Abstract
Background
Acute liver failure (ALF) is a rare syndrome of severe, rapid-onset hepatic dysfunction without prior advanced liver disease that is associated with high morbidity and mortality. Intensive care and liver transplantation provide support and rescue, respectively.
Objective
To determine whether changes in causes, disease severity, treatment, or 21-day outcomes have occurred in recent years among adult patients with ALF referred to U.S. tertiary care centers.
Design
Prospective observational cohort study. (ClinicalTrials.gov: NCT00518440)
Setting
31 liver disease and transplant centers in the United States.
Patients
Consecutively enrolled patients–without prior advanced liver disease–with ALF (n = 2070).
Measurements
Clinical features, treatment, and 21-day outcomes were compared over time annually for trends and were also stratified into two 8-year periods (1998 to 2005 and 2006 to 2013).
Results
Overall clinical characteristics, disease severity, and distribution of causes remained similar throughout the study period. The 21-day survival rates increased between the two 8-year periods (overall, 67.1% vs. 75.3%; transplant-free survival [TFS], 45.1% vs. 56.2%; posttransplantation survival, 88.3% vs. 96.3% [P < 0.010 for each]). Reductions in red blood cell infusions (44.3% vs. 27.6%), plasma infusions (65.2% vs. 47.1%), mechanical ventilation (65.7% vs. 56.1%), and vasopressors (34.9% vs. 27.8%) were observed, as well as increased use of N-acetylcysteine (48.9% vs. 69.3% overall; 15.8% vs. 49.4% [P < 0.001] in patients with ALF not due to acetaminophen toxicity). When examined longitudinally, overall survival and TFS increased throughout the 16-year period.
Limitations
The duration of enrollment, the number of patients enrolled, and possibly the approaches to care varied among participating sites. The results may not be generalizable beyond such specialized centers.
Conclusion
Although characteristics and severity of ALF changed little over 16 years, overall survival and TFS improved significantly. The effects of specific changes in intensive care practice on survival warrant further study.
Primary Funding Source
National Institutes of Health.
Acute liver failure (ALF) is defined as severe liver injury with rapid onset that results in hepatic encephalopathy (HE) and coagulopathy in persons without preexisting liver disease. The principal causes of ALF include acetaminophen (n-Acetyl-p-Aminophenol [APAP]) overdose, ischemic and pregnancy-associated liver injury, acute infection with hepatitis A or B virus, drug-induced liver injury, autoimmune hepatitis, Budd–Chiari syndrome, and Wilson disease (1, 2). For some causes, such as APAP toxicity, outcomes are favorable and transplant-free survival (TFS) approaches 70%, whereas other causes have unfavorable outcomes, including a much lower likelihood (<30%) of recovery without liver transplantation (2). One-year survival after emergency liver transplantation in patients with ALF in the United States and Europe is reportedly good but is lower than among patients with cirrhosis who receive a transplant (3).
Patients with ALF often deteriorate rapidly and therefore receive the most urgent ranking (status 1) in the United Network for Organ Sharing transplantation system. Treatment of ALF in the intensive care unit is largely supportive and includes ventilator and vasopressor support for respiratory and/or circulatory failure, renal replacement therapy, plasma and blood transfusions, antibiotics, and measures to decrease intracranial pressure (4–6). N-acetylcysteine is used to treat APAP overdose and has shown efficacy in patients with ALF not due to APAP toxicity, particularly those referred early and having only mild HE (7). However, few disease-specific or general treatments are available that yield improved outcomes.
In this study, our aim was to update the U.S. experience with ALF at specialized liver disease and transplant centers since the last published overview by the Acute Liver Failure Study Group (ALFSG) in 2002 (2). This group initiated its registry in January 1998 to better characterize the causes, clinical features, and outcomes of this “super-orphan” condition and aimed to enroll cases prospectively from participating liver transplant centers across North America. Accordingly, we analyzed data on all patients with ALF enrolled between 1998 and 2013, focusing on whether clinical features or outcomes of the ALF syndrome have changed over time. In addition, we sought to determine the relationship between ALF causes and rates of TFS and whether utilization of liver transplantation changed in the 16-year observation period.
Methods
Study Population
From 1 January 1998 through 31 December 2013, adult patients were consecutively enrolled in the ALFSG registry (2) from 31 U.S. academic liver centers (of which only 5 “legacy” sites participated continuously throughout the 16-year period). All enrolled patients had both coagulopathy (international normalized ratio [INR] ≥1.5) and any grade of HE (as clinically defined by the classic West Haven criteria [8]) within 26 weeks of the first symptoms and had no evidence of significant chronic liver disease, especially cirrhosis. Patients for whom prior liver transplantation failed (due to primary graft nonfunction or other causes) were excluded. During the 16-year period, the number of sites participating, their geographic locations, and the number of cases contributed per site varied depending on each site’s ability to continually identify and enroll patients over time (Appendix Figure 1, available at www.annals.org).
Patients were usually admitted to intensive care units; 82.4% were hospitalized before transfer to the referral tertiary care study site, and the remainder were admitted directly to the study site. All were screened for inclusion according to the ALF criteria defined earlier. Written informed consent was obtained from the legal next of kin. A log of “screen failures” and consent refusal was maintained. All centers complied with local institutional review board requirements.
Data Management and Integrity
At enrollment into the study, we prospectively collected patient demographic characteristics (age, sex, race, and ethnicity); a complete medical history, including the timing of the first symptom of ill health, onset of jaundice and HE, and the number of days between the first symptom, hospital admission, transfer to the study site (where relevant), and enrollment in the study; and clinical features, including blood pressure and need for vasopressor support, mechanical ventilation, and renal replacement therapy, which allowed calculation of the systemic inflammatory response syndrome (SIRS) score (9). We also collected standard liver and metabolic test results and clinical data daily for up to 7 days, as well as serologic and other tests to determine the cause.
All data were managed and housed on a central server at the Medical University of South Carolina. A data query system and periodic monitoring are in place to manage data integrity. In addition, ALFSG leadership conducted annual visits to clinical sites to verify data and ensure compliance with study procedures.
Statistical Analysis
Statistical analyses were performed using SAS, version 9.4 (SAS Institute). Missing values were not replaced or estimated. Patients with missing data were excluded from the respective analyses for those variables, and patients who were lost to follow-up before 21 days were excluded from the study. Descriptive statistics were used to characterize the demographic and other clinical variables. Categorical variables were compared using the chi-square test or the Fisher exact test (the latter when expected cell counts were <5). Medians were reported with interquartile ranges (IQRs) and were compared with the Wilcoxon rank-sum test. Survival and transplant outcomes at 21 days after study enrollment were classified as TFS (survival without liver transplantation), liver transplantation, or death (2). Outcomes were also determined at 1 and 2 years after study enrollment, but these data were less complete than the 21-day outcome data. Survival rates over time were assessed descriptively at the individual-site level to verify that changes in TFS were not affected by varying accrual of patients from different sites. Treatment utilization and survival and transplant outcomes were analyzed over time annually for trends and were also stratified into two 8-year periods: early (1998 to 2005) and later (2006 to 2013). Trends over time were analyzed using the Cochran–Armitage test. A significance level of less than 0.05 was used for all comparisons.
Role of the Funding Source
This study was funded by the National Institutes of Health. The funding source had no direct role in the design, conduct, or reporting of the study.
