Abstract
The use of interferon-alfa and allogeneic-stem cell transplantation, and more recently of tyrosine-kinase inhibitors (TKIs) has improved the outcome of patients with chronic myeloid leukemia (CML). We performed a population-based study of CML to evaluate relative survival (RS) trend by treatment eras. All instances of CML diagnosed between 1975 and 2009 reported in the Surveillance, Epidemiology, and End Results databases were reviewed. The incidence of CML was 1.75/100,000 persons per-year and increased with age. The incidence was highest in Detroit and lowest among Asians. The 5-year RS ratios increased from 0.26 in patients diagnosed in 1975–1989 to 0.36 in 1990–2000 and 0.56 in 2001–2009. There was a significant improvement in 5-year RS ratios in 2005–2009 calendar period compared to 2001–2004 period (P<0.05) corresponding to the introduction of second generation of TKIs. Age was the most important prognostic factor for RS, but the improvement in 5-year RS ratios was observed in all age groups except the group aged <15 years (P>0.05) including adolescent and young adults and elderly patient groups. There are ethnic and geographic variations in the incidence of CML. The RS improved with each treatment era, with the greatest improvement in all age groups occurring during the TKI era.
Keywords: CML, incidence, survival, imatinib, TKI, transplantation
Introduction
Chronic myeloid leukemia (CML), a myeloproliferative disorder of hematopoietic stem cells, arises from a balanced translocation between the long arms to chromosome 9 and 22 t(9;22)(q34;q11.2). Approximately 4870 new cases of CML were diagnosed in 2010 in the United States.1 Imatinib mesylate was the first selective Bcr-Abl tyrosine kinase inhibitor (TKI) introduced as targeted therapy for CML in 2000, revolutionized the treatment of this disease and has become the standard treatment for CML since 2001.2–4
During the 1970s and 1980s, palliative cytoreductive therapy with busulfan or hydroxyurea was the mainstay of therapy for CML. Treatment with either drug had a minimal effect on the rate of progression to blastic phase and induced a negligible rate of cytogenetic response.5 In the 1990s, allogeneic stem-cell transplant (allo-SCT) became the treatment choice for young eligible patients in chronic phase. For patients not eligible for allo-SCT, interferon-alfa alone or in combination with cytarabine was the frontline treatment.6,7
The purpose of this large scale population-based study is to provide comprehensive, up-to- date analysis of the short- and long-term RS of patients with CML over three different treatment eras, with particular focus on the era of TKI targeted therapy.
Patients and methods
Patient databases
Data from Surveillance, Epidemiology, and End Results (SEER) 9 registries database were selected for the present study. The 9 registries included Connecticut, Detroit (Michigan), Atlanta (Georgia), Iowa, Utah, New Mexico, San Francisco-Oakland (California), Seattle-Puget Sound (Washington) and Hawaii, covering about 10% of the US population. The SEER Program registries routinely record data on patient demographic, diagnosis and follow-up for vital status.
To evaluate the potential impact of allo-SCT on survival in CML, we obtained data from the Center for International Blood and Marrow Transplant Research (CIBMTR) to identify patterns of allo-SCT performed in patients with CML in the United States during the study period.
A total of 13,871 patients with an initial diagnosis of CML between 1975–2009 were reported to the SEER 9 registries. Two patients were excluded from this study because of unknown ages. The remaining 13,869 patients with CML were included for the incidence rate calculations. In 1740 reported cases, CML was not the first primary cancer, 188 cases were diagnosed by autopsy or reported by death certificate, and 53 cases were without active follow-up, leaving 11,888 cases for the survival analysis. Patients were grouped into 3 calendar periods according to year of diagnosis: 1975–1989, 1990–2000, and 2001–2009, representing the three main eras in the history for CML therapy: the era of cytotoxic therapy (busulfan and hydroxyurea); the era of interferon-alfa and allo-SCT; and the TKIs era. Patients were grouped into six age groups: <15 years, 15–29 years (adolescents and young adults, AYA), 30–49 years, 50–64 years, 65–74 years and ≥75 years.
Statistical Analysis
The patient characteristics were analyzed by descriptive statistics. Categorical variables were compared using the χ2 test for continuous variables. Age-adjusted incidence rate was expressed per 100,000 persons per-year.
We analyzed relative survival (RS) using the Kaplan-Meier method. SEER*Stat 7.0.4 statistical software and SPSS 16.0 software were used for data analysis. Expected survival was estimated according to Hakulinen’s methods using the 2000 US sex, age and race-specific life tables. RS is defined as the ratio of proportion of observed survival in a cohort of cancer patients (where all deaths are considered events) to the proportion of expected survival in a comparable age group of cancer free individuals, and is typically used for survival analysis using data from cancer registry databases. Typically, RS ratio is well below 1.00, reflecting excess mortality among cancer patients compared to the general population. RS thus measures the survival rate associated with the cancer in question, regardless of whether the excess mortality is due to cancer or non-cancer deaths. One-, 5- and 10-year RS were expressed as ratios of patients with CML who survived cancer at 1, 5, and 10 years, respectively.
Results
Patients Characteristics and Incidence of CML
Table 1 shows the patient sociodemographic parameters as well as the incidence and relative risk corresponding to age, sex, race, calendar year of diagnosis and registry. Among 13,869 patients with CML, 7941 were male (57%) with a median age at diagnosis of 66 years (range, 0 to 108 years); 85 % of patients were Caucasians. A total of 1006, 6958, and 2485 allo-SCT in the United States was reported to CIBMTR in the calendar periods 1975–1989, 1990–2000, and 2001–2009, respectively. The numbers of allo- SCTs in first chronic phase or in first complete remission during these calendar periods were 588 (58%), 4790 (69%) and 1445 (58%), respectively.
Table 1.
