Abstract
Objective
Imbalanced cytokine production by T cells characterizes both patients with SLE and lupus-prone mice and contributes to immune dysregulation. In this study, we further characterize in detail the production of IL-2, IFNγ, IL-4 and IL-17A by CD4+ subsets in healthy subjects and SLE patients and the signaling response of CD4+ T cells in response to exogenous IL-2.
Methods
Cytokine production by CD4+ T cell differentiated subsets was assessed by intracellular staining following stimulation with phorbol myristate acetate and ionomycin and by ELISA after anti-CD3/anti-CD28 stimulation. IL-2 signaling pathway was examined by assessing Jak3 and STAT5 phosphorylation. Cell proliferation in response to IL-2 was examined by CFSE dilution.
Results
Production of IL-2 was defective primarily among naïve CD4+ T cell, whereas the production of IFNγ, IL-4 and IL-17A was not significantly different among SLE patients and healthy subjects. Jak3 and STAT5 phosphorylation and proliferation of SLE CD4+ T cells in response to exogenous IL-2 were impaired compared to that of healthy subjects.
Conclusion
These data suggest that altered IL-2 production, as well as impaired IL-2-mediated signaling and proliferative responses characterize SLE CD4+ T cells. Our data suggest caution in designing IL-2 treatment trials for patients with SLE. Approaches to restore CD4+ T cell sensitivity to IL-2 should be considered along the way.
Keywords: SLE, Interleukine-2, CD4+ T lymphocyte, Autoimmunity
T cells are key players in the pathogenesis of systemic lupus erythematosus (SLE). A defect in IL-2 production by T cells is a hallmark of both murine lupus and human SLE [1, 2]. IL-2 is an important cytokine involved in normal T cell activation and critical for the control of the peripheral immune responses by promoting activation-induced cell death, a process required for deletion of autoreactive cells, and by favoring differentiation, survival and function of regulatory T cells [3, 4]. More importantly, IL-2 plays a significant role in cell-mediated immunity against infections, one of the leading causes of morbidity and mortality among SLE patients [3].
IL-2 signals through the IL-2 receptor (IL-2R) which is composed of three chains – the CD25 or IL-2Rα, the CD122 or IL-2Rβ, and the CD132 or IL-2R common γ-chain, the latter is an essential component of the receptors for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21. Binding of IL-2 to its receptor leads to the activation of the Janus family tyrosine kinases 1 and 3 (Jak1 and Jak3), followed by the phosphorylation of the STAT5A/B transcription factor. Once phosphorylated, STAT5 translocates to the nucleus and binds to IL-2 target genes, which are mainly involved in T cell growth, proliferation and differentiation [5].
Low-dose IL-2 treatment is being considered as a therapeutic approach in patients with SLE and other autoimmune diseases [6–9]. Notwithstanding the impressive response rates in these open label trials, it is important to understand the response behavior of T cell subsets when exposed to IL-2 [10, 11]. In this communication, we report cytokine production patterns by subsets of CD4+ T cells isolated from the peripheral blood of SLE patients and healthy subjects. CD4+ T cells from SLE patients produce significantly less IL-2 than CD4+ T cells isolated from healthy subjects. This defect is more pronounced in the naïve CD4+ T cell subset, compared to more differentiated subsets (central memory or effector memory CD4+ T cells). Moreover, CD4+ T cells from SLE patients responded poorly to recombinant IL-2 as manifested by decreased phosphorylation of Jak3 and STAT5 and impaired proliferation compared to healthy controls. Our data alert the possibility of poor responses of SLE patients to IL-2 treatment because of the compromised IL-2 > IL-2 receptor > Jak1/3 > STAT5 pathway.
Methods
Human SLE and control cells
All patients with SLE (n=36) included in this study were diagnosed according to the American College of Rheumatology classification criteria [12]. Patients with SLE were recruited from the Division of Rheumatology at Beth Israel Medical Center and provided consent, as approved by the Institutional Review Board. Age-, sex-, and ethnicity-matched healthy individuals were chosen as controls (n=37). Disease activity score for the patients with SLE was measured using the SLEDAI scoring system. Informed consent was obtained and approved by the Institutional Review Board after the nature and possible consequences of the studies were explained, in compliance with the Helsinki Declaration.
