Abstract
The aim of the present study was to examine the effect of CCAAT enhancer binding protein β (C/EBPβ) on human breast cancer cells. The plasmids pCDH-C/EBPβ and pLKO.1-shC/EBPβ were constructed and were infected into MDA-MB-468 cells, to provide C/EBPβ overexpressing and C/EBPβ knockdown cells, respectively. Cell viability, cell cycle and apoptosis were observed by MTT assay and flow cytometry analysis. Protein expression levels of C/EBPβ, TGF-β1, P-Smad3 and Smad3 were detected by western blotting. MTT assay showed that the absorbance of MDA-MB-468 cells in the pCDH-C/EBPβ group was increased, whereas that in the pLKO.1-shC/EBPβ group was decreased, compared with the respective control at 48 and 72 h. Flow cytometric analysis indicated that the percentage of cells in the G2 phase was significantly increased in the pCDH-C/EBPβ group (P<0.05) and decreased in the pLKO.1-shC/EBPβ group compared with the respective control group. The proportion of apoptotic cells was decreased in the pCDH-C/EBPβ group and increased in the pLKO.1-shC/EBPβ group compared with the controls. The scratch-wound assay revealed that MDA-MB-468 cells depleted of C/EBPβ exhibited reduced motility compared with the control cells. Moreover, western blotting demonstrated that pCDH-C/EBPβ increased transforming growth factor (TGF)β1 and P-Smad3 protein expression and decreased Smad3 protein expression, whereas pLKO.1-shC/EBPβ decreased TGFβ1 and P-Smad3 protein expression and increased Smad3 protein expression levels. The present study demonstrated that C/EBPβ has a crucial role in regulating breast cancer cell growth through activating TGF-β-Smad3 signaling. These findings suggest that C/EBPβ may be a potential therapeutic target for breast cancer; however, in vivo studies are required to confirm this.
Keywords: CCAAT enhancer binding protein β, breast cancer, MDA-MB-468 cells, transforming growth factor-β1, Smad3
Introduction
Breast cancer is one of the most common malignant tumors and the second most common cause of cancer-related fatality in the United States (1). Although the death rate of breast cancer has decreased with advances in prevention, surgical resection and adjuvant therapies, there were ~232,340 new cases of breast cancer and 39,620 associated fatalities in the United States in 2013 (1). Metastasis to vital organs such as lung, brain and bone is a major cause of mortality resulting from breast cancer (2). Therefore, it is essential to study the molecular mechanism of breast cancer and identify further effective diagnostic and treatment methods.
CCAAT enhancer binding protein β (C/EBPβ), a type of trans-acting factor, is one of the important members of the C/EBP family (including C/EBPα, β, γ, δ, ε and ζ) (3). C/EBPβ is able to bind to the DNA specific regulatory region and is involved in multiple cell processes, such as metabolism, hematopoiesis, adipogenesis, immune response and morphogenesis (4,5). Additionally, C/EBPβ serves as a key factor in neuronal differentiation and apoptosis (6) and is involved in inflammatory processes and brain injury by regulating the expression levels of several genes, such as GRO1/KC, 24p3/LCN2 and TM4SF1/L6 (7). As observed in C/EBPβ-null mice by Zhu et al (8), reduced levels of C/EBPβ result in cell apoptosis, and thus these mice display resistance to 7,12-dimethylbenz[a]anthracene-induced skin tumorigenesis (8). Moreover, C/EBPβ has been shown to promote cell survival downstream of DNA damage by repressing p53 expression and activity (9).
Considerable research has demonstrated that C/EBPβ is an essential mediator of breast tumorigenesis. C/EBPβ has been indicated to be overexpressed at late stages of breast carcinogenesis (10), suggesting its potential role in the metastatic progression of breast cancer. C/EBPβ also has an important role in the evasion of metastatic breast cancer cells from the cytostatic effects of transforming growth factor (TGF)-β (11). The loss of C/EBPβ promotes epithelial-mesenchymal transition (EMT) and invasion in breast cancer (12). Although C/EBPβ has been reported to be deregulated in breast cancer, the underlying mechanisms of the effects of C/EBPβ on breast cancer cells remain far from clear and require further elucidation.
C/EBP functionally and physically interacts with TGF-β1 signaling factors in astrocytes (13). TGF-β1 has a key role in tumor pathogenesis, contributes to cell growth, invasion and metastasis, and inhibits host antitumor immune responses (14). A previous study indicated that the TGF-β pathway may be considered a therapeutic target for tumor diseases (15). TGF-β super family ligands bind to serine/threonine kinase receptors type II, which phosphorylate receptor type I (16). The receptor type I phosphorylates Smad2/3 (R-Smads), which combines with coSmad-Smad4 and R-Smad/coSmad complexes and subsequently shuttles into the nucleus to regulate the expression of their downstream genes (16). Several studies have suggested that activation of TGF-β-Smad signaling has a deteriorative effect on glioblastoma, and that inhibition of TGF-β signaling reduces the growth and invasion of gliomas (17–19). However, studies concerning the interactions of C/EBPβ and the TGF-β signaling pathway are limited.