Patient demographics and incidence of chronic myeloid leukemia in1975–2009
| Characteristic | No. of patients | Incidence per 100,000 (95% CI) | Relative risk (95% CI) |
|---|---|---|---|
| Age, years | |||
| Median | 66 | ||
| Range | 0–108 | ||
| <15 | 175 | 0.09(0.08–0.11) | |
| 15–29 | 720 | 0.35(0.33–0.38) | 3.8(3.2–4.5) |
| 30–49 | 2,514 | 1.03(0.99–1.07) | 11.1(9.5–13.0) |
| 50–64 | 2,965 | 2.33(2.25–2.42) | 25.1(21.6–29.5) |
| 65–74 | 2,944 | 5.53(5.33–5.73) | 59.6(51.1–69.8) |
| >75 | 7,495 | 7.88(7.70–8.06) | 84.9(73.1–99.3) |
| Total | 13869 | 1.75(1.72–1.78) | |
| Sex | |||
| Female | 5,928 | 1.34(1.31–1.38) | |
| Male | 7,941 | 2.32(2.27–2.37) | 1.7(1.7–1.8) |
| Race | |||
| Other* | 809 | 1.19(1.11–1.28) | |
| Caucasian | 11,720 | 1.78(1.75–1.81) | 1.5(1.4–1.6) |
| African American | 1,234 | 1.77(1.67–1.87) | 1.5(1.4–1.6) |
| unknown | 106 | ||
| Calendar year of diagnosis | |||
| 1975–1989 | 5,001 | 1.78(1.73–1.83) | |
| 1990–2000 | 4,719 | 1.81(1.76–1.86) | 1.0(1.0–1.1) |
| 2001–2009 | 4,149 | 1.67(1.62–1.72) | 0.9(0.9–1.0) |
| Registry | |||
| Hawaii | 531 | 1.44(1.32–1.57) | |
| Atlanta (Metropolitan) | 945 | 1.49(1.39–1.59) | 1.0(0.9–1.1) |
| San Francisco-Oakland SMSA | 2,004 | 1.59(1.52–1.66) | 1.1(1.0–1.2) |
| Connecticut | 1,849 | 1.57(1.50–1.65) | 1.1(1.0–1.2) |
| Utah | 816 | 1.66(1.55–1.78) | 1.2(1.0–1.3) |
| New Mexico | 839 | 1.69(1.58–1.81) | 1.2(1.0–1.3) |
| Seattle (Puget Sound) | 2,060 | 1.84(1.76–1.93) | 1.3(1.2–1.4) |
| Iowa | 2,069 | 1.91(1.83–2.00) | 1.3(1.2–1.5) |
| Detroit (Metropolitan) | 2,756 | 2.13(2.05–2.21) | 1.5(1.3–1.6) |
American Indian/Alaska Native and Asians/Pacific populations
The incidence of CML was 1.75 cases/100,000 persons per-year, and was essentially stable during the study periods. The incidence increased with age from a rate of 0.09/100,000 among those ≤15 years old to 7.88/100,000 among those ≥75 years old with a relative risk of 85. The male to female ratio was 1.7. There were ethnic and geographic differences in the incidence on CML. The incidence was lowest among American Indian/Alaska Native and Asians/Pacific populations and was highest in Detroit (Table 1; P<0.05, data not shown). The registry included no codes on Hispanic origin.
Relative Survival
Table 2 shows the cumulative RS by age, sex, race and calendar year of diagnosis. Overall, 1-year, 5-year and 10-year RS ratios after diagnosis were 0.74, 0.36 and 0.21, respectively (Table 2). There were no significant differences in RS between male and female, and between Caucasian and African-American patients, but the 10-year RS ratios were considerably higher among Asians compared to Caucasian and African-American patients (P<0.05). A more comprehensive picture of RS within 10 years after diagnosis is shown in Figure 1A. The cumulative RS for all patients with CML under study improved significantly with each study period, with the greatest improvement among patients diagnosed during the 2001–2009 period. The cumulative RS were significantly higher in the 2005–2009 calendar period compared with the 2001–2004 calendar period (P<0.05; Fig 1B). The 5-year RS ratios were 0.26 for the calendar period 1975–1989, 0.36 for the calendar period 1990–2000, and 0.56 for the calendar period 2001–2009.
Table 2.
Cumulative relative survival in patients with chronic myeloid leukemia from 1975–2009 by age, sex, race, or calendar year
| characteristic | No. of patients | Relative Survival
|
||
|---|---|---|---|---|
| 1-year survival (95%CI) | 5-year survival (95%CI) | 10-year survival (95%CI) | ||
| Age, years | ||||
| <15 | 170 | 0.85(0.78–0.89) | 0.50(0.42–0.58) | 0.45(0.37–0.53) |
| 15–29 | 702 | 0.91(0.88–0.93) | 0.54(0.50–0.58) | 0.38(0.34–0.42) |
| 30–49 | 2,405 | 0.90(0.89–0.91) | 0.54(0.52–0.56) | 0.39(0.36–0.41) |
| 50–64 | 2,683 | 0.83(0.81–0.84) | 0.43(0.41–0.45) | 0.21(0.19–0.23) |
| 65–74 | 2,432 | 0.71(0.68–0.72) | 0.29(0.27–0.31) | 0.12(0.10–0.14) |
| >75 | 3,505 | 0.55(0.53–0.56) | 0.17(0.17–0.19) | 0.04(0.03–0.06) |
| Total | 11,888 | 0.74(0.73–0.75) | 0.36(0.35–0.37) | 0.21(0.20–0.22) |
| Sex | ||||
| male | 6,848 | 0.74(0.73–0.75) | 0.35(0.34–0.37) | 0.21(0.20–0.22) |
| female | 5,040 | 0.75(0.73–0.76) | 0.37(0.36–0.39) | 0.20(0.19–0.22) |
| Race | ||||
| Caucasian | 9,970 | 0.73(0.72–0.74) | 0.35(0.34–0.37) | 0.20(0.19–0.20) |
| African American | 1,104 | 0.78(0.75–0.80) | 0.36(0.33–0.40) | 0.22(0.18–0.25) |
| Other | 733 | 0.78(0.75–0.81) | 0.40(0.36–0.44) | 0.28(0.24–0.32) |
| unknown | 81 | 0.94(0.85–0.98) | 0.81(0.65–0.90) | 0.54(0.31–0.72) |
| Calendar year of diagnosis | ||||
| 1975–1989 | 4,496 | 0.71(0.69–0.72) | 0.26(0.24–0.27) | 0.09(0.08–0.10) |
| 1990–2000 | 4,001 | 0.73(0.71–0.74) | 0.36(0.35–0.38) | 0.25(0.24–0.27) |
| 2001–2009 | 3,391 | 0.80(0.79–0.82) | 0.56(0.54–0.58) | Not available |
American Indian/Alaska Native and Asians/Pacific populations
Fig 1.