Results
Demographic Characteristics and Comorbidities
During the 16-year study, 2070 patients (median age, 39.0 years [IQR, 29.0 to 52.0 years]) were enrolled in the ALFSG registry. Over the same interval, there were 660 confirmed ALF screen failures (286 due to failure to meet inclusion criteria, 212 for whom consent could not be obtained, and 162 for other reasons). Among enrolled patients, 69.3% were women and 76.4% were white (Table 1). Patients did not differ in sex, race, or ethnicity between the two 8-year periods but were significantly older and heavier in the later period. Prevalence of hypertension, heart disease, diabetes, psychiatric illness, and substance dependency all increased significantly between the early and later periods, whereas prevalence of renal disease did not.
Table 1.
Demographic Characteristics, Comorbidities, Clinical Severity, and Causes at Admission
| Variable | Overall (1998–2013) (n = 2070) |
Early (1998–2005) (n = 989) |
Later (2006–2013) (n = 1081) |
P Value† | |||
|---|---|---|---|---|---|---|---|
| Total Patients, n |
Value* | Total Patients, n |
Value* | Total Patients, n |
Value* | ||
| Median age (IQR), y | 2070 | 39.0 (29.0–52.0) | 989 | 38.0 (28.0–49.0) | 1081 | 41.0 (30.0–53.0) | <0.001 |
|
| |||||||
| Female | 2070 | 1434 (69.3) | 989 | 680 (68.8) | 1081 | 754 (69.8) | 0.62 |
|
| |||||||
| Race | 2070 | 989 | 1081 | 0.050 | |||
|
| |||||||
| White | 1581 (76.4) | 768 (77.7) | 813 (75.2) | ||||
|
| |||||||
| African American | 299 (14.4) | 124 (12.5) | 175 (16.2) | ||||
|
| |||||||
| Other | 190 (9.2) | 97 (9.8) | 93 (8.6) | ||||
|
| |||||||
| Ethnicity | 2066 | 989 | 1077 | 0.70 | |||
|
| |||||||
| Hispanic/Latino | 191 (9.2) | 94 (9.5) | 97 (9.0) | ||||
|
| |||||||
| Other | 1875 (90.8) | 895 (90.5) | 980 (91.0) | ||||
|
| |||||||
| Comorbidities | |||||||
| Hypertension | 2069 | 364 (17.6) | 989 | 133 (13.5) | 1080 | 231 (21.4) | <0.001 |
|
| |||||||
| Heart disease | 2069 | 168 (8.1) | 989 | 55 (5.6) | 1080 | 113 (10.5) | <0.001 |
|
| |||||||
| Renal disease | 2069 | 119 (5.8) | 989 | 52 (5.3) | 1080 | 67 (6.2) | 0.36 |
|
| |||||||
| Diabetes/endocrine disease | 2069 | 336 (16.2) | 989 | 118 (11.9) | 1080 | 218 (20.2) | <0.001 |
|
| |||||||
| Psychiatric illness | 2069 | 701 (33.9) | 989 | 266 (26.9) | 1080 | 435 (40.3) | <0.001 |
|
| |||||||
| Substance dependency | 2069 | 469 (22.7) | 989 | 205 (20.7) | 1080 | 264 (24.4) | 0.044 |
|
| |||||||
| Met SIRS criteria at admission | 2011 | 1293 (64.3) | 965 | 630 (65.3) | 1046 | 663 (63.4) | 0.37 |
|
| |||||||
| Median HE grade at admission (IQR) | 2020 | 2.0 (1.0–4.0) | 989 | 2.0 (1.0–4.0) | 1031 | 2.0 (1.0–4.0) | 0.42 |
|
| |||||||
| HE severity at admission | 2020 | 989 | 1031 | 0.48 | |||
|
| |||||||
| Mild (grade 1 or 2) | 1068 (52.9) | 515 (52.1) | 553 (53.6) | ||||
|
| |||||||
| Deep (grade 3 or 4) | 952 (47.1) | 474 (47.9) | 478 (46.4) | ||||
|
| |||||||
| Median time from symptom onset to initial hospitalization (IQR), d | 1996 | 3.0 (0.0–11.0) | 970 | 3.0 (1.0–14.0) | 1026 | 2.0 (0.0–8.0) | <0.001 |
|
| |||||||
| Median time from symptom onset to HE (IQR), d | 1989 | 4.0 (1.0–14.0) | 973 | 4.0 (1.0–15.0) | 1016 | 4.0 (1.0–12.0) | 0.062 |
|
| |||||||
| Median BMI (IQR), kg/m2 | 1663 | 26.8 (23.3–31.6) | 761 | 26.3 (22.9–31.2) | 902 | 27.3 (23.7–31.8) | 0.007 |
|
| |||||||
| Median systolic blood pressure at admission (IQR), mm Hg | 2059 | 124.0 (110.0–140.0) | 984 | 125.0 (110.0–140.0) | 1075 | 123.0 (110.0–140.0) | 0.33 |
|
| |||||||
| Median diastolic blood pressure at admission (IQR), mm Hg | 2059 | 68.0 (58.0–79.0) | 984 | 68.0 (58.0–79.0) | 1075 | 68.0 (57.0–78.0) | 0.79 |
|
| |||||||
| Primary cause | 2061 | 989 | 1072 | – | |||
|
| |||||||
| APAP toxicity | 955 (46.3) | 450 (45.5) | 505 (47.1) | ||||
|
| |||||||
| Pregnancy | 19 (0.9) | 7 (0.7) | 12 (1.1) | ||||
|
| |||||||
| Hepatitis A virus infection | 37 (1.8) | 28 (2.8) | 9 (0.8) | ||||
|
| |||||||
| Hepatitis B virus infection | 148 (7.2) | 76 (7.7) | 72 (6.7) | ||||
|
| |||||||
| Ischemia/shock | 117 (5.7) | 36 (3.6) | 81 (7.6) | ||||
|
| |||||||
| Autoimmune hepatitis | 145 (7.0) | 54 (5.5) | 91 (8.5) | ||||
|
| |||||||
| Drug-induced liver injury | 222 (10.8) | 123 (12.4) | 99 (9.2) | ||||
|
| |||||||
| Indeterminate | 251 (12.2) | 138 (14.0) | 113 (10.5) | ||||
|
| |||||||
| Wilson disease | 26 (1.3) | 17 (1.7) | 9 (0.8) | ||||
|
| |||||||
| Budd–Chiari syndrome | 15 (0.7) | 10 (1.0) | 5 (0.5) | ||||
|
| |||||||
| Mushroom toxicity | 12 (0.6) | 4 (0.4) | 8 (0.8) | ||||
|
| |||||||
| Other virus | 19 (0.9) | 8 (0.8) | 11 (1.0) | ||||
|
| |||||||
| Other cause‡ | 95 (4.6) | 38 (3.8) | 57 (5.3) | ||||
|
| |||||||
| Etiologic group | 2061 | 989 | 1072 | 0.46 | |||
|
| |||||||
| APAP toxicity | 955 (46.3) | 450 (45.5) | 505 (47.1) | ||||
|
| |||||||
| Non-APAP cause | 1106 (53.7) | 539 (54.5) | 567 (52.9) | ||||
|
| |||||||
| Favorability of cause | 2061 | 989 | 1072 | 0.072 | |||
|
| |||||||
| Favorable§ | 1128 (54.7) | 521 (52.7) | 607 (56.6) | ||||
|
| |||||||
| Unfavorable∥ | 934 | 933 (45.3) | 438 | 468 (47.3) | 496 | 465 (43.4) | 0.22 |
|
| |||||||
| APAP overdose intentionality | |||||||
|
| |||||||
| Suicidal | 367 (39.3) | 183 (41.8) | 184 (37.1) | ||||
|
| |||||||
| Unintentional | 485 (51.9) | 222 (50.7) | 263 (53.0) | ||||
|
| |||||||
| Unknown | 82 (8.8) | 33 (7.5) | 49 (9.9) | ||||
APAP = n-Acetyl-p-Aminophenol; BMI = body mass index; HE = hepatic encephalopathy; IQR = interquartile range; SIRS = systemic inflammatory response syndrome.
