All statistical results are expressed as the mean ± standard error of the mean. Neurological deficit assessment data were analyzed with one-way analysis of variance (ANOVA)-Tukey's multiple comparison test. Other data were analyzed with ANOVA followed by the Student-Newman-Keuls test. Statistically significant differences were considered to be those with P<0.05.
Results
TK improves neurological deficits in rats following cerebral ischemia
To examine whether TK exerted a neuroprotective effect on cerebral ischemia, neurologic deficit scoring was performed at 24 h after cerebral I/R in rats. Compared with the MCAO model group, the immediate and delayed TK treatment groups presented a significant improvement in neurological deficit scores (Fig. 1A; P<0.05), suggesting a neuroprotective effect of TK treatment in acute stroke. Notably, compared with the TK delayed treatment group, the TK immediate treatment group had a more evident reduction of neurological deficit scores at 24 h after cerebral I/R, with a significant difference observed between the two TK groups.
Figure 1.
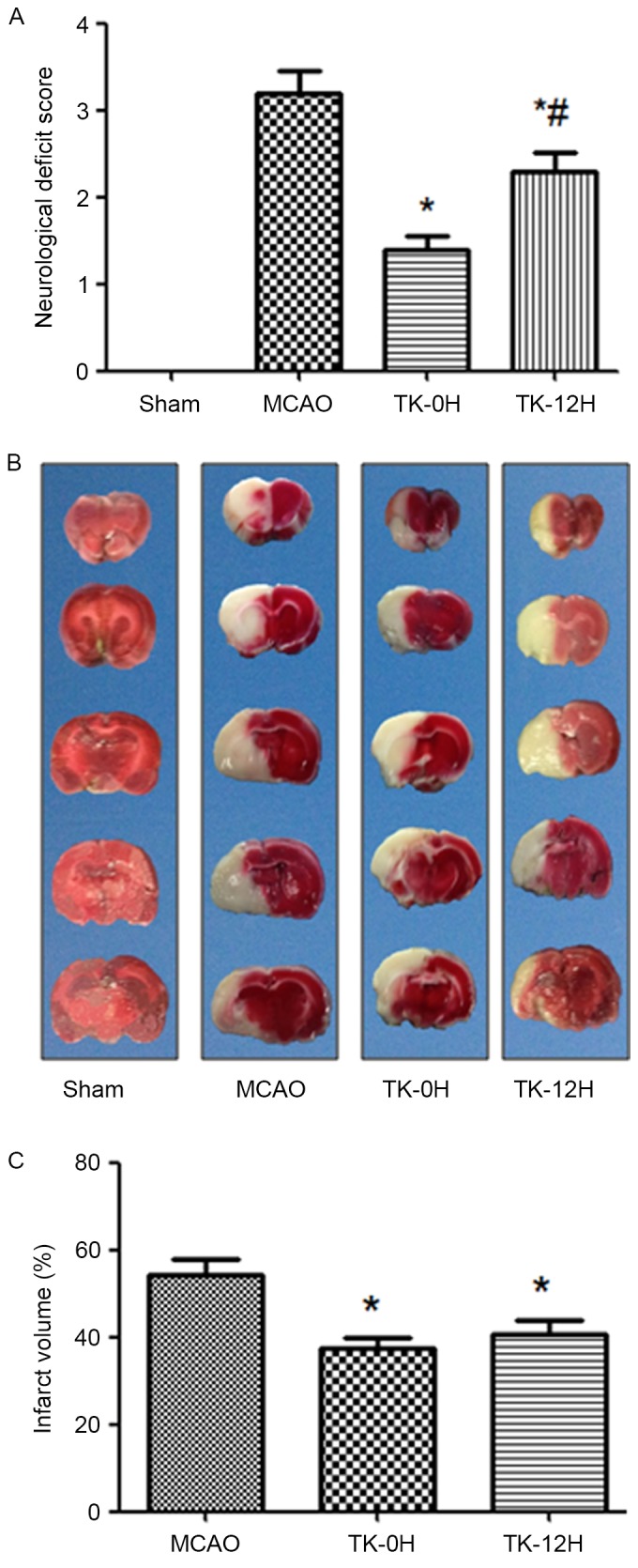
Effects of TK on neurological score and cerebral infarct area at 24 h after reperfusion. (A) Neurological deficit scores at 24 h after reperfusion were decreased upon TK treatment (n=10). (B) Representative images of serial coronal brain sections with TTC staining. The white area of the slices represents the ischemic area, while the red area represents the normal tissue. (C) Statistical results of infarct volumes are shown (n=5). *P<0.05 vs. MCAO group; #P<0.05 vs. TK-0H group. TK, tissue kallikrein; TTC, 2,3,5-triphenyltetrazolium chloride; MCAO, middle cerebral artery occlusion; TK-0H, immediate TK treatment after reperfusion; TK-12H, delayed TK treatment at 12 h after reperfusion.