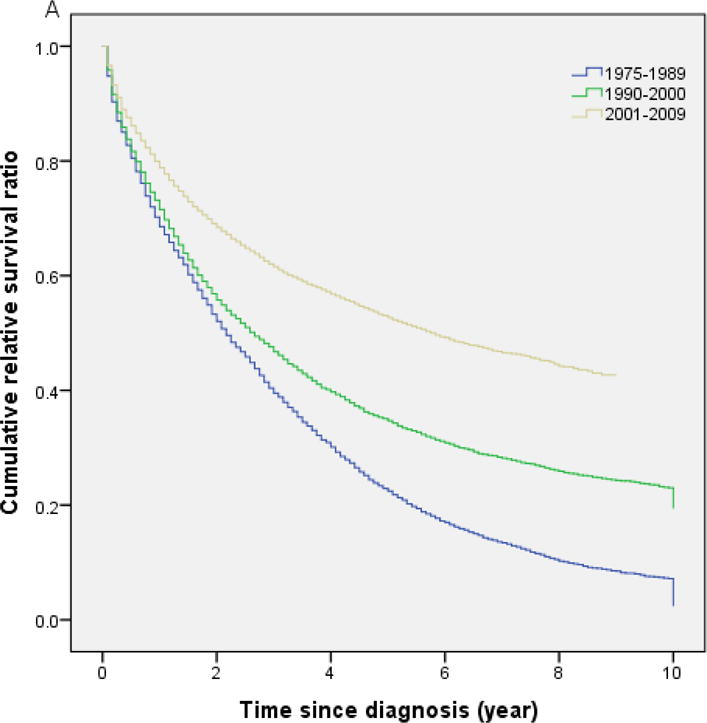
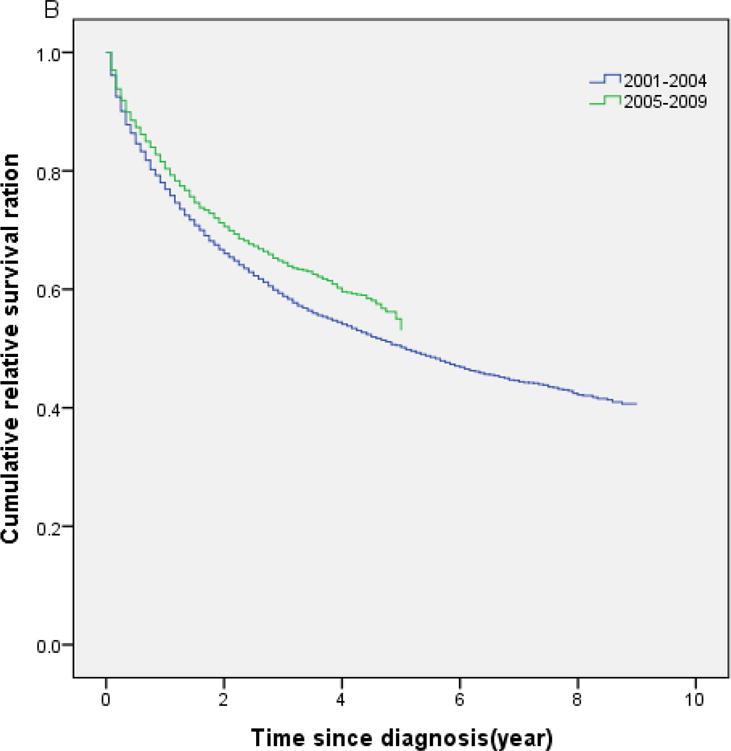
(A) Cumulative relative survival of all patients with chronic myeloid leukemia by calendar period of diagnosis. (B) Cumulative relative survival of patients with chronic myeloid leukemia during 2001–2004 versus 2005–2009
Table 3 shows the 1-year, 5-years and 10- year RS ratios by age and calendar period. As expected, age was a strong predictor of survival through all 3 calendar periods. The 5-year and 10-year RS ratios decreased rapidly for patients age greater than 64 years old (Figure 2). Patients diagnosed in 2001–2009 had the highest RS ratios among all age groups. Of note, the1-year and 5-year RS ratios in all calendar periods were highest in AYA (Figure 3, Table 3). In the last two calendar periods under study, the 5-year RS ratios improved significantly for all groups (P<0.05) except for the group aged <15 years (P>0.05). The increases were: from 0.56 to 0.70 for patients aged <15 years, from 0.56 to 0.86 for patients aged 15–29 years, from 0.53 to 0.84 for patients aged 30–49 years, from 0.45 to 0.70 for patients aged 50–64 years, from 0.29 to 0.47 for patients aged 65–74 years and 0.16 to 0.25 for patients aged ≥ 75 years. 1-year and 10-year RS ratios showed similar trends.
Table 3.
1-, 5-, and 10-year relative survival in patients with chronic myeloid leukemia by age and calendar period of diagnosis
| 1-year relative survival (95% CI) | ||||
| Age, years | 1975–1989 | 1990–2000 | 2001–2009 | |
| <15 | 0.81(0.70–0.89) | 0.82(0.68–0.90) | 0.92(0.80–0.97) | |
| 15–29 | 0.87(0.83–0.91) | 0.88(0.83–0.92) | 1.00(0.96–1.00) | |
| 30–49 | 0.88(0.86–0.90) | 0.87(0.85–0.89) | 0.96(0.94–0.97) | |
| 50–64 | 0.79(0.77–0.82) | 0.83(0.80–0.86) | 0.88(0.85–0.90) | |
| 65–74 | 0.67(0.64–0.70) | 0.69(0.65–0.72) | 0.79(0.76–0.83) | |
| >75 | 0.50(0.47–0.53) | 0.54(0.51–0.57) | 0.61(0.58–0.64) | |
| 5-year relative survival (95% CI) | ||||
| Age, years | 1975–1989 | 1990–2000 | 2001–2009 | |
| <15 | 0.35(0.24–0.47) | 0.56(0.41–0.68) | 0.70(0.53–0.81) | |
| 15–29 | 0.38(0.32–0.43) | 0.56(0.49–0.63) | 0.86(0.79–0.91) | |
| 30–49 | 0.37(0.34–0.41) | 0.53(0.49–0.56) | 0.84(0.80–0.87) | |
| 50–64 | 0.29(0.27–0.32) | 0.45(0.42–0.47) | 0.70(0.65–0.74) | |
| 65–74 | 0.21(0.18–0.24) | 0.29(0.26–0.32) | 0.47(0.41–0.52) | |
| >75 | 0.1340.11–0.16) | 0.16(0.13–0.19) | 0.25(0.21–0.29) | |
| 10-year relative survival (95% CI) | ||||
| Age, years | 1975–1989 | 1990–2000 | 2001–2009 | |
| <15 | 0.26(0.17–0.37) | 0.56(0.41–0.68) | Not available | |
| 15–29 | 0.17(0.13–0.22) | 0.49(0.42–0.56) | Not available | |
| 30–49 | 0.16(0.14–0.19) | 0.45(0.42–0.48) | Not available | |
| 50–64 | 0.08(0.06–0.10) | 0.29(0.25–0.32) | Not available | |
| 65–74 | 0.06(0.05–0.06) | 0.13(0.11–0.16) | Not available | |
| >75 | 0.02(0.01–0.04) | 0.04(0.02–0.07) | Not available | |
Fig 2.
