Abstract
Background
Immunotherapy targeting the PD-1 axis has activity in several tumor types. We aimed to determine the efficacy and safety of pembrolizumab in patients with untreated brain metastases. Here we present results from a Phase II trial of the PD-1 inhibitor pembrolizumab in patients with new or progressive brain metastases from melanoma or non-small cell lung cancer (NSCLC).
Methods
Thirty-six patients were enrolled, 18 with melanoma and 18 with NSCLC. Patients had at least one untreated or progressive brain metastasis between 5 and 20 mm in longest diameter without associated neurologic symptoms or the need for corticosteroids. NSCLC patients had tumor tissue demonstrating PD-L1 expression. Patients were treated with pembrolizumab 10 mg/kg every two weeks until progression, and brain metastasis response was assessed every eight weeks by modified RECIST. The primary endpoint was brain metastasis response rate and the analysis was performed on an intent-to-treat basis. The trial is ongoing and here we present an early analysis. The study is registered with clinicaltrials.gov, number NCT02085070.
Findings
Brain metastasis response rate was 22% and 33% among patients with melanoma and NSCLC, respectively. Responses were durable, with all but one patient who responded demonstrating an ongoing response at the time of data analysis. Treatment-related serious and grade 3–4 adverse events were rare and included transaminitis, colitis, pneumonitis, fatigue, endocrine abnormalities, and acute kidney injury (1 patient each). Serious neurological adverse events included cognitive dysfunction and seizures (1 and 3 patients, respectively), due to pembrolizumab, metastases or both.
Interpretation
Pembrolizumab demonstrates activity in brain metastases in patients with melanoma or NSCLC with an acceptable safety profile, indicating that there may be a role for systemic immunotherapy in patients with untreated or progressive brain metastases.
Funding
Merck and the Yale Cancer Center.
Introduction
Significant progress has been made in treating patients with various malignancies with immune checkpoint inhibitors. Ipilimumab (anti-CTLA-4) was the drug in this class to gain approval for advanced melanoma based on improved survival compared to a peptide vaccine.1 The second immune checkpoint to be widely targeted in clinical trials was the PD-1 axis. Two PD-1 inhibitors, pembrolizumab and nivolumab, have been approved for metastatic melanoma and NSCLC, having been studied in phase III trials in both diseases, resulting in improvement in overall survival compared to standard-of-care.2–6
In the United States ~50,000 patients with metastatic melanoma or NSCLC develop brain metastases annually.7 Melanoma in particular metastasizes to the brain frequently; the incidence on autopsy is 70%.8–10 At diagnosis, 10% of metastatic NSCLC patients have brain metastases, and another 30% develop brain involvement during their illness.11 Multifocal disease is common in both diseases; about half the patients with CNS involvement present with more than one brain lesion.12
Many effective drugs currently in development have not been well studied for CNS penetration, and patients with untreated brain metastases are excluded from most clinical trials. Frequently trials exclude patients with any history of brain metastasis, even if lesions have been irradiated and stable for a prolonged period of time due to concerns about potential neurologic sequelae.13,14 More recently, clinical trials for patients with metastatic melanoma or NSCLC have allowed enrollment of patients with untreated brain metastases, but these are rare and typically local CNS therapy is required prior to trial enrollment.13,14
With recent advances in local therapies, particularly stereotactic radiosurgery (SRS), local control of brain metastases and has improved with resultant prolongation of survival.15 However, SRS remains limited and long-term complications can occur.16 Whole brain radiation therapy (WBRT) is the primary radiotherapy modality for patients with over four lesions or when SRS is not feasible, while surgery is typically reserved for hemorrhage, large lesions and solitary brain metastases.14 Given the limitations of local therapies, systemic therapies might provide benefit for brain metastasis while simultaneously treating extracerebral disease.17
With the well-documented systemic benefit from immunotherapy in patients with metastatic melanoma and NSCLC, we aimed to study activity and safety of pembrolizumab in a phase II trial for patients with untreated or progressive brain metastases. Here we present initial results.
Patients and Methods
Study Design and Participants
This is a single-institution, two-cohort phase II trial evaluating activity and safety of pembrolizumab in patients with melanoma or NSCLC and untreated or progressive brain metastases.