The aim of the present study was to investigate whether C/EBPβ contributes to the development of breast cancer via the regulation of TGF-β1-Smad3 signaling. In this study, a recombinant lentiviral vector containing the C/EBPβ gene was constructed and the effect of C/EBPβ on cell viability, cell cycle, cell apoptosis and TGF-β1-Smad3 signaling in the MDA-MB-468 human breast cancer cell line was investigated.
Materials and methods
Cell lines
The human breast cancer cell line, MDA-MB-468, was purchased from The Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.) at 37°C in an atmosphere containing 5% CO2.
Construction of lentiviral vector
Human C/EBPβ gene was synthesized by Shanghai Sangon Biotech Co., Ltd. (Shanghai, China) and was cloned into pCDH lentiviral vector (System Biosciences, Mountain View, CA, USA). In the pCDH lentiviral vector, green fluorescent protein was a single transcript under the control of a CMV promoter and expressed after the transcription of the C/EBPβ gene. To knockdown C/EBPβ expression, the selected interfering [short hairpin (SH)] sequence 5′-CCTTTAGACCCATGGAAGTTT-3′ was cloned into pLKO.1 vector (Sigma-Aldrich; merck KGgA, Darmstadt, Germany) after the oligonucleotides were annealed.
Packaging and infection of lentivirus vector
The lentiviral vectors pCDH-C/EBPβ and pLKO.1-shC/EBPβ were co-transfected with the corresponding helper plasmids into 293T cells (Cell bank, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) After 6 h incubation at 37°C in a humidified atmosphere containing 5% CO2, DMEM was exchanged for complete medium (containing 10% FBS, Gibco; Thermo Fisher Scientific, Inc.). The supernatant was harvested after culturing for 48 h and concentrated by ultrafiltration. MDA-MB-468 cells were infected with recombinant lentivirus pCDH-C/EBPβ, lentivirus pCDH, lentivirus pLKO.1-shC/EBPβ and the negative control (NC) lentivirus pLKO.1-shNC, respectively. MDA-MB-468 cells without infection served as the blank group. Medium was replaced with fresh medium 24 h post-infection and cells were collected 72 h post-infection for subsequent analysis.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Cells were washed three times with PBS and total RNA was extracted using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was treated with DNase to remove genomic DNA contamination. The Revert Aid First-Strand RT-PCR kit (Invitrogen; Thermo Fisher Scientific, Inc.) was used to synthesize cDNA from 250 ng of each extracted RNA sample. cDNA was amplified in a 20-µl reaction mixture containing 10 µl of SYBR-Green PCR Supermix (Invitrogen; Thermo Fisher Scientific, Inc.), 100 ng of cDNA template and selected primers (200 nM; Table I). Each transcript was normalized to the amplification levels of GAPDH, which served as control. C/EBPβ mRNA levels were quantified by qPCR amplification. The following conditions were used: Pre-denaturing at 95°C for 5 min followed by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C for 10 sec, and finally elongation at 72°C for 10 min. Data analyses were conducted with the 2−ΔΔCq method (20). Each experiment was performed in triplicate.
Table I.
The primers for reverse transciption-quantitative polymerase chain reaction.
| Gene | Forward | Reverse |
|---|---|---|
| C/EBPβ | CCTCGCAGGTCAAGAGCAAG | GAACAAGTTCCGCAGGGTG |
| GAPDH | TGTTGCCATCAATGACCCCTT | CTCCACGACGTACTCAGCG |
C/EBPβ, CCAAT enhancer binding protein β.
MTT assay
1×104 cells were seeded into each well of a 96-well plate. On the second day, the cells were infected with the lentiviruses pCDH, pCDH-C/EBPβ, pLKO.1-shNC and pLKO.1-shC/EBPβ, respectively, to form the pCDH, pCDH-C/EBPβ, pLKO.1-shNC and pLKO.1-shC/EBPβ groups. Subsequently, the cells were incubated for 24, 48 or 72 h at 37°C in a humidified atmosphere containing 5% CO2. MTT (10 µl, 5 mg/ml; Shanghai Sangon Biotech Co., Ltd.) was added into each well at the same time of each day and the cells were then incubated for 4 h. Dimethyl sulfoxide (DMSO; 100 µl; Shanghai Sangon Biotech Co., Ltd.) was added to each well to solubilize the formazan crystals. Zero (DMEM, MTT and DMSO) and blank wells were established. The absorbance of each well was read at 570 nm using a microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Flow cytometry
For cell cycle detection, infected cells cultured in DMEM from all groups were digested with 0.25% trypsin (Invitrogen; Thermo Fisher Scientific, Inc.), collected by centrifuging at 377 × g for 6 min at 4°C and washed once with phosphate-buffered saline (PBS; Shanghai Sangon Biotech Co.). Cells were fixed with ice-cold 75% ethanol at 4°C overnight. Subsequently, cells were centrifuged (377 × g for 6 min at 4°C) and ethanol was removed by washing with PBS three times. Cells were slightly resuspended with 300 µl PBS and treated with 50 µg/ml RNase A (Shanghai Sangon Biotech Co., Ltd.) for 30 min at 37°C. Cells were stained with propidium iodide (PI; BioLegend, Inc., San Diego, CA, USA) in the dark for 15 min at 4°C and detected using a flow cytometer (BD Biosciences, San Jose, CA, USA). Data were analyzed by FCS Express 4 (De Novo Software, Los Angeles, CA, USA).