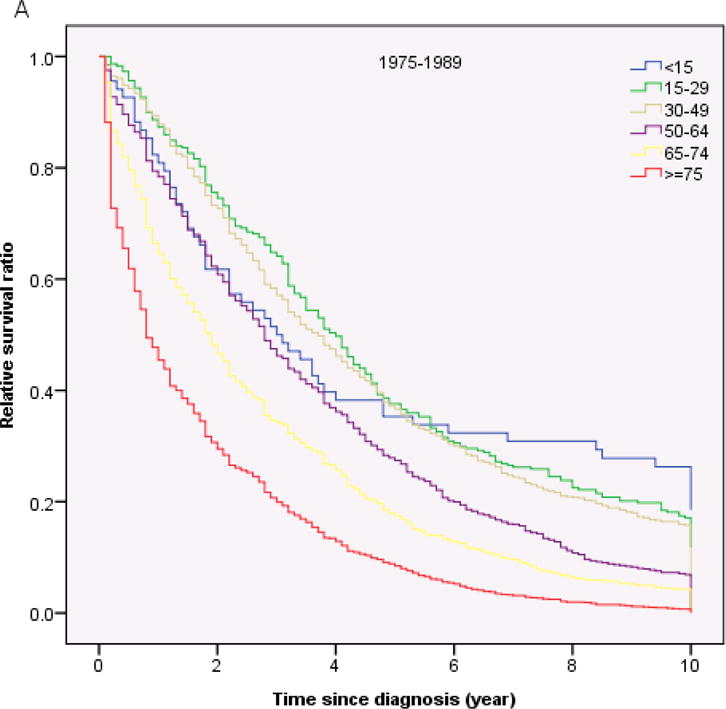
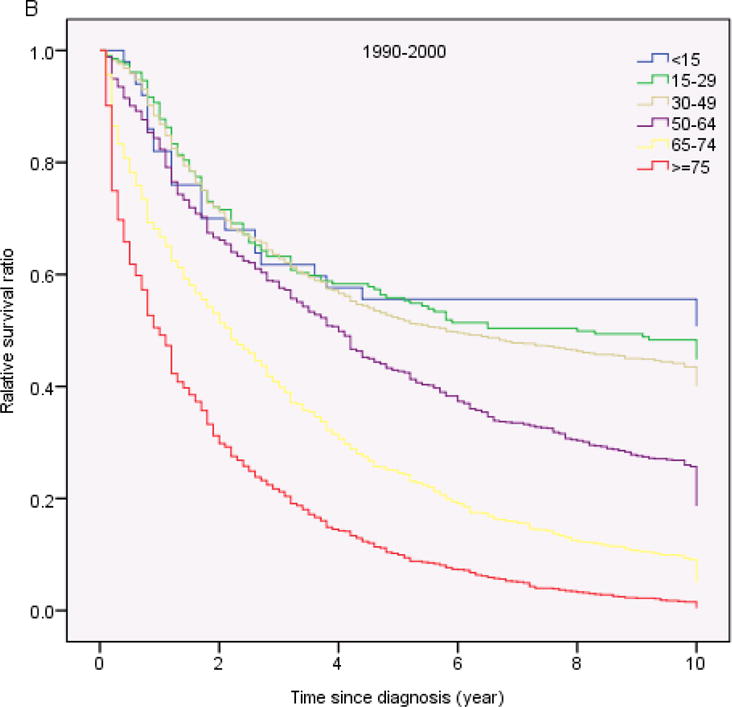
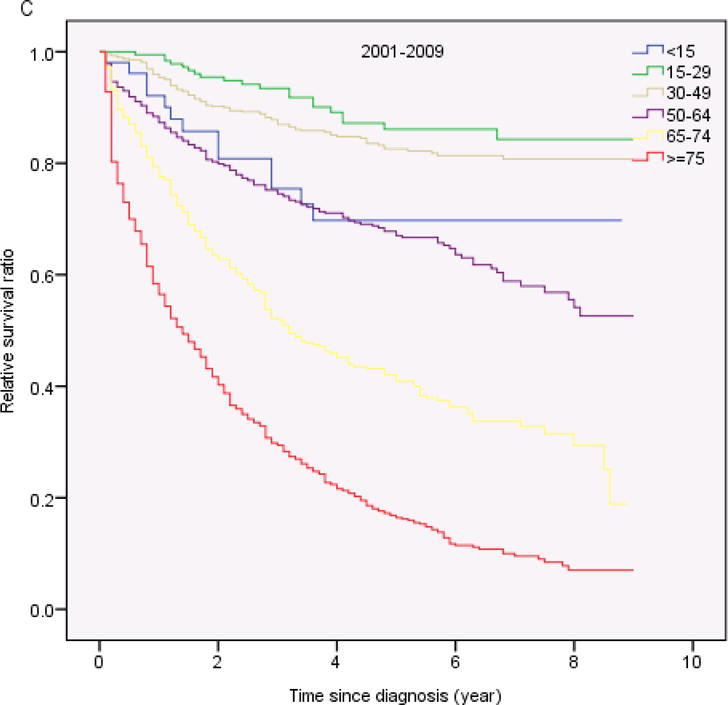
Relative survival in patients with chronic myeloid leukemia by age and calendar period of diagnosis
Fig 3.