Key eligibility criteria include stage IV melanoma or NSCLC, age >18, Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1, life expectancy > 3 months, and adequate organ function. Patients must have at least one brain metastasis between 5 and 20mm that is untreated or unequivocally progressing following radiation as assessed by brain MRI; there is no maximum number of brain metastases allowed. Tumor tissue from a brain metastasis prior to enrollment is required for melanoma patients; use of archival tissue is allowed. Tumor PD-L1 positivity is required for NSCLC patients only. PD-L1 staining is performed on tissue from any disease site following the most recent systemic therapy employing previously described methods,6 using 1% staining as a cut-point for positivity. Patients with neurologic symptoms attributable to brain metastases or who require corticosteroids to control neurologic symptoms or perilesional edema are excluded, as are patients with leptomeningeal disease, significant autoimmune disease, or prior treatment with agents targeting PD-1/PD-L1. Prior surgical resection or radiotherapy for brain metastases is allowed, but lesions present at the time of WBRT or included in the SRS field are not considered evaluable unless documented to have since progressed (defined as unequivocal growth of a metastasis following prior radiotherapy). Any number of prior systemic therapies is allowed. A two-week wash-out period for systemic therapies and radiotherapy is required prior to initiating trial therapy. All patients were given prophylactic anti-epileptics following seizure activity noted in a patient early in the trial.
Prior to activation, the study was approved by the Yale University Institutional Review Board, and it is being conducted in accordance with international standards of good practice. All patients provide written informed consent at enrollment.
Procedures
Pembrolizumab is administered at 10mg/kg intravenously every two weeks. Dose selection was based on data available when the protocol was written; subsequent studies showed that smaller doses are equally effective.6,18 Dose-reduction is not permitted but doses can be held for up to 12 weeks for toxicity, at which time patients are discontinued from study. Treatment continues until disease progression, toxicity that precludes continuing study drug, withdrawal from study, development of co-morbidities, complications following local therapy, termination of study, or death. Patients are allowed to continue on trial despite disease progression provided they are deriving clinical benefit, or if progressive lesions can be controlled with local therapy.
A brain MRI is obtained after four weeks on therapy for patient safety (to rule out imminent risk from progressing lesions, tumor hemorrhage, or peri-lesional edema). Evaluation for efficacy of systemic therapy is performed every eight weeks including brain gadolinium-enhanced MRI and CT scans of the chest, abdomen and pelvis. Cerebral metastases are assessed by the investigator with unidimensional evaluation using RECIST 1.1 criteria19 modified to allow target CNS lesions ≥5mm in maximum diameter (or at least twice the slice thickness if >2.5mm), and permitting up to five target brain metastases (modified RECIST, mRECIST). Systemic response is measured using standard RECIST 1.1. The National Cancer Institute Common Toxicity Criteria version 4·0 is used to grade adverse events. Laboratory monitoring including LDH is performed at baseline and at the start of each treatment cycle (i.e. every two weeks); thyroid function studies are performed every eight weeks.
Outcomes
The primary endpoint is brain metastasis response rate, defined as the percentage of patients with a complete or partial response per mRECIST among all treated patients, with the two cohorts analyzed independently. Secondary endpoints include toxicity (defined as the percentage of patients with adverse events as assessed by CTCAE v4.0 criteria), overall response rate (defined as the percentage of patients who experience a CR or PR as determined by mRECIST in the brain and RECIST in the body), progression-free and overall survival (calculated as the time from start of study treatment to progression or death, or start of treatment to death, respectively), and predictive biomarkers (including PD-L1 expression).
Statistical analysis
Analyses, including the primary endpoint of brain metastasis response rate, include all patients who received at least one dose of pembrolizumab on trial. The goal sample size was 20 patients with melanoma and 44 with NSCLC, based on the hypothesis that pembrolizumab would result in a similar response rate in the brain as the overall response rate seen in other trials that required treated brain metastases. We have at least 80% power to demonstrate that the best brain metastasis response rate exceeds that of a drug with minimal activity (10% response rate) at an overall one-sided 10% alpha level.
A sequential monitoring procedure was utilized to evaluate efficacy and futility based on the number of subjects with a brain metastasis response. Monitoring was performed in the melanoma and NSCLC cohorts after the first eight and eighteen patients, respectively.
Response rates were calculated with 95% confidence intervals (CIs) by the exact binomial method. Median progression-free and overall survival was estimated using the Kaplan-Meier method, with patients censored at the data cut-off, June 30, 2015. Analysis was performed using R 3.1.3 and Matlab R2015b.
This study is registered with ClinicalTrials.gov, NCT02085070.
Role of funding source
The funder of this study was involved in trial design and data interpretation. The funder did not have a role in the collection, analysis, or writing of the report. All authors had full access to the raw data and SBG had final responsibility for the decision to submit for publication.
Results
Patient Characteristics
Between March 31, 2014 and May 31, 2015, 52 patients with untreated or progressive brain metastases were screened (18 melanoma, 34 NSCLC), and 36 were treated with pembrolizumab (18 melanoma, 18 NSCLC, Table 1). The 16 NSCLC screen failures were due to PD-L1 negativity in 8, patient decision in 5, and small cell histology, leptomeningeal disease, and declining performance status in 1 each. Patient demographics are summarized in Table 1.
Table 1.
