Abstract
We examined the genetic implications and clinical impact of telomere length (TL) in 67 patients with acute myeloid leukemia (AML). There was a trend toward improved survival at 6 months in patients with longer TL. We found that patients with activating mutations, such as FLT3-ITD, had shorter TL, while those with mutations in epigenetic modifying enzymes, particularly IDH1 and IDH2, had longer TL. These are intriguing findings that warrant further investigation in larger cohorts. Our data show the potential of TL as a predictive biomarker in AML and identify genetic subsets that may be particularly vulnerable to telomere-targeted therapies.
Keywords: acute myeloid leukemia, telomere length, clinical outcomes, somatic mutations
Introduction
Perturbations of telomere homeostasis have been implicated in the pathogenesis of aplastic anemia, myelodysplastic syndromes (MDS), and acute myeloid leukemia (AML),1,2 but it is unknown if telomere length (TL) is associated with clinical outcomes or somatic mutations in AML. Critical loss of telomeric DNA in hematopoietic stem cells can lead to genomic instability and cytogenetic abnormalities, such as gain or loss of chromosomes and non-reciprocal translocations.3 Over half of AML patients present with an abnormal karyotype,4 and patients with complex cytogenetic abnormalities have shorter TL.5 Hematopoietic stress—such as chemotherapy and ionizing radiation—can accelerate physiological, age-related, telomere attrition leading to telomere crisis and chromosomal instability as reported in therapy-related myeloid neoplasms.6–7 Hypofunctional germ-line mutations in telomere-specific reverse transcriptase (TERT), the enzymatic component of the telomere repair complex, have also been identified in patients with cytogenetically-abnormal AML.8 While there are data suggesting that shorter TL may correlate with worse outcomes in MDS and acute promyelocytic leukemia,9,10 the degree to which TL is associated with clinical outcome in AML is unknown.
There are at least 30 recurrently mutated genes in AML that cannot be detected by routine cytogenetic testing.11,12 Many of these mutations are enriched in patients with normal karyotype-AML (NK-AML), which accounts for ~45% of AML.4 Outcomes in NK-AML can vary markedly, and it is not known why certain mutations or mutation combinations, such as FLT3-ITD mutations—particularly if co-occurring with TET2 or DNMT3A mutations—are associated with a poor prognosis, while others, such as NPM1/IDH co-mutations in the absence of FLT3-ITD, are associated with a much more favorable prognosis.11 Such mutations, by enhancing proliferation or through epigenetic alterations affecting telomerase expression or function, may be associated with shortened or preserved telomeres. This may account in part for the variations in outcome seen across genetically-defined AML subsets. The potential association between TL and specific mutations or mutation groups in AML has not been adequately studied.
Methods
We analyzed tumor samples from 67 AML patients treated at Memorial Sloan Kettering Cancer Center (MSKCC). Patients were consented for sample collection under a hematologic oncology tissue banking protocol approved by the MSKCC Institutional Review Board (IRB). DNA extraction was performed using viably frozen peripheral blood and bone marrow mononuclear cells. Targeted sequencing for exons of a set of 27 genes commonly mutated in AML was performed as previously described.13 TL was measured as mean telomere content by qPCR.2 FLT3 and NPM1 mutations, as well as cytogenetics by metaphase karyotyping, were extracted by clinical chart review.
For newly diagnosed (ND) patients, survival outcomes were assessed from sample collection. ND patient samples were collected during the initial diagnostic period and before any anti-leukemia therapy was given. Relapsed or refractory (RR) patient samples were collected from patients who had received prior AML chemotherapy, and either relapsed or had refractory disease. Patients with secondary AML were defined as having therapy-related AML or AML evolved from an antecedent hematologic disorder. Overall survival (OS) were calculated using standard Kaplan-Meier methods, with survival distributions compared using a log-rank test. Differences in TL across patient characteristics were evaluated by the Wilcoxon rank-sum test. All p-values presented are unadjusted for multiple comparisons unless otherwise noted.
Results
In the 67 patient AML cohort, 45 patients were ND (67.2%) and 22 RR (32.8%) at the time of sample collection. Median age was 64.1 years (range 26.2–84.4), and 36 (53.7%) of patients were female. Median WBC was 16.2 × 109/L (range 0.9–136.1). Twenty-three (34.3%) patients had secondary AML (therapy related or antecedent hematologic disorder). Cytogenetic risk groups included 5 (7.5%) patients with favorable risk, 43 (64.2%) patients with intermediate risk, 18 (26.9%) patients with adverse risk, and 1 (1.5%) patient with missing data.14
Of the 67 patients, median TL was 5.22 kb (range 3.73–8.76 kb). While in healthy cells TL shortens with age15, we found no association between TL and age in our cohort (R2=0.043, p=0.73), validating that we were analyzing the leukemic population and that there was, as expected, no association between blasts TL and age of patients (Figure 1). We found no difference in TL in ND vs. RR patients, or in patients with de novo vs. secondary AML. In the 45 ND patients, there was improved survival at 6 months in the longest TL tertile group compared to the middle and shortest tertiles groups (86.7% vs. 33.3% and 60.0%); however, this association was not statistically significant (p=0.662) (Figure 2A). When TL was separated into 2 groups, longer [range 6.02–8.76] and shorter [range 3.73–6.02], survival at 6 months was 86.7% vs. 46.7%, p=0.732 (Figure 2B). In the 22 RR patients, there was similar improved OS at 6 months in the longest TL tertile group (60.0% vs. 11.1% and 25.0%, p=0.284). The lack of statistical significance may have been due to the number of subjects examined (45 ND and 22 RR patients).