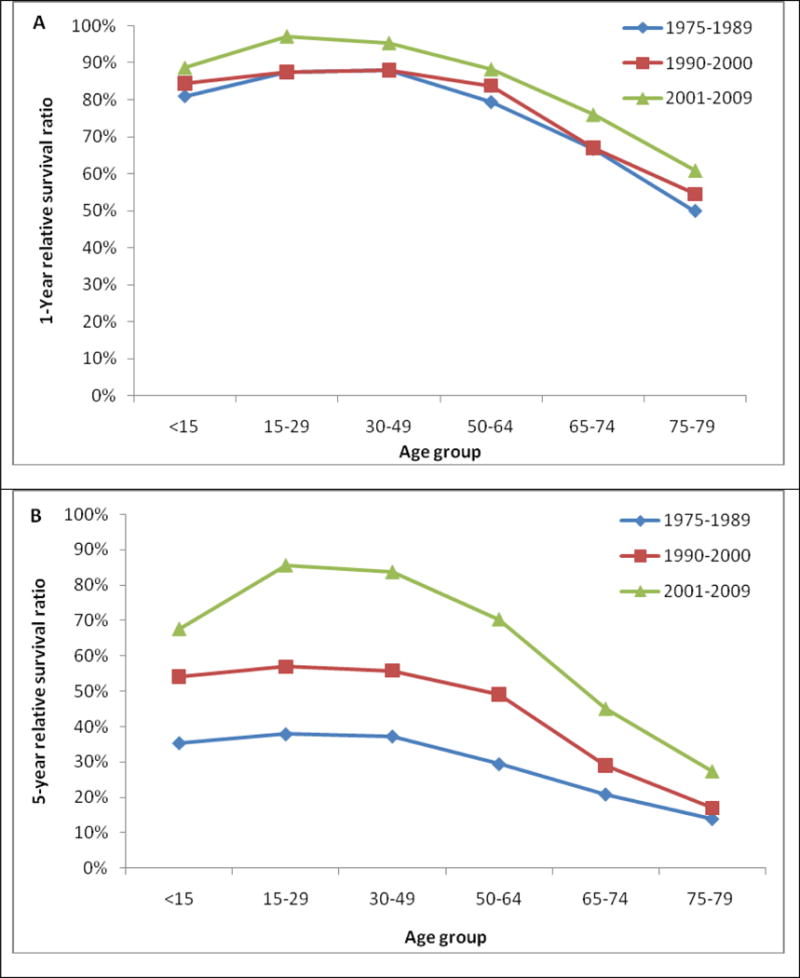
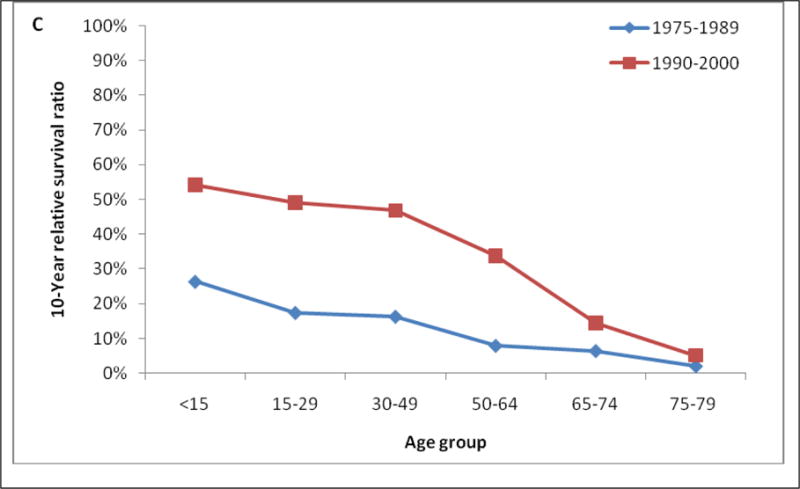
One-, 5-, and 10-year relative survival in patients with chronic myeloid leukemia by age group and calendar period of diagnosis
Discussion
In this comprehensive study of survival of patients with CML using the SEER database during 1975–2009, a significant improvement in RS was observed starting in calendar period 1990–2000 (Figure 1; Table 2).
The period 1990–2000 was characterized by the prevalent use of the allo-SCT and interferon alfa as the preferred modalities for treatment of CML. Allo-SCT was first reported in 1970s, first with identical twin donors and then from matched sibling donors in 1980s.8,9 It became the prevailing treatment in 1990s in eligible young patients (typically those age younger than 55 years old) with available matched sibling donors after reports that allo-SCT could induce long-term cytogenetic remissions. Long-term follow-up data confirmed that allo-SCT is curative in patients with CML in chronic phase.6,7 These patients can achieve 10-year survival rates of 50–70 percent.6,7,10 Unfortunately, only 15%–30% of patients with CML are eligible for allo-SCT from HLA-matched sibling donor given the limitation of age at diagnosis and availability of HLA-matched donor. The use of unrelated donors increased the percentage of candidates for transplantation up to 30%.11 For those patients who were unable to receive allo-SCT, Interferon-alfa plus hydroxyurea and/or cytarabine was a standard treatment during this period. In 1980s, a clinical study was first conducted and reported that interferon-alfa could induce cytogenetic response in patients with CML.12 Subsequently, other investigators confirmed the effect of interferon-alfa on CML.5,13 The finding that cytogenetic responses were associated with improved survival led to widespread use interferon-alfa in patient with CML not eligible for allo-SCT in 1990s. Long-term follow-up of clinical studies confirmed that interferon-alfa based therapy could induce complete cytogenetic remission in 17% to 36% of patients treated5,13,14 and improved survival in patients with CML as compared to cytostatic treatments.14–16 Accordingly, the overall 5-year RS ratio increased from 0.26 to 0.36 for the calendar period 1990–2000 (Table 2). This is lower than the 0.54 for 1994–2000 reported by Swedish study group using Swedish Cancer Registry data,17 but higher than 0.27 reported by Brenner et al. The latter used period analysis to estimate trends in RS among patients with CML from 1990–1992 and 2002–2004 using SEER data.18 Period analysis is statistically less accurate than cohort analysis and their validity is based on the validity of the underlying assumptions that previously observed trends will continue. In addition, such improvement results are also likely because of early diagnosis, better supportive care, and better management of patients with relapse or resistant disease during the calendar period (e.g. use of donor lymphocytes infusions in patients relapsing after allo-SCT).