Baseline Characteristics of All Treated Patients with Melanoma (N=18) or NSCLC (N=18)
| Melanoma | NSCLC | |||
|---|---|---|---|---|
| Characteristic | n | % | n | % |
| Age, years | ||||
| Median | 65 | 59 | ||
| Range | 41–85 | 33–82 | ||
| Sex | ||||
| Male | 12 | 67 | 6 | 33 |
| Female | 6 | 33 | 12 | 67 |
| ECOG PS | ||||
| 0 | 6 | 33 | 3 | 17 |
| 1 | 12 | 67 | 15 | 83 |
| Elevated LDH | 7 | 39 | N/A | N/A |
| Tumor Histology | ||||
| Adenocarcinoma | N/A | N/A | 14 | 78 |
| Squamous cell carcinoma | N/A | N/A | 3 | 17 |
| Other | N/A | N/A | 1 | 6 |
| Mutation | ||||
| BRAF | 6 | 33 | N/A | N/A |
| NRAS* | 3 | 19 | N/A | N/A |
| KRAS | N/A | N/A | 4 | 22 |
| EGFR | N/A | N/A | 1 | 5 |
| ALK | N/A | N/A | 1 | 5 |
| PD-L1 positive | N/A | N/A | 18 | 100 |
| Number of prior systemic therapy regimens | ||||
| 0 | 4 | 22 | 5 | 28 |
| 1 | 7 | 39 | 6 | 33 |
| 2 | 3 | 17 | 3 | 17 |
| ≥ 3 | 4 | 22 | 4 | 22 |
| Prior ipilimumab | 11 | 61 | N/A | N/A |
| Prior CNS therapy** | ||||
| None | 6 | 33 | 8 | 44 |
| Surgical resection | 8 | 44 | 2 | 11 |
| Whole brain radiotherapy | 3 | 17 | 6 | 33 |
| Stereotactic radiosurgery | 9 | 50 | 5 | 28 |
Abbreviations: NSCLC, non-small cell lung cancer; ECOG PS, Eastern Cooperative Oncology Group Performance Status; LDH, lactate dehydrogenase; CNS, central nervous system.
NRAS status was unknown for two melanoma patients
Some patients had more than one CNS therapy modality.
The size of untreated or progressive brain metastases was ≤20 mm in diameter; lesions that were larger, located in an eloquent area of the brain, or otherwise concerning were treated with local therapy prior to enrollment. The range of total and target lesions per patient, and sizes of previously treated and untreated lesions are summarized in Table 2. No target lesions were previously resected, while 9 had been treated with WBRT, 7 with SRS and 75 were untreated. Twenty patients received some form of local CNS therapy prior to enrollment.
Table 2.
Number and Size of Total and Target Brain Metastases in Patients with Melanoma or NSCLC
| Melanoma (n=18) | NSCLC (n=18) | |
|---|---|---|
| Number of total brain lesions per patient, median (range) | 8 (1–81) | 6 (2–78) |
| Number of target brain lesions per patient, median (range) | 2 (1–5) | 2 (1–5) |
| Size of all target lesions, median (range) | 11 mm (5–20 mm) | 8 mm (5–15 mm) |
| Previously untreated, median (range) | 10 mm (5–18 mm) | 8 mm (5–15 mm) |
| Progressing after prior SRS or WBRT, median (range) | 14 mm (8–20mm) | 10 mm (6–11 mm) |
| Number of target brain lesions in all patients | 45 | 46 |
| Previously untreated | 32 (13 patients) | 43 (16 patients) |
| Progressing after prior SRS or WBRT | 13 (5 patients) | 3 (2 patients) |
| Progressing after prior WBRT | 6 (2 patients) | 3 (2 patients) |
| Progressing after prior SRS | 7 (3 patients) | 0 |
Abbreviations: NSCLC, non-small cell lung cancer; SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy.
Clinical Activity in Brain Metastases and Systemic Disease
Of the 18 melanoma patients, four (22%) were unevaluable for brain metastasis response; three had rapid extracerebral progression necessitating removal from protocol (two treated with BRAF inhibitors within weeks before enrollment) and one had intralesional hemorrhage requiring SRS. Four of 18 patients (22% of all patients, 29% of evaluable patients, Table 3, Figure 1A) had confirmed brain metastasis partial response (PR) for a confirmed brain metastasis response rate of 22% (95% CI, 7–48). CNS responses were durable, ranging from four to ten months, and all responses are ongoing at the time of data analysis (Figure 1B). BRAF mutations were not detected in any of the responders; one patient with a response had an NRAS mutation. Among non-responders, two had stable disease (SD, one confirmed, one unconfirmed) and eight had progressive disease (PD) in the brain. One patient classified as PD in the brain had a mixed response with >30% shrinkage of target lesions but the development of new brain metastases. The best systemic responses included two complete responses (CR), two PRs, four with stable disease, eight with PD and two unevalauble (one not imaged due to rapid clinical deterioration and one without extra-cerebral metastases).