Number (percentage) unless otherwise indicated.
For comparison of early and later periods. Boldface values are statistically significant.
Includes 3 patients with hepatitis C virus infection and 3 with hepatitis E virus infection.
Includes APAP toxicity, ischemia, hepatitis A virus infection, and pregnancy.
Includes indeterminate cause, drug-induced liver injury, autoimmune hepatitis, hepatitis B virus infection, other viruses, Wilson disease, Budd–Chiari syndrome, mushroom toxicity, and other causes.
Causes and Clinical Severity of ALF
The percentage of enrollment as a reflection of the most common causes of ALF did not change during the two 8-year periods. Hepatotoxicity due to APAP accounted for almost half the cases of ALF for the entire 16-year period (Table 1), with the highest annual prevalence (53.0%) occurring in 2013. Unintentional APAP overdoses (those in which patients took excessive medication over several days for such ailments as pain, malaise, or fever [10, 11]) were more common than intentional (suicidal) overdoses. Hepatitis A virus infection was significantly less evident during the later period (9 cases [0.8%]) than the early period (28 cases [2.8%]) (P < 0.001). Hepatic ischemia and autoimmune hepatitis increased modestly, whereas hepatitis B virus infection, drug-induced liver injury, Wilson disease, and Budd–Chiari syndrome were less frequently noted.
Patients entered either the primary or the referral (study) site more rapidly after initial symptom onset in the later period (2.0 days [IQR, 0.0 to 8.0 days]) than the early period (3.0 days [IQR, 1.0 to 14.0 days]) (P < 0.001) (Table 1). However, the corresponding interval between symptom onset and HE onset was 4.0 days in both the early (IQR, 1.0 to 15.0 days) and later (IQR, 1.0 to 12.0 days) periods, and time from onset of jaundice to enrollment also was unchanged (3.0 days in each period [IQRs, 1.0 to 12.0 and 1.0 to 10.0 days, respectively]). Most patients with ALF were severely ill at study enrollment, with nearly 50% having grade 3 or 4 (that is, deep) HE throughout. Biochemical liver test results varied widely but indicated severe illness in most patients (Appendix Table 1, available at www.annals.org).
Laboratory Tests for ALF
Between the early and later periods, median bilirubin and creatinine levels at enrollment decreased slightly, whereas median INR was unchanged, resulting in a small but clinically insignificant reduction in the median Model for End-Stage Liver Disease (MELD) score. Nearly two thirds of patients met criteria for SIRS at study enrollment in both periods (P = 0.37). Based on clinical features and laboratory results, the severity of the ALF syndrome seemed similar over time.
Clinical Interventions and Complications
Certain therapeutic interventions were used more frequently during the later period than the early period, whereas others were used less often. Although the overall median blood pressure at admission was 124/68 mm Hg (Table 1), 14.9% of patients had median mean arterial pressures less than 70 mm Hg at admission, with no difference observed between the early and later periods. At study enrollment, vasopressors were used in 18.3% of patients, renal replacement therapy was used in 20.3%, and 47.3% had undergone intubation and mechanical ventilation, with no differences between the periods.
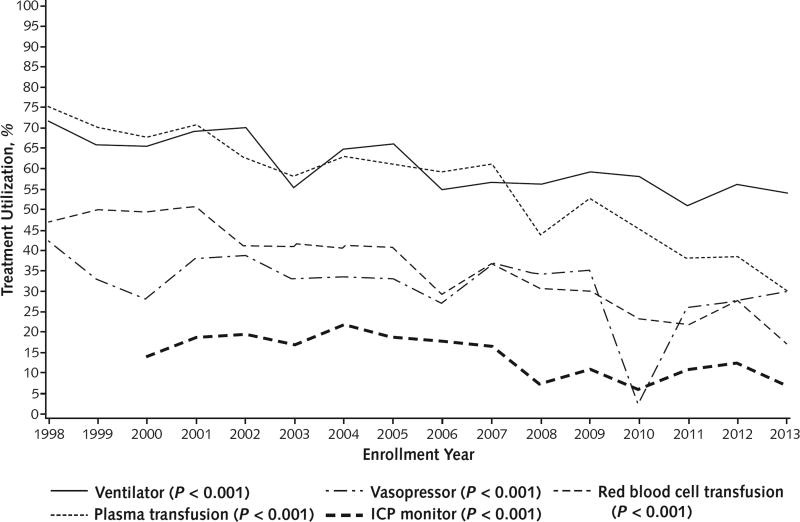
As shown in Table 2, however, vital function support increased during the 7 days after enrollment, to cumulative overall rates of 60.7% for assisted ventilation, 31.2% for vasopressors, and 32.2% for renal replacement therapy. Between the early and later periods, assisted ventilation and vasopressor use within 7 days after enrollment decreased significantly. Red blood cell and plasma infusions were also used less frequently (although the volumes transfused were not recorded) in the later period; 44.3% and 65.2% of patients, respectively, received red blood cells and plasma in the early period, compared with only 27.6% and 47.1%, respectively, in the later period (P < 0.001 for each). Intracranial pressure was monitored less often during the later period (11.4%) than the early period (18.7%) (P < 0.001). Use of renal replacement therapy was consistent across both periods.
Table 2.