The greatest improvement in survival for all age groups was noted in the calendar period 2001–2009. The 5-year RS ratios improved significantly in all age groups except <15 year age group in comparison to those in calendar period 1990–2000 (P<0.05). This dramatic improvement in outcomes can be mainly attributed to the increased use of TKIs. In the United States, treatment of CML with imatinib was first introduced in June 1998.19 Subsequently, a phase 2 trial in 532 CML patients who had inadequate response to interferon-alfa was conducted during 1999–2000.20 Between June 2000 and January 2001, a phase 3 International Randomized Study of imatinib versus Interferon-alfa plus cytarabine (IRIS) was conducted in 1,106 patients with newly diagnosed CML in chronic phase.21,22 In this trial, higher rates of complete cytogenetic response and major molecular response were observed in patients receiving imatinib (18 month rates of 76% versus 14%, and 39% versus 2%, respectively). Overall survival rate was 89% in the imatinib group at 5 years. In May 2001, imatinib was approved by the Food and Drug Administration for patients with CML. Thereafter, Imatinib became first-line therapy for newly diagnosed CML.2–4 Our results from this population-based study are in line with reports from single-institution historical comparisons showing survival benefit from imatinib versus interferon-alfa.23–25 Our overall 5-year RS ratio of 56% for 2001–2008 calendar period is lower than the 80% reported by Swedish study group,17 but higher than 49% reported by Brenner et al.18 The latter study did not identify a significant difference in survival between 1990–92 and 2002–04 in patients among age groups 65–74 years and older than 75 years.18 Of note, our analysis showed the AYA group has highest 1-year and 5-year RS ratios during 3 calendar periods among all groups. This is in contrast to a recent report that rates of complete cytogenetic, major molecular and complete molecular response with imatinib were significantly lower in AYA compared to older patients, although the overall survival were similar between the two groups.26 One possible explanation might be the use of SCT in this age group after failure to initial therapy, considering that this procedure has a particularly good outcome in this age group.27
We also observed a significant improvement in RS occurring in 2005–2009 compared to 2001–2004(P<0.05). This may be due to a better penetration of imatinib in community-practice therapy of CML, improved familiarity with the use of the drug and monitoring guidelines, or to the effect of salvage therapy with second-generation TKIs. Despite the efficacy of imatinib about 40% to 42% of patients discontinued imatinib due to lack of efficacy, loss of response, or intolerance.28 29 Dasatinib and nilotinib have a higher binding affinity and selectivity for the ABL kinase than does imatinib. Both agents are active against many imatinib-resistant kinase domain mutations of BCR-ABL. Dasatinib was first introduced to patients with CML following imatinib therapy failure in 2003,30 and nilotinib in 2004.31 Several trials further confirmed the efficacy and safety of dasatinib in patients with CML in accelerated phase,32 and blastic phase.33 Based on these results, the FDA approved dasatinib and nilotinib for treatment of CML in 2006 and 2007, respectively. Clinical trials have demonstrated that dasatinib or nilotinib can induce significantly higher and faster rates of complete cytogenetic response and major molecular response in newly diagnosed CML in chronic phase.34–37 Recently studies have suggested that achieving complete cytogenetic response and early response were associated with improved survival.29,38,39
Patients for our population-based study were obtained from SEER registries with no selection biases, and have a long-term follow-up. However, there is no individual treatment information in the SEER database. Thus, the conclusions of this study are entirely based on the assumption of changing patterns of treatment of CML with the FDA approval of TKIs, as well as data from relevant publications. There is no data in SEER database to categorize patients according to risk factors or clinical phases. Therefore, the analysis of survival according to risk groups or three phases could not be performed.
In our analysis the incidence of CML has been steady in the United States over the past 30 years with a rate of 1.75/100,000 person per year. CML is more common in male individuals and in older people. The incidence was 0.09 among population aged < 15 years compared to 7.88/100,000 among those aged >75 years. Our findings suggest that environmental or occupational exposures40 as well as immune senescence may play important roles in the etiology of CML. The incidence was significantly higher in Detroit compared to other registries. The incidence rate of 1.75 is higher than incidence rates of 0.9–1.10 reported from 44 European cancer registries41 and the United Kingdom Cancer Network42, but consistent with a report of 4,870 new cases with CML in the Unites States in 2010.1 We also noticed a significant low incidence rate in American Indians/Alaska Native and Asian/Pacific Islanders compared Caucasians and African-Americans (P<0.05). To our knowledge, this is first report of geographic and race variations in the incidence of CML.
In summary, in this large population-based study, we found that the incidence of CML was stable over time. The RS of patients with CML increased with each treatment eras, with the greatest improvement occurring in 2001–2009 for all age groups, presumably because of increasing use of TKIs. Future research should focus on methods to identify and to eliminate residual dormant CML stem cells that cure relapse, so we can achieve the ultimate goal of cure in CML. The efficacy of T-cell mediated immune mechanism on curing leukemia has clearly been demonstrated with the use of donor lymphocytes infusions.43–45 Combinations of imatinib and interferon-alfa increase molecular response rates46–48 and induce a proteinase-3 specific cytotoxic T lymphocyte that may eradicate residue disease.46 It will be interesting to see the effect of combination of TKIs with immunotherapy on the survival in CML in the future. Other strategies may employ older (decitabine, omacetaxine) or newer agents (hedgehog or JAK2 inhibitors) to eliminate dormant CML stem cells.
Acknowledgments
None
Footnotes
Previous presentations: Submitted to 2012 ASH Meeting.