Annexin V-APC Apoptosis Detection kit (BD Biosciences) was used to detect cell apoptosis. Infected cells were digested with 0.25% trypsin-EDTA (Invitrogen; Thermo Fisher Scientific, Inc.) and collected by centrifuging at 377 × g for 6 min at 4°C, then washed once with PBS. Cells were added to APC-Annexin V and PI in the dark for 15 min at 25°C after being slightly resuspended with 1X Binding Buffer. A total of 400 µl 1X Binding Buffer was added and the cells were detected using a flow cytometer.
Cell migration and wound healing assay
Cell motility was measured using a wound healing assay. Cells from all groups were seeded onto 60-mm plates and incubated in serum-free DMEM overnight at 37°C. A P200 pipette tip was used to create an artificial wound by scratching the confluent cell monolayer. Immediately, a photomicrograph was taken (time 0 h). Subsequently, at 24, 48 and 72 h post wounding, images were captured to observe the migrating cells and closure of the scratch wound. The wound areas were quantified using Muscale analysis software (Muscale LLC, Scottsdale, AZ, USA).
Western blotting
Cells from all groups were collected into 1.5-ml tubes, washed twice with PBS, and then placed on ice for 30 min in radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) containing 1 mM phenylmethylsulfonyl fluoride (Shanghai Sangon Biotech Co., Ltd.). Supernatant were acquired by centrifuging at 18894 × g for 15 min at 4°C. Subsequently, BCA protein quantitative assay was used to determine the protein concentration (Shanghai Sangon Biotech Co., Ltd.). A sample containing 40 µg total protein was separated using 12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes, which were blocked in 5% non-fat milk for 1 h. The membranes were incubated overnight at 4°C with mouse anti-human β-actin monoclonal antibody (1:1,000; sc-58673), rabbit anti-human C/EBPβ polyclonal antibody (1:500; sc-56637), mouse anti-human TGFβ1 monoclonal antibody (1:500; sc-146), rabbit anti-human Smad3 polyclonal antibody (1:800; sc-8332; all Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and rabbit anti-human P-Smad3 polyclonal antibody (1:1,000; ab52903; Abcam, Cambridge, MA, USA). Membranes were washed three times with PBS and then incubated with secondary antibodies goat anti-mouse IgG(H+L)-HRP (1:5,000) or goat anti-rabbit IgG (H+L)-HRP (1:5,000; both Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 2 h at room temperature, respectively. Proteins were detected using enhanced chemiluminescence (ECL; EMD Millipore, Billerica, MA, USA).
Statistical analysis
Statistical analysis in the present study was performed using SPSS 12.0 statistical analysis software (SPSS Inc., Chicago, IL, USA). All determinations were performed in triplicate. Data are expressed as the mean ± standard deviation and analyzed by one-way analysis of variance and multiple comparisons between groups were performed using Student-Newman-Keuls method. P<0.05 was considered to indicated a statistically significant difference.
Results
Identification of lentiviral vector pCDH-C/EBPβ
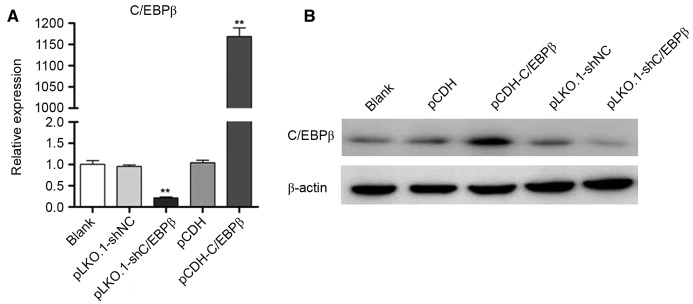
Recombinant lentiviruses pCDH-C/EBPβ and pLKO.1-shC/EBPβ were efficiently infected into MDA-MB-468 cells (Fig. 1A), respectively. Western blotting indicated that the protein expression level of C/EBPβ was markedly increased in the pCDH-C/EBPβ group, whereas the protein expression level of C/EBPβ was markedly decreased in the pLKO.1-shC/EBPβ group when compared with the blank control (Fig. 1B). These results demonstrated that lentiviral vector pCDH-C/EBPβ and pLKO.1-shC/EBPβ were successfully constructed and that the lentiviruses (including pCDH-C/EBPβ, pCDH, pLKO.1-shC/EBPβ and pLKO.1-shNC) had a high efficiency of infection.