Cell isolation
Peripheral blood was collected in heparin-lithium tubes from the study subjects. Peripheral blood mononuclear cells (PBMCs) were enriched by density gradient centrifugation (Lymphocyte Separation Medium, Corning Life Sciences). PBMCs were either used directly or cryopreserved in liquid nitrogen for future experiments. Total CD4+ T cells, naïve CD4+ T cells and memory CD4+ T cells were isolated using CD4+, naïve CD4+ or memory CD4+ T Cell Isolation Kit II (Miltenyi Biotec), respectively, according to the manufacturer’s instruction.
Assessment of CD4+ T cell cytokine production
PBMC (1×106 cells) were cultured in complete culture medium [RPMI (Corning) supplemented with 10% fetal bovine serum, 100mg/ml streptomycin and 100U/ml penicillin] and were stimulated with phorbol 12-myristate 13-acetate (PMA 25 ng/ml) and ionomycin (0.5 µg/ml) for 6 hours in the presence of Brefeldin A (GolgiPlug, BD). At the end of stimulation, cells were washed, stained for dead cells using the Zombie Aqua Fixable Viability Kit (20 min, 4°C) (Biolegend) and then labeled with the following antibodies: CD3 Brilliant Violet Horizon 395, CD4 PerCP eFluor 710, CD8 PerCP, CD45RA APC Cy7 and CCR7 Alexa Fluor 488 (30 min, 4°C). Subsequently, cells were permeabilized (20 min, 4°C)(Cytofix/Cytoperm, BD), washed twice (Perm/Wash; BD) and stained with IL-2 PE Cy7, IFNγ Pacific Blue, IL-17A Alexa Fluor 647 and IL-4 PE (30 min, 4°C). Antibodies were purchased from BD Bioscience, Biolegend and eBioscience.
Assessment of STAT5 phosphorylation
CD4+ T cells (0.2×106 cells) were stimulated in complete medium in 96-well plates with recombinant IL-2 (Peprotech) for 0 to 180 minutes. At the end of stimulation, cells were washed, stained for dead cells using the Zombie Aqua Fixable Viability Kit (20 min, 4°C) (Biolegend), washed, permeabilized and stained with CD45RA APC (Biolegend) and phospho-STAT5 Alexa Fluor 488 (Cell Signaling Technologies) using the Phospho-Epitopes Exposure kit (PerFix EXPOSE, Beckman Coulter) and following the manufacturer’s instructions.
Cell proliferation
Carboxyfluorescein succinimidyl ester (CFSE)-labeled naïve CD4+ T cells (0.2×106) were stimulated for 6 days in complete media in 96-well plates pre-coated (overnight) with anti-CD3 (0.5 µg/ml), anti-CD28 (5 µg/ml) with or without recombinant IL-2 (50 IU/ml) (Peprotech). At the end of the stimulation, the cells were stained for dead cells using the Zombie Aqua Fixable Viability Kit (20 min, 4°C). The CFSE dilution was assessed by flow cytometry.
Flow Cytometry
Data were acquired on a LSR II SORP (5 lasers 355, 405, 488, 561, 640 nm; BD Bioscience).
IL-2 ELISA
Total, naïve or memory CD4+ T cells were isolated and stimulated as described above. After 18h, IL-2 was measured in supernatants by ELISA (Biolegend).
Western blotting
Cells were lysed in RIPA buffer supplemented with phosphatase and protease inhibitors (Roche). Proteins were separated in 4 to 12% gradient Bis-Tris gels (Life Technologies) and transferred on PVDF (Millipore) membrane. Membranes were blocked for 1h with Tris-buffered saline solution containing 0.05% Tween and 5% non-fat dry milk. Membranes were incubated overnight at 4°C with the indicated antibody followed by incubation with an HRP-conjugated antibody. Detection was performed with the Clarity ECL Western Blotting reagents (Biorad) and visualization with the ChemiDoc XRS+ Molecular Imager (Biorad). Densitometric analysis was performed using the ImageJ software.