Figure 1.
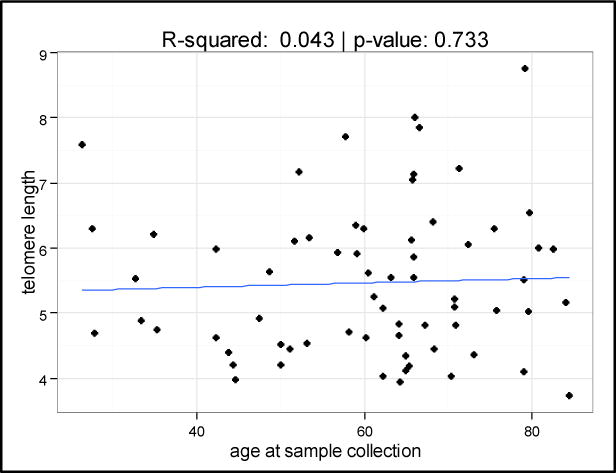
Scatter plot showing no relationship between telomere length and age at sample collection in leukemia blast samples.
Figure 2.
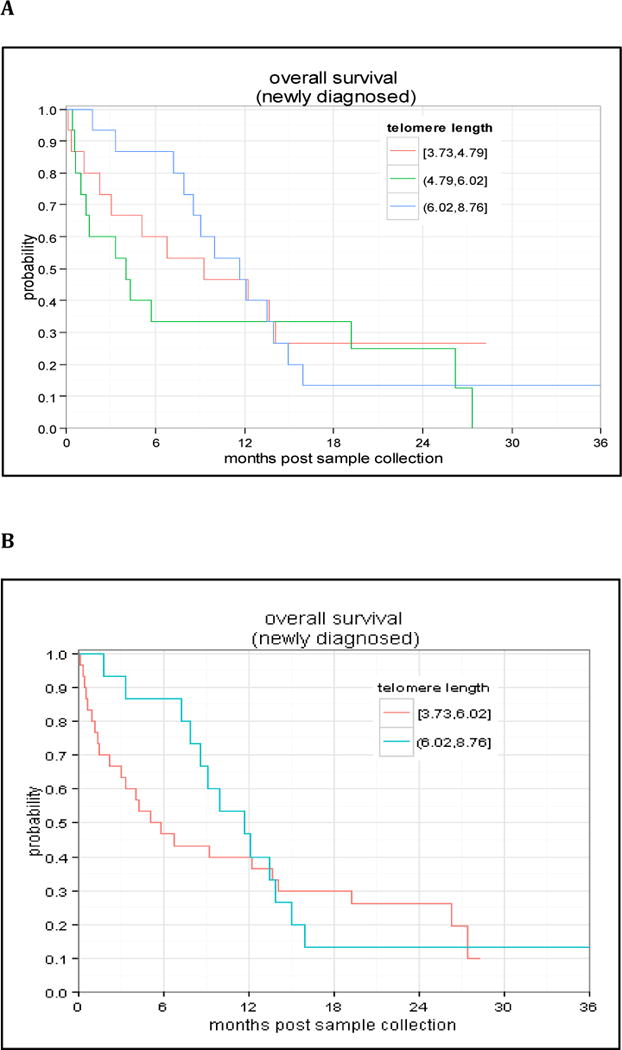
Overall survival of newly diagnosed AML patients when stratified by telomere length divided into tertiles (A) or into 2 groups (short vs. long; B). OS for the entire cohort at 3 years was approximately 12%.