Figure 1.
Lentiviral vectors pCDH-C/EBPβ and pLKO.1-shC/EBPβ were successfully constructed and had a high efficiency of infection. (A) Quantitative polymerase chain reaction analysis suggested that the mRNA expression levels of C/EBPβ were significantly increased in the pCDH-C/EBPβ group and significantly decreased in the pLKO.1-shC/EBPβ group compared with pCDH and pLKO.1-shNC, respectively. (B) Western blotting indicated that the expression of C/EBPβ was increased in the pCDH-C/EBPβ group and decreased in the pLKO.1-shC/EBPβ group compared with pCDH and pLKO.1-shNC, respectively. **P<0.01 vs. blank, pCDH and pLKO.1-shNC groups. C/EBPβ, CCAAT enhancer binding protein β; NC, negative control.
Effect of C/EBPβ on cell viability and cell cycle in MDA-MB-468 cells
MTT analysis was performed to observe the cell viability after infection. As Fig. 2A indicates, the absorbance of the MDA-MB-468 cells was significantly increased in the pCDH-C/EBPβ group at 48 and 72 h compared with that of the pCDH and blank groups (P<0.05). Conversely, cell viability was significantly diminished in the pLKO.1-shC/EBPβ group compared with that in the pLKO.1-shNC and blank groups (P<0.05). These data suggest that C/EBPβ has an important role in cell viability.
Figure 2.
C/EBPβ regulated MDA-MB-468 cell viability and the cell cycle. (A) MTT analysis showed that the absorbance of MDA-MB-468 cells was significantly increased in the pCDH-C/EBPβ group compared with the blank and pCDH groups after 48 and 72 h. Absorbance in the pLKO.1-shC/EBPβ group was significantly decreased compared with that in the blank and pLKO.1-shNC groups after 48 and 72 h. (B) Flow cytometric analysis indicated that, compared with the pCDH and blank groups, the proportion of cells of the pCDH-C/EBPβ group in the G1 phase decreased and that in the G2 phase increased. The proportion of cells in the G1 phase was increased and in the G2 phase was decreased in the pLKO.1-shC/EBPβ cells. *P<0.05 vs. the blank, pCDH and pLKO.1-shNC groups. C/EBPβ, CCAAT enhancer binding protein β; NC, negative control; O.D., optical density.
Cell cycle analysis was performed using flow cytometry, and the results demonstrated that the percentage of MDA-MB-468 cells in the G1 phase was significantly decreased and that in the G2 phase was significantly increased in the pCDH-C/EBPβ group compared with the blank and pCDH groups (P<0.05; Fig. 2B and Table II). Furthermore, the percentage of cells in the G1 phase in the pLKO.1-shC/EBPβ group was significantly increased compared with that in the blank and pLKO.1-shNC groups (P<0.05; Fig. 2B and Table II). These results suggest that cells in the pLKO.1-shC/EBPβ group were blocked at the G1 boundary, which concomitantly reduced the proportion of cells in the S phase (Fig. 2B and Table II).
Table II.
Cell cycle analysis in the blank, pCDH, pCDH-C/EBPβ, pLKO.1-shNC and pLKO.1-shC/EBPβ groups.
| Group | G1 phase (%) | S phase (%) | G2 phase (%) |
|---|---|---|---|
| Blank | 62.79±1.06 | 27.87±1.36 | 9.31±1.09 |
| pCDH | 64.83±1.69 | 28.18±1.35 | 8.42±0.75 |
| pCDH-C/EBPβ | 55.13±1.13a,b | 26.21±1.95 | 18.67±0.73a,b |
| pLKO.1-shNC | 66.08±3.50 | 27.91±1.02 | 5.75±0.62 |
| pLKO.1-shC/EBPβ | 91.62±2.83a,c | 4.21±0.23a,c | 4.13±0.29 |
P<0.05 vs. the blank group
P<0.05 vs. the pCDH group
P<0.05 vs. the pLKO.1-shNC group. C/EBPβ, CCAAT enhancer binding protein β; NC, negative control.
Effect of C/EBPβ on cell apoptosis in MDA-MB-468 cells
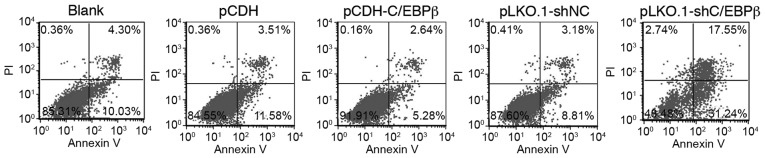
The apoptotic ratio of the cells was quantitatively analyzed using flow cytometry. As shown in Fig. 3 and Table III, the apoptotic cell ratio was significantly decreased in the pCDH-C/EBPβ group compared with the blank and pCDH groups (P<0.05); however, the apoptotic cell level was significantly increased in the pKLO.1-shC/EBPβ group compared with the blank and pLKO.1-shNC groups (P<0.05; Fig. 3 and Table III). These results suggest that the expression of C/EBPβ decreases cell apoptosis.