Statistics
Statistical analysis was performed using Student’s t-test, corrected with the Holm-Sidak method. For multiple comparisons, statistical analysis was performed using one-way Anova, followed by post hoc analysis with Tukey's test. Statistical comparison of cytokine producing CD4+ T cell subset was performed using χ2 tests in SPICE (version 5.35) [13]. Statistical analyses and illustrations were performed using FlowJo (version X), SPICE (version 5.35) and GraphPad Prism (version 6).
Results
IL-2 producing CD4+ T cells are reduced in SLE patients compared to healthy subjects
Circulating differentiated subsets of T cells were examined by assessing the expression of the chemokine receptor CCR7 and CD45RA. This allows to distinguish three subsets of CD4+ T cells: naïve (CD45RA+CCR7+), central memory (CM, CD45RA−CCR7+) and effector memory (EM, CD45RA−CCR7−) [14].
We examined the production of IL-2, IL-4, IL-17A and IFNγ by the aforementioned subsets of CD4+ T cells following PMA and ionomycin stimulation. The frequency of cytokine producing cells increased over differentiation status, as IFNγ, IL-2 and IL-4 production augmented over differentiation from naïve to CM and to EM (figure 1A). Production of IL-17A remained low in this experimental setting, as cells were not polarized toward Th17 differentiation before PMA-ionomycin stimulation.
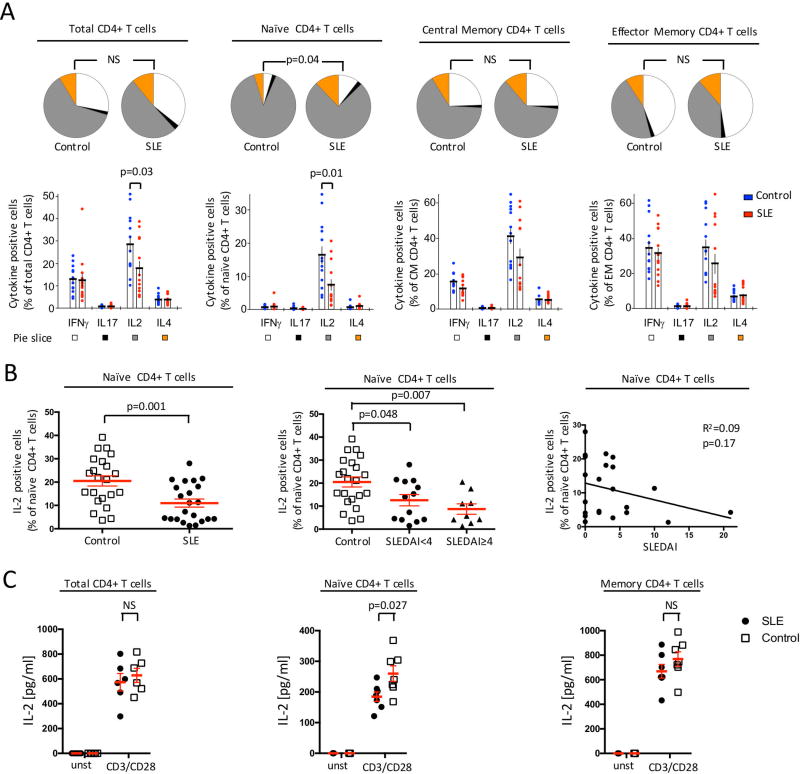
Figure 1. Cytokine production by CD4+ T cell-differentiated memory subsets from SLE and controls.
PBMC from SLE patients (n=13) or healthy subjects (n=13) were stimulated with PMA and ionomycin. Expression of IFNγ, IL-2, IL-4 and IL-17A by total CD4+ T cells, naïve CD4+ T cells, central memory (CM) CD4+ T cells and effector memory (EM) CD4+ T cells was measured by flow cytometry. (A) Frequency of cytokine producing CD4+ T cells and their differentiation status are depicted as pie and dot plots. (B) Frequency of IL-2 producing naïve CD4+ T cells was evaluated in accordance to the SLE disease activity score (SLEDAI). Left panel: control (n=22) vs. SLE (n=22). Middle panel: control (n=22), inactive SLE (SLEDAI<4; n=13) and active SLE (SLEDAI≥4; n=9). Right panel: Linear regression of IL-2 production by naïve CD4+ T cells vs. SLEDAI score (n=22). (C) IL-2 production from sorted total CD4+ T cells, naive CD4+ T cells and memory CD4+ T cells was assessed by ELISA in cell culture supernatant after 18h of stimulation (mean ± SEM).