Therapeutic Interventions During 7 d After Enrollment and Outcomes at 21 d
| Variable | Overall (1998–2013) (n = 2070) |
Early (1998–2005) (n = 989) |
Later (2006–2013) (n = 1081) |
P Value* | |||
|---|---|---|---|---|---|---|---|
| Total Patients, n |
Patients, n (%) |
Total Patients, n |
Patients, n (%) |
Total Patients, n |
Patients, n (%) |
||
| Therapeutic interventions during 7 d after enrollment | |||||||
| Treatments | |||||||
|
| |||||||
| Vasopressor support | 2070 | 645 (31.2) | 989 | 345 (34.9) | 1081 | 300 (27.8) | <0.001 |
|
| |||||||
| Renal replacement therapy | 2070 | 666 (32.2) | 989 | 322 (32.6) | 1081 | 344 (31.8) | 0.72 |
|
| |||||||
| Ventilator support | 2070 | 1256 (60.7) | 989 | 650 (65.7) | 1081 | 606 (56.1) | <0.001 |
|
| |||||||
| Red blood cell transfusion | 2070 | 737 (35.6) | 989 | 439 (44.3) | 1081 | 298 (27.6) | <0.001 |
|
| |||||||
| Frozen plasma transfusion | 2070 | 1154 (55.8) | 989 | 645 (65.2) | 1081 | 509 (47.1) | <0.001 |
|
| |||||||
| Intracranial pressure monitor | 1872 | 272 (14.5) | 804 | 150 (18.7) | 1068 | 122 (11.4) | <0.001 |
| N-acetylcysteine treatment | |||||||
|
| |||||||
| Overall | 2070 | 1233 (59.6) | 989 | 484 (48.9) | 1081 | 749 (69.3) | <0.001 |
|
| |||||||
| APAP toxicity | 955 | 862 (90.3) | 450 | 399 (88.7) | 505 | 463 (91.7) | 0.117 |
|
| |||||||
| Non-APAP cause | 1106 | 365 (33.0) | 539 | 85 (15.8) | 567 | 280 (49.4) | <0.001 |
|
| |||||||
| Antibiotic use | 2070 | 655 (31.6) | 989 | 298 (30.1) | 1081 | 357 (33.0) | 0.157 |
| Specific therapy | |||||||
|
| |||||||
| Prophylaxis | 2070 | 875 (42.3) | 989 | 370 (37.4) | 1081 | 505 (46.7) | <0.001 |
|
| |||||||
| Outcomes at 21 d | |||||||
| Liver transplantation status | |||||||
| Listed | 2070 | 778 (37.6) | 989 | 430 (43.5) | 1081 | 348 (32.2) | <0.001 |
|
| |||||||
| Received transplant (listed patients) | 778 | 461 (59.3) | 430 | 247 (57.4) | 348 | 214 (61.5) | 0.253 |
|
| |||||||
| Received transplant (all patients) | 2070 | 461 (22.3) | 989 | 247 (25.0) | 1081 | 214 (19.8) | 0.005 |
| TFS | |||||||
|
| |||||||
| Overall | 2070 | 1054 (50.9) | 989 | 446 (45.1) | 1081 | 608 (56.2) | <0.001 |
|
| |||||||
| Listed but did not receive transplant | 317 | 182 (57.4) | 183 | 89 (48.6) | 134 | 93 (69.4) | <0.001 |
|
| |||||||
| Not listed | 1292 | 872 (67.5) | 559 | 357 (63.9) | 733 | 515 (70.3) | 0.015 |
|
| |||||||
| Survival | |||||||
| Overall | 2070 | 1478 (71.4) | 989 | 664 (67.1) | 1081 | 814 (75.3) | <0.001 |
|
| |||||||
| Received transplant | 461 | 424 (92.0) | 247 | 218 (88.3) | 214 | 206 (96.3) | 0.002 |
APAP = n-Acetyl-p-Aminophenol; TFS = transplant-free survival.
For comparison of early and later periods. Boldface values are statistically significant.
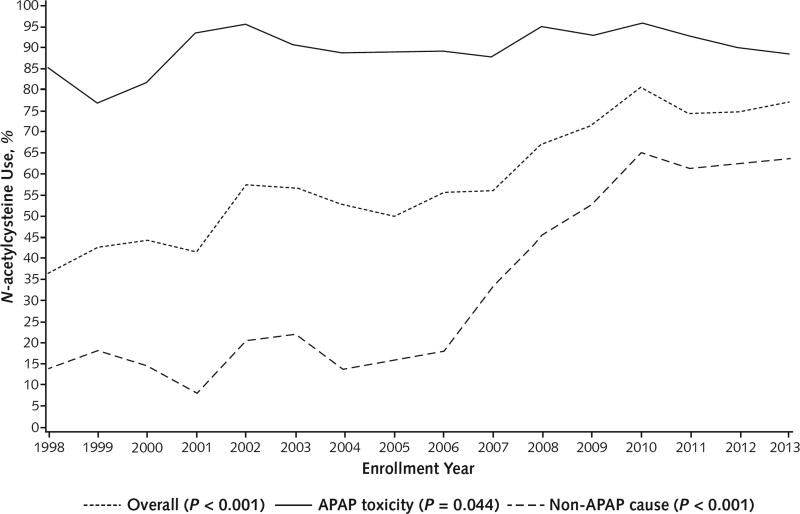
Over the entire 16-year period, use of ventilator support, plasma and red blood cell transfusions, and vasopressors decreased (P < 0.001 for all) (Figure 1). Only use of renal replacement therapy (at any time) was unchanged. Approximately 90% of patients with APAP overdose were consistently treated with intravenous or oral N-acetylcysteine overall (Table 2), and N-acetylcysteine use in patients without APAP toxicity showed a significant 3-fold increase between the early and later periods, due largely to a linear increase in administration from 18.2% in 2006 to 65.1% in 2010 that plateaued thereafter (Table 2 and Figure 2). Regardless of whether antibiotics were used during the initial 7 inpatient days after enrollment was recorded, information on the drug type and its duration of use as not collected. Antibiotics were given as specific therapy in 31.6% of patients during the 16-year period, whereas prophylactic use increased significantly between periods from 37.4% to 46.7% (P < 0.001).
Figure 1.
Treatment utilization over time.
P values represent trends over time and were calculated using the Cochran–Armitage test. ICP = intracranial pressure.
Figure 2.
N-acetylcysteine use over time.
P values represent trends over time and were calculated using the Cochran–Armitage test. APAP = n-Acetyl-p-Aminophenol.
Overall, 192 (9.3%) patients in the cohort had gastrointestinal bleeding as a complication during the initial 7-day period. Gastrointestinal bleeding occurred in 110 (11.1%) patients in the early period and 82 (7.6%) in the later period (P = 0.006).
Advanced life- and liver-support systems (such as extracorporeal membrane oxygenation, molecular adsorbent recycling, and extracorporeal liver-assist devices) were not used during the study.
ALF Outcomes: Overall Survival, TFS, and Liver Transplantation Status
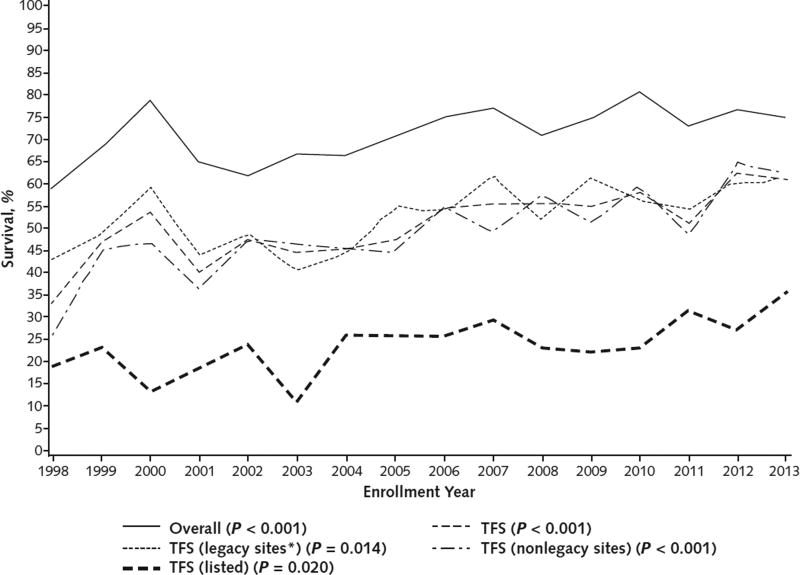
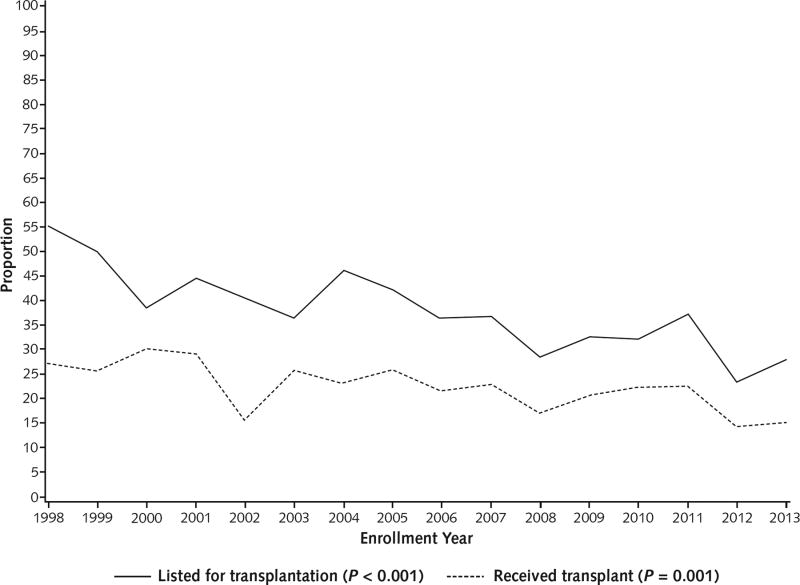
Overall, 22.3% of patients received a transplant; 21-day postoperative survival was 92.0% (Table 2). However, between the early and later periods, there was a reduction in liver transplantation listing from 43.5% to 32.2% (P < 0.001), accompanied by a slight but significant improvement in posttransplantation survival. Thus, the corresponding combined survival rate (TFS plus posttransplantation survival) increased, resulting in an overall survival for the 16-year period of 71.4% (50.9% for TFS and 20.5% for posttransplantation survival). Between the early and later periods, TFS increased significantly among patients who were listed for but did not receive a transplant, and outcomes also improved among those who were not listed (Figure 3 and Table 2).
