Abstract
OBJECTIVE
To determine the reliability of an objective measure of pain, agitation and sedation using the Neonatal Pain, Agitation and Sedation Scale (N-PASS) compared with nursing bedside assessment.
STUDY DESIGN
Neonates admitted in neonatal intensive care unit over a 6-month period were eligible. Pain and sedation were assessed with N-PASS, and a subjective questionnaire was administered to the bedside nurse.
RESULT
A total of 218 neonates were eligible (median: gestational age 34.6 weeks, age at assessment 7 days). N-PASS pain score correlated significantly with both nurses’ pain score (Spearman coefficient (r) = 0.37; P<0.001) and agitation score (r = 0.56; P<0.001). N-PASS sedation score correlated with nurses’ sedation score (r = − 0.39; P<0.001). Adjusting for gestational age, day of life, intrauterine drug exposure and use of high frequency ventilation only slightly attenuated the correlations (r = 0.36, 0.55 and − 0.31, respectively).
CONCLUSION
The N-PASS captures nursing assessment of pain, agitation and sedation in this broad population and provides a quantitative assessment of subjective descriptions that often drives patient therapy.
INTRODUCTION
Pain management has been increasingly studied in the neonates, but there is much debate about the quality and accuracy of the tools that are most frequently utilized.1–2 Adequate recognition of pain is essential to properly address the needs of each infant to allow safe yet compassionate care.3–5 Evaluation of agitation and sedation provides a further challenge and historically has been done by subjective assessment, which is not well quantified. Currently, bedside nurses provide the greatest input to the subjective evaluation as they are more able to detect minute-to-minute variability of clinical status.6 Consequently, their evaluation lends great influence to the management of pain and sedation in the clinical setting.
The Neonatal Pain, Agitation and Sedation Scale (N-PASS) has been developed for the purpose of implementing a scale that combines the assessment of pain, agitation and sedation levels in a critically ill infant with acute and/or ongoing pain.7–8 Although there are a variety of tools available for assessing pain in infants, there is not currently another tool that has been used in the assessment of sedation in critically ill neonates, particularly those who are premature.9–13 In a small patient population of ventilated and/or postoperative neonates, the N-PASS has been validated for inter-rater reliability and accuracy.7 Its use in a broader population of non-ventilated or sedated infants for routine assessment of pain has not been established.
The objective of this study was to determine the most clinically useful information that would help to guide therapy by comparing the N-PASS with the Neonatal Infant Pain Scale (NIPS), which is the current standard of routine pain assessment, to correlate it with a nursing subjective bedside assessment of pain and sedation, and to determine the impact of gestational age and illness on the N-PASS score.
METHODS
During a 6-month period, all infants admitted to the 45 bed, level III C, neonatal intensive care unit (NICU) at the Johns Hopkins Hospital were evaluated for enrollment in the study. Infants on day of birth and those with limited viability were excluded. The first day of life was potentially confounded by the events surrounding delivery and the natural tendency of infants to sleep more during the first 24 h. Limited viability was determined by the attending physician. If an infant was so critically ill that death appeared inevitable, the severity of illness may have an impaired response to pain or sedation in a way that would not allow extrapolation to the general NICU population.
All infants enrolled in the study had a single evaluation at greater than 1 day of age, using the N-PASS by study personnel. A single evaluation was done to allow broad sampling, across a wide range of gestational and chronological ages, varying degrees of severity of illness and to avoid clustering of data around sicker infants with longer lengths of stay. A subjective questionnaire assessing pain, agitation and sedation, and need for pain or sedative therapy was administered to the bedside nurse at the time of the performance of N-PASS. The documented NIPS score closest to the time of the study assessment was recorded. This study was approved by the Johns Hopkins Medicine Institutional Review Board.