When we compared SLE to healthy controls, no difference was observed in the frequency of IL-4, IL-17A and IFNγ producing CD4+ T cells (figure 1A). On the opposite, the percentage of IL-2 producing CD4+ T cells was reduced among SLE patients. This decrease was observed in all subsets (naïve, CM, EM), but was only statistically significant in the naïve CD4+ T cell population (figure 1A). We were not able to establish any correlation between the frequency of IL-2 producing cells and SLE disease activity score (SLEDAI) (figure 1B), suggesting that compromised IL-2 by SLE naïve CD4+ T cells is a hallmark of the disease that is not affected by its activity.
To confirm these results, we isolated total CD4+ T cells, naïve CD4+ T cells (CD4+CD45RA+) and memory CD4+ T cells (CD4+CD45RA−) from SLE patients and healthy controls. Cells were stimulated for 18h with anti-CD3 and anti-CD28 monoclonal antibodies. Production of IL-2 was assessed by ELISA in the supernatant of the cell culture. We observed a decrease in IL-2 production from all the tested subsets (figure 1C). The defect was more pronounced when we examined the naïve CD4+ T cell population compared to the memory CD4+ T cells (figure 1C).
CD4+ T cells from SLE patients display an impaired response to exogenous IL-2
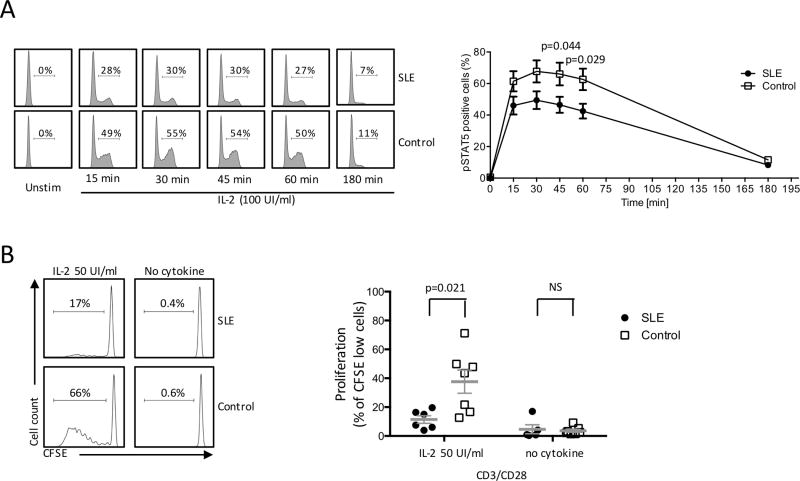
After showing that IL-2 production is compromised in SLE CD4+ T cells, we examined the response of SLE CD4+ T cells to exogenous IL-2. To explore this aspect, we studied the IL-2 signaling pathway in SLE CD4+ T cells by measuring STAT5 phosphorylation in response to IL-2. CD4+ T cells from SLE and controls were isolated and stimulated with recombinant IL-2. The IL-2/STAT5 pathway was examined by assessing the frequency of phospho-STAT5 (pSTAT5) positive cells over time. pSTAT5 expression in response to exogenous IL-2 increased over time and reached maximum levels after 30 minutes of cell stimulation and was almost completely abrogated after 3h of stimulation (figure 2A). Following stimulation with IL-2, the percentage of pSTAT5+ CD4+ T cells from patients with SLE was lower compared to healthy controls. The difference was statistically significant when pSTAT5 was assessed at 30 and 45min of stimulation (figure 2A). Differences in STAT5 phosphorylation between SLE and controls is not a result of a diminished IL-2R expression on SLE CD4+ T cells. As we have previously shown, CD25 and CD122 expression is similar in SLE and controls [15]. In summary, these data emphasize that the phosphorylation of STAT5 upon IL-2 stimulation is defective in CD4+ T cells isolated from SLE patients.