Figure 3.
Overall survival and TFS over time.
P values represent trends over time and were calculated using the Cochran–Armitage test. TFS = transplant-free survival.
* Legacy sites (Northwestern University; University of California, San Francisco; University of Michigan; University of Washington; and University of Texas Southwestern Medical Center) enrolled ≥1 patient in each of the 16 y of the registry.
Transplant-free survival increased across the 2 periods for patients with APAP toxicity and those without, as shown in Appendix Tables 2 to 4 (available at www.annals.org). For patients without APAP toxicity who had mild HE on admission (grade 1 or 2), TFS increased from 34.5% to 43.7% (P = 0.019) (Appendix Table 3), whereas there was no significant change in TFS in patients without APAP toxicity who had deep HE (grade 3 or 4).
Longitudinal Analysis for Trends
Overall survival and TFS rates improved progressively over the 16-year study period (Figure 3). Overall survival increased significantly from 58.8% in 1998 to 75.0% in 2013 (P < 0.001), and TFS increased from 32.9% in 1998 to 61.0% in 2013 (P < 0.001). Both the number of patients listed and the number who received a transplant decreased significantly (Appendix Figure 2, available at www.annals.org). Transplant-free survival among patients listed for transplantation improved from 19.2% in 1998 to 35.7% in 2013 (P < 0.001). Longitudinal TFS rates were assessed descriptively for differences by site, and none were found (data not shown). Annual TFS rates at legacy sites (those that participated throughout the entire study period), which contributed 825 of 2070 (39.9%) patients, were indistinguishable from those observed at the remaining sites (Figure 3). Timelines for patient enrollment are shown for all sites over the 16-year study period in Appendix Figure 1.
Outcomes Related to ALF Causes and Other Risk Factors
Improvement in overall outcomes was apparent across most causes (Appendix Table 2). Rates of TFS increased and liver transplantation listing decreased for most of the causes; however, these were statistically significant only for TFS in patients with APAP hepatotoxicity or drug-induced liver injury (2 of the 3 most prevalent causes). Transplant-free survival among the combined non-APAP causes also improved.
Causes of Death
Causes of death from ALF (as assigned by the site principal investigator) were as follows: multiorgan failure (20.6%), neurologic cause (13.9%), multifactorial cause (11.2%), unspecified “liver-related” cause (10.8%), sepsis or infection (7.9%), and cardiac cause (5.3%). In 25% of cases, the cause of death was unknown or unspecified. The only noticeable change was a decrease in neurologic deaths, from 19.0% to 7.6%.
Discussion
Outcomes of ALF in specialized centers in the United States, as exemplified by these data (Figure 3), have improved steadily in recent years, both without and with liver transplantation, whereas clinical features of the syndrome have mostly remained unchanged. The short-term liver transplantation survival rate of 96.3% in the later period compares favorably with survival rates reported in the same period by the United Network for Organ Sharing, representing all 132 liver transplant centers in the United States (12). With few exceptions, demographic features of patients with ALF varied little during the 16-year study. Patients were generally young, female, and white. Changes in comorbidities observed during the study likely reflect changes in prevalence in the general population (13). Overdose of APAP remained the most frequently identified cause of ALF; the proportion of such patients who enrolled in our registry increased from 31.8% in 1998 to 53.0% in 2013. These data contrast sharply with those from the United Kingdom, where APAP-induced severe liver injury has decreased by approximately 40.0% since 1998 (4). Acute viral hepatitis, the most common cause of ALF worldwide (14), accounts for only a small number of U.S. cases; the overall proportion of enrolled patients with hepatitis B virus infection was 7.2% and remained steady during the 16-year study period, whereas the proportion with hepatitis A virus infection decreased significantly from 2.8% to 0.8% during the later period. Changes in the proportions of other causes, such as drug-induced liver injury (10.8%), autoimmune hepatitis (7.0%), and ischemic liver disease (5.7%), were minimal.
Clinically severe ALF was common throughout the 16-year study period. Most patients were referred to tertiary care centers with advanced-grade HE, elevated serum creatinine levels (≥50% of patients), and SIRS features (64.3% of patients). The slight but statistically significant reduction in median MELD scores between the early and later periods is unlikely to be biologically important. Patients in the later period were just as ill (according to most criteria) as earlier enrollees. There were no changes in the distribution of specific causes or the speed of ALF evolution, as judged by the interval between the onset of symptoms and the appearance of HE (Table 1).
The frequency of intensive care unit interventions reflects severity of illness as well as temporal changes in practice patterns during the study. Although intervention frequencies were notably high, mechanical ventilation and vasopressor use decreased significantly between the early and later periods, with no change in use of renal replacement therapy (Table 2 and Figure 1). Blood products were given less frequently over time, possibly as the deleterious effects of unnecessary blood transfusion in intensive care (15) and the futility of routine use and overuse of plasma administration in ALF (16) became better appreciated. Although the prevalence of patients with advanced encephalopathy remained unchanged, we observed less reliance on intracranial pressure monitoring during the later period, which may reflect concern over its limited clinical utility and associated risks (17, 18). The increased prophylactic use of antibiotics seen in the later period preceded the recognition (19) that this therapy does not seem to reduce bloodstream infections in ALF. This practice may be modified in the future.
Use of N-acetylcysteine for APAP toxicity, which is considered a standard treatment, was almost universal. In contrast, although N-acetylcysteine was used infrequently for patients without APAP toxicity in the early period, its use increased markedly after completion of a trial of its use in such patients in 2006 (7). The results of that trial suggested a benefit for N-acetylcysteine administration only in patients with mild (grade 1 or 2) HE, who were enrolled twice as often as those with deeper HE. The frequent administration of N-acetylcysteine to patients without APAP toxicity with all grades of HE in our current study may have been due to the providers’ assessment that benefit was possible and harm was unlikely. Transplant-free survival increased between the 8-year periods for patients with mild or deep HE regardless of APAP toxicity; on the basis of the outcomes with N-acetylcysteine use (Appendix Tables 3 and 4), we postulate that increased use of this medication as well as other factors, such as improved care in the intensive care unit, contributed to the overall improvement in TFS for patients with ALF.