The N-PASS is comprised of two measurements, each of which uses five criteria: crying/irritability, behavioral state, facial expression, extremity tone and vital signs. The pain/agitation score is assessed through observation without intervention, with a score range of 0 to 10, with 0 to 2 points available for each criterion. The sedation score is typically assessed for patients receiving sedating medications and requires stimulation, with a score range of 0 to − 10, with points of − 2 to 0 assigned for each criterion. For this study, all patients were assessed with both segments of the tool in order to evaluate the contribution of clinical factors that may impact the sedation portion of the N-PASS in patients who were not receiving sedating medications, such as neurologic conditions rendering infants, were less responsive than expected.
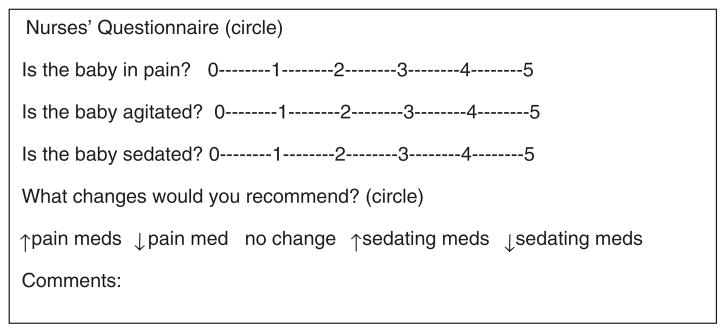
A questionnaire as shown in Figure 1 was designed to capture a nurse’s subjective assessment of pain, agitation and sedation that is often verbally communicated to care providers. Each patient’s nurse was asked to assess the level of pain, sedation and agitation of the patient using 6-point Likert scales ranging from 0 (none) to 5 (most affected). They were also asked if they would adjust pain and/or sedating medications by increasing, decreasing or initiating therapy, if the patient was not currently receiving any therapy, and given an opportunity to explain the rationale for their recommendation.
Figure 1.
Nurses’ questionnaire. Key for Likert scale: 0 indicates no evidence of pain, agitation or sedation. 5 indicates severe pain, agitation or sedation.
Infant demographic and medical data were collected and it included gestational age, birth weight, gender, mode of delivery and day of life on which the assessment occurred. Additionally, information about disease states, medications, including narcotics and sedatives, intrauterine drug exposure (IUDE) and types of ventilator support was collected.
Patient characteristics were summarized using frequencies and percents, means and standard deviations or medians and ranges as appropriate. The association of patient characteristics and the nurses’ ratings of pain, agitation and sedation with the N-PASS measures were assessed using Spearman rank correlations for ordinal or continuous measures and the Jonckheere-Terpstra test for categorical measures. Partial correlation, controlling for gestational age, day of life, IUDE and ventilation was also used to assess the association of the nurses’ ratings with the N-PASS ratings. N-PASS scores and the nurses’ recommendation for medication changes were coded as agreeing if the N-PASS score was ≤2 and the nurse recommended a decrease/no change, or if the score was >2 and the nurse recommended an increase; otherwise they were coded as disagreeing. Analyses were performed using SAS version 9.22 (SAS Institute Inc., Cary, NC, USA). All statistical tests were two-sided and significance was set at P<0.05.
RESULTS
Patient population
During the 6-month study period, 263 infants were admitted to the NICU. Eighteen patients were ineligible for the study because they were born less than 24 h before the time of admission and their stay was less than 24 h in the NICU (n = 17) or they were critically ill and died prior to the nursing assessment (n = 1). Twenty-seven patients who met eligibility criteria were either transferred or discharged prior to enrollment. Demographic and clinical characteristics of the 218 enrolled infants are shown in Table 1.
Table 1.