TK reduces the infarct volume in rats following cerebral ischemia
The cerebral infarction following I/R was detected by TTC staining, as shown in Fig. 1B and C. No evident infarction was observed in the sham group, while an extensive lesion was observed in both the striatum and the cortex in the MCAO model group (Fig. 1B). Compared with the MCAO group, the infarct volume in the TK immediate and delayed treatment groups was significantly decreased (Fig. 1C; P<0.05). However, a tendency towards higher reduction of the infarct volume was observed in the TK immediate treatment group when compared with TK delayed treatment group, but this difference was not statistically significant.
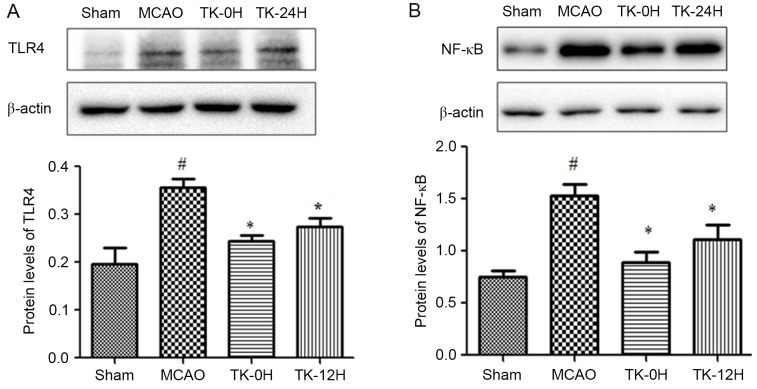
TK decreases inflammatory responses in ischemic brain
Since inflammation is closely associated with cerebral infarction, the present study next sought to examine whether TK exerts an anti-inflammatory effect during the process of cerebral I/R. As depicted in Fig. 2, 24 h after cerebral I/R, the expression levels of TLR4 and NF-κB in ischemic brains were detected by western blot analysis. The results revealed that the protein levels of TLR4 and NF-κB (Fig. 2A and B) were significantly increased following MCAO. However, this effect was markedly ameliorated by TK immediate treatment (Fig. 2A and B; P<0.05). Meanwhile, TK delayed treatment was also able to significantly inhibit the expression levels of TLR4 and NF-κB.
Figure 2.
Effect of TK on the protein levels of (A) TLR4 and (B) NF-κB, demonstrated in the western blots and quantified results. TK reduced TLR4 and NF-κB levels in the ischemic cortex. Data are expressed as the mean ± standard error (n=5 in each group). *P<0.05 vs. MCAO group; #P<0.05 vs. sham group. TK, tissue kallikrein; MCAO, middle cerebral artery occlusion; TK-0H, immediate TK treatment after reperfusion; TK-12H, delayed TK treatment at 12 h after reperfusion; TLR4, Toll-like receptor 4; NF, nuclear factor.