Figure 3.
C/EBPβ affected the apoptosis of MDA-MB-468 cells. Flow cytometric analysis revealed that the apoptotic cell ratio was decreased in the pCDH-C/EBPβ group compared with the pCDH and blank groups; however, the apoptotic cell ratio was increased in the pLKO.1-shC/EBPβ group. C/EBPβ, CCAAT enhancer binding protein β; NC, negative control; PI, propidium iodide.
Table III.
Cell apoptosis in the blank, pCDH, pCDH-C/EBPβ, pLKO.1-shNC and pLKO.1-shC/EBPβ groups.
| Group | Apoptotic cell ratio (%) |
|---|---|
| Blank | 14.46±0.89 |
| pCDH | 15.32±1.86 |
| pCDH-C/EBPβ | 7.49±0.51a,b |
| pLKO.1-shNC | 12.14±0.94 |
| pLKO.1-shC/EBPβ | 48.21±1.97a,c |
P<0.05 vs. the blank group
P<0.05 vs. the pCDH group
P<0.05 vs. the pLKO.1-shNC group. C/EBPβ, CCAAT enhancer binding protein β; NC, negative control.
C/EBPβ depletion inhibits MDA-MB-468 cell motility
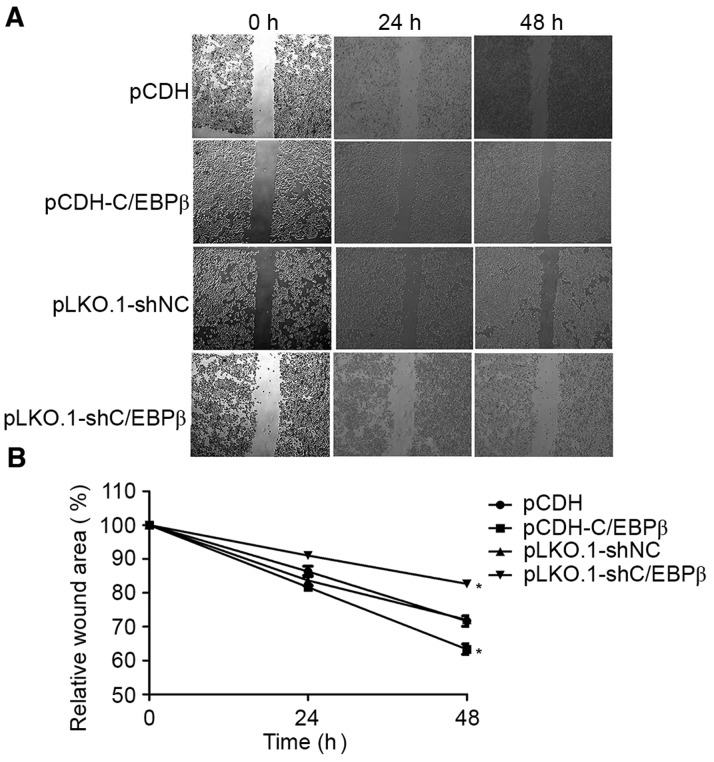
Scratch-wound assays were performed to assess the role of C/EBPβ in MDA-MB-468 cell motility. These assays showed that MDA-MB-468 cells with overexpressed C/EBPβ exhibited significantly increased motility, whereas the cells depleted of C/EBPβ exhibited significantly decreased motility as they did not fill in the scratch as extensively as did cells from the control groups (P<0.05; Fig. 4).
Figure 4.
C/EBPβ depletion inhibits MDA-MB-468 cell motility. (A) Scratch-wound assays showed that MDA-MB-468 cells depleted of C/EBPβ filled in less of the scratch area compared with the cells in the pLKO.1-shNC group, while increased C/EBPβ expression increased the motility of MDA-MB-468 cells. (B) Relative wound area in each group. C/EBPβ, CCAAT enhancer binding protein β; NC, negative control. *P<0.05 vs. the pLKO.1-shNC and pCDH groups.