Infant characteristics on day of assessment
| Patient characteristics (n = 218) | No. (%) | Median (range) |
|---|---|---|
| Male gender | 120 (55) | |
| Gestational age (weeks) | 34.6 (23–41.7) | |
| Birth weight (g) | 1890 (390–5540) | |
| DOL of assessment (days) | 7 (1–171) | |
| Post conceptional age (weeks) | 36.6 (24.3–47.6) | |
| Respiratory support | ||
| None | 101 (46.3) | |
| Oxyhood | 1 (0.5) | |
| Nasal cannula | 61 (28) | |
| CPAP | 9 (4.1) | |
| SIMV | 38 (17.4) | |
| JET | 2 (0.9) | |
| HFOV | 6 (2.8) | |
| Sepsis | ||
| None | 107 (49.1) | |
| Suspected | 108 (49.5) | |
| Confirmed | 3 (1.4) | |
| GERD | 13 (6) | |
| Intraventricular hemorrhage | ||
| None | 180 (82.6) | |
| Grade 1 | 14 (6.4) | |
| Grade 2 | 3 (1.4) | |
| Grade 3 | 14 (6.4) | |
| Grade 4 | 7 (3.2) | |
| Hydrocephalus | 27 (12.4) | |
| Periventricular leukomalacia | 7 (3.2) | |
| IUDE | 8 (3.7) | |
| Hypoxic ischemic encephalopathy | 6 (2.7) | |
Abbreviations: CPAP, continuous positive airway pressure; DOL, day of life; GERD, gastroesophageal reflux disease; HFOV, high frequency oscillator ventilation; IUDE, intrauterine drug exposure; JET, jet ventilation; SIMV, synchronized intermittent mandatory ventilation.
N-PASS score
The N-PASS pain score was significantly positively associated with the use of high frequency ventilation (median N-PASS pain score = 2 for high frequency ventilation compared with scores of 1 for non-high frequency invasive and 0 for noninvasive ventilation; P = 0.002), and there was suggestive evidence of a positive association with IUDE (median N-PASS pain score = 2 for IUDE compared with 0 for no IUDE; P = 0.07) and a negative association with day of life (median N-PASS pain score = 0 for less than 7 days compared with 1 for ≥7 days; P = 0.05). N-PASS pain score was not correlated with gestational age (Spearman rank correlation coefficient (r) = − 0.07; P = 0.31). The N-PASS sedation score was significantly negatively associated with the use of high frequency ventilation (median N-PASS sedation score = − 2.5 for high frequency ventilation compared with scores of − 1 for non-high frequency invasive, and 0 for noninvasive ventilation; P<0.001), but was not associated with gestational age (r = 0.01; P = 0.91), day of life (median = 0 for <7 and ≥7 days; P = 0.40) or IUDE (median = 0 for IUDE and no IUDE; P = 0.83). Both pain and sedation scores were analyzed by stratification by gestational age at birth, postmenstrual age at the time of assessment and postnatal age, in days, at the time of assessment, with no differences found.
The N-PASS pain score was significantly correlated with the nurses’ subjective rating of pain (r = 0.37; P<0.001) and the nurses’ agitation score (r = 0.56; P<0.001). The N-PASS sedation score was significantly correlated with the nurses’ sedation score (r = − 0.39; P<0.001). Adjusting for gestational age, day of life, IUDE and the use of high frequency ventilation, only slightly attenuated the correlations (r = 0.36, 0.55 and − 0.31, respectively; P<0.001 for all). In contrast, the NIPS score provided no information on level of pain in the patient population because all 218 neonates were scored as 0.
Using an N-PASS score of greater than 2 for pain as an indication of requiring intervention, the nurses’ assessment of need to increase, decrease or continue therapy unchanged was consistent with the N-PASS score obtained in 178 of 218 (81.7%) infants. Similarly, using a score of less than − 2 for sedation as an indication of requiring intervention, the N-PASS score was consistent with the nursing recommendation in 185 of 218 (84.9%) infants as shown in Table 2. The majority of disagreements between N-PASS and the nursing intervention assessment occurred when scores were greater than 2 and the nurse indicated they would not change the current therapy. Comments indicated that disagreement in management may reflect diagnosis, which mimics pain or sedation, such as gastroesophageal reflux disease or those that mimic sedation, such as neurologic disease.