Figure 2. SLE CD4+ T cell response is impaired in SLE patients compared to healthy subjects.
CD4+ T cells from SLE and healthy controls were stimulated with IL-2. (A) pSTAT5 was assessed by flow cytometry at mentioned time points. Left panel: representative flow plot. Right panel: cumulative data (SLE n=8 and controls n=8). (B) CD4+ T cells isolated from SLE patients or controls were stimulated with anti-CD3/anti-CD28 with or without recombinant IL-2. Proliferation was expressed as CFSE-low cells. Left panel: representative flow plot. Right panel: cumulative data (SLE n=6 and controls n=7) (mean ± SEM).
Proliferation of SLE CD4+ T in response to exogenous IL-2 is compromised
Because SLE CD4+ T cells display a defective response to exogenous IL-2 in terms of STAT5 phosphorylation, we examined the proliferation of CD4+ T cells in response to anti-CD3/anti-CD28 stimulation with or without exogenous IL-2. CD4+ T cells were labeled with CFSE and proliferation was expressed as the percentage of CFSE-low cells after 6 days of stimulation. The percentage of proliferating CD4+ T cells from SLE patients was significantly reduced compared to healthy controls in response to exogenous IL-2 (figure 2B).
SLE T cells display reduced Jak3 phosphorylation in response to IL-2 administration
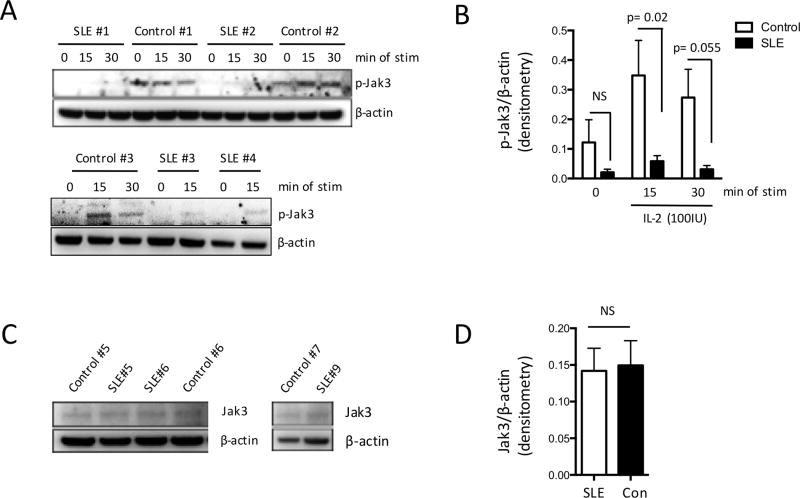
As shown above, SLE CD4+ T cells demonstrate decreased STAT5 phosphorylation and reduced proliferative responses following stimulation with exogenous IL-2. We sought to further delineate the events that precede STAT5 activation by assessing phosphorylation of Jak3 upon stimulation with IL-2. To this end, we isolated T cells from the peripheral blood of healthy subjects and patients with SLE and either kept them unstimulated or stimulated them with recombinant IL-2 for 15 and 30 min. Jak3 phosphorylation was examined by Western immunoblotting. Highest levels of Jak3 phosphorylation were seen after 15min of stimulation. When we treated SLE T cells with recombinant IL-2, phosphorylation of Jak3 was significantly lower compared to healthy controls (figure 3A and B). Reduced Jak3 phosphorylation was not due to diminished total Jak3 levels, as we observed no statistically significant differences in the levels of total Jak3 among SLE patients and healthy controls (figure 3C and D).
Figure 3. Impaired Jak3 phosphorylation of SLE T cells in response to IL-2 stimulation.