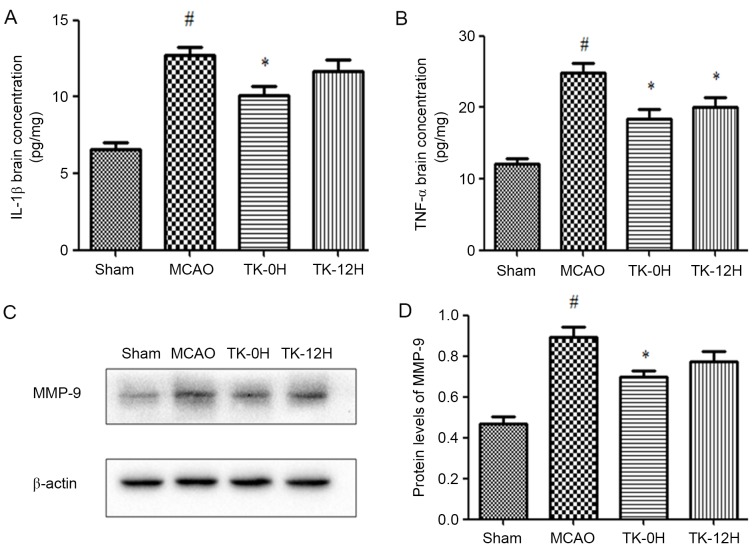
Next, the protein levels of IL-1β and TNF-α in brain tissue were measured by ELISA. The presented results reveal that IL-1β and TNF-α levels were significantly increased in the MCAO model group, in comparison with the sham group (Fig. 3A and B). In consistency with the aforementioned modulation of TLR4 and NF-κB, the levels of IL-1β and TNF-α were significantly decreased in the TK immediate treatment group. Similarly, TK delayed treatment significantly inhibited the level of TNF-α, while exerting no significant effect on the level of IL-1β.
Figure 3.
TK reduces the expression levels of IL-1β, TNF-α and MMP-9. (A) IL-1β and (B) TNF-α concentrations in the brain cortex were examined by ELISA at 24 h after reperfusion. (C) Western blots and (D) quantified results of MMP-9 expression in the ischemic cortex are also demonstrated. Values are expressed as the mean ± standard error (n=5 in each group). *P<0.05 vs. MCAO group; #P<0.05 vs. sham group. TK, tissue kallikrein; MCAO, middle cerebral artery occlusion; TK-0H, immediate TK treatment after reperfusion; TK-12H, delayed TK treatment at 12 h after reperfusion; IL, interleukin; TNF, tumor necrosis factor; MMP, matrix metallopeptidase.
Involvement of MMP-9 in the protective effects of TK against ischemic stroke
To investigate the potential effect of TK on BBB, the expression of MMP-9 in the ischemic brain was detected by western blot analysis at 24 h after MCAO. As shown in Fig. 3C and D, MMP-9 expression was markedly increased in the MCAO group compared with the sham group (P<0.05). Following the immediate TK treatment, the level of MMP-9 was significantly reduced (P<0.05). In addition, delayed TK treatment displayed some effect on inhibiting the expression of MMP-9 in the ischemic brain, but this effect was not significant when compared with the MCAO group.
TK increases the expression levels of Nrf2 and HO-1 proteins in ischemic brain
To further elucidate the mechanisms underlying the neuroprotective effect of TK, the influence of TK treatment on the activity of Nrf2 pathway was examined at 24 h after MCAO using western blot analysis. Compared with the sham group, the levels of Nrf2 and HO-1 proteins were significantly elevated in the ischemic brain of the MCAO model group (Fig. 4), suggesting the activation of endogenous antioxidative system in brain ischemia. Furthermore, TK immediate treatment significantly increased the protein levels of Nrf2 and HO-1. In the TK delayed treatment group, Nrf2 protein expression was significantly increased, however, the HO-1 expression was not significantly affected when compared with the MCAO group.
Figure 4.