Table 3.
Clinical Activity of Pembrolizumab in Brain Metastases and Systemic Disease in Patients with Melanoma or NSCLC
| Melanoma (n=18) | NSCLC (n=18) | |||||
|---|---|---|---|---|---|---|
| No. of Patients | % | 95% CI | No. of Patients | % | 95% CI | |
| Brain Metastasis RR (CR + PR) | 4 | 22 | 7–48 | 6* | 33 | 14–59 |
| Individual duration of brain metastasis response (months)^ | 4.0+, 4.0+, 7.0+, 10+ | 3.2+, 6.0+, 6.1+, 6.6, 7.0+ | ||||
| Systemic RR (CR + PR) | 4 | 22 | 7–48 | 6* | 33 | 14–59 |
Abbreviations: NSCLC, non-small cell lung cancer; RR, response rate; CR, complete response; PR, partial response; CI, confidence interval.
5 confirmed, 1 unconfirmed as of data cut-off. Of the 6 patients with NSCLC who had a response, 4 had both brain and systemic response, 1 patient had a systemic response with progression in the brain, and 1 patient had a brain metastasis response with systemic progression.
Response duration for all confirmed responders; time from first response to time of documented progression, death, or data cut-off; continued responders at time of data cut-off denoted by +.
Figure 1.
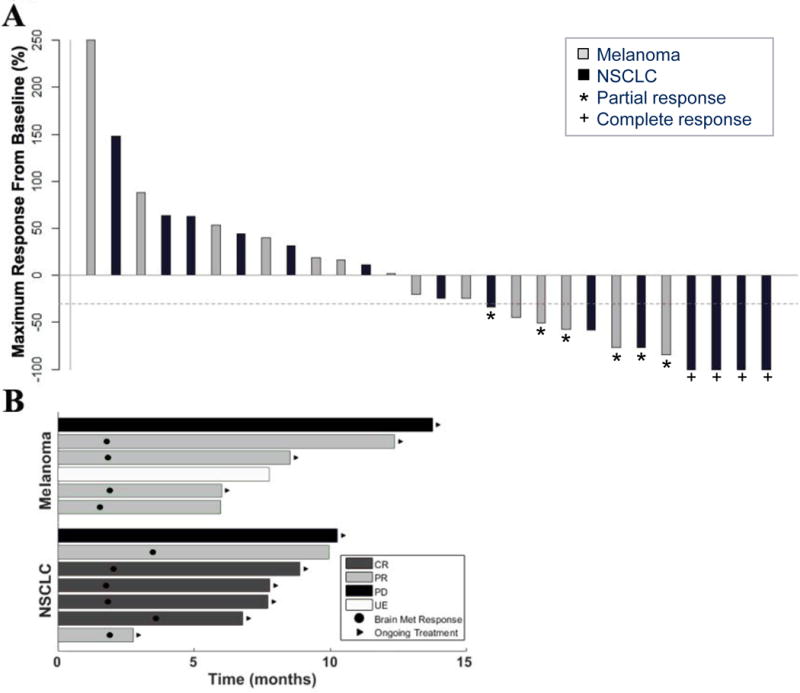
Characteristics of brain metastasis responses in patients with melanoma or NSCLC who received pembrolizumab. A. Best brain metastasis response by mRECIST in evaluable patients. Bars represent individual patients, with percent change in tumor burden in the brain from baseline to the best response demonstrated by the amplitude of the bars. Bar colors indicate patients with melanoma (gray) or NSCLC (black). The dashed line demonstrates the −30% cut-off that defines an objective response. Stars denote partial responses (PRs) and crosses denote complete responses (CRs). Two patients, one with melanoma and one with NSCLC, had PD despite >30% shrinkage of target lesions due to the development of new brain metastases and unequivocal progression of non-target lesions, respectively. The number of target (and total) untreated lesions among melanoma patients with a CNS response were 1 (1), 2 (6), 3 (5) and 3 (4) and among NSCLC patients 1 (3), 5 (58), 1 (2), 5 (50), 1 (3) and 1 (4). Eight patients were unevaluable in the brain, seven due to rapid systemic progression and one due to intralesional hemorrhage. B. Time to brain metastasis response and duration of therapy. Bars represent individual patients who achieved a brain metastasis response or remained on trial ≥ six months. Ten patients demonstrated an objective response in the brain by mRECIST (four with melanoma and six with NSCLC), and three additional patients remained on therapy ≥ six months due to clinical benefit despite having either disease progression or being unevaluable due to treatment of hemorrhagic lesions in the brain. Black circles indicate the time of first brain metastasis response, and black arrows indicate patients who continued on study treatment (regardless of response) at the time of data analysis.