Although the extent to which the ALF syndrome described here is representative of the U.S. experience as a whole is not clear, we believe that it is representative because the study cohort was drawn from heterogeneous and geographically diverse sites. Given an estimate of 2000 instances of ALF in the United States annually (3), the 2070 patients enrolled represent only about 6.5% of all potential ALF cases during this period. Therefore, the improved outcomes we observed are probably the best that can currently be achieved given the specific expertise and interest of the ALFSG investigators, which may not be available elsewhere. We could not identify any pattern of outcomes attributed to specific centers despite variability across sites in terms of the number of cases contributed each year.
The outcomes in our study compare favorably with those observed in an earlier retrospective study (4) from King’s College Hospital that also suggested improvement in outcomes over 35 years (1973 to 2008). Unlike that study, we showed only a minimal (if any) reduction in ALF severity over time and a slight decrease in the time from illness onset to admission to specialized care (6). However, our cohort did not include patients with acute liver injury (severe liver injury without HE), which we will report separately. We speculate that better intensive care may have been instrumental in improving TFS and possibly posttransplantation survival. Improved survival may have been the result of other factors. However, because the severity of ALF remained constant throughout the study period and comorbidities increased, there is no a priori reason to suspect that decreased interventions were the product of the improvement in outcomes; rather, they may have contributed to this improvement by averting iatrogenic misadventure.
In conclusion, ALF, which affects only 2000 patients annually in the United States, seemed stable with respect to causes, demographic characteristics, and severity over 16 years of study by the U.S. ALFSG. Nevertheless, ALF outcomes improved considerably over time, in association with slightly improved survival after liver transplantation and especially with improved survival without transplantation, which we speculate relates mainly to more effective intensive care unit management. The potentially beneficial role of N-acetylcysteine and more judicious use of ventilator support, vasopressors, and blood products should be further evaluated in future studies.
EDITORS’ NOTES.
Context
Whether changes have occurred in the causes of acute liver failure (ALF), its management, or the survival of patients with the condition (with or without liver transplantation) is not known.
Contribution
This large cohort study found that despite similar causes and severity of ALF among patients referred to specialty centers from 1998 to 2013, the proportion of patients listed for liver transplantation decreased and survival improved among those who did not receive a transplant as well as those who did.
Implication
More study is warranted to better understand the specific changes in care that may have led to improved survival of patients with ALF.
Acknowledgments
Participating site investigators are listed as authors. The authors gratefully acknowledge the contributions of the many site coordinators and the patients and their families who made the study possible, as well as the ongoing support of the staff of the National Institute of Diabetes and Digestive and Kidney Diseases, including Sherry Brown and Rebecca Torrance. The University of Texas Southwestern Administrative Group included Ezmina Lalani, Carla Pezzia, Corron Sanders, Nahid Attar, Linda S. Hynan, and Angela Bowling. Members of the Data Coordination Unit of the Medical University of South Carolina included Wenle Zhao, Catherine Riley, Jaime Speiser, Michelle Gottfried, Sarah Williams, and Caitlyn Ellerbe.
Grant Support: By the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DK-U-01-58369).
Disclosures: Dr. Reuben reports grants from the National Institutes of Health during the conduct of the study. Ms. Tillman reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study. Dr. Fontana reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study. Dr. Durkalski reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study. Dr. Larson reports grants from the National Institutes of Health during the conduct of the study. Dr. Schilsky reports grants from Wilson Therapeutics and personal fees from Gilead Sciences and Kadmon outside the submitted work. Dr. Shaikh reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study. Dr. Han reports grants from UT Southwestern during the conduct of the study. Dr. Brown reports grants and personal fees from Gilead Sciences, AbbVie, Bristol-Myers Squibb, Janssen, and Merck outside the submitted work. Dr. Crippin reports personal fees from Vertex Pharmaceuticals, Gilead Sciences, Genentech, AbbVie, and Merck outside the submitted work. Dr. Harrison reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study. Dr. Hanje reports personal fees from Salix Pharmaceuticals outside the submitted work. Dr. Olson reports other support from the National Institutes of Health during the conduct of the study and personal fees from Baxter Healthcare outside the submitted work. Dr. Lee reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study and grants from Bristol-Myers Squibb, Gilead Sciences, Merck, and Ocera outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M15-2211.
Appendix
Appendix Figure 1.
Site enrollment over time.
Appendix Figure 2.
Listing for transplantation and receipt of transplant over time.
P values represent trends over time and were calculated using the Cochran–Armitage test.
Appendix Table 1.
Laboratory Values at Study Enrollment
| Variable | Overall (1998–2013) (n = 2070) |
Early (1998–2005) (n = 989) |
Later (2006–2013) (n = 1081) |
P Value* | |||
|---|---|---|---|---|---|---|---|
| Patients, n |
Median Value (IQR) | Patients, n |
Median Value (IQR) | Patients, n |
Median Value (IQR) | ||
| Bilirubin level (mg/dL) | 2042 | 7.1 (3.7–19.7) | 985 | 7.7 (4.0–21.0) | 1057 | 6.6 (3.4–17.8) | <0.001 |
|
| |||||||
| Aspartate aminotransferase level (IU/L) | 2043 | 1552.0 (451.0–5123.0) | 983 | 1516.0 (483.0–5165.0) | 1060 | 1584.0 (414.5–4974.0) | 0.43 |
|
| |||||||
| Alanine aminotransferase level (IU/L) | 2037 | 1949 (632.0–4417.0) | 980 | 2003.0 (632.0–4801.5) | 1057 | 1913.0 (632.0–4174.0) | 0.48 |
|
| |||||||
| Alkaline phosphatase level (IU/L) | 1998 | 136.0 (99.0–189.0) | 954 | 143.5 (103.0–198.0) | 1044 | 129.5 (97.0–182.0) | <0.001 |
|
| |||||||
| Total protein level (g/dL) | 1516 | 5.3 (4.8–6.0) | 698 | 5.5 (4.9–6.1) | 818 | 5.2 (4.7–5.8) | <0.001 |
|
| |||||||
| INR | 2025 | 2.7 (2.0–4.1) | 969 | 2.7 (2.0–4.3) | 1056 | 2.7 (2.0–3.9) | 0.33 |
|
| |||||||
| Creatinine level (mg/dL) | 2058 | 1.6 (1.0–3.1) | 988 | 1.7 (1.0–3.1) | 1070 | 1.5 (1.0–3.0) | 0.053 |
|
| |||||||
| MELD score | 2003 | 32.0 (26.0–38.5) | 964 | 32.8 (26.6–39.9) | 1039 | 31.1 (25.3–37.2) | <0.001 |
|
| |||||||
| Hemoglobin level (g/dL) | 2049 | 11.1 (9.5–12.7) | 983 | 11.3 (9.7–13.0) | 1066 | 10.8 (9.4–12.4) | <0.001 |
|
| |||||||
| Leukocyte count (× 1000/mm3) | 2051 | 10.3 (6.9–15.0) | 985 | 10.8 (7.3–15.9) | 1066 | 9.6 (6.6–14.1) | <0.001 |
|
| |||||||
| Platelet count (× 1000/mm3) | 2044 | 129.0 (84.0–190.0) | 983 | 133.0 (87.0–197.0) | 1061 | 126.0 (82.0–187.0) | 0.020 |
|
| |||||||
| Glucose level (mg/dL) | 1828 | 117.0 (91.5–149.0) | 783 | 115.0 (89.0–149.0) | 1045 | 119.0 (93.0–149.0) | 0.126 |
|
| |||||||
| Amylase level (IU/L) | 890 | 96.0 (54.0–185.0) | 522 | 97.0 (54.0–189.0) | 368 | 92.0 (55.0–183.0) | 0.54 |
|
| |||||||
| Bicarbonate level (mmol/L) | 1725 | 22.0 (19.0–25.0) | 855 | 22.0 (18.0–25.0) | 870 | 22.0 (19.0–25.0) | 0.92 |
|
| |||||||
| pH | 1558 | 7.4 (7.4–7.5) | 802 | 7.4 (7.4–7.5) | 756 | 7.4 (7.4–7.5) | <0.001 |
|
| |||||||
| Arterial ammonia level (µmol/L) | 441 | 95.0 (58.0–162.0) | 285 | 97.0 (61.0–164.0) | 156 | 93.5 (54.0–161.5) | 0.50 |
|
| |||||||
| Venous ammonia level (µmol/L) | 690 | 92.0 (60.0–139.0) | 198 | 106.5 (68.0–159.0) | 492 | 88.5 (58.0–129.5) | <0.001 |
|
| |||||||
| Lactate level (mmol/L) | 1070 | 4.3 (2.5–8.8) | 485 | 5.2 (2.9–10.4) | 585 | 3.7 (2.1–6.9) | <0.001 |
|
| |||||||
| Phosphate level (mg/dL) | 1787 | 3.0 (2.1–4.4) | 842 | 3.0 (1.9–4.6) | 945 | 3.1 (2.2–4.3) | 0.185 |
INR = international normalized ratio; IQR = interquartile range; MELD = Model for End-Stage Liver Disease.