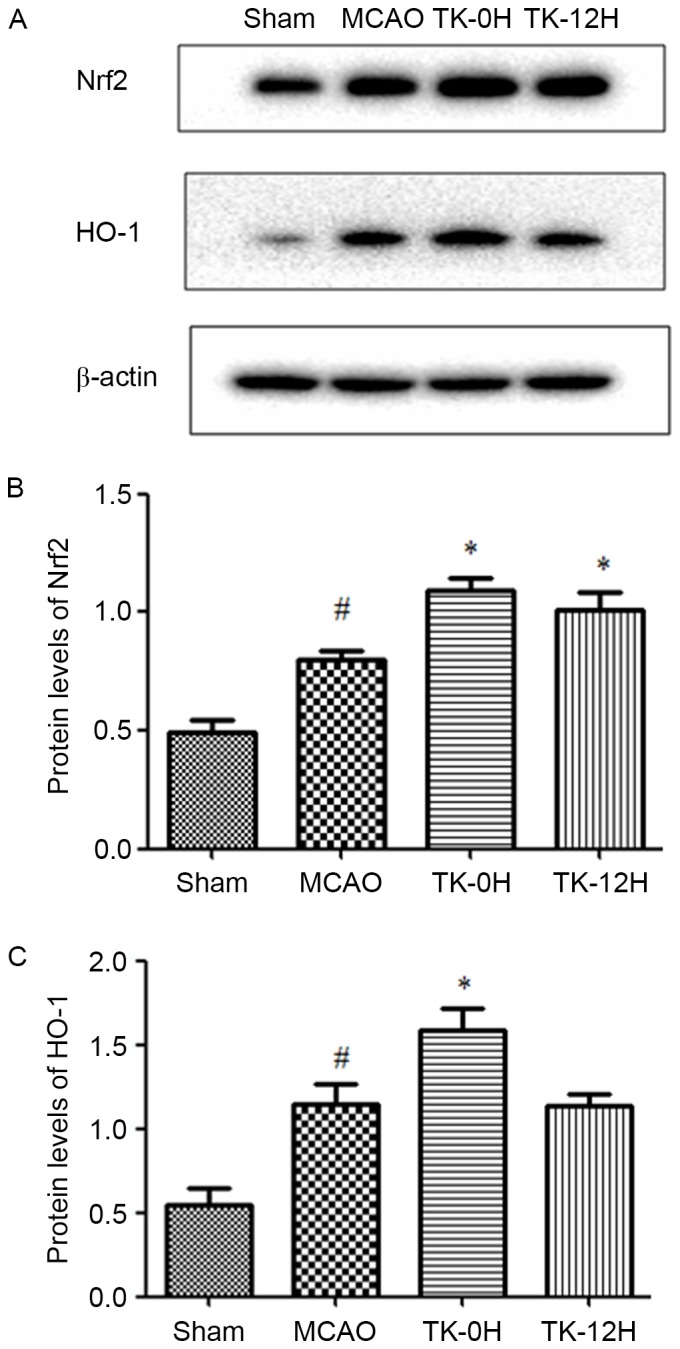
Effect of TK on protein expression of Nrf2 and HO-1. (A) Representative western blots of Nrf2 and HO-1, and quantified results for (B) Nrf2 and (C) HO-1 protein levels. Compared with the MCAO group, the Nrf2 expression was significantly increased in the two TK treatment groups, while HO-1 expression was significantly increased only in the TK-0H group. β-actin was used as an internal control. Data Values are expressed as the mean ± standard error (n=5 in each group). *P<0.05 vs. MCAO group; #P<0.05 vs. sham group. TK, tissue kallikrein; MCAO, middle cerebral artery occlusion; TK-0H, immediate TK treatment after reperfusion; TK-12H, delayed TK treatment at 12 h after reperfusion; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase 1.
Discussion
In the present study, direct evidence that TK protected rats from I/R-induced brain damage was provided. The reported data were obtained with an MCAO model, a well-characterized and classical model used to study the mechanisms of cerebral ischemic damage in rats (6,26). Using this model, the present study demonstrated that TK exerted a neuroprotective effect on cerebral ischemia, which may be partially due to its anti-inflammatory signaling pathway via downregulation of TLR4 and NF-κB, accompanied by the activation of antioxidant protein regulator Nrf2 and HO-1, and decrease of MMP-9 expression.