Table 2.
Nursing management agreement with Neonatal Pain, Agitation and Sedation Scale (N-PASS) pain scores
| Nursing recommendation for therapy
|
|||
|---|---|---|---|
| Decrease | No change | Increase | |
| Pain: disagree (n = 40) | |||
| N-PASS score ≤2 (n) | 0 | 0 | 3 |
| N-PASS score >2 (n) | 0 | 37 | 0 |
| Pain: agree (n = 178) | |||
| N-PASS score ≤2 (n) | 6 | 170 | 0 |
| N-PASS score >2 (n) | 0 | 0 | 2 |
| Sedation: disagree (n = 33) | |||
| N-PASS score ≤2 (n) | 0 | 0 | 3 |
| N-PASS score >2 (n) | 1 | 29 | 0 |
| Sedation: agree (n = 185) | |||
| N-PASS score ≤2 (n) | 2 | 182 | 0 |
| N-PASS score >2 (n) | 0 | 0 | 1 |
DISCUSSION
Management of pain and sedation in the NICU can be driven by the subjective impression of the bedside nurse, which is not consistently documented or applied. The N-PASS provides the NICU with an assessment tool that is easy to use, minimally invasive or noninvasive, and can be administered for all patients in the unit. Our study shows that it is a more clinically relevant tool than the NIPS for assessing ongoing pain and discomfort that occurs in this population. We additionally have demonstrated the usefulness of N-PASS as the routine screening tool for the routine pain assessment required for all patients, regardless of the need for sedation or severity of illness. It additionally assesses sedation, which has not been previously systematically assessed in any neonatal tool. Assessment of sedation without this tool is based primarily on subjective impression, which is difficult to quantify. We have shown that the N-PASS is able to quantify this impression and correlates with the bedside judgment of need for alteration of therapy. This assessment tool thus has the potential to prevent over sedation and help to identify the most adequate level of sedation for each individual patient. While the N-PASS was initially hypothesized to require point adjustment for prematurity,7 we found no association of gestational age with the scores, and thus found adjustment for prematurity unnecessary.
A common tool for assessing pain is the NIPS, and is frequently used for documenting pain assessment.11 Although the scale has been validated, it was developed to address procedural pain. Discomfort in neonates who are mechanically ventilated or who have illnesses that are associated with sustained and persistent pain, such as necrotizing enterocolitis, may not be captured when using a scale such as the NIPS for routine assessment. Ongoing pain can also be challenging to distinguish from agitation in this patient population. No existing pain scales distinguish between pain and agitation.
A number of tools have been created to address the assessment of pain in infants. The N-PASS is a more suitable tool than many of the previously used tools in the NICU because it was created to address a broad range of infants, allowing assessment of the extreme premature infant as well as the postdates infant. It may be used in a range of chronologic ages, from newborn infants to older infants of varying postconceptional ages. While both the NIPS and the premature infant pain profile use physiologic and behavioral indicators to identify and address postoperative or procedural pain, the premature infant pain profile incorporates a scoring adjustment for gestational age.10,14 Another tool, Crying, Requires oxygen, Increased vital signs, Expression, Sleep tool (CRIES), was developed for acute postoperative pain in infants less than 6 months of age, which is not valid for the premature infant.12 The N-PASS provides the use of a single tool across the varying population of both term and preterm infants served in the NICU and addresses ongoing and acute pain. Unlike the Echell Douleur Inconfort Nouveau-Ne (EDIN), a tool which is used to assess chronic pain in newborns by assessing five behavioral indicators: facial activity, body movements, quality of sleep, quality of contact with nurses and consolability,13 the N-PASS assesses sedation as well. The EDIN scale was developed and tested in preterm infants, thus lacking the ability to be generalized to the broader patient population in the NICU.