Among NSCLC patients, six of 18 had brain metastasis responses (Table 3, Figure 1A) including four CRs and two PRs (one unconfirmed), for a brain metastasis response rate of 33% (95% CI, 14–59). CNS responses were similarly durable for NSCLC, ranging from three to seven months, with five of the six continuing to respond at the time of data analysis (Figure 1B). None of the four patients with a KRAS mutation had a CNS response. Two additional patients had SD in the CNS (both unconfirmed), six had PD, and four were unevaluable in the brain due to rapid systemic progression requiring early removal from protocol therapy. One patient had a mixed response with >30% shrinkage of target lesions but unequivocal progression of non-target lesions. The best systemic responses in patients with NSCLC included six PRs (one unconfirmed), one SD, and ten PDs; one patient’s systemic disease was unevaluable due to rapid clinical decline from cancer progression. None of the patients with either disease who had target lesions previously treated with SRS or WBRT had a CNS response.
Overall there was strong concordance between CNS and systemic responses. Among the nine patients with a confirmed systemic response, all but one had a confirmed CNS response. The one patient with discordance had a transient brain metastasis response followed by asymptomatic disease progression in several brain lesions. Because she was otherwise benefitting from pembrolizumab, she underwent radiotherapy to the growing brain metastases and continued on study with a sustained systemic response after ten months.
Six patients with melanoma and nine with NSCLC died, all but one due to disease progression (one with unknown cause of death many months after discontinuing treatment). Median OS among patients with melanoma was not reached (NR) and among patients with NSCLC was 7.7 months (95% CI, 3.5 to NR). Median follow-up time for the melanoma cohort was11.6 months (IQR 8.5–13.9) and for NSCLC 6.8 months (IQR 3.1–7.8). Additional efficacy endpoints will be reported when data are mature.
Safety
Overall, pembrolizumab was safe and well-tolerated in this patient population (Table 4). The majority of treatment-related adverse events (AEs) were grade 1–2. One patient developed grade 3 autoimmune hepatitis; treatment was discontinued due to clinical deterioration. Three (8%) developed grade 1–2 seizure activity controlled by anti-epileptic medication and transient use of corticosteroids. An additional two patients (5%, both with melanoma) developed other neurological symptoms from peri-lesional edema resulting in removal from study, one in the setting of disease progression and one with inflammation and minimal tumor cells,20 histologically documented in both cases. One patient (3%) required treatment discontinuation due to toxicity, a NSCLC patient with grade 3 colitis. Only mild neurologic AEs were observed among NSCLC patients, none requiring treatment discontinuation. There were no treatment-related deaths.
Table 4.
Neurologic AEs and Treatment-Related Non-Neurologic AEs in All Treated Patients with Melanoma or NSCLC
| Melanoma (n=18) | NSCLC (n=18) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Neurologic* | ||||||||||||
| Cognitive dysfunction | 0 | 0 | 1 | 6 | 0 | 0 | 1 | 6 | 0 | 0 | 0 | 0 |
| Headache | 3 | 17 | 0 | 0 | 0 | 0 | 4 | 22 | 0 | 0 | 0 | 0 |
| Dizziness | 1 | 6 | 0 | 0 | 0 | 0 | 2 | 11 | 0 | 0 | 0 | 0 |
| Stroke | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 | 0 | 0 |
| Seizure | 3 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Treatment-Related Non-Neurologic | ||||||||||||
| Gastrointestinal | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 17 | 1 | 6 | 0 | 0 |
| Pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 |
| Interstitial nephritis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 | 0 | 0 |
| Constitutional symptoms | 8 | 44 | 0 | 0 | 0 | 0 | 5 | 28 | 1 | 6 | 0 | 0 |
| Anorexia | 1 | 6 | 0 | 0 | 0 | 0 | 2 | 11 | 0 | 0 | 0 | 0 |
| Dermatologic | 6 | 33 | 0 | 0 | 0 | 0 | 4 | 22 | 0 | 0 | 0 | 0 |
| Arthralgias | 2 | 11 | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 | 0 | 0 |
| Endocrine | 1 | 6 | 0 | 0 | 0 | 0 | 5 | 28 | 0 | 0 | 1 | 6 |
| Hematologic | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 11 | 0 | 0 | 0 | 0 |
| Elevated transaminases | 0 | 0 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: AE, adverse event; NSCLC, non-small cell lung cancer.