T cells from patients with SLE (n=5) and healthy controls (n=5) were treated with IL-2 (100IU/ml) for 15 or 30 min or were left untreated. Levels of phosphorylated Jak3 (pJak3) were examined by immunoblotting. Representative experiment is shown in (A) and cumulative results in (B). (C) Whole cell lysates were extracted from patients with SLE (n=17) and healthy individuals (n=8) and levels of Jak3 were examined with western immunoblotting. Cumulative results are shown in (D). Results are expressed as the densitometric ratio of pJak3 or Jak3 density over β-actin (mean ± SEM).
Altogether, these data suggest that SLE CD4+ T cells, and especially naïve CD4+ T cells, not only display decreased IL-2 production following stimulation, but also demonstrate impaired IL-2 signaling and response.
Conclusion
Aberrant cytokine production is a hallmark of SLE immune dysregulation. Compromised IL-2 production has been consistently described in SLE. In this study, we confirmed that IL-2 production is impaired in T cells from SLE patients, yet in our cohort, we did not observe any significant differences in the production of IL-4, IL-17A and IFNγ by CD4+ T cells.
We further characterized this abnormality by examining IL-2 production by CD4+ T cells and CD4+ T cell-differentiated subsets. Our data emphasize that IL-2 production is reduced in each differentiated subset of CD4+ T cells and that the defect was more pronounced in the naïve compartment. More importantly, this abnormality is associated with a defect in IL-2 signaling, as illustrated by reduced Jak3 phosphorylation, leading to decreased phosphorylation of STAT5, a downstream transcription factor of the IL-2 signaling pathway. At a functional level, we also observed a decreased CD4+ T cell proliferation in response to recombinant IL-2. Moreover, compromised IL-2 production is barely affected by disease activity, suggesting that the IL-2 defect is a primary defect in SLE, as it is already apparent in naïve CD4+ T cells population.
Recent reports from uncontrolled clinical trials emphasized that low-dose IL-2 administration could be beneficial in SLE [7–9]. In this context, our results suggest that strategy aiming at restoring IL-2 sensitivity of the CD4+ T cells, for example through increasing the expression of the IL-2R on CD4+T cell surface [15], should be considered in conjunction with low dose IL-2 treatment, because CD4+ T cells not only display decreased IL-2 production but also a defective response to IL-2.
Acknowledgments
This work was supported by National Institutes of Health grant PO1 AI065687, RO1 AI42269 and R37 AI49954. This work was also supported by a SICPA foundation grant (to D.C.).
References
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Comte D, Karampetsou MP, Tsokos GC. T cells as a therapeutic target in SLE. Lupus. 2015;24(4–5):351–63. doi: 10.1177/0961203314556139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann MF, Oxenius A. Interleukin 2: from immunostimulation to immunoregulation and back again. EMBO Rep. 2007;8(12):1142–8. doi: 10.1038/sj.embor.7401099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malek TR, et al. IL-2 family of cytokines in T regulatory cell development and homeostasis. J Clin Immunol. 2008;28(6):635–9. doi: 10.1007/s10875-008-9235-y. [DOI] [PubMed] [Google Scholar]
- 5.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38(1):13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieberman LA, Tsokos GC. The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. J Biomed Biotechnol. 2010;2010:740619. doi: 10.1155/2010/740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22(9):991–3. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
- 8.Humrich JY, et al. Rapid induction of clinical remission by low-dose interleukin-2 in a patient with refractory SLE. Ann Rheum Dis. 2015;74(4):791–2. doi: 10.1136/annrheumdis-2014-206506. [DOI] [PubMed] [Google Scholar]
- 9.von Spee-Mayer C, et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2015-207776. [DOI] [PubMed] [Google Scholar]
- 10.Ohl K, Tenbrock K. Inflammatory cytokines in systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:432595. doi: 10.1155/2011/432595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apostolidis SA, et al. The dysregulation of cytokine networks in systemic lupus erythematosus. J Interferon Cytokine Res. 2011;31(10):769–79. doi: 10.1089/jir.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan EM, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 13.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79(2):167–74. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallusto F, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 15.Comte D, et al. Engagement of SLAMF3 enhances CD4+ T-cell sensitivity to IL-2 and favors regulatory T-cell polarization in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1605081113. [DOI] [PMC free article] [PubMed] [Google Scholar]