Our data indicate that the N-PASS accurately captures the nursing assessment of pain, agitation and sedation and is more effective than the NIPS in our patient population. The N-PASS score provided a quantitative assessment to the previous subjective nursing description that often drives patient therapy. It was useful across gestational ages, chronologic ages and postconceptional ages, and consistent across the varied medical conditions common to the NICU. The N-PASS is easy to administer and is noninvasive. It can be used before and after a stimulus or with routine care. The pain portion requires only observation of the infant’s clinical status. The sedation assessment was designed to be performed on infants who are medically sedated and requires minimal stimulation to determine the level of sedation. By providing an objective score, response to sedation can be more easily compared across different caregivers and provides an assessment of changes in response to therapy.
Our treatment goal is often to sedate, especially, infants who are mechanically ventilated. This tool gives us the ability to determine appropriate levels of sedation based on individual need. Both over and under sedation can have negative impact on clinical outcomes, however infants who are properly sedated for mechanical ventilation have improved oxygenation, decreased complications of ventilation and decreased morbidity.15 Excess sedation can also lead to increased time on the ventilator.16–19
The N-PASS’s use may be expanded to assessment prior to procedures such as lumbar punctures and controlled intubations. In patients who will have elective painful procedures, the N-PASS can be employed to achieve a sedative goal and thereby maximize success of the intervention by allowing for a more cooperative infant and possibly decreasing the complication rates and increase the success rate. A trial assessing the N-PASS for acute pain associated with heel stick indicated that it was a reliable tool when compared with the premature infant pain profile in a broad range of gestational ages.8
In earlier studies, prematurity points were added to the observed pain score owing to the concern that premature infants might have inadequate neurologic development to adequately demonstrate measurable components of pain.7,20,21 In our study however, their mean scores were similar between groups without the use of the additional points for prematurity. Based on our study, the N-PASS score required no adjustment for prematurity, thus improving the usability of the tool.
Limitations of this observational study include: enrollment at a single institution and each infant having only one assessment. The N-PASS was previously shown to have inter-rater reliability; however, the original findings were confined to 46 patients, all mechanically ventilated.7 A strength of our study is the inclusion of a larger number of patients in a diverse NICU population, assessing the tool in ventilated and non-ventilated patients, regardless of pharmacologic interventions being used. This broad range of all infants admitted to the NICU allows validation of the tool for using the N-PASS as a standard assessment tool for assessing pain and sedation. The nursing assessment for medical intervention was consistent with the N-PASS scoring tool, which would allow improved quantification and documentation of their assessments.
CONCLUSIONS
Pain and sedation management continue to be a difficult balance in infants. Our research shows that the N-PASS is a more clinically useful tool for the assessment of pain compared to the currently used NIPS for our patient population and provides an objective assessment of sedation that correlates with the clinical subjective assessment that may influence therapy. This is a valuable tool for all infants admitted to the NICU, not only those requiring narcotic drips. Further evaluation of the N-PASS to assess its impact on guiding therapy is needed, especially among preterm infants.