Conflict-of-interest disclosure: none
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. Prepublished on 2010/07/09 as DOI caac.20073 [pii] 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Schiffer CA. BCR-ABL tyrosine kinase inhibitors for chronic myelogenous leukemia. N Engl J Med. 2007;357(3):258–265. doi: 10.1056/NEJMct071828. Prepublished on 2007/07/20 as DOI 357/3/258 [pii] 10.1056/NEJMct071828. [DOI] [PubMed] [Google Scholar]
- 3.Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370(9584):342–350. doi: 10.1016/S0140-6736(07)61165-9. Prepublished on 2007/07/31 as DOI S0140-6736(07)61165-9 [pii] 10.1016/S0140-6736(07)61165-9. [DOI] [PubMed] [Google Scholar]
- 4.Peggs K, Mackinnon S. Imatinib mesylate–the new gold standard for treatment of chronic myeloid leukemia. N Engl J Med. 2003;348(11):1048–1050. doi: 10.1056/NEJMe030009. Prepublished on 2003/03/15 as DOI 10.1056/NEJMe030009348/11/1048 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Interferon alfa-2a as compared with conventional chemotherapy for the treatment of chronic myeloid leukemia. The Italian Cooperative Study Group on Chronic Myeloid Leukemia. N Engl J Med. 1994;330(12):820–825. doi: 10.1056/NEJM199403243301204. Prepublished on 1994/03/24. [DOI] [PubMed] [Google Scholar]
- 6.Kalidas M, Kantarjian H, Talpaz M. Chronic myelogenous leukemia. JAMA. 2001;286(8):895–898. doi: 10.1001/jama.286.8.895. Prepublished on 2001/08/31 as DOI jct10003 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340(17):1330–1340. doi: 10.1056/NEJM199904293401706. Prepublished on 1999/04/29. [DOI] [PubMed] [Google Scholar]
- 8.Fefer A, Cheever MA, Thomas ED, et al. Disappearance of Ph1-positive cells in four patients with chronic granulocytic leukemia after chemotherapy, irradiation and marrow transplantation from an identical twin. N Engl J Med. 1979;300(7):333–337. doi: 10.1056/NEJM197902153000702. Prepublished on 1979/02/15. [DOI] [PubMed] [Google Scholar]
- 9.Clift RA, Buckner CD, Thomas ED, et al. Treatment of chronic granulocytic leukaemia in chronic phase by allogeneic marrow transplantation. Lancet. 1982;2(8299):621–623. doi: 10.1016/s0140-6736(82)92735-0. Prepublished on 1982/09/18 as DOI. [DOI] [PubMed] [Google Scholar]
- 10.van Rhee F, Szydlo RM, Hermans J, et al. Long-term results after allogeneic bone marrow transplantation for chronic myelogenous leukemia in chronic phase: a report from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1997;20(7):553–560. doi: 10.1038/sj.bmt.1700933. Prepublished on 1997/10/23. [DOI] [PubMed] [Google Scholar]
- 11.Hansen JA, Gooley TA, Martin PJ, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338(14):962–968. doi: 10.1056/NEJM199804023381405. Prepublished on 1998/04/02. [DOI] [PubMed] [Google Scholar]
- 12.Talpaz M, McCredie KB, Mavligit GM, Gutterman JU. Leukocyte interferon-induced myeloid cytoreduction in chronic myelogenous leukemia. Blood. 1983;62(3):689–692. Prepublished on 1983/09/01 as DOI. [PubMed] [Google Scholar]
- 13.Mahon FX, Faberes C, Pueyo S, et al. Response at three months is a good predictive factor for newly diagnosed chronic myeloid leukemia patients treated by recombinant interferon-alpha. Blood. 1998;92(11):4059–4065. Prepublished on 1998/12/03 as DOI. [PubMed] [Google Scholar]
- 14.Kantarjian HM, Smith TL, O’Brien S, Beran M, Pierce S, Talpaz M. Prolonged survival in chronic myelogenous leukemia after cytogenetic response to interferon-alpha therapy. The Leukemia Service. Ann Intern Med. 1995;122(4):254–261. doi: 10.7326/0003-4819-122-4-199502150-00003. Prepublished on 1995/02/15 as DOI. [DOI] [PubMed] [Google Scholar]
- 15.Allan NC, Richards SM, Shepherd PC. UK Medical Research Council randomised, multicentre trial of interferon-alpha n1 for chronic myeloid leukaemia: improved survival irrespective of cytogenetic response. The UK Medical Research Council’s Working Parties for Therapeutic Trials in Adult Leukaemia. Lancet. 1995;345(8962):1392–1397. doi: 10.1016/s0140-6736(95)92596-1. Prepublished on 1995/06/03 as DOI. [DOI] [PubMed] [Google Scholar]
- 16.Guilhot F, Chastang C, Michallet M, et al. Interferon alfa-2b combined with cytarabine versus interferon alone in chronic myelogenous leukemia. French Chronic Myeloid Leukemia Study Group. N Engl J Med. 1997;337(4):223–229. doi: 10.1056/NEJM199707243370402. Prepublished on 1997/07/24. [DOI] [PubMed] [Google Scholar]
- 17.Bjorkholm M, Ohm L, Eloranta S, et al. Success story of targeted therapy in chronic myeloid leukemia: a population-based study of patients diagnosed in Sweden from 1973 to 2008. J Clin Oncol. 2011;29(18):2514–2520. doi: 10.1200/JCO.2011.34.7146. Prepublished on 2011/05/18 as DOI JCO.2011.34.7146 [pii] 10.1200/JCO.2011.34.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner H, Gondos A, Pulte D. Recent trends in long-term survival of patients with chronic myelocytic leukemia: disclosing the impact of advances in therapy on the population level. Haematologica. 2008;93(10):1544–1549. doi: 10.3324/haematol.13045. Prepublished on 2008/07/22 as DOI haematol.13045 [pii] 10.3324/haematol.13045. [DOI] [PubMed] [Google Scholar]
- 19.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–1037. doi: 10.1056/NEJM200104053441401. Prepublished on 2001/04/05. [DOI] [PubMed] [Google Scholar]
- 20.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346(9):645–652. doi: 10.1056/NEJMoa011573. Prepublished on 2002/03/01 as DOI 10.1056/NEJMoa011573346/9/645 [pii] [DOI] [PubMed] [Google Scholar]
- 21.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. Prepublished on 2003/03/15 as DOI 10.1056/NEJMoa022457348/11/994 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. Prepublished on 2006/12/08 as DOI 355/23/2408 [pii]10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 23.Kantarjian H, O’Brien S, Jabbour E, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119(9):1981–1987. doi: 10.1182/blood-2011-08-358135. Prepublished on 2012/01/10 as DOI blood-2011-08-358135 [pii] 10.1182/blood-2011-08-358135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy L, Guilhot J, Krahnke T, et al. Survival advantage from imatinib compared with the combination interferon-alpha plus cytarabine in chronic-phase chronic myelogenous leukemia: historical comparison between two phase 3 trials. Blood. 2006;108(5):1478–1484. doi: 10.1182/blood-2006-02-001495. Prepublished on 2006/04/22 as DOI blood-2006-02-001495 [pii]10.1182/blood-2006-02-001495. [DOI] [PubMed] [Google Scholar]