Regardless of attribution to study drug
Discussion
Here we show that pembrolizumab has activity in melanoma and NSCLC brain metastases. This early analysis demonstrates a brain metastasis response rate of 22% in 18 melanoma patients and 33% in 18 NSCLC patients. Prior clinical trials of patients with advanced melanoma or NSCLC treated with pembrolizumab have shown excellent activity with some durable responses. In a frontline phase III trial for melanoma patients studying two dosing schedules of pembrolizumab compared to ipilimumab, patients on the pembrolizumab arms had superior response and overall survival.2 A phase I trial of pembrolizumab in patients with advanced NSCLC demonstrated an overall response rate of 19·4%, with a median duration of response of 12·5 months.6 However, these and other trials of immune checkpoint inhibitors generally either exclude brain metastasis patients, or require local therapy and a period of documented stabilization prior to initiating study therapy. Our trial specifically aims to study pembrolizumab in patients with untreated or progressive brain metastases, enabling the first evaluation, to our knowledge, of the activity of a PD-1 axis inhibitor in the CNS in melanoma or NSCLC patients.
Local treatment of melanoma or NSCLC brain metastases usually involves radiotherapy, either SRS or WBRT. Although radiotherapy typically results in good local CNS control, it can cause toxicity, such as cognitive decline or symptomatic radiation necrosis, particularly in patients with prolonged survival.21–23 Moreover, local CNS therapy can result delayed initiation of systemic therapy which might compromise outcomes.
Historically, systemic agents for melanoma or NSCLC have had limited activity in the CNS. More recent trials of systemic therapies in melanoma patients have shown CNS responses similar to extracerebral responses, as reviewed.17,24, 25 The highest response rates have been in patients with BRAF mutant melanoma receiving BRAF inhibitors, but duration of response is limited.26 Newer agents for NSCLC have shown promise in patients with CNS metastases.27–31 However, trials that specifically study activity in brain metastases are rare, and even those agents with activity in the brain often do not have durable benefit.
Notably, in our study there was high concordance between systemic and brain metastasis responses, with eight of the nine patients with a confirmed systemic response also demonstrating an intracerebral response. Brain metastasis responses were generally durable; all but one responder continued to respond at the data cut-off. Importantly, we obtained a brain MRI after one month of study treatment to confirm lack of significant progression, worsening peri-lesional edema, or other concerning findings, a practice that we recommend for patients with untreated or progressive brain metastases treated with systemic therapy.
The relatively low response rate of 22% in patients with melanoma compared to other studies of pembrolizumab may under-represent clinical benefit and may reflect complexities of clinical trials in this patient population. For example, four patients were unevaluable for CNS response, three due to rapid extracerebral response (two within weeks of cessation of BRAF inhibitor therapy) and one due to intralesional hemorrhage. None of the four responders had tumor BRAF mutations, however, this is likely due to the small number of cases enrolled, as BRAF mutation status is not generally predictive of response to immunotherapy. The patient with hemorrhage required SRS to control bleeding, but remains on standard-of-care pembrolizumab without new lesions or progression 14 months after enrollment. Another melanoma patient who met mRECIST criteria for progression remains on study with clinical benefit 20 months after enrollment.
Tumor PD-L1 expression was not required for melanoma patients and a low cutpoint for PD-L1 positivity (1%) was selected for NSCLC. Although in both diseases there is an association between response and PD-L1 expression, responses have been seen in PD-L1 negative patients. These eligibility criteria were intended to provide access to pembrolizumab for this patient population with limited treatment options.
None of the patients with target lesions growing after prior irradiation (SRS or WBRT) responded. While radiation and immunotherapy can be synergistic, the number of patients in this subset (8 of 36) was too small to draw conclusions regarding synergism in CNS lesions. Our data show, however, that pembrolizumab alone can clearly be active in brain metastases. Additional studies are needed to determine the safety and activity of combinations of radiation and immunotherapy compared to single modality approaches.
Treatment-related non-neurologic toxicity is consistent with prior reports, with few serious adverse events. Although grade 1–2 seizures and other neurologic toxicities were seen, pembrolizumab is generally safe in these patients. After the first seizure episode was observed, all patients were treated with prophylactic anti-epileptics. Neurologic toxicities were generally due to peri-lesional edema and were controlled with transient steroid use. Pembrolizumab was held until patients were neurologically stable off steroids, and retreatment proved to be safe. One melanoma patient who developed mental status changes after a single dose of pembrolizumab had an MRI that showed growth in all lesions, consistent with disease progression by mRECIST criteria. Biopsy of one lesion revealed inflammation, indicating that tumor growth in this setting might not always represent progression.20 Imaging studies using alternative PET and MRI modalities are needed to better determine disease status in these patients.