For comparison of early and later periods. Boldface values are statistically significant.
Appendix Table 2.
Transplantation Listing Status and TFS, by Predominant Cause of ALF
| Variable | Overall (1998–2013) (n = 2070) |
Early (1998–2005) (n = 989) |
Later (2006–2013) (n = 1081) |
P Value* | |||
|---|---|---|---|---|---|---|---|
| Total Patients, n |
Patients, n (%) |
Total Patients, n |
Patients, n (%) |
Total Patients, n |
Patients, n (%) |
||
| APAP toxicity | |||||||
|
| |||||||
| Listed for transplantation | 955 | 228 (23.9) | 450 | 126 (28.0) | 505 | 102 (20.2) | 0.005 |
|
| |||||||
| TFS | 955 | 668 (70.0) | 450 | 286 (63.6) | 505 | 382 (75.6) | <0.001 |
| Non-APAP cause | |||||||
|
| |||||||
| Listed for transplantation | 1106 | 547 (49.5) | 539 | 304 (56.4) | 567 | 243 (42.9) | <0.001 |
|
| |||||||
| TFS | 1106 | 381 (34.5) | 539 | 160 (29.7) | 567 | 221 (39.0) | 0.001 |
| Pregnancy | |||||||
|
| |||||||
| Listed for transplantation | 19 | 6 (31.6) | 7 | 2 (28.6) | 12 | 4 (33.3) | - |
|
| |||||||
| TFS | 19 | 14 (73.7) | 7 | 4 (57.1) | 12 | 10 (83.3) | - |
| Ischemia | |||||||
|
| |||||||
| Listed for transplantation | 117 | 7 (6.0) | 36 | 6 (16.7) | 81 | 1 (1.2) | 0.003 |
|
| |||||||
| TFS | 117 | 84 (71.8) | 36 | 24 (66.7) | 81 | 60 (74.1) | 0.41 |
| Hepatitis A virus infection | |||||||
|
| |||||||
| Listed for transplantation | 37 | 21 (56.8) | 28 | 14 (50.0) | 9 | 7 (77.8) | 0.25 |
|
| |||||||
| TFS | 37 | 20 (54.1) | 28 | 15 (53.6) | 9 | 5 (55.6) | 1.0 |
| Drug-induced liver injury | |||||||
|
| |||||||
| Listed for transplantation | 222 | 121 (54.5) | 123 | 73 (59.4) | 99 | 48 (48.5) | 0.106 |
|
| |||||||
| TFS | 222 | 74 (33.3) | 123 | 33 (26.8) | 99 | 41 (41.4) | 0.022 |
| Hepatitis B virus infection | |||||||
|
| |||||||
| Listed for transplantation | 148 | 84 (56.8) | 76 | 51 (67.1) | 72 | 33 (45.8) | 0.009 |
|
| |||||||
| TFS | 148 | 41 (27.7) | 76 | 22 (29.0) | 72 | 19 (26.4) | 0.73 |
| Indeterminate cause | |||||||
|
| |||||||
| Listed for transplantation | 251 | 143 (57.0) | 138 | 79 (57.3) | 113 | 64 (56.6) | 0.92 |
|
| |||||||
| TFS | 251 | 69 (27.5) | 138 | 32 (23.2) | 113 | 37 (32.7) | 0.092 |
| Autoimmune hepatitis | |||||||
|
| |||||||
| Listed for transplantation | 145 | 96 (66.2) | 54 | 39 (72.2) | 91 | 57 (62.6) | 0.24 |
|
| |||||||
| TFS | 145 | 35 (24.1) | 54 | 12 (22.2) | 91 | 23 (25.3) | 0.68 |
ALF = acute liver failure; APAP = n-Acetyl-p-Aminophenol; TFS = transplant-free survival.
For comparison of early and later periods. Boldface values are statistically significant.
Appendix Table 3.
TFS and N-acetylcysteine Use Over Time, by Cause and HE Severity at Admission
| Variable | Early (1998–2005)
|
Later (2006–2013)
|
P Value* | ||
|---|---|---|---|---|---|
| Total Patients, n |
Patients, n (%) |
Total Patients, n |
Patients, n (%) |
||
| TFS | |||||
| APAP toxicity | |||||
|
| |||||
| Mild HE | 219 | 179 (81.7) | 221 | 188 (85.1) | 0.35 |
|
| |||||
| Deep HE | 231 | 107 (46.3) | 262 | 176 (67.2) | <0.001 |
| Non-APAP cause | |||||
|
| |||||
| Mild HE | 296 | 102 (34.5) | 325 | 142 (43.7) | 0.019 |
|
| |||||
| Deep HE | 243 | 58 (23.9) | 214 | 66 (30.8) | 0.094 |
| N-acetylcysteine use | |||||
| APAP toxicity | |||||
|
| |||||
| Mild HE | 219 | 194 (88.6) | 221 | 204 (92.3) | 0.184 |
|
| |||||
| Deep HE | 231 | 205 (88.7) | 262 | 243 (92.8) | 0.124 |
| Non-APAP cause | |||||
|
| |||||
| Mild HE | 296 | 36 (12.2) | 325 | 161 (49.5) | <0.001 |
|
| |||||
| Deep HE | 243 | 49 (20.2) | 214 | 102 (47.7) | <0.001 |
APAP = n-Acetyl-p-Aminophenol; HE = hepatic encephalopathy; TFS = transplant-free survival.
Boldface values are statistically significant.
Appendix Table 4.