Effect of overexpression of C/EBPβ on TGFβ1-Smad3 signaling in MDA-MB-468 cells
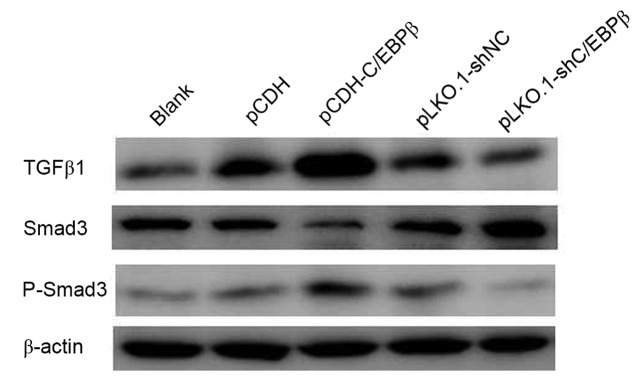
To investigate the mechanism by which C/EBPβ affects MDA-MB-468 cells, the protein expression levels of TGFβ1, P-Smad3 and Smad3 were detected. Western blotting indicated that TGFβ1 and P-Smad3 protein expression levels were increased in the pCDH-C/EBPβ group and decreased in pLKO.1-shC/EBPβ group when compared with those in the respective control groups (Fig. 5). Conversely, a marked reduction in the protein expression level of Smad3 was observed in the pCDH-C/EBPβ group, whereas Smad3 expression was notably increased in the pLKO.1-shC/EBPβ group compared with the respective control groups (Fig. 5).
Figure 5.
C/EBPβ affected the expression of TGF-β1 by Smad3 expression. Western blotting indicated that the expression levels of TGFβ1 and P-Smad3 were increased and the expression level of Smad3 was decreased in the pCDH-C/EBPβ group compared with the pCDH and blank groups. By contrast, the TGFβ1 and P-Smad3 expression levels were decreased and the expression level of Smad3 was increased in the pLKO.1-shC/EBPβ group. C/EBPβ, CCAAT enhancer binding protein β; TGFβ1, transforming growth factor β1; NC, negative control.
Discussion
In recent years, the morbidity of breast cancer has increased, despite considerable achievements being made in tumor therapy (21). It has been reported that C/EBPβ expression may be used to predict the overall survival in breast cancer patients, since it affects tumor growth and metastasis formation in mice (22). In the present study, the effect of C/EBPβ on a breast cancer cell line and the molecular mechanism of its effect were investigated. The present results showed that overexpression of C/EBPβ significantly increased breast cancer cell viability and concomitantly decreased the cell apoptosis rate. Western blotting results suggested that overexpression of C/EBPβ increased the protein expression levels of TGFβ1 and P-Smad3 and repressed the expression of Smad3.
The present study demonstrated that the overexpression of C/EBPβ promoted viability and inhibited apoptosis, and knockdown of C/EBPβ inhibited cell viability and promoted apoptosis in MDA-MB-468 cells. The role of C/EBPβ in the regulation of cell viability and apoptosis in the present study is partly consistent with previous studies in other cancer cell lines. For example, Buck et al (23) observed that the expression of C/EBPβ had an important role in the survival of hepatic stellate cells with DNA damage caused by CCl4-induced free radicals. The ability of cancer cells to invade into surrounding tissue is affected by their motility as well as their ability to penetrate through tissue barriers, such as the extracellular matrix. Wound assay results in the present study suggested that the overexpression of C/EBPβ increased cell motility, suggesting the potential role of C/EBPβ in cancer metastasis.
Previous studies have suggested that C/EBPβ has antiproliferative effects in various normal cells. For example, C/EBPβ was demonstrated to inhibit cell proliferation through interacting with the retinoblastoma protein family to suppress the expression of E2F target genes (S-phase genes) in primary fibroblasts (24). Furthermore, decreased expression of C/EBPβ enabled primary keratinocytes to resist calcium-induced growth arrest (25). The results of the present study are consistent with other data. In a previous study, knockdown of C/EBPβ significantly inhibited glioblastoma cell proliferation and invasion, and also prolonged survival in a murine brain tumor model (26). Additionally, another study demonstrated that C/EBPβ−/− mice were completely resistant to tumorigenic agents applied to the skin due to the Ras-dependent promotion of apoptosis (8). Also, growth-promoting activity of C/EBPβ has been observed in mammary epithelial cells (27) and hepatic cells (28). By consideration of the previous and present study results, it may be proposed that C/EBPβ has an accelerative role in breast cancer development by controlling cell proliferation and apoptosis.
The present study investigated the molecular mechanism of C/EBPβ and indicated that C/EBPβ promoted cell viability in MDA-MB-468 cells. Furthermore, the results indicated that it affected the expression levels of proteins associated with the TGF-β1-Smad3 signaling pathway. A previous study showed that increased C/EBPβ expression elevated transcription activity of the TGF-β1 promoter in human primary astrocytes and microglial cells (29). The present results demonstrated that overexpression of C/EBPβ increased the expression of TGF-β1 and P-Smad3, suggesting that C/EBPβ promoted TGF-β-Smad3 signaling by activating the TGFβ1 promoter in breast cancer. Notably, the present study also revealed that the protein expression levels of Smad3 were strongly inhibited in the pCDH-C/EBPβ group. Smad3 has previously been demonstrated to inhibit the activity of C/EBPβ on transcribing monocyte chemoattractant protein-1, inducible nitric oxide synthase and haptoglobin promoter by interacting with C/EBPβ (30–32). Additionally, the MH2 domain of Smad3 has been shown to combine with C/EBPβ, and subsequently decrease the association between C/EBPβ and TGF-β1 promoter, suggesting that the increased expression of Smad3 inhibited the activity of C/EBPβ on the TGF-β1 promoter (29,33). Thus, it may be speculated that inhibited Smad3 expression further promoted the activity of C/EBPβ on the TGF-β1 promoter via a positive feedback mechanism. A previous study indicated that TGFβ had an antiproliferative effect on epithelial cells, astrocytes and immune cells; however, in certain malignant tumors the capacity of TGFβ to inhibit proliferation is selectively lost (34) and TGF-β1 is considered as an oncogenic factor (16). The present results indicated that C/EBPβ may promote MDA-MB-468 cell growth through activating TGF-β signaling.