Targeted sequencing data were available in 62 patients. Analysis of single mutation association with TL showed that patients with IDH1 mutations had significantly longer TL than those without IDH1 mutations (unadjusted p=0.02; Bonferroni adjusted p=0.24; Table 1). Patients with either IDH1 or IDH2 mutations had longer TL (unadjusted p=0.04; Bonferroni adjusted p=0.48). There was also a suggestion that mutations in a set of genes associated with epigenetic regulation (IDH1/2, ASXL1, DNMT3A, and TET2) were associated with longer TL when examined as a group (p=0.073). As previously reported,5 we also found that FLT3 mutations may be associated with shorter TL [FLT3-ITD p=0.084; FLT3-ITD or FLT3-TKD mutation p=0.092]. FLT3-mutated patients had a higher white blood cell count (WBC) than FLT3 wild-type patients (p<0.001). When we examined mutations in the signaling pathway genes NRAS, KIT, and FLT3-ITD/TKD together as a group, there was a trend toward shorter TL (p=0.089). The median ages of patients with IDH or FLT3 mutations were not statistically different from the rest of the cohort, again supporting that age was not related to TL (and thus not a confounder) in this AML cohort. There was no evidence of an association between TL and NPM1 mutations or any other mutations examined.
Table 1.
Single gene mutation and mutation class correlation with telomere length (TL) in AML.
| Mutation | N* | Median TL (range) | p-value** |
|---|---|---|---|
| IDH1 | 0.020 | ||
| negative | 56 | 5.09 (3.73,8.76) | |
| positive | 6 | 6.32 (4.81,7.72) | |
| IDH2 | 0.870 | ||
| negative | 59 | 5.16 (3.73,8.76) | |
| positive | 3 | 5.63 (4.10,6.54) | |
| IDH1 or IDH2 | 0.040 | ||
| negative | 53 | 5.08 (3.73,8.76) | |
| positive | 9 | 6.29 (4.10,7.72) | |
| DNMT3A | 0.697 | ||
| negative | 53 | 5.08 (3.73,8.76) | |
| positive | 9 | 5.53 (4.53,6.29) | |
| TET2 | 0.423 | ||
| negative | 55 | 5.16 (3.73,7.85) | |
| positive | 7 | 5.53 (4.40,8.76) | |
| ASXL1 | 0.219 | ||
| negative | 59 | 5.10 (3.73,7.85) | |
| positive | 3 | 5.56 (5.52,8.76) | |
| IDH1, IDH2, TET2, ASXL1 or DNMT3A | 0.073 | ||
| negative | 39 | 5.03 (3.73,7.85) | |
| positive | 23 | 5.56 (4.10,8.76) | |
| FLT3-ITD | 0.084 | ||
| negative | 46 | 5.54 (3.95,8.76) | |
| positive | 12 | 4.72 (3.97,8.00) | |
| FLT3-ITD/TKD | 0.092 | ||
| negative | 43 | 5.53 (3.95,8.76) | |
| positive | 15 | 4.74 (3.97,8.00) | |
| NRAS | 0.512 | ||
| negative | 59 | 5.26 (3.73,8.76) | |
| positive | 3 | 4.89 (4.81,5.03) | |
| KIT | 0.870 | ||
| negative | 59 | 5.22 (3.73,8.76) | |
| positive | 3 | 4.93 (4.36,6.30) | |
| FLT3-ITD/TKD, NRAS or KIT | 0.089 | ||
| negative | 36 | 5.54 (3.95,8.76) | |
| positive | 20 | 4.85 (3.97,8.00) | |
| NPM1 | 0.937 | ||
| negative | 38 | 5.24 (3.95,8.76) | |
| positive | 18 | 5.12 (4.02,8.00) |
A small number of patients had missing data for certain genes.
P-values presented are unadjusted for multiple comparisons.
Discussion
This was a high-risk AML cohort, with 3-year OS of 11.7% in ND patients. Nevertheless, there was a suggestion of increased survival at 6 months in AML patients with longer TL, a difference that diminished over time (Figure 2). This trend did not achieve statistical significance, possibly due to our limited sample size and poor-risk AML population. However, we hypothesize that longer TL may be associated with improved outcomes in AML. Whether this is due to increased sensitivity to chemotherapy, duration of aplasia, or other disease or host factors needs to be further elucidated in subsequent studies. Mechanistically, in the setting of shorter TL, telomere-associated proteins such as telomeric repeat-binding factor 2 (TRF2) and others are known to disassociate from the telomere complex, and these enzymes have other non-telomeric functions, such as upregulation of the DNA damage response and repair mechanisms.16 Thus, it follows that shorter TL could confer resistance to cytotoxic chemotherapy by affecting DNA repair mechanisms. This potential association warrants further study of TL as a predictive biomarker of chemotherapy response in larger AML cohorts, with additional analyses of telomere-associated proteins such as TRF2.