Several aspects unique to this patient population need to be considered when assessing the results. Patients eligible for this trial are those with brain metastases between 5 and 20mm, asymptomatic and not requiring corticosteroids to control symptoms. Larger lesions had little or no surrounding edema and were located in non-eloquent areas of the brain. Therefore it is unknown whether pembrolizumab is effective or safe in patients with symptomatic or larger brain metastases. Additionally, several patients on this trial were considered unevaluable in the brain due to rapid systemic progression reflecting aggressive disease, particularly after cessation of targeted therapy, and progression may have no association with the treatment received. Additional limitations of this early report include the immature survival data and small sample size. Correlative studies are pending and will be conducted upon study completion. Finally, the heterogeneity in terms of prior local and systemic therapies, volume of CNS disease and variability of histologic and genetic subtypes limit the conclusions. Nonetheless, our data clearly demonstrate that systemic PD-1 inhibition can be effective in treating non-irradiated or progressing brain metastases.
In summary, pembrolizumab can have therapeutic activity in CNS metastases in patients with melanoma or NSCLC based on this early analysis of a Phase II trial which focuses on patients with untreated or progressive brain metastases. Toxicity is consistent with prior trials of pembrolizumab in these diseases and neurologic adverse events associated with drug or disease are uncommon and manageable. Despite these encouraging data, many patients failed to respond to pembrolizumab in the brain or in extracerebral sites. Additional combination therapy strategies and biomarker development will be important as this field evolves, particularly in patients with brain metastases who may have a different disease biology than patients with extra-cerebral disease. This trial continues to accrue patients and predictive biomarker studies are ongoing; results of biomarker studies and updated response and survival data will be reported when accrual is complete and data are mature.
Research in Context.
Evidence before this study
We performed a PubMed search through January 12, 2016 using the following terms: Brain metastases and PD-1 or PD-L1 and melanoma or NSCLC. Although inhibitors of the PD-1 axis are being studied extensively in patients with various malignancies with extremely encouraging outcomes, there are very limited data focusing on the activity in the central nervous system (CNS), as patients with untreated brain metastases have largely been excluded from these trials. The CTLA4 inhibitor ipilimumab has demonstrated activity in patients with untreated melanoma brain metastases, but no studies have been published on activity of PD-1 or PD-L1 inhibitors in patients with untreated brain lesions.
Added value of this study
To our knowledge, this is the first study assessing the activity and safety of a PD-1 inhibitor in brain metastases from melanoma or NSCLC. In this Phase II trial, we found that pembrolizumab is safe in patients with small, asymptomatic brain metastases, and has evidence of activity in the central nervous system that is comparable to activity in systemic disease.
Implications of all the available evidence
Brain metastases from melanoma or NSCLC often present a clinical challenge, and very few trials focus on systemic therapies to control their disease. Prior studies have demonstrated that pembrolizumab has clinical activity in patients with melanoma or NSCLC with a good toxicity profile. Here we demonstrate early evidence that pembrolizumab has activity in the CNS in patients with small, asymptomatic, untreated brain metastases. Further studies are required to confirm this activity and delineate the patient population in which pembrolizumab is most likely to be effective.
Acknowledgments
This study was funded by Merck and the Yale Cancer Center. Additional support was provided NIH grants R0-1 CA158167 and K24CA172123 (H. Kluger, PI), Yale SPORE in Skin Cancer, P50 CA121974 (R. Halaban, PI), CTSA Grant Number KL2 TR000140 (L. Jilaveanu, PI) from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), NIH roadmap for Medical Research and a grant from the Lung Cancer research Foundation-LUNGevity and Melanoma Research Alliance, Award#308721 (L. Jilaveanu, PI), and by the Office of the Secretary of Defense for Health Affairs, through the Lung Cancer Research Program under Award No. W81XWH-15-1-0203 (S. Goldberg, PI). Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions:
SBG, VC, MS, RSH, AJT, LJ, and HMK designed the study. SBG, SNG, ACC, RSH, MS, UH, JY, and HMK recruited and treated patients. SBG, SNG, ACC, AM, VC, MS, AR, MM, SS, RSH, ER, HG, HMK, JC, UH, and AV collected the data. SBG, XY, and HMK analyzed the data. SBG, AM, VC, MS, RSH, HMK, and XY were responsible for interpretation of data. SBG and HMK wrote the first draft of the manuscript. All authors reviewed the manuscript and approved the final version.