Cerebral ischemic/reperfusion injury is accompanied with inflammatory responses and oxidative stress, which serve a crucial role in the dysfunction and death of neurons in the brain regions (6–8,28). Brain ischemia triggers inflammatory responses, which has been associated with the activation of TLR signaling. Several studies suggest that TLR4 may serve a more important role in comparison with other Toll-like receptors during the course of brain damage caused by ischemia/reperfusion. A previous study demonstrated that the expression of TLR4 was increased in the ischemic brain (17). In addition, TLR4 knockout mice had significantly smaller infarct area and volume, and exhibited improved neurological outcome at 24 h after cerebral I/R compared with wild-type mice (17). In previous in vitro studies, TLR4 expression level was also increased in cultured neurons subjected to glucose deprivation, and neurons deficient in TLR4 were resistant to death induced by energy deprivation (29,30). Furthermore, it has been demonstrated that a constitutively active mutant of human TLR4 transfection into human cell lines could induce the activation of NF-κB and the expression of NF-κB-mediated inflammatory cytokines, including IL-1, IL-6, IL-8 and TNF-α (13). The transcription factor NF-κB, a key regulator of a variety of genes involved in cell survival and inflammation, is activated following cerebral ischemia in neurons, endothelial cells, astrocytes, microglia and infiltrating inflammatory cells (31). Studies have suggested that NF-κB activation is primarily mediated by the TLR signaling pathway, while the activation of NF-κB is required for the induction of numerous inflammatory cytokines (32). The mammalian NF-κB family comprises of five members: p65 (RelA), RelB, c-Rel, p50/p105 (NF-κB1), and p52/p100 (NF-κB2), among which the p65/RelA and p50 are known to be responsible for a detrimental effect in cerebral ischemia (31). For instance, NF-κB subunit p50 knockout mice displayed a significant reduction in infarct size in focal cerebral ischemia (33). In agreement with these studies, the results of the present study demonstrated that cerebral I/R induced the upregulation of TLR4 and NF-κB protein expression levels, while immediate and delayed TK treatment deactivated the activity of TLR4 and NF-κB, emphasizing the neuroprotective effect of TK on cerebral I/R injury.
It is well documented that oxidative stress is generated and serves a detrimental role in cerebral I/R injury (8,34). Nrf2 and its targets genes, known as phase-II enzymes, function in synergy to remove reactive oxygen species/reactive nitrogen species through sequential enzymatic reactions (35). Among phase-II enzymes, HO-1, a redox-sensitive inducible stress protein, has been proposed to serve a crucial role in the endogenous anti-oxidative defense system (36). Studies have revealed that increasing the activity of Nrf2 pathways and gene targets exerts highly neuroprotective effect against oxidative and excitotoxic insults relevant to ischemic stroke in cell culture and animal models (37,38). Nrf2 knockout mice presented more severe neurologic dysfunction and larger infarct size caused by MCAO in comparison with wild-type mice (39). The present study indicated that systemic administration of TK significantly increased the expression levels of Nrf2 and HO-1 in the ischemic cortex at 24 h after MCAO, suggesting the involvement of Nrf2 and HO-1 in the protective effect of TK in ischemic stroke.
MMPs degrade the extracellular matrix around the blood vessels, as well as the matrix around neurons, which have been shown to be associated with increased BBB paracellular permeability (40). In the early phase subsequent to cerebral ischemia, MMPs disrupt the BBB, leading to BBB leakage, leukocyte infiltration, brain edema and hemorrhage (41). Within the MMP family, MMP-2 and MMP-9 have been regarded as the key enzymes associated with secondary damage following ischemic cerebral stroke (42). Clinical data support that the activated MMP-9 can be considered as an effective marker for negative outcome within the early phase of ischemic stroke development (41). Therefore, MMP-9 is a potential therapeutic target for BBB disruption following thrombolysis (43). In the present study, it was observed that MMP-9 expression was increased at 24 h after ischemic stroke, and TK significantly attenuated the expression of MMP-9. It is suggested that the MMP-9 suppression was involved in the neuroprotective effect of TK against stroke, which may be, at least partly, due to the improvement of the BBB integrity.
However, certain studies have demonstrated that the KKS serves a detrimental role in the course of brain injury in stroke, as well as other brain disease animal models (23). The early activation of the KKS following cerebral ischemia increased brain vessel permeability, edema and spread of the ischemic lesion, as kinins and their receptors have been documented to induce proinflammatory responses (44). In addition, early administration of a kinin B2 receptor antagonist may improve the neurological recovery after focal cerebral I/R (45). Therefore, the present results appear to contradict previous findings regarding the role of kinin in inflammation. However, numerous other studies support the results of the present study, indicating that TK exerts a neuroprotective effect in ischemic injury (24,25,46,47). This type of protective effect may be associated with the following factors: i) The actions of TK can be mediated by kinin B2 receptor activation without kinin formation (48); ii) kallikrein-induced anti-inflammation is dependent on kinin B2 receptor activation and nitric oxide formation (24); iii) upregulation of endothelial nitric oxides resulted in dilation of cerebral arterial vessels, which is critical in maintaining the cerebral blood flow and conducive to the removal of inflammatory mediators (25); and iv) in the early period following cerebral I/R, kallikrein induced angiogenesis and improved the regional cerebral blood flow in the peri-infarction region, and further reduced the infarction volume and improved the neurological deficits (46).