Acknowledgments
We thank the NICU staff nurses at Johns Hopkins Hospital for their cooperation in this research. We also thank Christoph Lehmann, MD, for informatics support and Lauren Jansson, MD, and W Christopher Golden, MD, for their support as members of the B. Hillman’s scholarship oversight committee. Kathryn A Carson’s work on this publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1 TR 000424-06 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS or NIH. None of the authors have financial relationships relevant to this article to disclose.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Anand KJ. Pharmacological approaches to the management of pain in the neonatal intensive care unit. J Perinatol. 2007;27(Suppl 1):S4–S11. doi: 10.1038/sj.jp.7211712. [DOI] [PubMed] [Google Scholar]
- 2.Anand KJ. Pain assessment in preterm neonates. Pediatrics. 2007;119:605–607. doi: 10.1542/peds.2006-2723. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJ, Aranda JV, Berde CB, Buckman S, Capparelli EV, Carlo W, et al. Summary proceedings from the neonatal pain-control group. Pediatrics. 2006;117:S9–S22. doi: 10.1542/peds.2005-0620C. [DOI] [PubMed] [Google Scholar]
- 4.Anand KJ. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155:173–180. doi: 10.1001/archpedi.155.2.173. [DOI] [PubMed] [Google Scholar]
- 5.Batton DG, Barrington KJ, Wallman C. Prevention and management of pain in the neonate: an update. Pediatrics. 2006;118:2231–2241. doi: 10.1542/peds.2006-2277. [DOI] [PubMed] [Google Scholar]
- 6.Taylor BJ, Robbins JM, Gold JI, Logsdon TR, Bird TM, Anand KJ. Assessing postoperative pain in neonates: a multicenter observational study. Pediatrics. 2006;118:e992–1000. doi: 10.1542/peds.2005-3203. [DOI] [PubMed] [Google Scholar]
- 7.Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. J Perinatol. 2008;28:55–60. doi: 10.1038/sj.jp.7211861. [DOI] [PubMed] [Google Scholar]
- 8.Hummel P, Lawlor-Klean P, Weiss MG. Validity and reliability of the N-PASS assessment tool with acute pain. J Perinatol. 2010;30:474–478. doi: 10.1038/jp.2009.185. [DOI] [PubMed] [Google Scholar]
- 9.Johansson M, Kokinsky E. The COMFORT behavioural scale and the modified FLACC scale in paediatric intensive care. Nurs Crit Care. 2009;14:122–130. doi: 10.1111/j.1478-5153.2009.00323.x. [DOI] [PubMed] [Google Scholar]
- 10.Ballantyne M, Stevens B, McAllister M, Dionne K, Jack A. Validation of the premature infant pain profile in the clinical setting. Clin J Pain. 1999;15:297–303. doi: 10.1097/00002508-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Netw. 1993;12:59–66. [PubMed] [Google Scholar]
- 12.Krechel SW, Bildner J. CRIES: a new neonatal postoperative pain measurement score. Initial testing of validity and reliability. Paediatr Anaesth. 1995;5:53–61. doi: 10.1111/j.1460-9592.1995.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 13.Debillon T, Zupan V, Ravault N, Magny JF, Dehan M. Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2001;85:F36–F41. doi: 10.1136/fn.85.1.F36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens B, Johnston C, Taddio A, Gibbins S, Yamada J. The premature infant pain profile: evaluation 13 years after development. Clin J Pain. 2010;26:813–830. doi: 10.1097/AJP.0b013e3181ed1070. [DOI] [PubMed] [Google Scholar]
- 15.Pokela ML. Pain relief can reduce hypoxemia in distressed neonates during routine treatment procedures. Pediatrics. 1994;93:379–383. [PubMed] [Google Scholar]
- 16.Anand KJ, Barton BA, McIntosh N, Lagercrantz H, Pelausa E, Young TE, et al. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Arch Pediatr Adolesc Med. 1999;153:331–338. doi: 10.1001/archpedi.153.4.331. [DOI] [PubMed] [Google Scholar]
- 17.Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–1682. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 18.Simons SH, van Dijk M, van Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA. 2003;290:2419–2427. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- 19.Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst Rev. 2000:CD002052. doi: 10.1002/14651858.CD002052.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Hummel P, van Dijk M. Pain assessment: current status and challenges. Semin Fetal Neonatal Med. 2006;11:237–245. doi: 10.1016/j.siny.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Johnston CC, Stevens BJ, Franck LS, Jack A, Stremler R, Platt R. Factors explaining lack of response to heel stick in preterm newborns. J Obstet Gynecol Neonatal Nurs. 1999;28:587–594. doi: 10.1111/j.1552-6909.1999.tb02167.x. [DOI] [PubMed] [Google Scholar]