Conflict of interest statements:
SBG reports grants from Merck, during the conduct of the study; and grants from AstraZeneca and personal fees from Clovis, outside the submitted work. RSH reports other from Merck, outside the submitted work. MS reports grants and personal fees from Merck, during the conduct of the study; personal fees from Bristol-Myers, Genentech-Roche, Astra-Zeneca-Medimmune, Anaeropharma, Merus, Symphogen, Nektar, Amgen, Kyowa-Kirin, Astellas-Agensys, Lion Biotechnologies, Neostem, Seattle Genetics, Novartis, Pfizer,, Vaccinex, Immune Design, Lilly, Prometheus, and Biodesix, and other from Immunova, Intensity, and Amphivena, outside the submitted work; and TRM Oncology, Physicians Education Resource, Imedex, Research to Practice, AcademicCME, Haymarket Media, Dava, Clinical Care Options. JY reports grants from 21st Century Oncology, outside the submitted work. HMK reports grants from Merck, during the conduct of the study; and personal fees from Regeneron, Bioclinica, Promethius and Alexion, outside of the submitted work.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 7.Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro-oncology. 2012;14:1171–7. doi: 10.1093/neuonc/nos152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Retsas S, Gershuny AR. Central nervous system involvement in malignant melanoma. Cancer. 1988;61:1926–34. doi: 10.1002/1097-0142(19880501)61:9<1926::aid-cncr2820610934>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Sloan AE, Nock CJ, Einstein DB. Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control. 2009;16:248–55. doi: 10.1177/107327480901600307. [DOI] [PubMed] [Google Scholar]
- 10.de la Monte SM, Moore GW, Hutchins GM. Patterned distribution of metastases from malignant melanoma in humans. Cancer Res. 1983;43:3427–33. [PubMed] [Google Scholar]
- 11.Sorensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1474–80. doi: 10.1200/JCO.1988.6.9.1474. [DOI] [PubMed] [Google Scholar]
- 12.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. Journal of neuro-oncology. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 13.Flanigan JC, Jilaveanu LB, Faries M, et al. Melanoma brain metastases: is it time to reassess the bias? Curr Probl Cancer. 2011;35:200–10. doi: 10.1016/j.currproblcancer.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flanigan JC, Jilaveanu LB, Chiang VL, Kluger HM. Advances in therapy for melanoma brain metastases. Clin Dermatol. 2013;31:264–81. doi: 10.1016/j.clindermatol.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Mathieu D, Kondziolka D, Cooper PB, et al. Gamma knife radiosurgery for malignant melanoma brain metastases. Clin Neurosurg. 2007;54:241–7. [PubMed] [Google Scholar]
- 16.Redmond AJ, Diluna ML, Hebert R, et al. Gamma Knife surgery for the treatment of melanoma metastases: the effect of intratumoral hemorrhage on survival. J Neurosurg. 2008;109(Suppl):99–105. doi: 10.3171/JNS/2008/109/12/S16. [DOI] [PubMed] [Google Scholar]
- 17.Yushak ML, Chiang VL, Kluger HM. Clinical trials in melanoma patients with brain metastases. Pigment Cell Melanoma Res. 2015;28:741–3. doi: 10.1111/pcmr.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribas A, Puzanov I, Drummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refracgtory melanoma (KEYNOTE-002): a randomized, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 11) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Cohen JV, Alomari AK, Vortmeyer AO, et al. Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res. 2015;4:179–182. doi: 10.1158/2326-6066.CIR-15-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene-Schloesser D, Robbins ME. Radiation-induced cognitive impairment–from bench to bedside. Neuro Oncol. 2012;14(Suppl 4):iv37–44. doi: 10.1093/neuonc/nos196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan AJ, Dicker AP. On the merits and limitations of whole-brain radiation therapy. J Clin Oncol. 2013;31:11–3. doi: 10.1200/JCO.2012.46.0410. [DOI] [PubMed] [Google Scholar]
- 23.Alomari A, Rauch PJ, Orsaria M, Minja FJ, Chiang VL, Vortmeyer AO. Radiologic and histologic consequences of radiosurgery for brain tumors. J Neurooncol. 2014;117:33–42. doi: 10.1007/s11060-014-1359-8. [DOI] [PubMed] [Google Scholar]
- 24.Di Giacomo AM, Ascierto PA, Pilla L, et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol. 2012;13:879–86. doi: 10.1016/S1470-2045(12)70324-8. [DOI] [PubMed] [Google Scholar]
- 25.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459–65. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 26.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–95. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 27.Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01) Ann Oncol. 2011;22:2466–70. doi: 10.1093/annonc/mdr003. [DOI] [PubMed] [Google Scholar]
- 28.Bearz A, Garassino I, Tiseo M, et al. Activity of Pemetrexed on brain metastases from Non-Small Cell Lung Cancer. Lung Cancer. 2010;68:264–8. doi: 10.1016/j.lungcan.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Wu YL, Zhou C, Cheng Y, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803) Ann Oncol. 2013;24:993–9. doi: 10.1093/annonc/mds529. [DOI] [PubMed] [Google Scholar]
- 30.Gerber NK, Yamada Y, Rimner A, et al. Erlotinib versus radiation therapy for brain metastases in patients with EGFR-mutant lung adenocarcinoma. Int J Radiat Oncol Biol Phys. 2014;89:322–9. doi: 10.1016/j.ijrobp.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol. 2015;33:1881–8. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]