In the present study, the protective effect in the TK delayed treatment group was not as effective as the protection observed in the TK immediate treatment group, which was not consistent with the results of Xia et al (24). A possible reason for the discrepancy between these results was the differential time point selected. In the present study, 24 h after reperfusion was selected as the only observation point, not extending to 1–2 weeks after reperfusion. Furthermore, the pharmaceutical dosage may be another reason for these different findings. Xia et al (24) used an adenovirus carrying human TK cDNA, while the present study used the injection of TK directly. Therefore, additional studies are required to precisely define how long after ischemic stroke the start of the therapeutic window can be delayed, and for how long a treatment should be given to achieve a beneficial prognosis.
In conclusion, the current study demonstrated that immediate systemic administration of TK decreased the infarct size, oxidative and inflammatory responses in cerebral ischemia, and further enriched its anti-inflammatory mechanism against ischemic stroke. These data also provide important new evidence that delayed systemic TK treatment following cerebral vascular insult may achieve neuroprotective effects against ischemia-induced brain damage. Finally, these results indicated that the upregulation of the Nrf2 pathway and downregulation of the TLR4/NF-κB pathway subsequent to ischemia by administration of TK was a potential mechanism of the neuroprotective effect of TK.
Acknowledgements
This study was supported by a major project from the Jiangsu Province (grant no. BS2006007), the 333 Program for high-level personnel training foundation of Jiangsu province of China (grant no. BRA2012050), funding for the development of medical clinical science and technology from Jiangsu University (grant no. JLY2010053), and funding from Changzhou Municipal Bureau of Health (grant no. WZ201043).
References
- 1.Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: Huge burden, significant workload, and a national priority. Stroke. 2011;42:3651–3654. doi: 10.1161/STROKEAHA.111.635755. [DOI] [PubMed] [Google Scholar]
- 2.Song D, Cho AH. Previous and recent evidence of endovascular therapy in acute ischemic stroke. Neurointervention. 2015;10:51–59. doi: 10.5469/neuroint.2015.10.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amar AP, Griffin JH, Zlokovic BV. Combined neurothrombectomy or thrombolysis with adjunctive delivery of 3K3A-activated protein C in acute ischemic stroke. Front Cell Neurosci. 2015;9:344. doi: 10.3389/fncel.2015.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reuter B, Grudzenski S, Chatzikonstantinou E, Meairs S, Ebert A, Heiler P, Schad LR, Staufenbiel M, Hennerici MG, Fatar M. Thrombolysis in experimental cerebral amyloid angiopathy and the risk of secondary intracerebral hemorrhage. Stroke. 2014;45:2411–2416. doi: 10.1161/STROKEAHA.113.004483. [DOI] [PubMed] [Google Scholar]
- 5.Chechneva OV, Mayrhofer F, Daugherty DJ, Krishnamurty RG, Bannerman P, Pleasure DE, Deng W. A Smoothened receptor agonist is neuroprotective and promotes regeneration after ischemic brain injury. Cell Death Dis. 2014;5:e1481. doi: 10.1038/cddis.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Zhang X, Wang Y, Lei H, Su H, Zeng J, Pei Z, Huang R. Inhibition of immunoproteasome reduces infarction volume and attenuates inflammatory reaction in a rat model of ischemic stroke. Cell Death Dis. 2015;6:e1626. doi: 10.1038/cddis.2014.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad M, Graham SH. Inflammation after stroke: Mechanisms and therapeutic approaches. Transl Stroke Res. 2010;1:74–84. doi: 10.1007/s12975-010-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima H, Kubo T, Ihara H, Hikida T, Danjo T, Nakatsuji M, Shahani N, Itakura M, Ono Y, Azuma YT, et al. Nuclear-translocated Glyceraldehyde-3-phosphate dehydrogenase promotes poly (ADP-ribose) polymerase-1 activation during oxidative/nitrosative stress in stroke. J Biol Chem. 2015;290:14493–14503. doi: 10.1074/jbc.M114.635607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yainoy S, Houbloyfa P, Eiamphungporn W, Isarankura-Na-Ayudhya C, Prachayasittikul V. Engineering of chimeric catalase-Angiopep-2 for intracellular protection of brain endothelial cells against oxidative stress. Int J Biol Macromol. 