For the first time, we have demonstrated that mutations in the IDH1/2 genes may be associated with longer TL (unadjusted p-value 0.04) (Table 1). IDH mutations have been associated with favorable clinical outcomes in AML,11 which might be consistent with longer TL. We also showed that TL may be associated with specific classes of AML mutations; for example, a group of commonly mutated epigenetic modifiers showed a trend towards longer TL, while mutations in FLT3 and other signaling mutations were associated with shorter TL. Patients with mutations in signaling pathways often have proliferative AML, which could explain the shorter TL in these patients (due to rapid cell turnover). However, it is less clear why patients with mutations in IDH1/2 or other epigenetic regulators such as DNMT3A, TET2, and ASXL1 might have longer telomeres, but potential explanations include dysregulated methylation of the TERT gene promoter or altered epigenetic control of genes controlling expression of telomerase, shelterin, or other proteins in the telomere complex. Clearly signaling and epigenetic class mutations overlap and TL will likely be impacted by the combination of a patient’s mutations, and larger studies are being planned to power a multivariate analysis of gene mutation status and TL. However, it is very intriguing that TL may reflect a balance of the sum of a patient’s driver mutations, which could be important for assessing outcome and understanding disease biology in genetically heterogeneous AML patients.
In summary, we present novel and intriguing findings that warrant further study of TL in AML, in particular associations with somatic mutations and epigenetic alterations and impact on outcome. Lastly, in addition to potentially being a predictive biomarker, TL measurement, with concurrent mutational profiling, may help identify the best candidates for treatment with telomerase inhibitors, which are currently in both pre-clinical and clinical development.
Acknowledgments
This research was supported by an American Society of Clinical Oncology (ASCO) Young Investigator Award (YIA) (recipient: Justin Watts, mentor: Martin Tallman). This work was also supported by and completed at Memorial Sloan Kettering Cancer Center and the National Heart, Lung, and Blood Institute in equal collaboration.
Footnotes
All authors have no conflicts of interests to declare.
Authorship Contributions:
JMW designed research, performed research, analyzed data, and wrote the paper; BD designed research, performed research, analyzed data, and wrote the paper; PH performed research, analyzed data, and edited the paper; AK performed research, analyzed data, and edited the paper; FR performed research, analyzed data, and edited the paper; CC performed research and edited the paper; JA performed research and edited the paper; RR performed research, analyzed data, and edited the paper; SMD performed research, analyzed data, and edited the paper; EMT performed research, analyzed data, and edited the paper; RLL performed research, analyzed data, and edited the paper; NY designed research, performed research, analyzed data, and edited the paper; MST designed research, performed research, analyzed data, and edited the paper.
References
- 1.Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775–83. doi: 10.1182/blood-2014-05-526285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheinberg P, Cooper JN, Sloand EM, et al. Association of telomere length of peripheral blood leukocytes with hematopoietic relapse, malignant transformation, and survival in severe aplastic anemia. JAMA. 2010;304:1358–64. doi: 10.1001/jama.2010.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swiggers SJ, Kuijpers MA, de Cort MJ, et al. Critically short telomeres in acute myeloid leukemia with loss or gain of parts of chromosomes. Genes Chromosomes Cancer. 2006;45:247–56. doi: 10.1002/gcc.20286. [DOI] [PubMed] [Google Scholar]
- 4.Mrózek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood. 2004;18:115–36. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann U, Brummendorf TH, Balabanov S, et al. Telomere length and hTERT expression in patients with acute myeloid leukemia correlates with chromosomal abnormalities. Haematologica. 2005;90:307–16. [PubMed] [Google Scholar]
- 6.Lansdorp PM. Telomeres, stem cells, and hematology. Blood. 2008;111:1759–66. doi: 10.1182/blood-2007-09-084913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J Clin Invest. 2004;113:160–8. doi: 10.1172/JCI20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calado RT, Regal JA, Hills M, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. PNAS. 2009;106:1187–92. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sieglova Z, Zilovcova S, Cermak J, et al. Dynamics of telomere erosion and its association with genome instability in myelodysplastic syndromes (MDS) and acute myelogenous leukemia arising from MDS: a marker of disease prognosis? Leukemia Res. 2004;28:1013–21. doi: 10.1016/j.leukres.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Ghaffari SH, Shayan-Asl N, Jamialahmadi AH, et al. Telomerase activity and telomere length in patients with acute promyelocytic leukemia: indicative of proliferative activity, disease progression, and overall survival. Ann Oncol. 2008;19:1927–34. doi: 10.1093/annonc/mdn394. [DOI] [PubMed] [Google Scholar]
- 11.Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–89. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlenk RF, Döhner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 13.Cull EH, Watts JM, Tallman MS, et al. Acute myeloid leukemia presenting with panhypopituitarism or diabetes insipidus: a case series with molecular genetic analysis and review of the literature. Leuk Lymphoma. 2014;55:2125–9. doi: 10.3109/10428194.2013.869327. [DOI] [PubMed] [Google Scholar]
- 14.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–36. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 15.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–65. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto K, Bartocci C, Ouzounov I, et al. A two-step mechanism for TRF2-meidated chromosome-end protection. Nature. 2013;494:502–5. doi: 10.1038/nature11873. [DOI] [PMC free article] [PubMed] [Google Scholar]