- 25.Kantarjian HM, Talpaz M, O’Brien S, et al. Survival benefit with imatinib mesylate versus interferon-alpha-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood. 2006;108(6):1835–1840. doi: 10.1182/blood-2006-02-004325. Prepublished on 2006/05/20 as DOI blood-2006-02-004325 [pii]10.1182/blood-2006-02-004325. [DOI] [PubMed] [Google Scholar]
- 26.Pemmaraju N, Kantarjian H, Shan J, et al. Analysis of outcomes in adolescents and young adults with chronic myelogenous leukemia treated with upfront tyrosine kinase inhibitor therapy. Haematologica. 2012 doi: 10.3324/haematol.2011.056721. Prepublished on 2012/01/25 as DOI haematol.2011056721 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saussele S, Lauseker M, Gratwohl A, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010;115(10):1880–1885. doi: 10.1182/blood-2009-08-237115. Prepublished on 2009/12/08 as DOI blood-2009-08-237115 [pii]10.1182/blood-2009-08-237115. [DOI] [PubMed] [Google Scholar]
- 28.Cortes J, Hochhaus A, Hughes T, Kantarjian H. Front-line and salvage therapies with tyrosine kinase inhibitors and other treatments in chronic myeloid leukemia. J Clin Oncol. 2011;29(5):524–531. doi: 10.1200/JCO.2010.31.3619. Prepublished on 2011/01/12 as DOI JCO.2010.31.3619 [pii]10.1200/JCO.2010.31.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30(3):232–238. doi: 10.1200/JCO.2011.38.6565. Prepublished on 2011/11/10 as DOI JCO.2011.38.6565 [pii]10.1200/JCO.2011.38.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354(24):2531–2541. doi: 10.1056/NEJMoa055229. Prepublished on 2006/06/16 as DOI 354/24/2531 [pii]10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 31.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354(24):2542–2551. doi: 10.1056/NEJMoa055104. Prepublished on 2006/06/16 as DOI 354/24/2542 [pii]10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 32.Apperley JF, Cortes JE, Kim DW, et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START a trial. J Clin Oncol. 2009;27(21):3472–3479. doi: 10.1200/JCO.2007.14.3339. Prepublished on 2009/06/03 as DOI JCO.2007.14.3339 [pii]10.1200/JCO.2007.14.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortes J, Rousselot P, Kim DW, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109(8):3207–3213. doi: 10.1182/blood-2006-09-046888. Prepublished on 2006/12/23 as DOI blood-2006-09-046888 [pii]10.1182/blood-2006-09-046888. [DOI] [PubMed] [Google Scholar]
- 34.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–2270. doi: 10.1056/NEJMoa1002315. Prepublished on 2010/06/08 as DOI NEJMoa1002315 [pii]10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 35.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. doi: 10.1056/NEJMoa0912614. Prepublished on 2010/06/08 as DOI NEJMoa0912614 [pii]10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 36.Cortes JE, Jones D, O’Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28(3):398–404. doi: 10.1200/JCO.2009.25.4920. Prepublished on 2009/12/17 as DOI JCO.2009.25.4920 [pii]10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortes JE, Jones D, O’Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28(3):392–397. doi: 10.1200/JCO.2009.25.4896. Prepublished on 2009/12/17 as DOI JCO.2009.25.4896 [pii]10.1200/JCO.2009.25.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortes JE. Not only response but early response to tyrosine kinase inhibitors in chronic myeloid leukemia. J Clin Oncol. 2012;30(3):223–224. doi: 10.1200/JCO.2011.39.6655. Prepublished on 2011/12/21 as DOI JCO.2011.39.6655 [pii]10.1200/JCO.2011.39.6655. [DOI] [PubMed] [Google Scholar]
- 39.Quintas-Cardama A, Kantarjian H, Jones D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood. 2009;113(25):6315–6321. doi: 10.1182/blood-2008-07-166694. Prepublished on 2009/04/17 as DOI blood-2008-07-166694 [pii]10.1182/blood-2008-07-166694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vlaanderen J, Lan Q, Kromhout H, Rothman N, Vermeulen R. Occupational benzene exposure and the risk of chronic myeloid leukemia: A meta-analysis of cohort studies incorporating study quality dimensions. Am J Ind Med. 2012 doi: 10.1002/ajim.22087. Prepublished on 2012/06/26. [DOI] [PubMed] [Google Scholar]
- 41.Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724–3734. doi: 10.1182/blood-2010-05-282632. Prepublished on 2010/07/29 as DOI blood-2010-05-282632 [pii]10.1182/blood-2010-05-282632. [DOI] [PubMed] [Google Scholar]
- 42.Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105(11):1684–1692. doi: 10.1038/bjc.2011.450. Prepublished on 2011/11/03 as DOI 10.1038/bjc.2011.450bjc2011450 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76(12):2462–2465. Prepublished on 1990/12/15 as DOI. [PubMed] [Google Scholar]
- 44.Pavlu J, Szydlo RM, Goldman JM, Apperley JF. Three decades of transplantation for chronic myeloid leukemia: what have we learned? Blood. 2011;117(3):755–763. doi: 10.1182/blood-2010-08-301341. Prepublished on 2010/10/23 as DOI blood-2010-08-301341 [pii]10.1182/blood-2010-08-301341. [DOI] [PubMed] [Google Scholar]
- 45.Mackinnon S, Papadopoulos EB, Carabasi MH, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. 1995;86(4):1261–1268. Prepublished on 1995/08/15 as DOI. [PubMed] [Google Scholar]
- 46.Burchert A, Muller MC, Kostrewa P, et al. Sustained molecular response with interferon alfa maintenance after induction therapy with imatinib plus interferon alfa in patients with chronic myeloid leukemia. J Clin Oncol. 2010;28(8):1429–1435. doi: 10.1200/JCO.2009.25.5075. Prepublished on 2010/02/10 as DOI JCO.2009.25.5075 [pii]10.1200/JCO.2009.25.5075. [DOI] [PubMed] [Google Scholar]
- 47.Preudhomme C, Guilhot J, Nicolini FE, et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med. 2010;363(26):2511–2521. doi: 10.1056/NEJMoa1004095. Prepublished on 2010/12/24 as DOI 10.1056/NEJMoa1004095. [DOI] [PubMed] [Google Scholar]
- 48.Simonsson B, Gedde-Dahl T, Markevarn B, et al. Combination of pegylated IFN-alpha2b with imatinib increases molecular response rates in patients with low- or intermediate-risk chronic myeloid leukemia. Blood. 2011;118(12):3228–3235. doi: 10.1182/blood-2011-02-336685. Prepublished on 2011/06/21 as DOI blood-2011-02-336685 [pii]10.1182/blood-2011-02-336685. [DOI] [PubMed] [Google Scholar]