2014;68:60–66. doi: 10.1016/j.ijbiomac.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Li Z, Zhang X, Wang S, Zhu C, Miao J, Chen L, Cui L, Qiao H. Protective effect of shikonin in experimental ischemic stroke: Attenuated TLR4, p-p38MAPK, NF-κB, TNF-α and MMP-9 expression, up-regulated claudin-5 expression, ameliorated BBB permeability. Neurochem Res. 2014;39:97–106. doi: 10.1007/s11064-013-1194-x. [DOI] [PubMed] [Google Scholar]
- 11.Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell PM, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 13.Medzhitov R, Preston-Hurlburt P and Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 14.Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Ge P, Zhu Y. TLR2 and TLR4 in the brain injury caused by cerebral ischemia and reperfusion. Mediators Inflamm. 2013;2013:124614. doi: 10.1155/2013/124614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou N, Ao L, Cleveland JC, Jr, Yang X, Su X, Cai GY, Banerjee A, Fullerton DA, Meng X. Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H2805–H2813. doi: 10.1152/ajpheart.00299.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyakkoku K, Hamanaka J, Tsuruma K, Shimazawa M, Tanaka H, Uematsu S, Akira S, Inagaki N, Nagai H, Hara H. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171:258–267. doi: 10.1016/j.neuroscience.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 18.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 19.Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 20.Rao J, Qian X, Li G, Pan X, Zhang C, Zhang F, Zhai Y, Wang X, Lu L. ATF3-mediated NRF2/HO-1 signaling regulates TLR4 innate immune responses in mouse liver ischemia/reperfusion injury. Am J Transplant. 2015;15:76–87. doi: 10.1111/ajt.12954. [DOI] [PubMed] [Google Scholar]
- 21.Surh YJ, Na HK. NF-kappaB and Nrf2 as prime molecular targets for chemoprevention and cytoprotection with anti-inflammatory and antioxidant phytochemicals. Genes Nutr. 2008;2:313–317. doi: 10.1007/s12263-007-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah ZA, Li RC, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, Doré S. The flavanol (−)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J Cereb Blood Flow Metab. 2010;30:1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albert-Weißenberger C, Sirén AL, Kleinschnitz C. Ischemic stroke and traumatic brain injury: The role of the kallikrein-kinin system. Prog Neurobiol 101–102. 2013:1–82. doi: 10.1016/j.pneurobio.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Xia CF, Yin H, Yao YY, Borlongan CV, Chao L, Chao J. Kallikrein protects against ischemic stroke by inhibiting apoptosis and inflammation and promoting angiogenesis and neurogenesis. Hum Gene Ther. 2006;17:206–219. doi: 10.1089/hum.2006.17.206. [DOI] [PubMed] [Google Scholar]
- 25.Xia CF, Yin H, Borlongan CV, Chao L, Chao J. Kallikrein gene transfer protects against ischemic stroke by promoting glial cell migration and inhibiting apoptosis. Hypertension. 2004;43:452–459. doi: 10.1161/01.HYP.0000110905.29389.e5. [DOI] [PubMed] [Google Scholar]
- 26.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 27.Tatlisumak T, Carano RA, Takano K, Opgenorth TJ, Sotak CH, Fisher M. A novel endothelin antagonist, A-127722, attenuates ischemic lesion size in rats with temporary middle cerebral artery occlusion: A diffusion and perfusion MRI study. Stroke. 1998;29:850–858. doi: 10.1161/01.STR.29.4.850. [DOI] [PubMed] [Google Scholar]