TFS, by Cause, HE Severity at Admission, and N-acetylcysteine Use
| Variable |
N-acetylcysteine Use
|
No N-acetylcysteine Use
|
P Value | ||
|---|---|---|---|---|---|
| Patients, n | TFS, n (%) | Patients, n | TFS, n (%) | ||
| APAP toxicity | |||||
|
| |||||
| Mild HE | 398 | 332 (83.4) | 42 | 35 (83.3) | 0.99 |
|
| |||||
| Deep HE | 448 | 258 (57.6) | 45 | 25 (55.6) | 0.79 |
| Non-APAP cause | |||||
|
| |||||
| Mild HE | 197 | 87 (44.2) | 424 | 157 (37.0) | 0.090 |
|
| |||||
| Deep HE | 151 | 49 (32.5) | 306 | 75 (24.5) | 0.073 |
APAP = n-Acetyl-p-Aminophenol; HE = hepatic encephalopathy; TFS = transplant-free survival.
Footnotes
Previously published in abstract form in Reuben A, Battenhouse H, Fontana RJ, Davern TJ, Durkalski V, Lee WM. Improved outcomes of acute liver failure (ALF) in the United States (US): updated overview of results in a prospective study of ALF 1998–2011. Hepatology. 2012;56(suppl):965A. Abstract no. 1658.
Reproducible Research Statement: Study protocol, statistical code, and data set: Available from Dr. Lee (william.lee@utsouthwestern.edu).
Author Contributions: Conception and design: A. Reuben, H. Tillman, R.J. Fontana, B. McGuire, O.S. Shaikh, R. Brown, L. Rossaro, P. Robuck, W.M. Lee.
Analysis and interpretation of the data: A. Reuben, H. Tillman, R.J. Fontana, B. McGuire, R.T. Stravitz, V. Durkalski, M. Schilsky, D. Ganger, S.B. Han, S. Munoz, L. Rossaro, R. Satyanarayana, A.H. Sherker, P. Robuck, W.M. Lee.
Drafting of the article: A. Reuben, R.J. Fontana, R.T. Stravitz, D. Ganger, C. Karvellas, P. Robuck, W.M. Lee.
Critical revision of the article for important intellectual content: A. Reuben, H. Tillman, R.J. Fontana, B. McGuire, R.T. Stravitz, V. Durkalski, M. Schilsky, S.B. Han, R.T. Chung, A. Smith, R. Brown, D. Koch, S. Munoz, K.R. Reddy, R. Subramanian, A.H. Sherker, P. Robuck, W.M. Lee.
Final approval of the article: A. Reuben, H. Tillman, R.J. Fontana, T. Davern, B. McGuire, R.T. Stravitz, V. Durkalski, A.M. Larson, I. Liou, O. Fix, M. Schilsky, T. McCashland, J.E. Hay, N. Murray, O.S. Shaikh, D. Ganger, A. Zaman, S.B. Han, R.T. Chung, A. Smith, R. Brown, J. Crippin, M.E. Harrison, D. Koch, S. Munoz, K.R. Reddy, L. Rossaro, R. Satyanarayana, T. Hassanein, A.J. Hanje, J. Olson, R. Subramanian, C. Karvellas, B. Hameed, A.H. Sherker, P. Robuck, W.M. Lee.
Provision of study materials or patients: R.J. Fontana, T. Davern, B. McGuire, R.T. Stravitz, A.M. Larson, I. Liou, O. Fix, M. Schilsky, O.S. Shaikh, D. Ganger, A. Zaman, R.T. Chung, R. Brown, M.E. Harrison, D. Koch, S. Munoz, K.R. Reddy, L. Rossaro, J. Olson, R. Subramanian, B. Hameed.
Statistical expertise: H. Tillman, V. Durkalski.
Obtaining of funding: O.S. Shaikh, W.M. Lee.
Administrative, technical, or logistic support: A.M. Larson, A.H. Sherker, P. Robuck, W.M. Lee.
Collection and assembly of data: A. Reuben, H. Tillman, R.J. Fontana, T. Davern, B. McGuire, R.T. Stravitz, A.M. Larson, O. Fix, M. Schilsky, T. McCashland, O.S. Shaikh, A. Zaman, S.B. Han, R. Brown, J. Crippin, M.E. Harrison, S. Munoz, K.R. Reddy, L. Rossaro, R. Satyanarayana, J. Olson, R. Subramanian, W.M. Lee.
References
- 1.Ichai P, Samuel D. Etiology and prognosis of fulminant hepatitis in adults. Liver Transpl. 2008;14(Suppl 2):S67–79. doi: 10.1002/lt.21612. [DOI] [PubMed] [Google Scholar]
- 2.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, et al. U.S. Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 3.Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: summary of a workshop. Hepatology. 2008;47:1401–15. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernal W, Hyyrylainen A, Gera A, Audimoolam VK, McPhail MJ, Auzinger G, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013;59:74–80. doi: 10.1016/j.jhep.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Stravitz RT, Kramer AH, Davern T, Shaikh AO, Caldwell SH, Mehta RL, et al. Acute Liver Failure Study Group. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med. 2007;35:2498–508. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 6.Bernal W, Auzinger G, Sizer E, Wendon J. Intensive care management of acute liver failure. Semin Liver Dis. 2008;28:188–200. doi: 10.1055/s-2008-1073118. [DOI] [PubMed] [Google Scholar]
- 7.Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–64. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atterbury CE, Maddrey WC, Conn HO. Neomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy. A controlled, double-blind clinical trial. Am J Dig Dis. 1978;23:398–406. doi: 10.1007/BF01072921. [DOI] [PubMed] [Google Scholar]
- 9.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. SCCM/ESICM/ACCP/ATS/SIS. SCCM/ESICM/ACCP/ATS/ SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 10.Schiødt FV, Rochling FA, Casey DL, Lee WM. Acetaminophen toxicity in an urban county hospital. N Engl J Med. 1997;337:1112–7. doi: 10.1056/NEJM199710163371602. [DOI] [PubMed] [Google Scholar]
- 11.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 12.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR 2013 Annual Data Report: liver. Am J Transplant. 2015;15(Suppl 2):1–28. doi: 10.1111/ajt.13197. [DOI] [PubMed] [Google Scholar]
- 13.Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–34. doi: 10.1056/NEJMra1208937. [DOI] [PubMed] [Google Scholar]
- 15.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 16.Stravitz RT, Lisman T, Luketic VA, Sterling RK, Puri P, Fuchs M, et al. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol. 2012;56:129–36. doi: 10.1016/j.jhep.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaquero J, Fontana RJ, Larson AM, Bass NM, Davern TJ, Shakil AO, et al. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005;11:1581–9. doi: 10.1002/lt.20625. [DOI] [PubMed] [Google Scholar]
- 18.Karvellas CJ, Fix OK, Battenhouse H, Durkalski V, Sanders C, Lee WM U S Acute Liver Failure Study Group. Outcomes and complications of intracranial pressure monitoring in acute liver failure: a retrospective cohort study. Crit Care Med. 2014;42:1157–67. doi: 10.1097/CCM.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karvellas CJ, Cavazos J, Battenhouse H, Durkalski V, Balko J, Sanders C, et al. US Acute Liver Failure Study Group. Effects of antimicrobial prophylaxis and blood stream infections in patients with acute liver failure: a retrospective cohort study. Clin Gastroenterol Hepatol. 2014;12:1942–9. doi: 10.1016/j.cgh.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]