In conclusion, the present study demonstrated that C/EBPβ has a crucial role in regulating breast cancer cell growth through the activation of TGF-β-Smad3 signaling. These findings suggest that C/EBPβ may be a potential therapeutic target for breast cancer, although in vivo studies in animal models are required to confirm this.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen DX, Bos PD, Massagué J. Metastasis: From dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 3.Osada S, Yamamoto H, Nishihara T, Imagawa M. DNA binding specificity of the CCAAT/enhancer-binding protein transcription factor family. J Biol Chem. 1996;271:3891–3896. doi: 10.1074/jbc.271.7.3891. [DOI] [PubMed] [Google Scholar]
- 4.Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- 5.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: Structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/bj20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortés-Canteli M, Pignatelli M, Santos A, Perez-Castillo A. CCAAT/enhancer-binding protein beta plays a regulatory role in differentiation and apoptosis of neuroblastoma cells. J Biol Chem. 2002;277:5460–5467. doi: 10.1074/jbc.M108761200. [DOI] [PubMed] [Google Scholar]
- 7.Cortes-Canteli M, Wagner M, Ansorge W, Pérez-Castillo A. Microarray analysis supports a role for ccaat/enhancer-binding protein-beta in brain injury. J Biol Chem. 2004;279:14409–14417. doi: 10.1074/jbc.M313253200. [DOI] [PubMed] [Google Scholar]
- 8.Zhu S, Yoon K, Sterneck E, Johnson PF, Smart RC. CCAAT/enhancer binding protein-beta is a mediator of keratinocyte survival and skin tumorigenesis involving oncogenic Ras signaling; Proc Natl Acad Sci USA; 2002; pp. 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewing SJ, Zhu S, Zhu F, House JS, Smart RC. C/EBPbeta represses p53 to promote cell survival downstream of DNA damage independent of oncogenic Ras and p19 (Arf) Cell Death Differ. 2008;15:1734–1744. doi: 10.1038/cdd.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zahnow CA. CCAAT/enhancer binding proteins in normal mammary development and breast cancer. Breast Cancer Res. 2002;4:113–121. doi: 10.1186/bcr428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomis RR, Alarcón C, Nadal C, Van Poznak C, Massagué J. C/EBPbeta at the core of the TGFbeta cytostatic response and its evasion in metastatic breast cancer cells. Cancer Cell. 2006;10:203–214. doi: 10.1016/j.ccr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Johansson J, Berg T, Kurzejamska E, Pang MF, Tabor V, Jansson M, Roswall P, Pietras K, Sund M, Religa P, Fuxe J. MiR-155-mediated loss of C/EBPβ shifts the TGF-β response from growth inhibition to epithelial-mesenchymal transition, invasion and metastasis in breast cancer. Oncogene. 2013;32:5614–5624. doi: 10.1038/onc.2013.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyle-Rink J, Sweet T, Abraham S, Sawaya B, Batuman O, Khalili K, Amini S. Interaction between TGFbeta signaling proteins and C/EBP controls basal and Tat-mediated transcription of HIV-1 LTR in astrocytes. Virology. 2002;299:240–247. doi: 10.1006/viro.2002.1439. [DOI] [PubMed] [Google Scholar]
- 14.Kaminska B, Wesolowska A, Danilkiewicz M. TGF beta signalling and its role in tumour pathogenesis. Acta Biochim Pol. 2005;52:329–337. [PubMed] [Google Scholar]
- 15.Seoane J. The TGFBeta pathway as a therapeutic target in cancer. Clin Transl Oncol. 2008;10:14–19. doi: 10.1007/s12094-008-0148-2. [DOI] [PubMed] [Google Scholar]
- 16.Wrana JL, Attisano L, Cárcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-S. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Yu J, Yin Q, Li W, Ren X, Hao X. Rosiglitazone suppresses glioma cell growth and cell cycle by blocking the transforming growth factor-beta mediated pathway. Neurochem Res. 2012;37:2076–2084. doi: 10.1007/s11064-012-0828-8. [DOI] [PubMed] [Google Scholar]