- 28.Arumugam TV, Tang SC, Lathia JD, Cheng A, Mughal MR, Chigurupati S, Magnus T, Chan SL, Jo DG, Ouyang X, et al. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death; Proc Natl Acad Sci USA; 2007; pp. 14104–14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, Kao RL, Browder IW, Schweitzer JB, Kalbfleisch JH, Li C. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190:101–111. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits; Proc Natl Acad Sci USA; 2007; pp. 13798–13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridder DA, Schwaninger M. NF-kappaB signaling in cerebral ischemia. Neuroscience. 2009;158:995–1006. doi: 10.1016/j.neuroscience.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 33.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 34.Morita-Fujimura Y, Fujimura M, Yoshimoto T, Chan PH. Superoxide during reperfusion contributes to caspase-8 expression and apoptosis after transient focal stroke. Stroke. 2001;32:2356–2361. doi: 10.1161/hs1001.097241. [DOI] [PubMed] [Google Scholar]
- 35.Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells; Proc Natl Acad Sci USA; 1997; pp. 10925–10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satoh T, Izumi M, Inukai Y, Tsutsumi Y, Nakayama N, Kosaka K, Shimojo Y, Kitajima C, Itoh K, Yokoi T, Shirasawa T. Carnosic acid protects neuronal HT22 cells through activation of the antioxidant-responsive element in free carboxylic acid- and catechol hydroxyl moieties-dependent manners. Neurosci Lett. 2008;434:260–265. doi: 10.1016/j.neulet.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 38.Shi H, Jing X, Wei X, Perez RG, Ren M, Zhang X, Lou H. S-allyl cysteine activates the Nrf2-dependent antioxidant response and protects neurons against ischemic injury in vitro and in vivo. J Neurochem. 2015;133:298–308. doi: 10.1111/jnc.12986. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Zhang X, Cui L, Wang L, Liu H, Ji H, Du Y. Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice. Brain Res. 2013;1497:32–39. doi: 10.1016/j.brainres.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 40.Kunze R, Urrutia A, Hoffmann A, Liu H, Helluy X, Pham M, Reischl S, Korff T, Marti HH. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp Neurol. 2015;266:99–111. doi: 10.1016/j.expneurol.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 41.Sapojnikova N, Kartvelishvili T, Asatiani N, Zinkevich V, Kalandadze I, Gugutsidze D, Shakarishvili R, Tsiskaridze A. Correlation between MMP-9 and extracellular cytokine HMGB1 in prediction of human ischemic stroke outcome. Biochim Biophys Acta. 2014;1842:1379–1384. doi: 10.1016/j.bbadis.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 42.Clark AW, Krekoski CA, Bou SS, Chapman KR, Edwards DR. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett. 1997;238:53–56. doi: 10.1016/S0304-3940(97)00859-8. [DOI] [PubMed] [Google Scholar]
- 43.Cai Y, Liu X, Chen W, Wang Z, Xu G, Zeng Y, Ma Y. TGF-β1 prevents blood-brain barrier damage and hemorrhagic transformation after thrombolysis in rats. Exp Neurol. 2015;266:120–126. doi: 10.1016/j.expneurol.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Kamiya T, Katayama Y, Kashiwagi F, Terashi A. The role of bradykinin in mediating ischemic brain edema in rats. Stroke. 1993;24:571–576. doi: 10.1161/01.STR.24.4.571. [DOI] [PubMed] [Google Scholar]
- 45.Zausinger S, Lumenta DB, Pruneau D, Schmid-Elsaesser R, Plesnila N, Baethmann A. Effects of LF 16–0687 Ms, a bradykinin B(2) receptor antagonist, on brain edema formation and tissue damage in a rat model of temporary focal cerebral ischemia. Brain Res. 2002;950:268–278. doi: 10.1016/S0006-8993(02)03053-6. [DOI] [PubMed] [Google Scholar]
- 46.Lu RY, Luo DF, Xiao SH, Yang LH, Zhao J, Ji EN, Tao EX, Xing YG, Zhu FY, Luan P, Liu J. Kallikrein gene transfer induces angiogenesis and further improves regional cerebral blood flow in the early period after cerebral ischemia/reperfusion in rats. CNS Neurosci Ther. 2012;18:395–399. doi: 10.1111/j.1755-5949.2012.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen ZB, Huang DQ, Niu FN, Zhang X, Li EG, Xu Y. Human urinary kallidinogenase suppresses cerebral inflammation in experimental stroke and downregulates nuclear factor-kappaB. J Cereb Blood Flow Metab. 2010;30:1356–1365. doi: 10.1038/jcbfm.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chao J, Shen B, Gao L, Xia CF, Bledsoe G, Chao L. Tissue kallikrein in cardiovascular, cerebrovascular and renal diseases and skin wound healing. Biol Chem. 2010;391:345–355. doi: 10.1515/bc.2010.042. [DOI] [PubMed] [Google Scholar]