- 18.Eichhorn PJ, Rodón L, Gonzàlez-Juncà A, Dirac A, Gili M, Martínez-Sáez E, Aura C, Barba I, Peg V, Prat A, et al. USP15 stabilizes TGF-β receptor I and promotes oncogenesis through the activation of TGF-β signaling in glioblastoma. Nat Med. 2012;18:429–435. doi: 10.1038/nm.2619. [DOI] [PubMed] [Google Scholar]
- 19.Kaminska B, Kocyk M, Kijewska M. TGF beta signaling and its role in glioma pathogenesis. Adv Exp Med Biol. 2013;986:171–187. doi: 10.1007/978-94-007-4719-7_9. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Parks RM, Cheung KL. Patient pathway for breast cancer: Turning points and future aspirations. Future Oncol. 2015;11:1059–1070. doi: 10.2217/fon.15.21. [DOI] [PubMed] [Google Scholar]
- 22.Kurzejamska E, Johansson J, Jirström K, Prakash V, Ananthaseshan S, Boon L, Fuxe J, Religa P. C/EBPβ expression is an independent predictor of overall survival in breast cancer patients by MHCII/CD4-dependent mechanism of metastasis formation. Oncogenesis. 2014;3:e125. doi: 10.1038/oncsis.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buck M, Poli V, Hunter T, Chojkier M. C/EBPbeta phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol Cell. 2001;8:807–816. doi: 10.1016/S1097-2765(01)00374-4. [DOI] [PubMed] [Google Scholar]
- 24.Sebastian T, Malik R, Thomas S, Sage J, Johnson PF. C/EBPbeta cooperates with RB:E2F to implement Ras(V12)-induced cellular senescence. EMBO J. 2005;24:3301–3312. doi: 10.1038/sj.emboj.7600789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu S, Oh HS, Shim M, Sterneck E, Johnson PF, Smart RC. C/EBPbeta modulates the early events of keratinocyte differentiation involving growth arrest and keratin 1 and keratin 10 expression. Mol Cell Biol. 1999;19:7181–7190. doi: 10.1128/MCB.19.10.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguilar-Morante D, Cortes-Canteli M, Sanz-Sancristobal M, Santos A, Perez-Castillo A. Decreased CCAAT/enhancer binding protein β expression inhibits the growth of glioblastoma cells. Neuroscience. 2011;176:110–119. doi: 10.1016/j.neuroscience.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Bundy LM, Sealy L. CCAAT/enhancer binding protein beta (C/EBPbeta)-2 transforms normal mammary epithelial cells and induces epithelial to mesenchymal transition in culture. Oncogene. 2003;22:869–883. doi: 10.1038/sj.onc.1206216. [DOI] [PubMed] [Google Scholar]
- 28.Buck M, Poli V, van der Geer P, Chojkier M, Hunter T. Phosphorylation of rat serine 105 or mouse threonine 217 in C/EBP beta is required for hepatocyte proliferation induced by TGF alpha. Mol Cell. 1999;4:1087–1092. doi: 10.1016/S1097-2765(00)80237-3. [DOI] [PubMed] [Google Scholar]
- 29.Abraham S, Sweet T, Khalili K, Sawaya BE, Amini S. Evidence for activation of the TGF-beta1 promoter by C/EBPbeta and its modulation by Smads. J Interferon Cytokine Res. 2009;29:1–7. doi: 10.1089/jir.2008.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham S, Sweet T, Sawaya BE, Rappaport J, Khalili K, Amini S. Cooperative interaction of C/EBP beta and Tat modulates MCP-1 gene transcription in astrocytes. J Neuroimmunol. 2005;160:219–227. doi: 10.1016/j.jneuroim.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Feinberg MW, Watanabe M, Lebedeva MA, Depina AS, Hanai J, Mammoto T, Frederick JP, Wang XF, Sukhatme VP, Jain MK. Transforming growth factor-beta1 inhibition of vascular smooth muscle cell activation is mediated via Smad3. J Biol Chem. 2004;279:16388–16393. doi: 10.1074/jbc.M309664200. [DOI] [PubMed] [Google Scholar]
- 32.Zauberman A, Lapter S, Zipori D. Smad proteins suppress CCAAT/enhancer-binding protein (C/EBP) beta- and STAT3-mediated transcriptional activation of the haptoglobin promoter. J Biol Chem. 2001;276:24719–24725. doi: 10.1074/jbc.M005813200. [DOI] [PubMed] [Google Scholar]
- 33.Wotton D, Massagué J. Smad transcriptional corepressors in TGF beta family signaling. Curr Top Microbiol Immunol. 2001;254:145–164. [PubMed] [Google Scholar]
- 34.Seoane J. Escaping from the TGFbeta anti-proliferative control. Carcinogenesis. 2006;27:2148–2156. doi: 10.1093/carcin/bgl068. [DOI] [PubMed] [Google Scholar]